Co-Adsorption of Formic Acid and Hexane Selenol on Cu
Abstract
1. Introduction
2. Materials and Methods
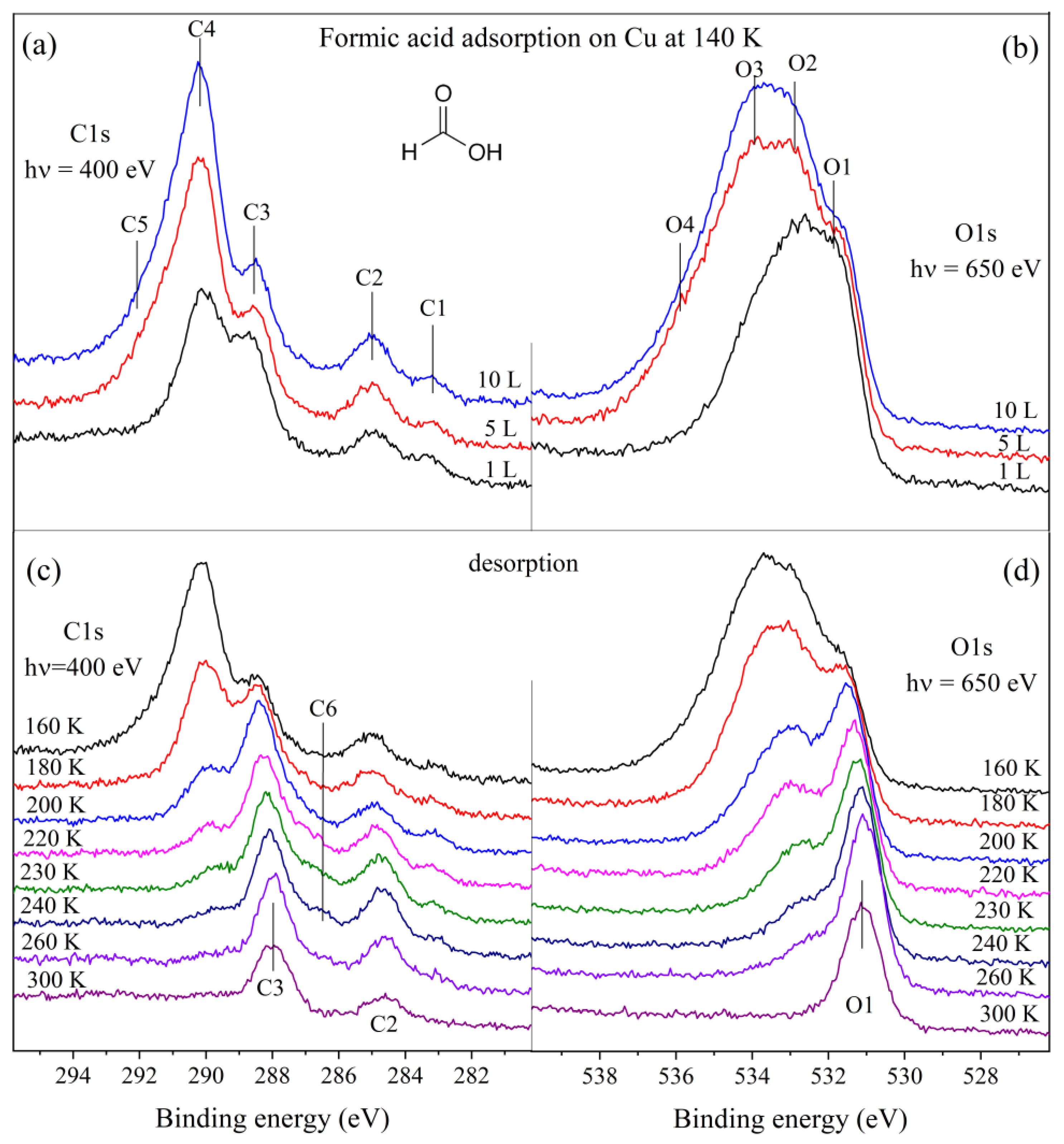
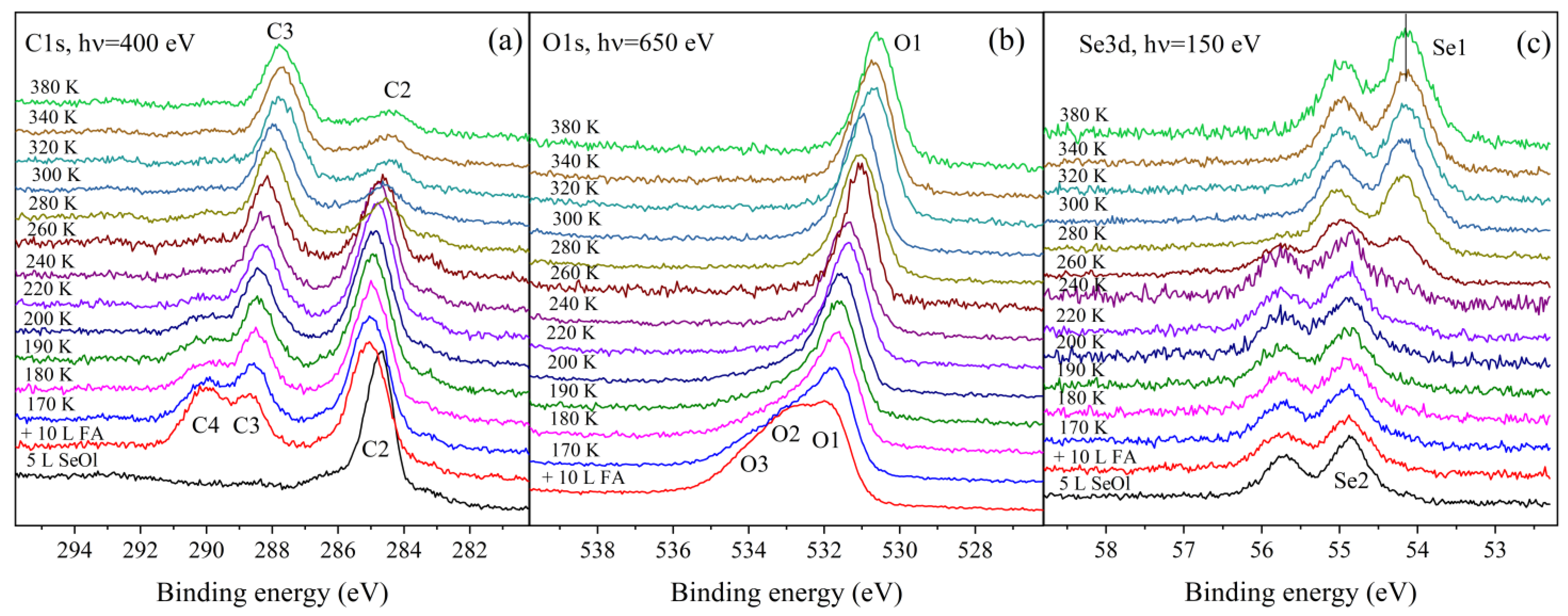
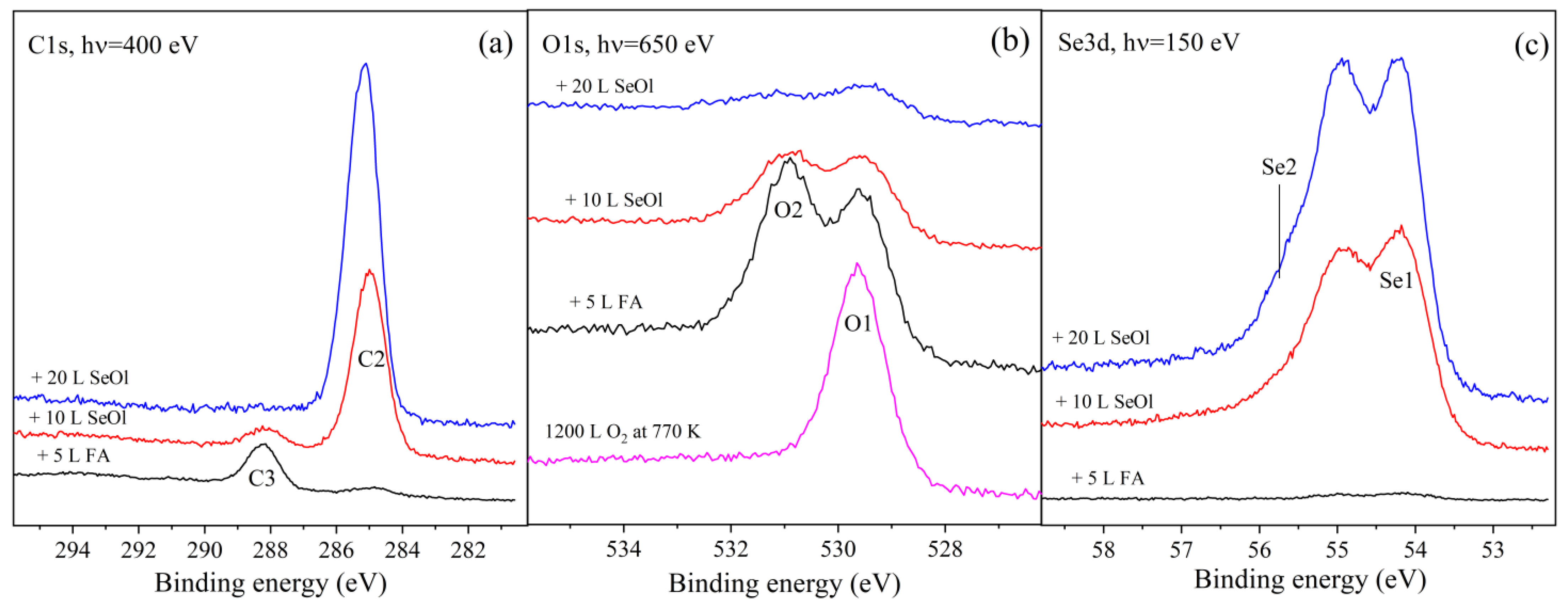
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| SAM | Self-assembled monolayer |
| XPS | X-ray photoelectron spectroscopy |
| L | Langmuir |
| UHV | Ultra-high vacuum |
References
- Love, J.C.; Estroff, L.A.; Kriebel, J.K.; Nuzzo, R.G.; Whitesides, G.M. Self-Assembled Monolayers of Thiolates on Metals as a Form of Nanotechnology. Chem. Rev. 2005, 105, 1103–1170. [Google Scholar] [CrossRef]
- Schreiber, F. Structure and growth of self-assembling monolayers. Prog. Surf. Sci. 2000, 65, 151–257. [Google Scholar] [CrossRef]
- Ulman, A. Formation and Structure of Self-Assembled Monolayers. Chem. Rev. 1996, 96, 1533–1554. [Google Scholar] [CrossRef] [PubMed]
- Hosseinpour, S.; Hedberg, J.; Baldelli, S.; Leygraf, C.; Johnson, M. Initial oxidation of alkanethiol-covered copper studied by vibrational sum frequency spectroscopy. J. Phys. Chem. C 2011, 115, 23871–23879. [Google Scholar] [CrossRef]
- Hosseinpour, S.; Schwind, M.; Kasemo, B.; Leygraf, C.; Johnson, M. Integration of Quartz Crystal Microbalance with Vibrational Sum Frequency Spectroscopy−Quantification of the initial Oxidation of Alkanethiol-Covered Copper. J. Phys. Chem. C 2012, 116, 24549–24557. [Google Scholar] [CrossRef]
- Hosseinpour, S.; Johnson, C.M.; Leygraf, C. Alkanethiols as inhibitors for the atmospheric corrosion of copper induced by formic acid: Effect of chain length. J. Electrochem. Soc. 2013, 160, C270–C276. [Google Scholar] [CrossRef]
