Graphenes for Corrosion Protection in Electrochemical Energy Technology
Abstract
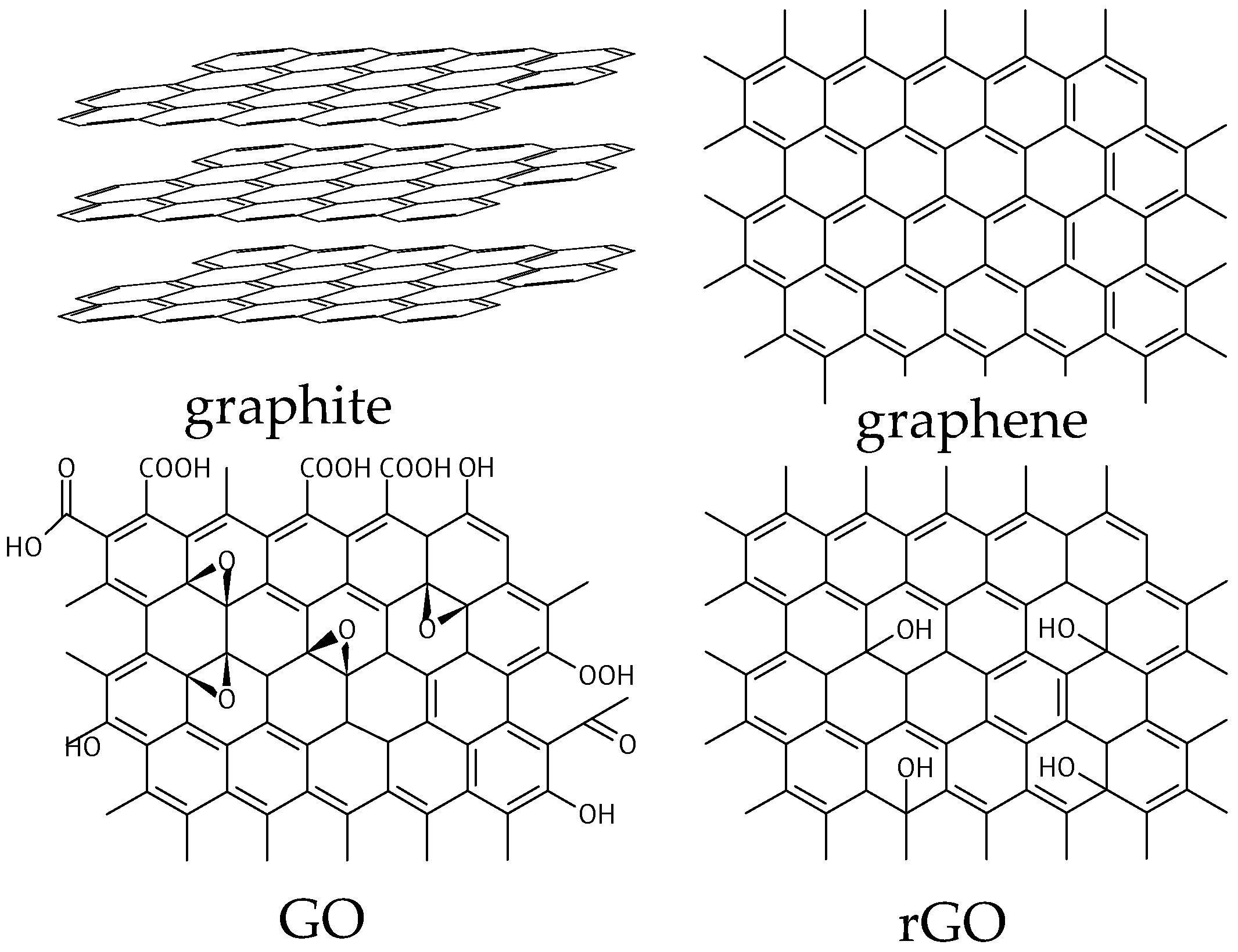
1. Introduction
2. The Applications
2.1. In Secondary Batteries
2.2. In Other Storage Systems
2.3. In Fuel Cells and Electrolyzers
3. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Qu, Q.; Fu, L.; Liu, L.; Kondratiev, V.; Holze, R. Functional graphene coatings in batteries and supercapacitors—Beyond corrosion protection. Molecules 2025, 30, 1436. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Zeng, M.; Li, J. Graphene-wrapped Cr2O3 hollow nanospheres with enhanced electrochemical performances for lithium-ion batteries. Int. J. Electrochem. Sci. 2015, 10, 7361–7370. [Google Scholar] [CrossRef]
- Ul Hoque, M.I.; Donne, S.W.; Holze, R. Graphene Nanocomposite Materials for Supercapacitor Electrodes. Encyclopedia 2024, 4, 101–116. [Google Scholar] [CrossRef]
- Kaesche, H. Die Korrosion der Metalle, 3rd ed.; Springer: Berlin, Germany, 1990. [Google Scholar]
- El-Meligi, A.A. Corrosion preventive strategies as a crucial need for decreasing environmental pollution and saving economics. Rec. Pat. Corros. Sci. 2010, 2, 22–33. [Google Scholar] [CrossRef]
- Foroulis, Z.A. Corrosion and corrosion inhibition in the petroleum industry. Mater. Corros. 1982, 33, 121–131. [Google Scholar] [CrossRef]
- Kaesche, H. The Corrosion of Metals; Springer: Berlin, Germany, 2003. [Google Scholar]
- Kelly, R.G.; Scully, J.R.; Shoesmith, D.W.; Buchheit, R.G. Electrochemical Techniques in Corrosion Science and Engineering; Marcel Dekker: New York, NY, USA, 2003. [Google Scholar]
- Groysman, A. Corrosion for Everybody; Springer: Dordrecht, The Netherlands, 2010. [Google Scholar]
- Roberge, P.R. Corrosion Engineering Principles and Practice; McGraw Hill: New York, NY, USA, 2008. [Google Scholar]
- Uhlig’s Corrosion Handbook; Revie, R.W., Uhlig, H.H., Eds.; Wiley: New York, NY, USA, 2000. [Google Scholar]
- Perez, N. Electrochemistry and Corrosion Science; Kluwer Academic Publisher: New York, NY, USA, 2004. [Google Scholar]
- Revie, R.W.; Uhlig, H.H. Corrosion and Corrosion Control; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Sastri, V.S. Green Corrosion Inhibitors; Wiley: Hoboken, NJ, USA, 2011. [Google Scholar]
