This chapter presents the results of the conducted experiments, discussing the recorded sensor data and the correlations with plant parameters, as well as the chemical analysis of the retrieved electrodes. The sensor data analysis is based on the sensor set currently mounted in the plant and corresponding plant data. The chemical analysis was performed in the second sensor set, which was in operation from July 2023 to August 2024. As discussed in
Section 2.3, the data does not allow for a quantification of the sensor data as described in previous publications [
17,
18]. Instead, correlations with operational events such as shower cleaning, plant data and the chemical analysis are employed for validation of the sensor data.
3.1. Analysis of Sensor Data
This section discusses the data recorded directly by the sensors and the interpretation thereof. The following analysis is based on the data recorded with the third sensor set, starting with its installation in September 2024. The data set serves as an example for the entirety of the performed measurements. Discussed observations and presented conclusions can also be found in the data from the other sensor sets. As a first step, the time resolved corrosion data recorded by the monitoring system is analyzed.
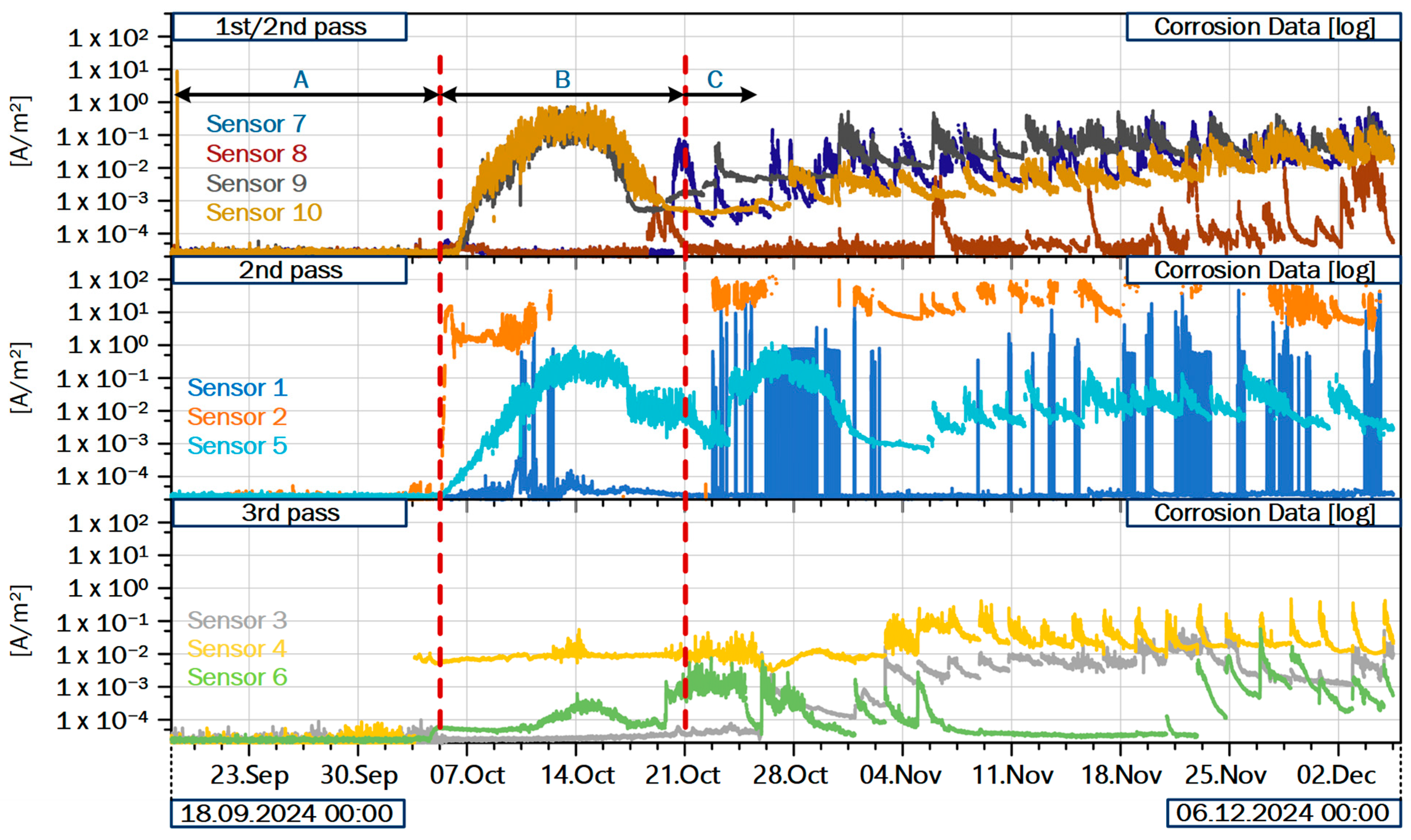
Figure 6 provides and overview of the sensor data recorded during commissioning of the third sensor set in 2024, as well as the following months. The sensors are grouped based on their location in the boiler, with the top graph showing the sensors placed first along the flue gas path (compare
Figure 4). In interval A, before the first plant startup, all sensors show the default signal. After startup, about two to three weeks are needed to form significant deposits (interval B). Following the initial deposit build-up, the data from almost all sensors shows the expected patterns and magnitudes of corrosion (interval C).
Comparing the corrosion signals of sensors within the same group, we usually find comparable patterns. For example, in the first group, consisting of sensors S7 to S10, the recorded CCD trend towards a base level between 10 × 10−2 and 10 × 10−1 A/m2 in interval C. All sensors show peaks with a steep ascending flank, increasing the signal of up to one order of magnitude. The descending flanks are commonly flatter, forming a characteristic saw tooth pattern. These peaks are comparable in frequency and magnitude for the sensors in each respective group, indicating that the corrosion attack is comparable. Notable outliers from their respective groups are firstly sensors S1 and S8 and secondly the two sensors equipped with the alternative electrode geometry, S2 and S4. The signals from S1 likely originate from an electrical issue. Comparable patterns were observed in previous experiments and could be attributed to conductive components in the deposits on the electrodes. Subsequent tests showed that short circuits between electrode connectors would produce similar results. In the present case, a detailed analysis can only be conducted after dismounting the sensor. Sensor S8 delivers plausible recordings and continuous measurements, albeit much a lower CCD than sensor S7, which is mounted just about twenty centimeters further upwards in the boiler wall. During the measurement campaigns, a pronounced line of thicker deposits was identified with an infrared (IR) camera during the second measurement campaign. The line of deposits crossed the area where S7 and S8 are mounted, providing a plausible explanation of this behavior. Due to the flue gas flow field of the plant, which is being redirected from upwards flow in the first pass to downwards flow in the second in that area, sensor S8 might be located in an area with low deposition rates.
