High-Temperature Oxidation Behavior of an Additively Manufactured Alumina-Forming Austenitic Stainless Steel
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. High-Temperature Oxidation Test
2.2.2. Microstructural Characterization
3. Results and Discussion
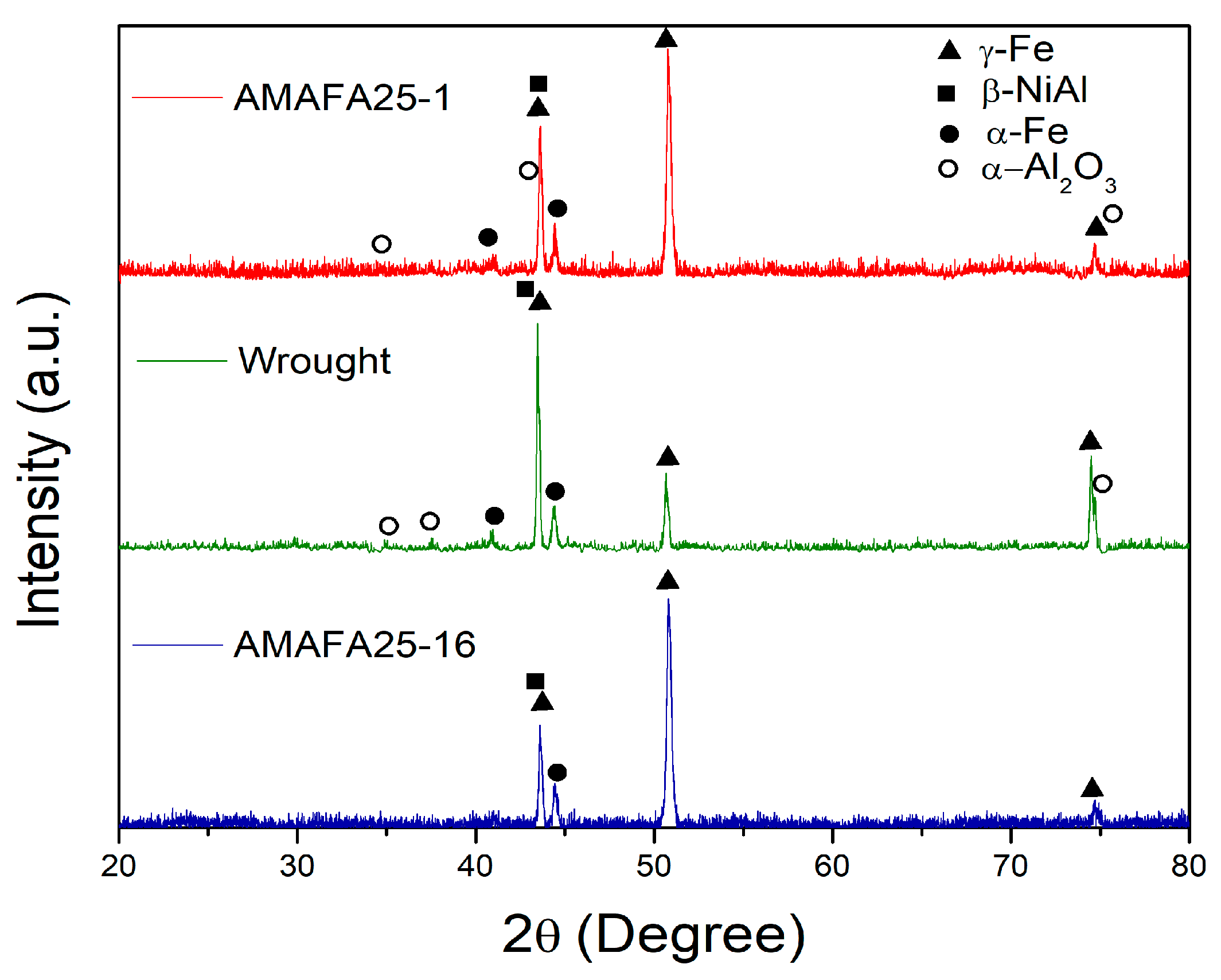
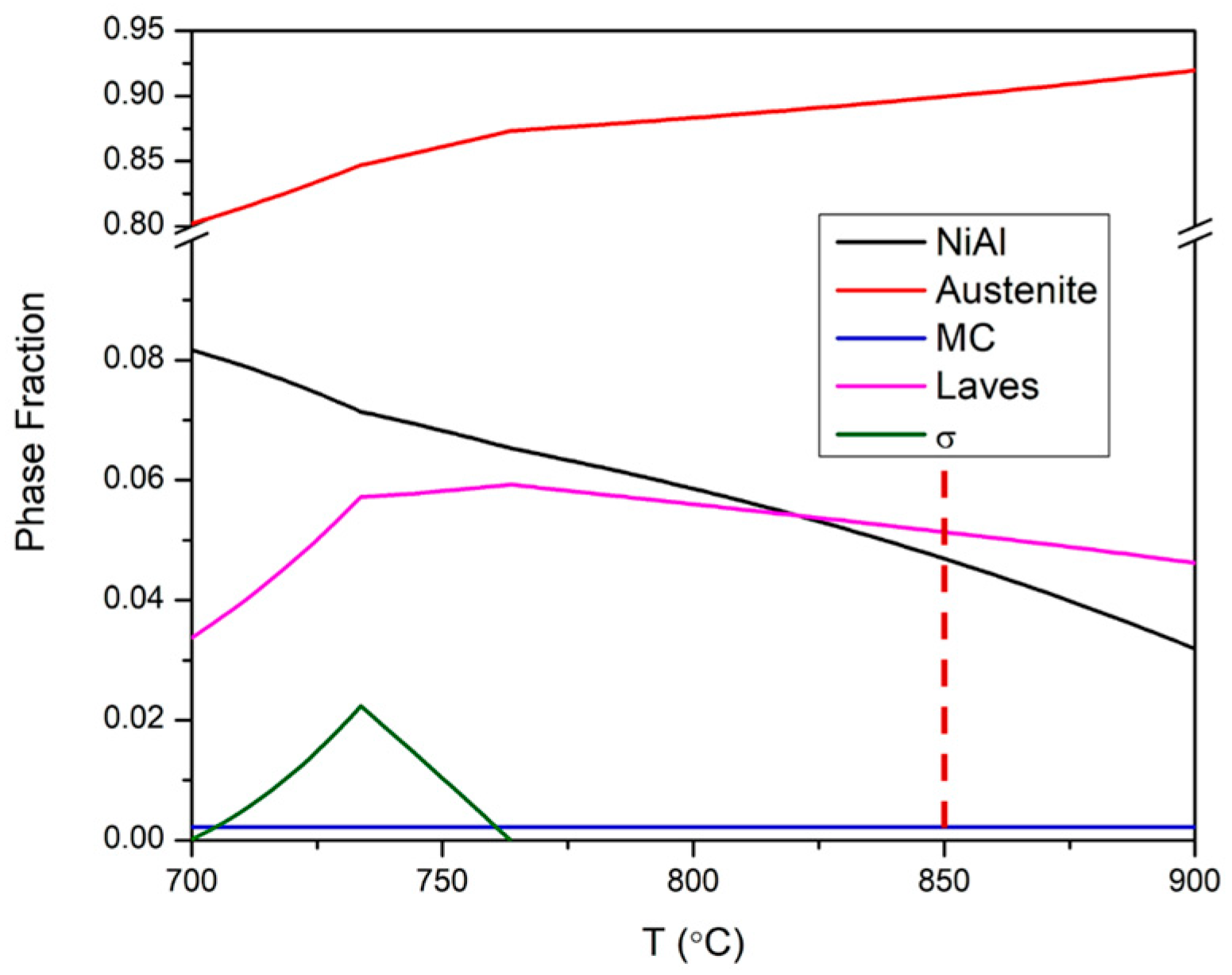
3.1. Pre-HTO Characterization
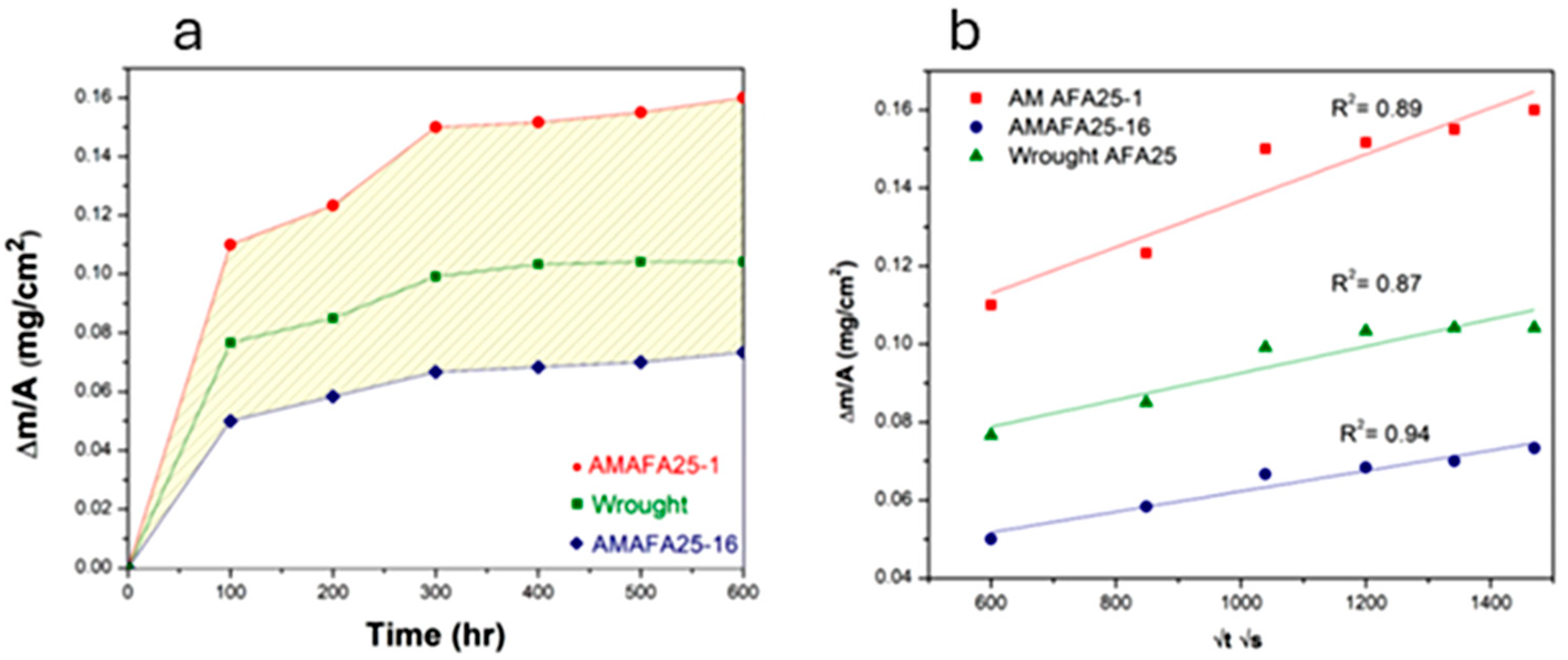
3.2. HTO Test and Oxidation Kinetics
3.3. Post-HTO Characterization
3.3.1. Surface Morphology