- Roberge, P.R. Corrosion Basics: An Introduction, 2nd ed.; NACE International: Houston, TX, USA, 2006. [Google Scholar]
- APV-Corrosion Handbook; APV: Getzville, NJ, USA, 2008.
- Jones, D.A. Principles and Prevention of Corrosion; Prentice Hall: Upper Saddle River, NJ, USA, 1996. [Google Scholar]
- Lawal, A.T. Graphene-based nano composites and their applications. A review. Biosens. Bioelectron. 2019, 141, 111384. [Google Scholar] [CrossRef] [PubMed]
- Roberge, P.R. Corrosion Inspection and Monitoring; John Wiley & Sons: Hoboken, NJ, USA, 2007. [Google Scholar]
- Talbot, D.; Talbot, J. Corrosion Science and Technology; CRC Press: Boca Raton, FL, USA, 1998. [Google Scholar]
- Li, Y.F.; Wang, S.G.; Shi, Y.H.; Fan, C.Y.; Lin, J.; Wu, X.L.; Sun, H.Z.; Zhang, J.P.; Xie, H.M. In situ chemically encapsulated and controlled SnS2 nanocrystal composites for durable lithium/sodium-ion batteries. Dalton Trans. 2020, 49, 15874–15882. [Google Scholar] [CrossRef] [PubMed]
- Duan, Z. Application of graphene in metal corrosion protection. IOP Conf. Ser. Mater. Sci. Engin. 2019, 493, 012020. [Google Scholar] [CrossRef]
- Gergely, A. A review on corrosion protection with single-layer, multilayer, and composites of graphene. Corros. Rev. 2018, 36, 155–225. [Google Scholar] [CrossRef]
- Singh Raman, R.K.; Tiwari, A. Graphene: The thinnest known coating for corrosion protection. JOM 2014, 66, 637–642. [Google Scholar] [CrossRef]
- Akhtar, S.; Laoui, T.; Ibrahim, A.; Kumar, A.M.; Ahmed, J.; Toor, I.U.H. Few-Layers Graphene Film and Copper Surface Morphology for Improved Corrosion Protection of Copper. J. Mater. Engin. Perform. 2019, 28, 5541–5550. [Google Scholar] [CrossRef]
- Paterakis, G.; Sygellou, G.; Sygellou, L.; Galiotis, C. Protection of Aluminum Foils against Environmental Corrosion with Graphene-Based Coatings. J. Coat. Sci. Technol. 2021, 8, 18–28. [Google Scholar] [CrossRef]
- Singh Raman, R.K.; Arya, A.K.; Tomy, K.; Dip, F.A.; Lai, E.; Al-Saadi, S. Graphene coatings for corrosion resistance of nickel and copper in acidic, alkaline and neutral environments. J. Mater. Sci. Technol. 2023, 142, 124–133. [Google Scholar] [CrossRef]