The two sensors equipped with the alternative electrode geometry, as displayed in
Figure 3, also exhibit different behavior compared to the other sensors in the same group. In both cases, the CCD are of a higher level and recorded peaks are more pronounced, supporting the hypothesis that the alternative design increases sensor sensitivity. In the second pass, this increase unfortunately results in insufficient data quality, because the upper measurement range limit is reached frequently. In the third pass, sensor S4 confirms increased sensitivity of the alternative geometry, while also recording continuous corrosion signals. This allows a detailed analysis of the cleaning measures employed in the plant, as discussed below. Both sensor signals seem to jump from the base signal level to the operation signal at the first plant startup, suggesting that the initial deposition formation phase might also be shortened by the alternative geometry. From the first data set, we can conclude that the test of the alternative geometry was successful. Elongating the opposing edges of the working- and auxiliary electrodes shows promising results for the application in low-corrosion environments and might also help in situations where deposits form only slowly. This can be seen from the following analysis of the cleaning activity, which was enabled by the increased sensitivity of the alternative geometry. Overall, the standard geometry remains the more robust and versatile geometry for the application at hand, allowing measurements and general interpretation of the data in the low-corrosion area of the third pass, while not exceeding measurement limits in the areas with higher corrosion attack.
Comparing the signals from the three groups to each other, we notice a decrease in absolute indicated CCD along the flue gas path. All sensors show peaks with the characteristic saw tooth pattern described above. In the sensor data, CCD peaks are frequently accompanied by corresponding peaks in temperature and heat flux. The deposits on the electrodes do not only act as electrolyte in the measurement principle, but also influence heat transfer from the flue gas into the membrane wall and consequently, the water cycle of the plant. Since the deposits separate the electrodes from the hot flue gas and provide an additional heat transfer resistance, sensor temperatures and heat flux tend to decrease beneath thick deposits. A peak with a steep ascending flank in both temperature and CCD thus signals detaching deposits. In the first two groups, these peaks appear randomly distributed for each sensor, suggesting that the deposits detach, for example, after growing too thick or due to variations in the flue gas flow. There are incidents where all sensors in the first two groups simultaneously show a peak, for example, on 5 November at around 18:00. The incident is easily identified in
Figure 6 as the first peak of sensor S8 in interval C. The substantial increase in CCD on a large number of sensors indicates a large event in the plant. A plausible explanation would be an event influencing the combustion on the grate, such as the fall of a substantial quantity of waste from the fuel slider onto the combustion bed. The material suddenly falling onto the burning bed can partially extinguish the fire, before igniting and releasing significant amounts of volatiles, thus causing a peak in temperature and unburnt components in the flue gas. Another possible event is the detachment of a significant amount of deposits from the membrane walls. In the first pass, the deposits have been observed to reach thicknesses of up to 0.5 m, covering the entire width of the membrane wall and reaching several meters in height. If such a large deposit detaches, it also has the potential to temporarily extinguish the fire, impeding clean combustion of the fuel on the grate. Both kinds of events have been observed in the boiler during the two measurement campaigns, and are thus possible explanations for the recorded signals. It is evident that both events have the potential to temporarily impede combustion on the bed, thereby resulting in the subsequent distribution of substantial quantities of unburned or corrosive species within the boiler.
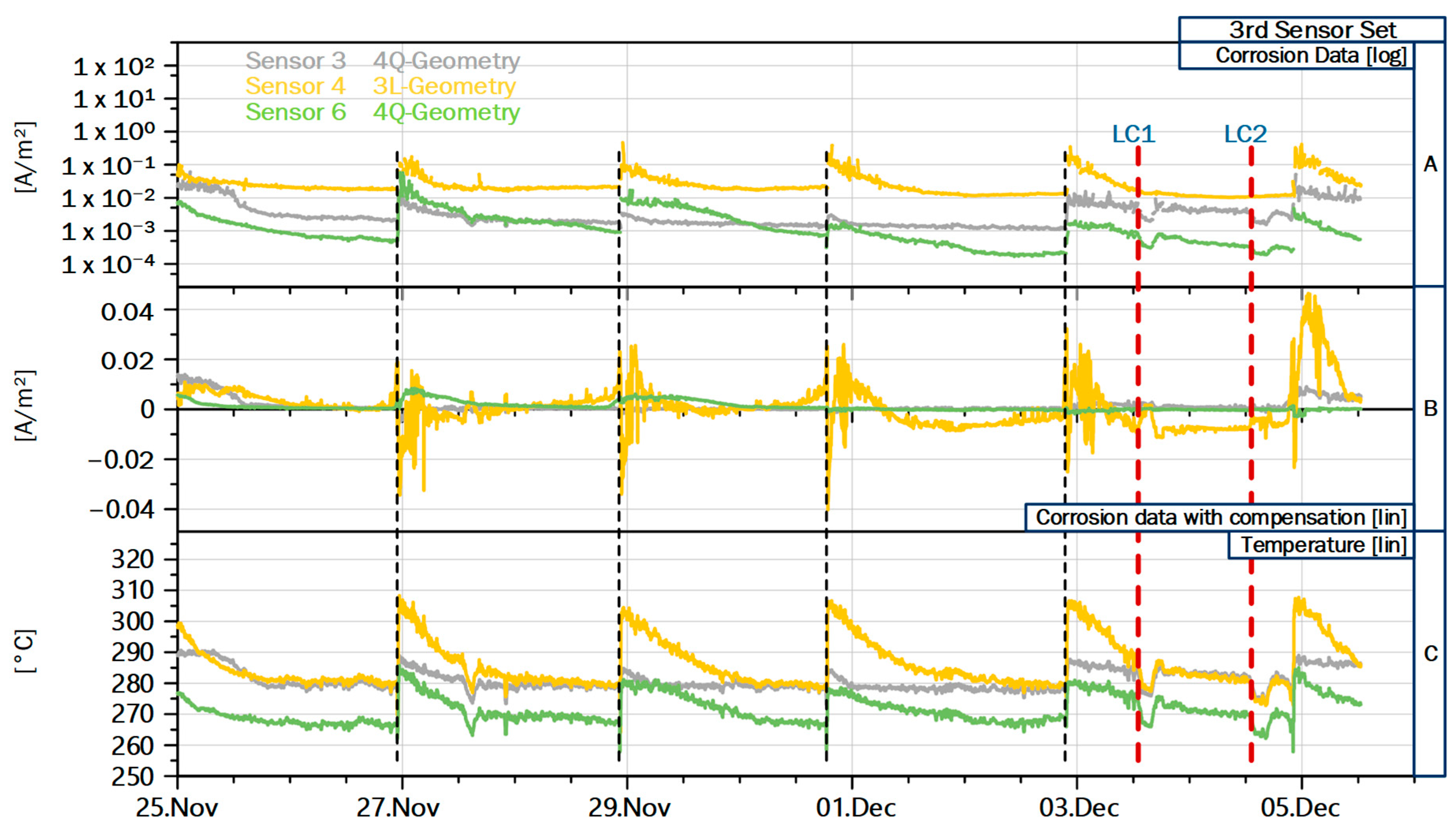
In the third group, which is mounted in the third pass, the peaks exhibit a noticeable synchronicity, as detailed in
Figure 7. Here, the recorded CCD of sensors are shown in the top diagram, the CCD with temperature compensation (compare
Section 2.5) appear in the middle, and the recorded temperatures are displayed at the bottom. As discussed, temperature has a major influence on the corrosion attack, which becomes obvious, since the graphs for temperature and CCD exhibit the same saw-tooth pattern. The interpretation of this pattern, which also appears qualitatively in the heat-flux measurements, is the slow build-up of deposits over time, followed by rapid detachment. Since deposits act as an insulation layer, decreasing wall temperature and heat flux, and form a diffusion barrier for corrosive species from the gas phase, deposit growth can explain the coupled developments of the sensor data. Since all sensors in the third group show regularly spaced and synchronized peaks indicating deposit detachment, a common cause is likely. From the operator, we received the information that shower cleaning is used to remove excessive deposits in the third pass and thus keep the flue gas temperatures before the first superheater at an acceptable level. The shower cleaning is performed every second night shift, which matches with the peaks recorded by the third sensor group.