- Hosseinpour, S.; Göthelid, M.; Leygraf, C.; Johnson, C.M. Self-Assembled Monolayers as Inhibitors for the Atmospheric Corrosion of Copper Induced by Formic Acid: A Comparison between Hexanethiol and Hexaneselenol. J. Electrochem. Soc. 2014, 161, C50–C56. [Google Scholar] [CrossRef]
- Zhao, W.; Göthelid, M.; Hosseinpour, S.; Johansson, M.; Li, G.; Leygraf, C.; Johnsson, M. The nature of self-assembled octadecylphosphonic acid (ODPA) layers on copper substrates. J. Colloid Interface Sci. 2021, 581, 816–825. [Google Scholar] [CrossRef]
- Martinovic, I.; Zlatic, G.; Pilic, Z.; Susie, L.; Kowalska, O.; Petrovic, D.; Falak, F.; Miskovic, J. Self-Assembled Monolayers of Alkanethiol as Inhibitors against Copper Corrosion in Synthetic Acid Rain. Int. J. Electrochem. Sci. 2019, 14, 4206–4215. [Google Scholar] [CrossRef]
- Feng, L.; Zheng, S.; Zhu, H.; Ma, X.; Hu, Z. Detection of corrosion inhibition by dithiane self-assembled monolayers (SAMs) on copper. J. Taiwan Inst. Chem. Eng. 2023, 142, 104610. [Google Scholar] [CrossRef]
- Mendoza, A.R.; Corvo, F. Outdoor and indoor atmospheric corrosion of non-ferrous metals. Corros. Sci. 2000, 42, 1123–1147. [Google Scholar] [CrossRef]
- Bastidas, J.M.; López-Delgado, A.; Cano, E.; Polo, J.L.; López, F.A. Copper Corrosion Mechanism in the Presence of Formic Acid Vapor for Short Exposure Times. J. Electrochem. Soc. 2000, 147, 999. [Google Scholar] [CrossRef]
- Notoya, T. Localized corrosion in copper tubes and the effect of anti-tarnishing pretreatment. J. Mater. Sci. Lett. 1991, 10, 389–391. [Google Scholar] [CrossRef]
- Bastidas, D.M.; Cayuela, I.; Bastidas, J.M. Ant-nest corrosion of copper tubing in air-conditioning units. Rev. Metal. 2006, 42, 367–381. [Google Scholar] [CrossRef]
- Li, H.; Liu, X.; Li, D.; Wang, Z.; Zhang, X.; Wang, S. An Investigation on the Mechanisms of Ant Nest Corrosion of Copper Tube in Formic Acid Environment. Mater. Corros. 2023, 74, 138–144. [Google Scholar] [CrossRef]