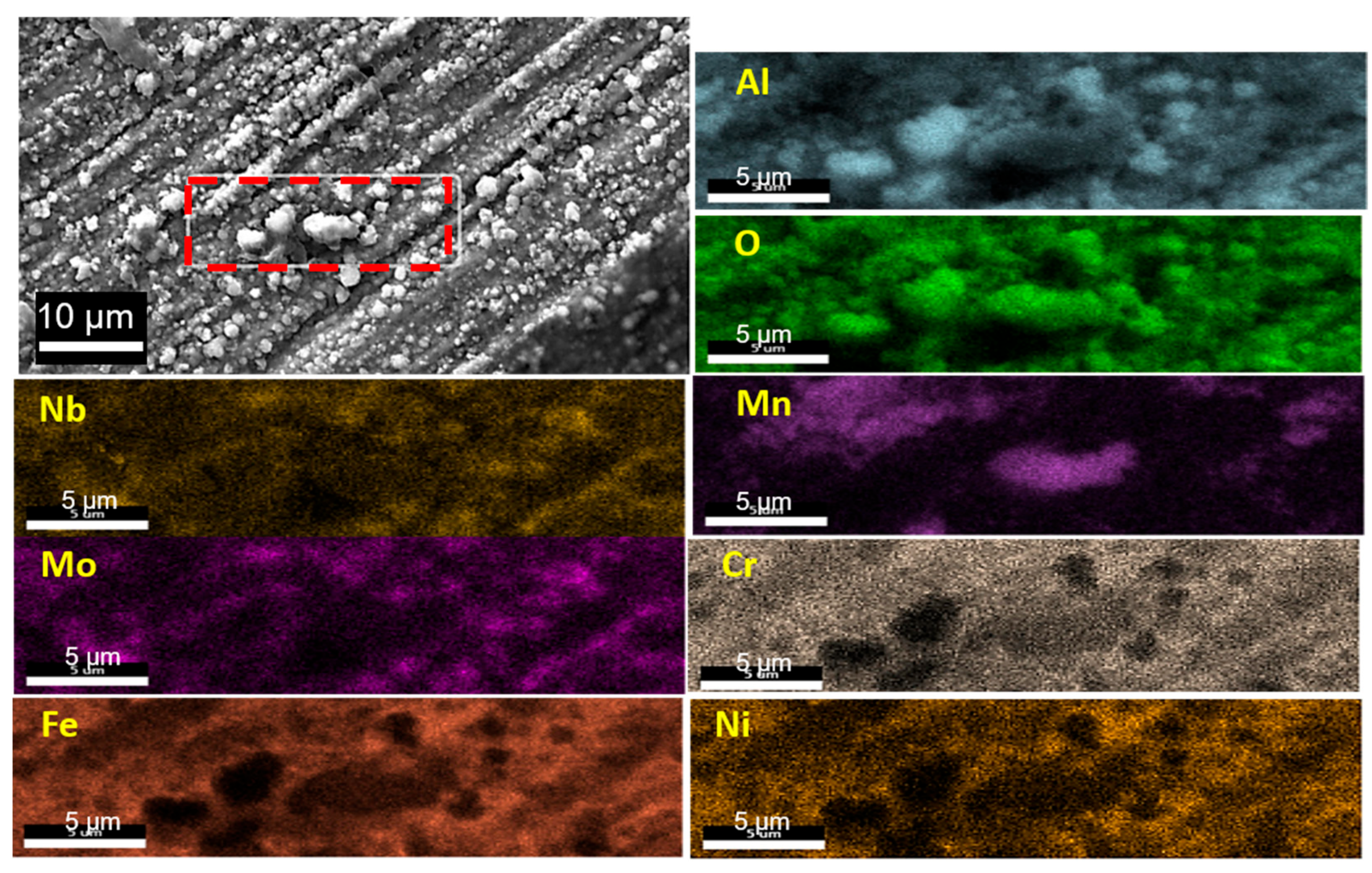
3.3.2. Identification of Oxide Phases
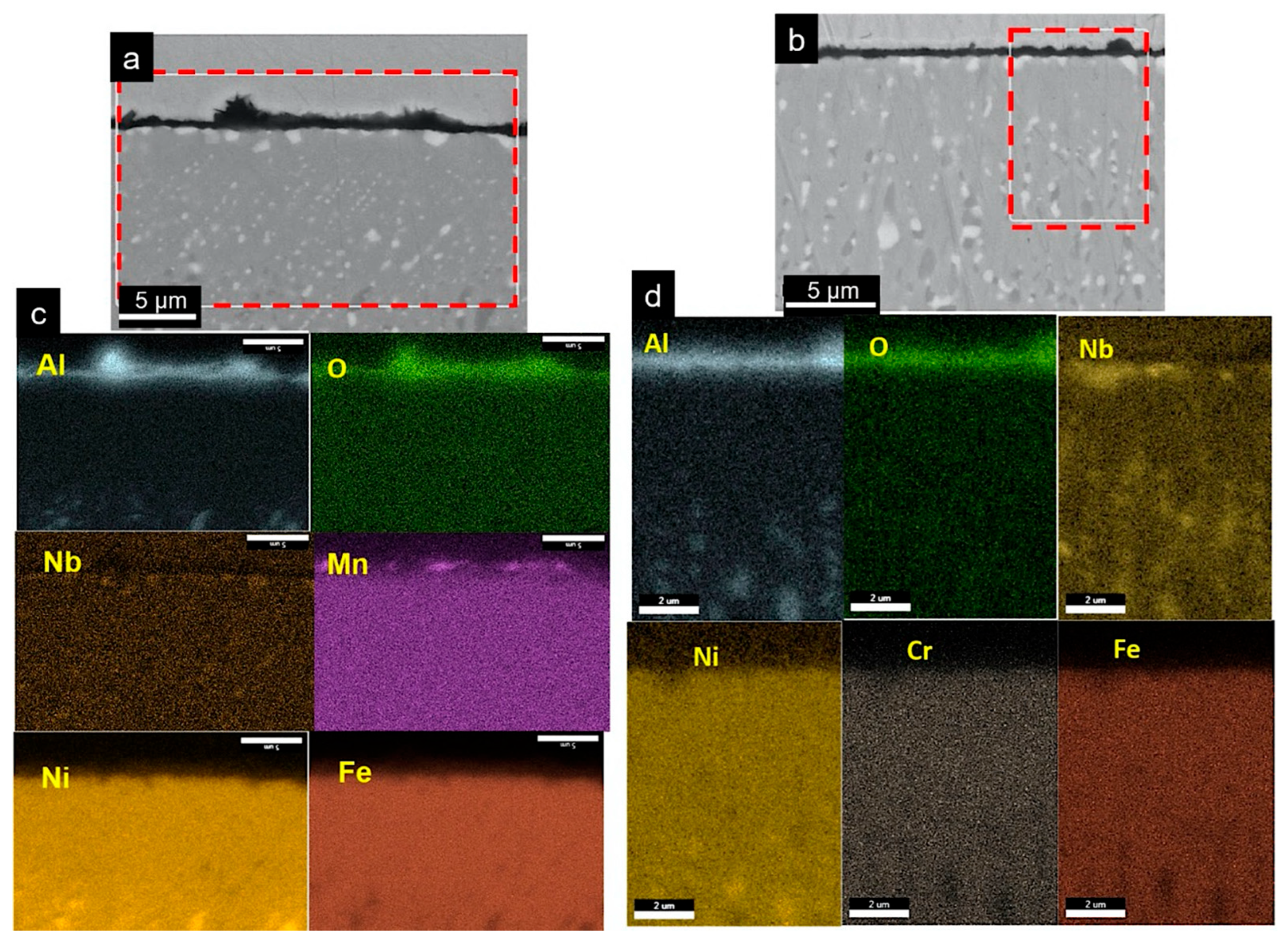
3.3.3. Cross-Sectional Microstructure Study
4. Conclusions
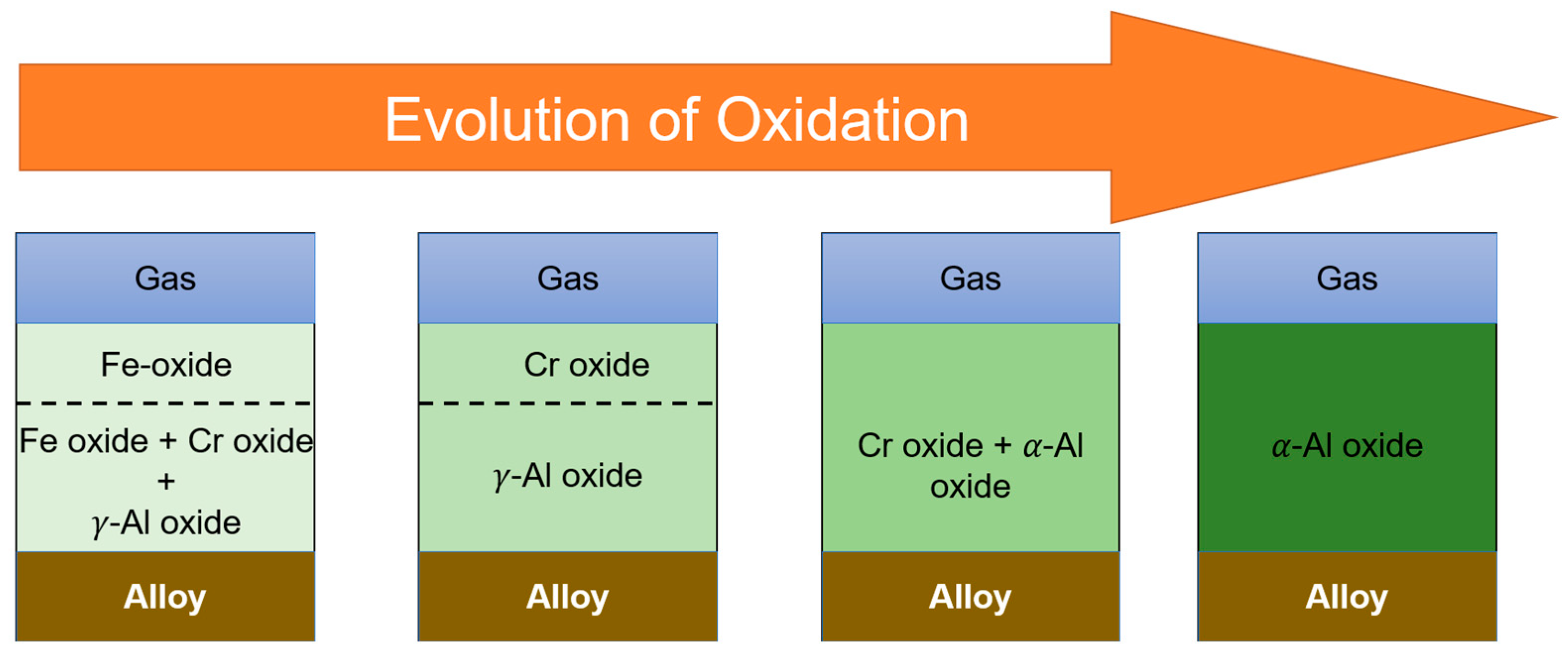
- The oxide scale formed on the AM and wrought AFA25 alloys is similar in nature and is composed of Al2O3. The continuous external Al2O3 scale acts as a barrier for oxygen diffusion from the oxide surface towards the matrix of the alloy and provides a superior high-temperature oxidation resistance.