Figure 7 also illustrates the added value of the temperature compensated CCD: Since temperature and CCD are directly related as discussed above, correctly attributing an increasing corrosion signal to either temperature changes or operational influences is not trivial. The temperature compensation combines both information and highlights intervals with CCD which are atypical for the current sensor temperature. Thanks to the added sensitivity of the 3L geometry, a more detailed analysis of the cleaning measures is possible at the example of the data from sensor S4. The two day interval between shower cleaning shows a recurring pattern: Directly after cleaning, both CCD and temperature increase, but the compensated CCD is negative, indicating that the freshly cleaned sensor records a below average corrosion attack for the increased temperature. During the initial deposit build up in the first hours after the cleaning event, the compensated CCD swings back into the positive, most likely because the freshly deposited corrosive species can directly interact with the electrodes base material, without having to diffuse into a stable deposit layer. After about six to twelve hours, the compensated CCD returns to near zero values or even becomes slightly negative until the next cleaning event is registered. This behavior indicates the growing deposits continue to increase heat transfer resistance and also act as a diffusion barrier between the freshly deposited corrosive species and the electrode base material. Towards the end of the intervals between cleaning events, the compensated CCD often starts to increase again, because the corrosion does not reduce as much as the temperature. This indicates that at some point, increasing thickness of the deposits does not increase the resistance to diffusion of corrosive species anymore, but continues to impede heat transfer. In practice, the interval for the shower cleaning in the third pass was determined by the flue gas temperature at the end of the third pass, which has to stay below a certain threshold to protect the superheater tubes from excessive temperatures and thus corrosion attack. Taking both corrosion measurements and experience of the operator into account, the chosen cleaning interval appears a good compromise, because more frequent cleaning would induce more corrosion peaks at the membrane wall during initial deposit build up, while less frequent cleaning would likely result in increased corrosion of the superheater tubes.
Figure 7 also displays the two short load changes performed for the second measurement campaign on the 3rd and 4th December, marked with LC1 and LC2. The load changes are visible in the temperature readings from all sensor groups, with drop by about 10 °C during the partial load state. In the third group, sensors S3 and S6 react to the first load change with decreased CCD, which is expected given the lower temperature. Sensor S3 shows no reduction in CCD for the second load change, where the recorded corrosion signal is already low and the decrease in the other two sensors is also lower than for the first load change. Similar behavior is observed in the other sensor groups, supporting the conclusion that stable deposits can mask short term influences from operation, especially in the case of stable deposits in the third pass. This also indicates that for thorough analysis of partial load states, longer periods are necessary to account for the change in deposit structure and growth.
3.2. Analysis of Plant Data
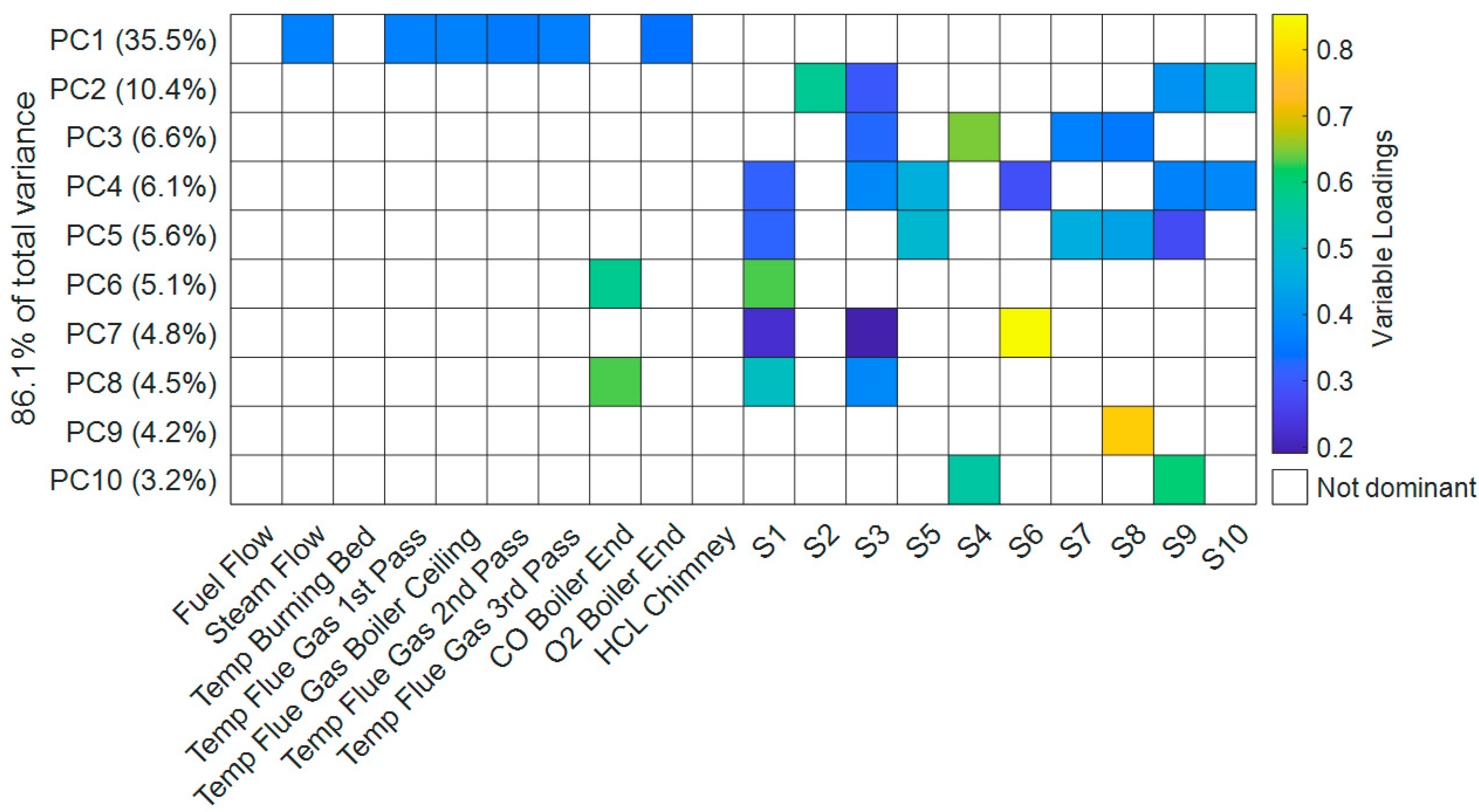
To understand the structure of the available data set, a principal component analysis (PCA) is performed with operational plant data and the compensated CCD from the third sensor set. In
Figure 8, the initial ten original variables represent the available measurements from the power plant. The following ten variables are the compensated CCD computed from the measurement system’s data. The resulting set contains 43,475 observations for the 20 analyzed variables, providing a solid basis for analysis. To assess the stability of the PCA, 100 new data sets are derived from the original set using a bootstrap algorithm. The stability of the PCA is assessed by analyzing the explained variance by the PC for each sample. The results of this evaluation are presented in
Table 3, which displays the variance explained for the first ten PC of the original data set, as well as the 97.5 and 2.5 percentiles and the mean of all bootstrap samples. The resulting 95% confidence intervals for each PC are relatively small, showing no overlaps down to the tenth PC and indicating good stability of the overall analysis. The contributions of the original variables to the respective PC are also analyzed, and exemplary results for the dominant contributors to PC1 are shown in