- de la Llave, E.; Scherlis, D.A. Selenium-Based Self-Assembled Monolayers: The Nature of Adsorbate−Surface Interactions. Langmuir 2010, 26, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Ossowski, J.; Wächter, T.; Silies, L.; Kind, M.; Noworolska, A.; Blobner, F.; Gnatek, D.; Rysz, J.; Bolte, M.; Feulner, P.; et al. Thiolate vs. selenolate: Structure, stability and charge transfer properties. ACS Nano 2015, 9, 4508–4526. [Google Scholar] [CrossRef]
- Tong, Y.; Jiang, T.; Bendounan, A.; Kotresh Harish, M.N.; Giglia, A.; Kubsky, S.; Sirotti, F.; Pasquali, L.; Sampath, S.; Esaulov, V.A. Case studies on the formation of chalcogenide self-assembled monolayers on surfaces and dissociative processes. Bielstein J. Nanotechnol. 2016, 7, 263–277. [Google Scholar] [CrossRef]
- Göthelid, M.; Hosseinpour, S.; Ahmadi, S.; Leygraf, C.; Johnson, C.M. Hexane selenol dissociation on Cu: The protective role of oxide and water. Appl. Surf. Sci. 2017, 423, 716–720. [Google Scholar] [CrossRef]
- Osada, W.; Tanaka, S.; Mukai, K.; Hyun Choi, Y.; Yoshinobu, J. Adsorption, desorption and decomposition of formic acid on Cu(977); the importance of facet of the step. J. Phys. Chem. C 2022, 126, 8354–8363. [Google Scholar] [CrossRef]
- Hohmann, L.; Dahlmann, F.; Braghin, G.B.; Laviron, L.; Hussein, L.; Martinez, J.; Harrer, A.; Robertson, H.; Guiborat, J.; Hu, X.; et al. Naphthalene Decomposition on Fe(110): Adsorption, Dehydrogenation, Surface Carbon Formation and the Influence of Coadsorbed Oxygen. J. Phys. Chem. C 2025, 129, 2441–2452. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Zaera, F. Adsorption and thermal chemistry of formic acid on clean and oxygen-predosed Cu(110) single crystal surfaces revisited. Surf. Sci. 2016, 646, 37–44. [Google Scholar] [CrossRef]