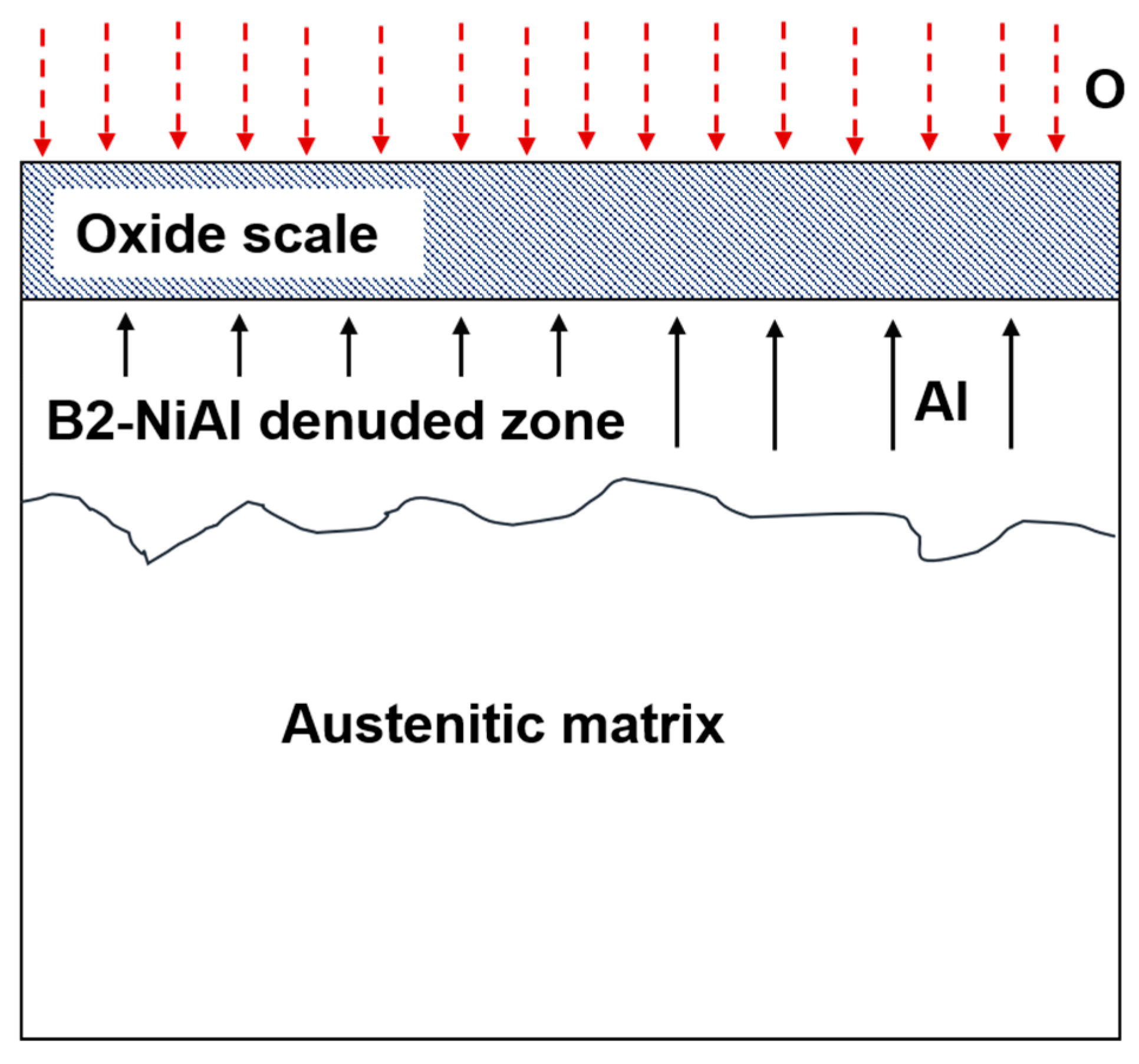
- An Al-denuded zone of β-NiAl phase was observed in the region underneath the oxide scale in the AM and wrought alloys. The depth of β-NiAl in AMAFA25-16 was much lower compared to the wrought and AMAFA25-1 alloys after 600 h of oxidation. Moreover, a dense and protective oxide scale formed on the surface of AMAFA25-16. This shows that AMAFA25-16 possesses not only good oxidation resistance after 600 h of exposure but also has enough Al reservoir due to lower depletion of the β-NiAl precipitate. Since the layer height and scan velocity were the same for both alloys, it appears that a variation in VED has a significant impact on the oxidation properties. The VED is expected to be higher for the AMAFA25-1 alloy, which shows inferior oxidation properties.
- Internal oxide was observed in both wrought and AM-AFA25 alloys. Wrought alloys showed the presence of intergranular alumina, whereas oxide particles were observed inside the AM-AFA25 alloy.