Table 4. In this case, the average relative difference between the variable loadings of the original and the mean of all Bootstrap PCA amounted to only 0.48%, while the 2.5 and 97.5 percentiles differed by only 9% from the results of the original data set. With average differences below 5%, the second to tenth PC also show good agreement again, indicating sufficient reliability for further analysis. Regarding the significance of the individual PC for analysis, an often-cited criterion is to discard any PC with an eigenvalue below one, originally proposed by Kaiser [
25]. According to this criterion, the first six PC are significant for further analysis, as shown in
Table 5.
Figure 8 displays a heat map of the PCA, detailing the contributions of the original variables to the first ten PC. To identify the most influential variables for each PC, only the most dominant variables are colorized, until a cumulative share of 85% of the cumulative squared variable loadings is reached. The variance explained by the individual principal components (PC) drops off quickly, which results in the first 10 components explaining about 86% of the total variance in the data set. As displayed in
Figure 8, the first PC explains about 36% of the total data variance and is dominated by the variables from the plant control system. The most dominant variables include the total steam flow, flue gas temperatures and the O
2 content at the end of the boiler. This set of variables can be interpreted as a description of the fireside control loop of the plant, which is supposed to provide the desired amount of steam and maintain a stable combustion of the fuel. From the coloration in the heat map, we can see that the dominant variables in PC1 are evenly contributing, indicating a strong correlation between the plant variables. This is plausible, since the data set mostly consists of intervals with stable plant operation under full load. Notably, the fuel flow and the burning bed temperature are not among the most influential variables of the first PC, indicating a weaker correlation in the measurement interval. Due to the high volatility of the fuels heating value, this appears plausible. In addition, the movement of the waste on the grate is complex and non-trivial to predict or simulate. Both grate bars and fuel sliders are necessary to introduce and stoke fuel in the boiler, which is needed to fully convert the fuel. However the movement of the waste particles on the grade depends on many parameters, such as particle shape and adhesive forces, and not every movement of the bars leads directly to waste movement [
26]. These inconsistencies in waste transport support the weaker link between the parameters describing the combustion bed and the temperature data from the boiler.
The following PC2 to PC10 consist mainly of differing combinations of the recorded sensor data, the only exception being the CO content of the flue gas measured at the end of the boiler. Such a connection is plausible, since CO is an indicator for reducing conditions, which can lead to accelerated corrosion attack [
27]. This relation appears in PC6 and PC8 for sensors S1 and S3, which are located in the second pass. The higher PC (ten to twenty) indicate relations between the burning bed temperature, the fuel flow and the content of HCl at the chimney. This connection could be interpreted to originate from incontinuities in the fuel flow or the fuel itself, both of which have been observed during the measurement campaigns on site. During the measurement campaigns on site, irregular fuel flow was frequently observed. The lead to significant quantities of the waste accumulating on the fuel sliders and then falling onto the grate, partially covering the burning material and leading to a peak in the release of volatiles and corrosive species. The importance of chlorine and sulfur species for corrosion phenomena in waste incineration plants is well discussed in the literature [
1,
22,
28]. However, the available data reveal no conclusive connection, probably because the corrosive species are measured at the chimney and the gas cleaning plant is interposed. This positioning is logical for the operator, who intends to prove compliance with emission protection laws. Comparison with the corrosion data from the boiler is unlikely to yield conclusive results, however, due to the effects of the gas cleaning plant on flue gas composition.