- Tillborg, H.; Nilsson, A.; Mårtensson, N. Shake-up and shake-off structures in core level photoemission spectra from adsorbates. J. Electron Spectrosc. Relat. Phenom. 1993, 62, 73–93. [Google Scholar] [CrossRef]
- Önsten, A.; Stoltz, D.; Palmgren, P.; Yu, S.; Göthelid, M.; Karlsson, U.O. Water adsorption on ZnO(0001): Transition from triangular reconstructions to hydroxyl termination. J. Phys. Chem. C 2010, 114, 11157–11161. [Google Scholar] [CrossRef]
- Tissot, H.; Halldin Stenlid, J.; Wang, C.; Panahi, M.; Kaya, S.; Brinck, T.; Sassa, Y.; Johansson, F.O.L.; Weissenrieder, J. Acetic acid conversion to ketene on Cu2O(100): Reaction mechanism deduced from experimental observations and theoretical computations. J. Catal. 2021, 402, 154–165. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Götelid, M.A.; Götelid, S.A.; Hosseinpour, S.; Leygraf, C.; Johnson, C.M. Co-Adsorption of Formic Acid and Hexane Selenol on Cu. Corros. Mater. Degrad. 2025, 6, 48. https://doi.org/10.3390/cmd6040048
Götelid MA, Götelid SA, Hosseinpour S, Leygraf C, Johnson CM. Co-Adsorption of Formic Acid and Hexane Selenol on Cu. Corrosion and Materials Degradation. 2025; 6(4):48. https://doi.org/10.3390/cmd6040048
Chicago/Turabian StyleGötelid, Mats Ahmadi, Sareh Ahmadi Götelid, Saman Hosseinpour, Christofer Leygraf, and C. Magnus Johnson. 2025. "Co-Adsorption of Formic Acid and Hexane Selenol on Cu" Corrosion and Materials Degradation 6, no. 4: 48. https://doi.org/10.3390/cmd6040048
APA StyleGötelid, M. A., Götelid, S. A., Hosseinpour, S., Leygraf, C., & Johnson, C. M. (2025). Co-Adsorption of Formic Acid and Hexane Selenol on Cu. Corrosion and Materials Degradation, 6(4), 48. https://doi.org/10.3390/cmd6040048