- Further research is required to understand how variation in each AM parameter can influence the underlying microstructures and their effect on high-temperature oxidation of AFA steels. Additionally, oxidation at each temperature interval needs to be investigated to better understand the oxidation mechanism of AM-based AFA alloys. The effect of the ferrite volume fraction in the AFA steels on oxidation behavior has not been investigated in this work and can be the focus of future research.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yamamoto, Y.; Brady, M.P.; Santella, M.L.; Bei, H.; Maziasz, P.J.; Pint, B.A. Overview of strategies for high-temperature creep and oxidation resistance of alumina-forming austenitic stainless steels. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2011, 42, 922–931. [Google Scholar] [CrossRef]
- Brady, M.P.; Yamamoto, Y.; Muralidharan, G.; Rogers, H.; Pint, B.A. Deployment of Alumina Forming Austenitic (AFA) Stainless Steel; Oak Ridge National Laboratory: Oak Ridge, TN, USA, 2013. [Google Scholar] [CrossRef]
- Rashidi, S.; Choi, J.P.; Stevenson, J.W.; Pandey, A.; Gupta, R.K. Effect of Aluminizing on the High-Temperature Oxidation Behavior of an Alumina-Forming Austenitic Stainless Steel. JOM 2019, 71, 109–115. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Brady, M.P.; Lu, Z.P.; Maziasz, P.J.; Liu, C.T.; Pint, B.A.; More, K.L.; Meyer, H.M.; Payzant, E.A. Creep-resistant, Al2O3-forming austenitic stainless steels. Science 2007, 316, 433–436. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Brady, M.P.; Lu, Z.P.; Liu, C.T.; Takeyama, M.; Maziasz, P.J.; Pint, B.A. Alumina-Forming Austenitic Stainless Steels Strengthened by Laves Phase and MC Carbide Precipitates. Metall. Mater. Trans. A 2007, 38, 2737–2746. [Google Scholar] [CrossRef]
- Rashidi, S.; Chatterjee, A.; Pandey, A.; Gupta, R. High-Temperature Oxidation Behavior of Additively Manufactured Inconel 625. 2021; in progress. [Google Scholar]
- Brady, M.P.; Yamamoto, Y.; Santella, M.L.; Walker, L.R. Composition, Microstructure, and Water Vapor Effects on Internal/External Oxidation of Alumina-Forming Austenitic Stainless Steels. Oxid. Met. 2009, 72, 311–333. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, X.; Chen, G.; Lu, Z. Improvement of high-temperature oxidation resistance and strength in alumina-forming austenitic stainless steels. Mater. Lett. 2011, 65, 3285–3288. [Google Scholar] [CrossRef]
- Brady, M.P.; Unocic, K.A.; Lance, M.J.; Santella, M.L.; Yamamoto, Y.; Walker, L.R. Increasing the Upper Temperature Oxidation Limit of Alumina Forming Austenitic Stainless Steels in Air with Water Vapor. Oxid. Met. 2011, 75, 337–357. [Google Scholar] [CrossRef]
- Brady, M.P.; Magee, J.; Yamamoto, Y.; Helmick, D.; Wang, L. Co-optimization of wrought alumina-forming austenitic stainless steel composition ranges for high-temperature creep and oxidation/corrosion resistance. Mater. Sci. Eng. A 2014, 590, 101–115. [Google Scholar] [CrossRef]
- Brady, M.P.; Yamamoto, Y.; Santella, M.L.; Maziasz, P.J.; Pint, B.A.; Liu, C.T.; Lu, Z.P.; Bei, H. The development of alumina-forming austenitic stainless steels for high-temperature structural use. JOM 2008, 60, 12–18. [Google Scholar] [CrossRef]
- Brady, M.P.; Yamamoto, Y.; Santella, M.L.; Pint, B.A. Effects of minor alloy additions and oxidation temperature on protective alumina scale formation in creep-resistant austenitic stainless steels. Scr. Mater. 2007, 57, 1117–1120. [Google Scholar] [CrossRef]
- Brady, M.P.; Yamamoto, Y.; Santella, M.L.; Bei, H.; Maziasz, P.J. Development of Alumina-Forming Austenitic (AFA) Stainless Steels = Multi-Phase High-Temperature Alloys. In Proceedings of the 22nd Annual Conference on Fossil Energy Materials; Pittsburgh, PA, USA, 8–10 July 2008. Available online: https://netl.doe.gov/sites/default/files/event-proceedings/2008/fem/Yamamoto.pdf (accessed on 30 May 2018).