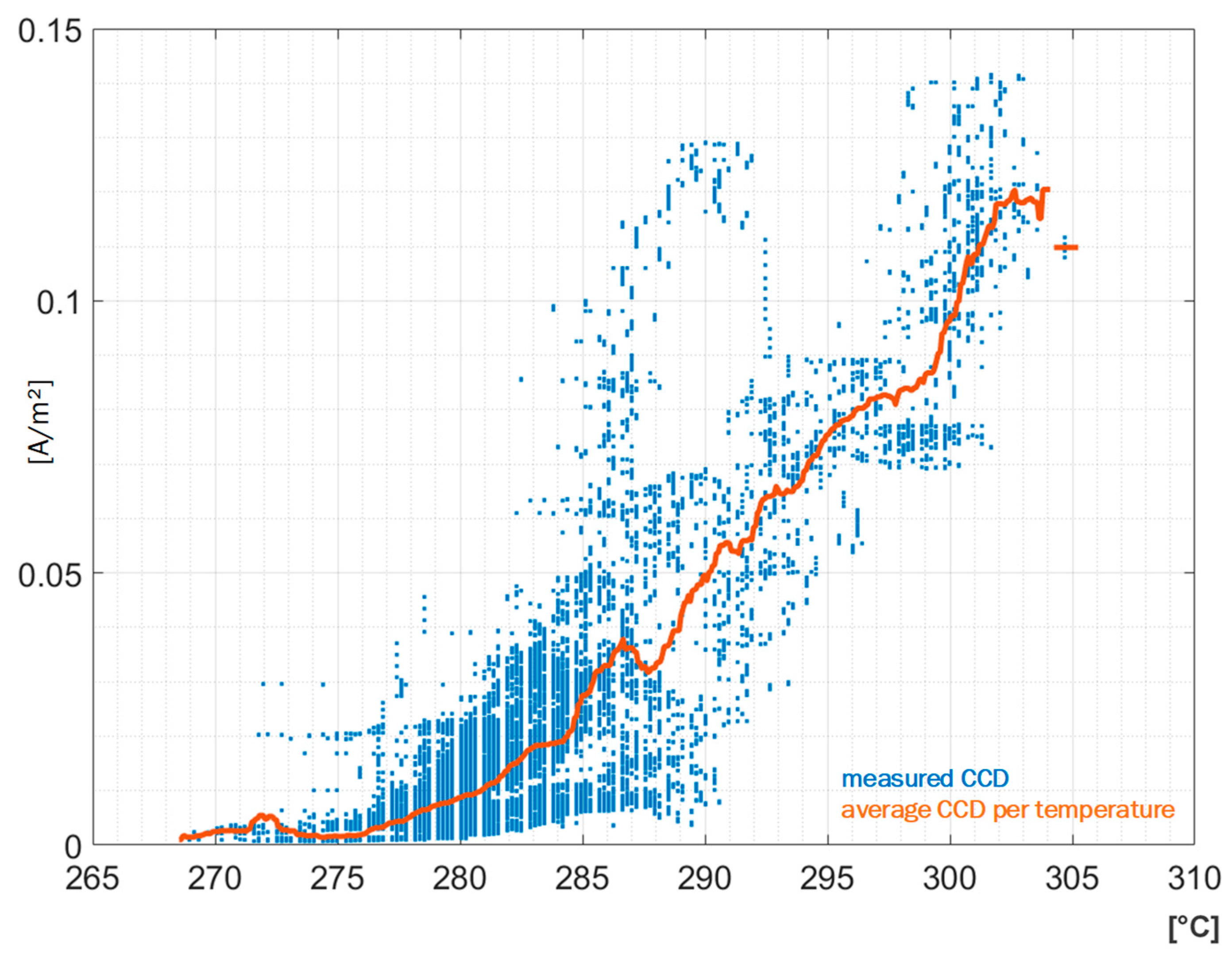
The relation between the CO content and the corrosion attack can be visualized by a scatter plot, which is displayed in
Figure 9A. Here, the normalized CCD recorded by sensor S3 is plotted over the CO content in the flue gas at the boiler end. The samples recorded during full load are shown as green dots; the red dots represent partial load states. To identify the trend, an average of the scattered data is computed for evenly spaced intervals of the CO content, resulting in a running average shown as black graph within the scatter plot. The blue graph in
Figure 9B displays the number of values used to compute the average in the individual intervals. The data clearly shows that increased CO content leads to increased normalized CCD for this sensor. A similar, albeit less pronounced, correlation is exhibited by other sensors in the second and third pass. The absence of a correlation for the sensors mounted in the first pass could suggest that the CO content has a negligible effect in the corrosion phenomena taking place there. Evaluation of the deposits from sensors S7 and S8 reveal a chlorine based corrosion mechanism, where the effect of reducing atmospheres might be negligible. To summarize, the plant control data shows limited correlation with the recorded CCD by the measurement system, suggesting that the major influence on corrosion is not the operation of the plant, but the fuel and the resulting deposit composition on the boiler walls. Some sensors suggest an influence of CO content in the flue gas on the corrosion attack.
3.3. Chemical Analysis of the Deposits
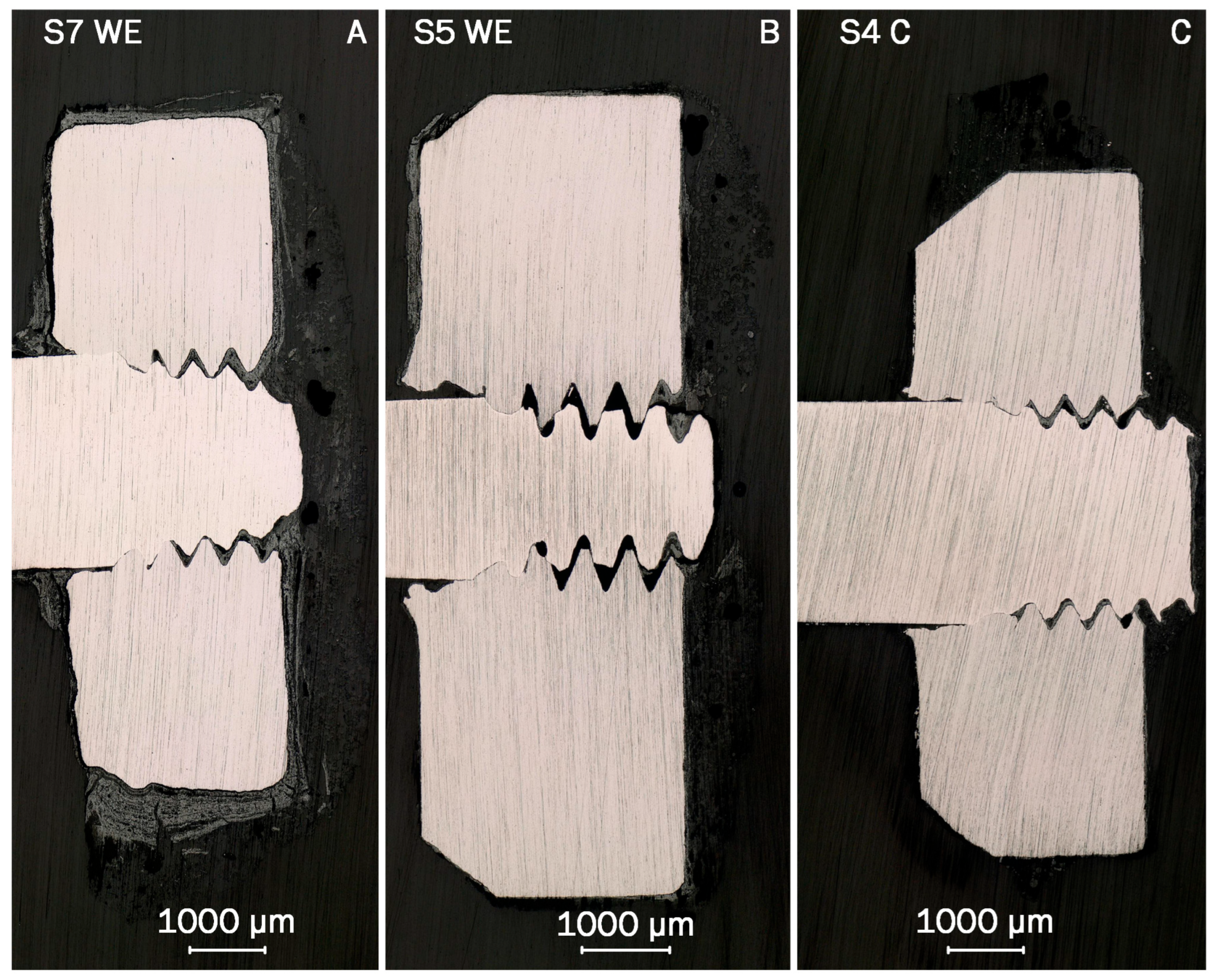
The images obtained from incident light microscopy (ILM) of sensors S7, S5 and S4 are displayed in
Figure 10, representing the results from each sensor group. In the images, the base material of the electrodes and connectors is displayed in bright gray. The material used for embedding the electrodes and deposits appears at the edges of the image in the dark gray. The deposits are displayed in various tones, darker than the base material, but brighter than the matrix. Comparing the shape of the base material of the cross sections, sensor S7 exhibits the most deformed shape. The shape of the electrode of sensor S4, visible as bright gray in the Figure, shows almost no change in the shape in comparison to the manufactured state. The cross section of sensor S5 is closer to the shape of sensor S4, but shows signs of the corrosion attack at the top of the electrode, mainly in the area of the thread. Although the base shape of the cross section remains virtually unchanged for S5, the surface at the top exhibits singular recesses and appears coarser in direct comparison the surface of S4. In addition, the thread connecting the electrode plate to the connector appears more rounded than that of S4, also indicating material loss. The thickest adhering deposits are observed on the surfaces of the electrode from S7, surprisingly including the base surface, which was facing the ceramic carrier during operation. This was likely caused by a gap from manufacturing, which allowed the initial deposition of particles during operation. The shape of the cross section suggests that this also allowed corrosion attack on the bottom of the electrode. The general trend observed from the ILM analysis is thus decreasing corrosion attack in the direction of the flue gas path, with thicker deposits on the surfaces of the electrodes from the first pass. Despite being based on a different sensor set, these observations are in good agreement with the evaluation of the sensor signals presented in
Section 3.1. In both sensor sets two and three, the highest signals were observed in the first pass, matching the position of the electrode with the highest observed mass loss.
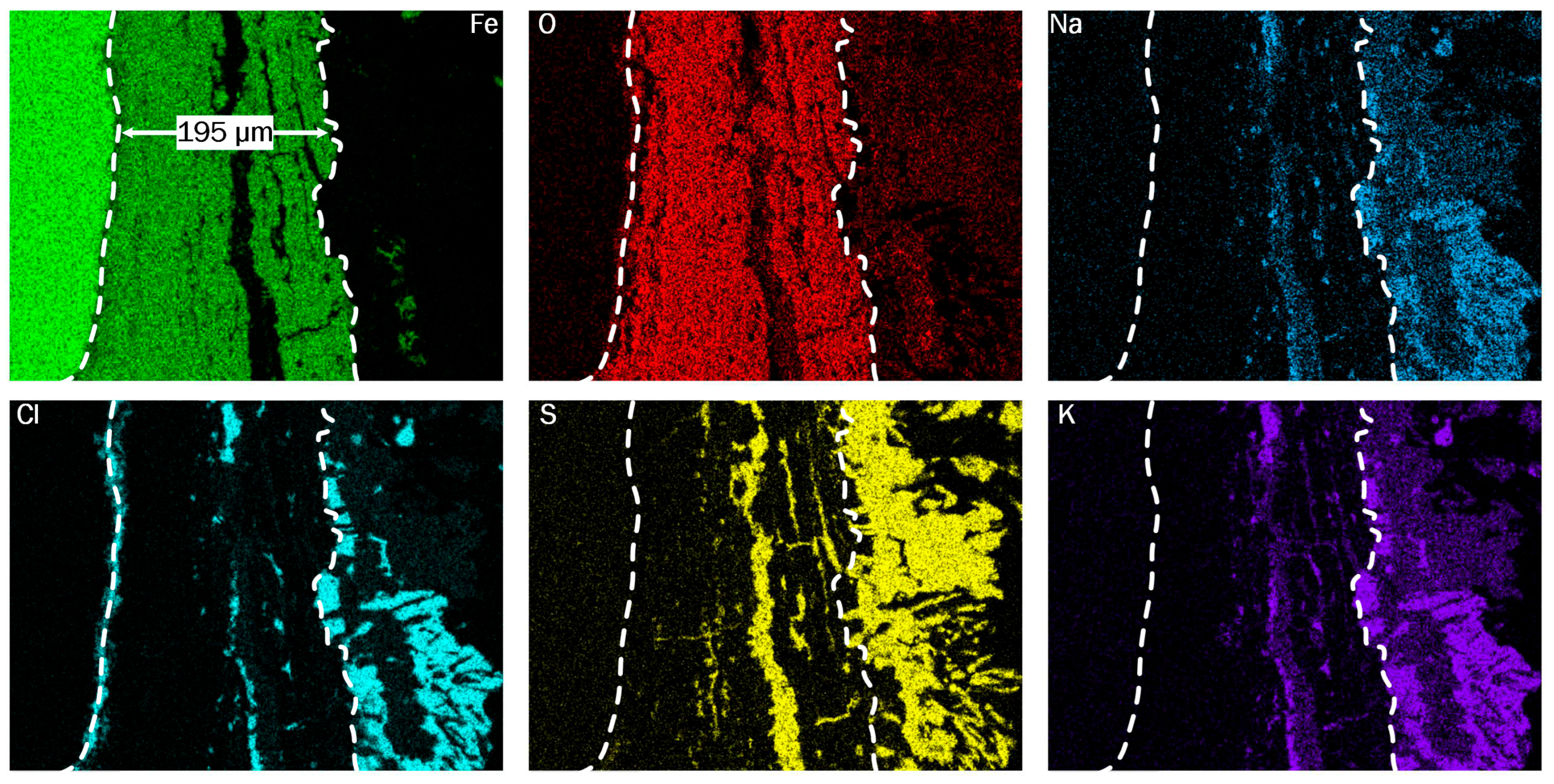
Figure 11 displays the results of the EDX scans on the working electrode of S7, showing the distributions of iron, oxygen, chlorine, sulfur, sodium and potassium in the corrosion products and deposits. S7 shows overall similar structures with three main layers, comparable to the results of EDX scans on the other electrodes. The first layer from the left is the base material, where iron is detected exclusively. Next, an intermediate layer is displayed, which consists mainly of iron and oxygen and varies in thickness from 19 to 195 µm. The outermost layer contains a variety of elements, including alkali metals, as well as sulfur and chlorine. The relatively homogeneous deposit layer in the first pass suggests either partially molten deposits during operation or condensation of gaseous salts as the deposition mechanism, as opposed to the deposition of solid particles. Additionally, the presence of molten chlorine salts would also facilitate the diffusion of chlorine into the oxygen layer, providing the precondition to start the active oxidation. For reference, the interfaces between these layers are marked by white dashed lines.