- Hu, B.; Baker, I. High temperature deformation of Laves phase precipitates in alumina-forming austenitic stainless steels. Mater. Lett. 2017, 195, 108–111. [Google Scholar] [CrossRef]
- Gao, Q.; Liu, Z.; Li, H.; Zhang, H.; Jiang, C.; Hao, A.; Qu, F.; Lin, X. High-temperature oxidation behavior of modified 4Al alumina-forming austenitic steel: Effect of cold rolling. J. Mater. Sci. Technol. 2021, 68, 91–102. [Google Scholar] [CrossRef]
- Luo, R.; Yang, Y.; Bian, H.; Chen, L.; Ouyang, L.; Peng, C.T.; Gao, P.; Xu, G.; Cheng, X. High Temperature Deformation Characteristics of an Alumina-Forming Stainless Steel. Steel Res. Int. 2019, 90, 1900022. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Santella, M.L.; Liu, C.T.; Evans, N.D.; Maziasz, P.J.; Brady, M.P. Evaluation of Mn substitution for Ni in alumina-forming austenitic stainless steels. Mater. Sci. Eng. A 2009, 524, 176–185. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Pint, B.A.; Terrani, K.A.; Field, K.G.; Yang, Y.; Snead, L.L. Development and property evaluation of nuclear grade wrought FeCrAl fuel cladding for light water reactors. J. Nucl. Mater. 2015, 467, 703–716. [Google Scholar] [CrossRef]
- Pint, B.A.; Shingledecker, J.P.; Brady, M.P.; Maziasz, P.J. Alumina-forming austenitic alloys for advanced recuperators. In Proceedings of the ASME Turbo Expo 2007: Power for Land, Sea, and Air, Montreal, QC, Canada, 14–17 May 2007; Volume 3, pp. 995–1002. [Google Scholar] [CrossRef]
- Chen, L.; Wang, M.; Wang, Q.; Gao, Y.; Dong, H.; Che, H.; Zhou, Z. Microstructure and mechanical property evolution of an AFA alloy with simple composition design during ageing at 700 °C. Mater. Sci. Eng. A 2020, 779, 139157. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, L.; Guo, X.; Kane, S.; Deng, Y.; Jung, Y.-G.; Lee, J.-H.; Zhang, J. Additive Manufacturing of Metallic Materials: A Review. J. Mater. Eng. Perform. 2018, 27, 1–13. [Google Scholar] [CrossRef]
- Sander, G.; Tan, J.; Balan, P.; Gharbi, O.; Feenstra, D.R.; Singer, L.; Thomas, S.; Kelly, R.G.; Scully, J.R.; Birbilis, N. Corrosion of Additively Manufactured Alloys: A review. Corrosion 2018, 74, 1318–1350. [Google Scholar] [CrossRef] [PubMed]
- Gu, D. Laser Additive Manufacturing of High-Performance Materials; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar] [CrossRef]
- Yu, Z.; Chen, M.; Shen, C.; Zhu, S.; Wang, F. Oxidation of an austenitic stainless steel with or without alloyed aluminum in O2 + 10% H2O environment at 800 °C. Corros. Sci. 2017, 121, 105–115. [Google Scholar] [CrossRef]
- Shi, H. Alumina forming alloys (steels, high entropy materials) for the mitigation of compatibility issues with liquid metals and steam in energy related, high-temperature applications. Ph.D. Thesis, Karlsruher Instituts für Technologie (KIT), Karlsruhe, Germany, 2020. [Google Scholar] [CrossRef]
- Mu, N.; Jung, K.; Yanar, N.M.; Pettit, F.S.; Holcomb, G.R.; Howard, B.H.; Meier, G.H. The Effects of Water Vapor and Hydrogen on the High-Temperature Oxidation of Alloys. Oxid. Met. 2013, 79, 461–472. [Google Scholar] [CrossRef]
- Shi, H.; Tang, C.; Jianu, A.; Fetzer, R.; Weisenburger, A.; Steinbrueck, M.; Grosse, M.; Stieglitz, R.; Müller, G. Oxidation behavior and microstructure evolution of alumina-forming austenitic & high entropy alloys in steam environment at 1200 °C. Corros. Sci. 2020, 170, 108654. [Google Scholar] [CrossRef]
- Birks, N.; Meier, G.H.; Pettit, F.S. Introduction to the High-Temperature Oxidation of Metals; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar] [CrossRef]
- Jia, Q.; Gu, D. Selective laser melting additive manufactured Inconel 718 superalloy parts: High-temperature oxidation property and its mechanisms. Opt. Laser Technol. 2014, 62, 161–171. [Google Scholar] [CrossRef]
- Gu, C.; Liu, R.; Wang, C.; Sun, Y.; Zhang, S. Effect of Aluminum on Microstructure and High-Temperature Oxidation Resistance of Austenitic Heat-Resistant Steel. Metals 2020, 10, 176. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, X.; Sun, X.; Lu, Z.P. Effects of silicon additions on the oxide scale formation of an alumina-forming austenitic alloy. Corros. Sci. 2012, 65, 317–321. [Google Scholar] [CrossRef]
- Stott, F.H.; Wood, G.C.; Stringer, J. The influence of alloying elements on the development and maintenance of protective scales. Oxid. Met. 1995, 44, 113–145. [Google Scholar] [CrossRef]
- Kofstad, P. High Temperature Oxidation of Metals; John Wiley & Sons, Inc.: New York, NY, USA, 1967. [Google Scholar] [CrossRef]
- Hyer, C.H.; Dryepondt, S.; Su, Y.-F.; Yamamoto, Y.; Pint, B.A.; Massey, C.P. Strength stability at high temperatures for additively manufactured alumina forming austenitic alloy. Scr. Mater. 2024, 253, 116286. [Google Scholar] [CrossRef]
- Du, Y.; Zhang, C.; Ma, Q.; Zheng, Y.; Li, H.; Gao, Q. Precipitation behavior and strengthening mechanism of NbC phase in alumina-forming austenitic steel. Mater. Sci. Eng. A 2025, 936, 148421. [Google Scholar] [CrossRef]
- Kain, V.; De, P.K. Transformation of delta ferrite during high heat input welding of austenitic stainless steels. Mater. Perform. 2003, 42, 50–54. [Google Scholar]
- Arh, B.; Tehovnik, F.; Vode, F. Transformation of the δ-Ferrite in SS2343 Austenitic Stainless Steel upon Annealing at 1050 °C, 1150 °C and 1250 °C. Metals 2021, 11, 935. [Google Scholar] [CrossRef]
- Yap, C.Y.; Chua, C.K.; Dong, Z.L.; Liu, Z.H.; Zhang, D.Q.; Loh, L.E.; Sing, S.L. Review of selective laser melting: Materials and applications. Appl. Phys. Rev. 2015, 2, 041101. [Google Scholar] [CrossRef]
- Sames, W.J.; List, F.A.; Pannala, S.; Dehoff, R.R.; Babu, S.S. The metallurgy and processing science of metal additive manufacturing. Int. Mater. Rev. 2016, 61, 315–360. [Google Scholar] [CrossRef]
- Khan, R.M. Problem Solving and Data Analysis Using Minitab Problem Solving and Data Analysis Using Minitab: A Clear and Easy Guide; Wiley & Sons: New York, NY, USA, 2013; Available online: https://onlinelibrary.wiley.com/doi/book/10.1002/9781118307502 (accessed on 27 August 2025).