Chlorine and sulfur, are present in most of the deposit layer, where the patterns match with indications of alkali-metals. In the case of S7, indications of sulfur are also displayed penetrating the intermediate layer in a delamination zone, which is visible in the Fe image as crack-like structure parallel to the base material’s surface. Strikingly, chlorine is not only displayed in the delaminated area, but also as continuous layer at the interface between base material and oxide layer. Local EDX-scans at the interface show peaks for iron and chlorine exclusively, suggesting the presence of iron-chloride. Since S7 also features the thickest oxide scale and also shows the most deformation of the cross section, the oxide scales likely do not protect against the dominant corrosion mechanism. Both distribution of chlorine and the presence of non-protective oxide scales align with the description of the active oxidation mechanism found in the literature, which may also be known as chorine catalyzed corrosion [
5].
It is theorized that chlorine forms iron-chloride at the interface to the base material, which is stable due to a low oxygen partial pressure. The high steam pressure of iron chloride at elevated temperatures enables outwards diffusion into the oxide layer and provides potential to damage the protective oxide layer in the process. With sufficient oxygen availability in the outer oxide layers, iron-oxide becomes the stable phase again. This results in free chlorine in the oxide layer, which can again diffuse towards the base material, restarting the catalytic cycle [
22].
Since sulfur and chlorine are present in the intermediate layer, both have to be considered when discussing the dominant corrosion mechanism. In the literature, chlorine has been found to trigger an accelerated corrosion attack, compared to the sulfur based mechanisms [
22]. The presence of sulfur is viewed as an explanation for the initial release of chlorine within the deposits by sulphation of the initially deposited chlorine salts, providing a substantially increased corrosion potential compared to gas-phase chlorine [
3]. The release of chlorine is also discussed to allow bubble formation, causing damage the oxide layer and facilitating delamination, which is also observed in this study [
22]. The EDX scans on the electrodes from the first pass show all necessary prerequisites for both mechanisms: The presence of chlorine salts and sulfur in the outer deposit layer, as well as chlorine penetration into the interface between intermediate layer and base material. Thus, it can be deduced that the active oxidation mechanism is most likely the dominant cause for corrosion in the present study.
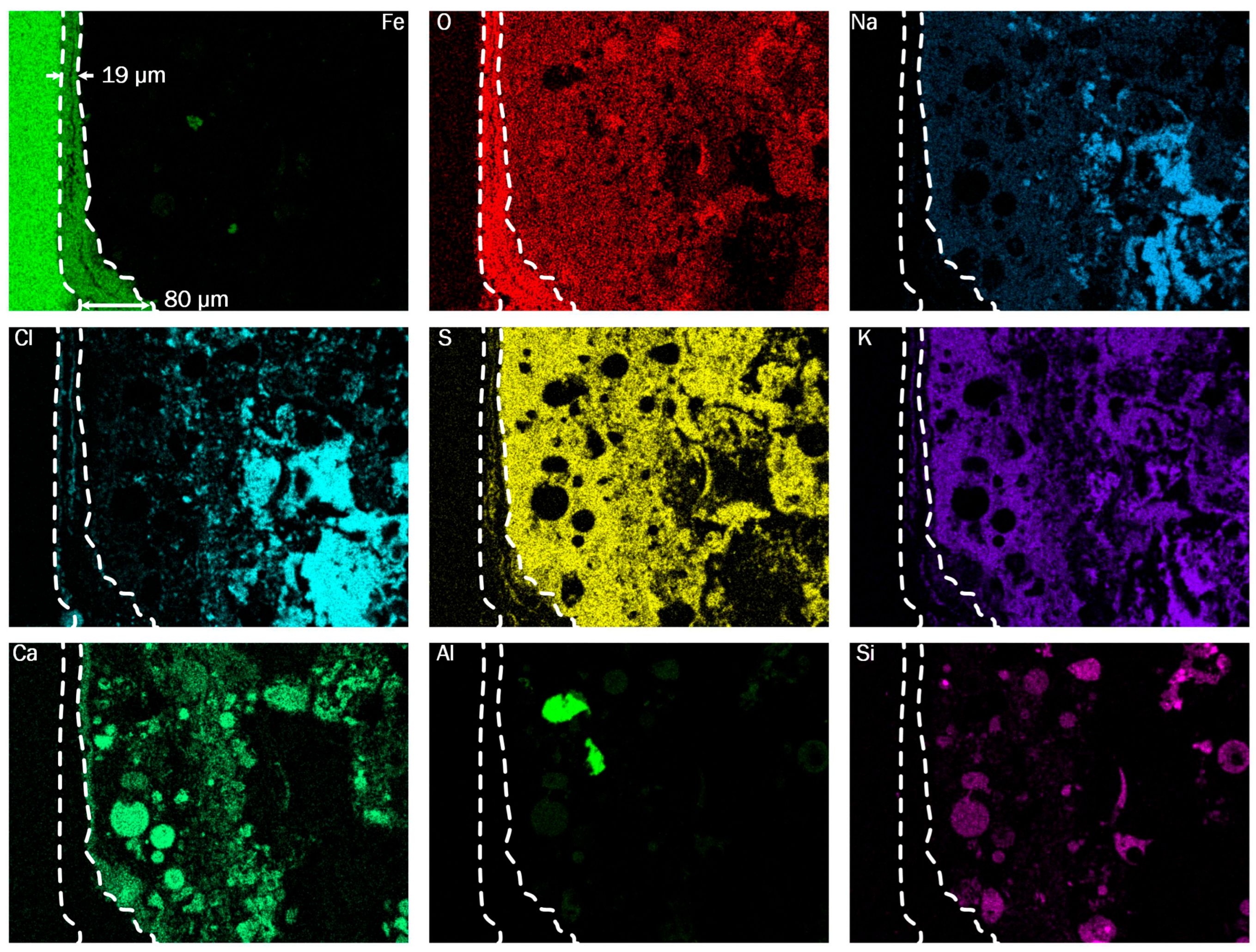