- Rashidi, S.; Choi, J.P.; Stevenson, J.W.; Pandey, A.; Gupta, R.K. High temperature oxidation behavior of aluminized Haynes 230. Corros. Sci. 2020, 174, 108835. [Google Scholar] [CrossRef]
- Pham, M.S.; Dovgyy, B.; Hooper, P.A. Twinning induced plasticity in austenitic stainless steel 316L made by additive manufacturing. Mater. Sci. Eng. A 2017, 704, 102–111. [Google Scholar] [CrossRef]
- Melia, M.A.; Nguyen, H.D.A.; Rodelas, J.M.; Schindelholz, E.J. Corrosion properties of 304L stainless steel made by directed energy deposition additive manufacturing. Corros. Sci. 2019, 152, 20–30. [Google Scholar] [CrossRef]
- Zhao, W.X.; Zhou, D.Q.; Jiang, S.H.; Wang, H.; Wu, Y.; Liu, X.J.; Wang, X.Z.; Lu, Z.P. Ultrahigh stability and strong precipitation strengthening of nanosized NbC in alumina-forming austenitic stainless steels subjecting to long-term high-temperature exposure. Mater. Sci. Eng. A 2018, 738, 295–307. [Google Scholar] [CrossRef]
- Marasco, A.L.; Young, D.J. The oxidation of iron-chromium-manganese alloys at 900 C. Oxid. Met. 1991, 36, 157–174. [Google Scholar] [CrossRef]
- Jozaghi, T.; Wang, C.; Arroyave, R.; Karaman, I. Design of alumina-forming austenitic stainless steel using genetic algorithms. Mater. Des. 2020, 186, 108198. [Google Scholar] [CrossRef]
- Rashidi, S.; Ataie, A. A comparison study of polymer / cobalt ferrite nano-composites synthesized by mechanical alloying route. J. Ultrafine Grained Nanostruct. Mater. 2016, 48, 59–67. [Google Scholar] [CrossRef]
- Sauer, J.P.; Rapp, R.A.; Hirth, J.P. Oxidation of iron-manganese-aluminum alloys at 850 and 1000 °C. Oxid. Met. 1982, 18, 285–294. [Google Scholar] [CrossRef]
- Prescott, R.; Graham, M.J. The formation of aluminum oxide scales on high-temperature alloys. Oxid. Met. 1992, 38, 233–254. [Google Scholar] [CrossRef]






| Scheme | Speed [mm/s] | Power [W] | Hatch [µm] | Density [g/cc] | % from Target |
|---|---|---|---|---|---|
| AM-AFA25-1 | 1000 | 175 | 100 | 7.73 | 98.03 |
| AM-AFA25-16 | 1000 | 250 | 150 | 7.74 | 98.10 |
| Sample | kp (mg/cm−2s−1/2) |
|---|---|
| AM-AFA25-1—850 °C | 5.94 × 10−5 |
| W-AFA25—850 °C | 3.45 × 10−5 |
| AM-AFA25-16—850 °C | 2.62 × 10−5 |
| Sample | Oxide Thickness (µm) | Denuded β-NiAl (µm) |
|---|---|---|
| AM-AFA25-1—850 °C | 1.5 ± 0.5 | 9.4 ± 0.1 |
| W-AFA25—850 °C | 1 ± 0.2 | 10.4 ± 0.3 |
| AM-AFA25-16—850 °C | 0.6 ± 0.09 | 4.5 ± 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rashidi, S.; Chatterjee, A.; Pandey, A.; Gupta, R.K. High-Temperature Oxidation Behavior of an Additively Manufactured Alumina-Forming Austenitic Stainless Steel. Corros. Mater. Degrad. 2025, 6, 47. https://doi.org/10.3390/cmd6040047
Rashidi S, Chatterjee A, Pandey A, Gupta RK. High-Temperature Oxidation Behavior of an Additively Manufactured Alumina-Forming Austenitic Stainless Steel. Corrosion and Materials Degradation. 2025; 6(4):47. https://doi.org/10.3390/cmd6040047
Chicago/Turabian StyleRashidi, Sedigheh, Arnab Chatterjee, Amit Pandey, and Rajeev K. Gupta. 2025. "High-Temperature Oxidation Behavior of an Additively Manufactured Alumina-Forming Austenitic Stainless Steel" Corrosion and Materials Degradation 6, no. 4: 47. https://doi.org/10.3390/cmd6040047
APA StyleRashidi, S., Chatterjee, A., Pandey, A., & Gupta, R. K. (2025). High-Temperature Oxidation Behavior of an Additively Manufactured Alumina-Forming Austenitic Stainless Steel. Corrosion and Materials Degradation, 6(4), 47. https://doi.org/10.3390/cmd6040047