In the sensor group mounted in the third pass, the fundamental composition of the deposits is similar. The oxide layer is only 19 to 80 µm thick on S4, as
Figure 12 illustrates. In terms of element distribution within the corrosion products and deposits, there are two key differences between the sensors from the first and third passes: Firstly, the oxide layer on S4 shows less penetration of sulfur and chlorine. Secondly, the deposit layer of S4 features a more heterogeneous structure compared to S7, including the presence of rounded particles composed mainly of calcium and silicon. These likely originate from the combustion process and adhere to the wall in the third pass. The heterogeneous structure in proximity to the iron oxide layer thus suggests that the deposits remain solid in the third pass and form as a result of particles sticking to the wall upon impact. The sensors from the second pass show an intermediate pattern compared to the two other positions, as shown in
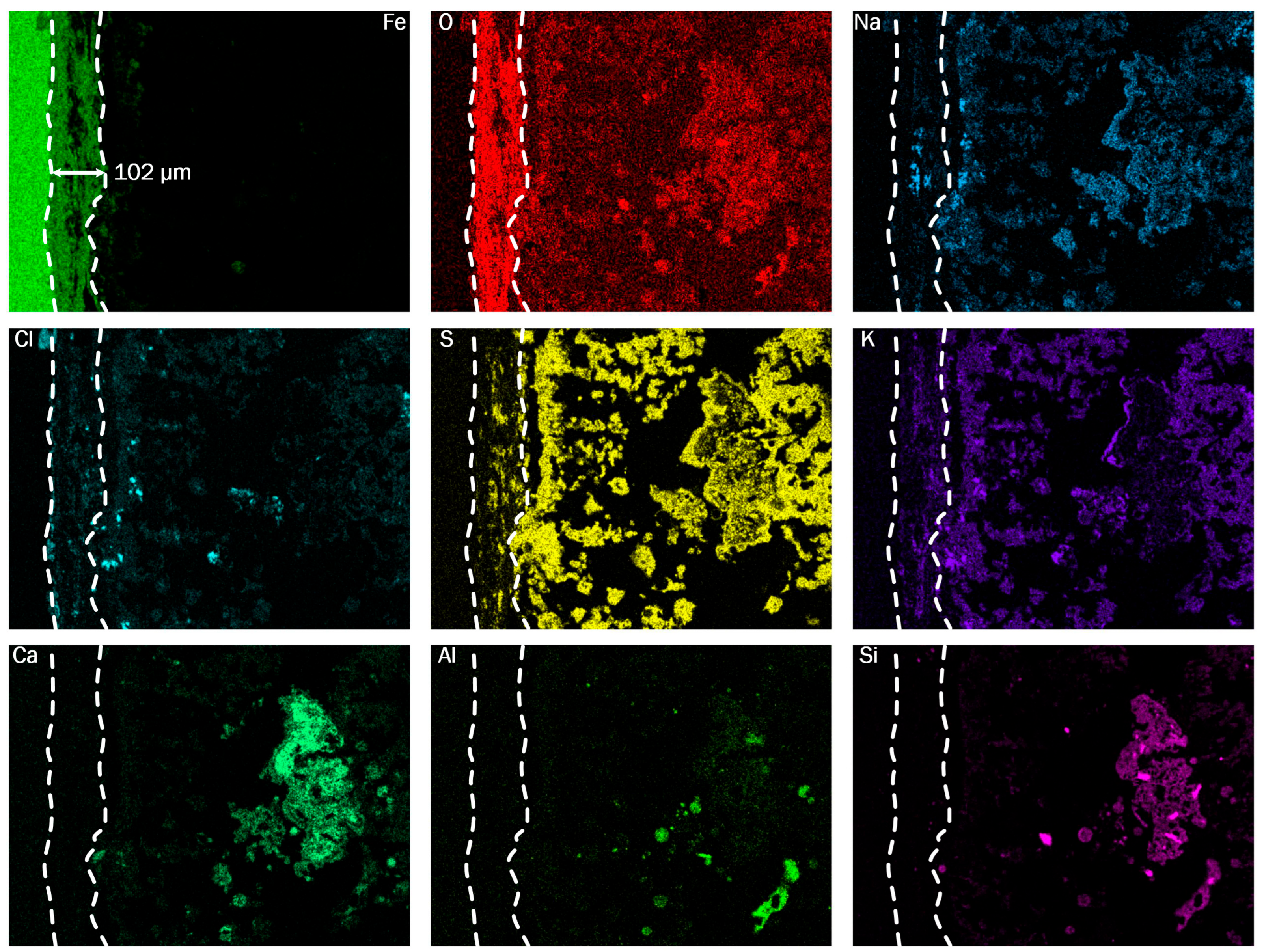
Figure 13. The oxide scales are about half as thick as in the first pass and show initial signs of delamination. Chlorine is present at the interface between base material and oxide layer, indicating the same dominant corrosion mechanism. The deposit layer is more homogeneous near the oxide layer than in the third pass, but still shows particle-like structures further away from the base material. This indicates a gradual transition of the deposit structures and decrease in the corrosion attack along the flue gas path. Due to the decreasing severity of the corrosion attack in the second and third passes, the influence of CO-rich atmospheres on the recorded CCD appears plausible. In the literature, chlorine catalyzed active oxidation is found to dominate the effects of other corrosion mechanisms, such as the effect of corrosion caused by oxygen deficiency [
5]. This could explain why the correlation between CO content in the flue gas is only found for sensors mounted in the second and third pass, where the chlorine based mechanism is evidently less pronounced than in the first sensor groups.
These results agree well with the recorded CCD from the sensors of third set, which also identify the groups in the first pass as the sensors with the highest corrosion attack. Electrodes with higher recorded CCD show higher deformation of the cross section of the base material after operation, as well as thicker oxide layers with higher contents of chlorine and sulfur. Thus, the results of the chemical analysis support the measurement data recorded by the online corrosion monitoring system, providing an additional confirmation of the recorded data. The good agreement of the results over long measurement periods and multiple sensor sets suggests stable operation of the plant and consistent levels of the corrosion attack.