- Quadri, T.W.; Olasunkanmi, L.O.; Fayemi, O.E.; Ebenso, E.E. Functionalized Carbon Allotropes as Corrosion Inhibitors. ACS Sympos. Ser. 2022, 1418, 87–114. [Google Scholar]
- Xu, X.; Yi, D.; Wang, Z.; Yu, J.; Zhang, Z.; Qiao, R.; Sun, Z.; Hu, Z.; Gao, P.; Peng, H.; et al. Greatly Enhanced Anticorrosion of Cu by Commensurate Graphene Coating. Adv. Mater. 2018, 30, 1702944. [Google Scholar] [CrossRef] [PubMed]
- Du, X.Q.; Liu, Y.W.; Chen, Y. Enhancing the corrosion resistance of aluminum by superhydrophobic silane/graphene oxide coating. Appl. Phys. A 2021, 127, 580. [Google Scholar] [CrossRef]
- Aneja, K.S.; Böhm, H.L.M.; Khanna, A.S.; Böhm, S. Functionalised graphene as a barrier against corrosion. FlatChem 2017, 1, 11–19. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Y.; Cai, H.; Shao, Y.; Xu, Z.; Wang, Y.; Wang, J. Effect of Graphene on Corrosion Resistance of Low Zinc Epoxy Coatings Applied to Low-Carbon Steel. Corrosion 2024, 80, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Hayatdavoudi, H.; Rahsepar, M. A mechanistic study of the enhanced cathodic protection performance of graphene-reinforced zinc rich nanocomposite coating for corrosion protection of carbon steel substrate. J. Alloys Compds. 2017, 727, 1148–1156. [Google Scholar] [CrossRef]
- Liu, J.; Liu, T.; Guo, Z.; Guo, N.; Lei, Y.; Chang, X.; Yin, Y. Promoting barrier performance and cathodic protection of zinc-rich epoxy primer via single-layer graphene. Polymers 2018, 10, 591. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Holze, R. Corrosion in supercapacitors—An Overview. Univ. J. Electrochem. 2023, 1, 1. [Google Scholar] [CrossRef]
- Chen, X.; Wu, Y.; Holze, R. Ag(e)ing and Degradation of Supercapacitors: Causes, Mechanisms, Models and Countermeasures. Molecules 2023, 28, 5028. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wu, Y.; Holze, R. Corrosion and Its Control in Redox-Flow Batteries. In Corrosion and Degradation in Fuel Cells, Supercapacitors and Batteries; Saji, V.S., Ed.; Springer: Cham, Switzerland, 2024; pp. 485–496. [Google Scholar]
- Chen, X.; Wu, Y.; Holze, R. Corrosion and Degradation in Supercapacitors and Mitigation Approaches. In Corrosion and Degradation in Fuel Cells, Supercapacitors and Batteries; Saji, V.S., Ed.; Springer: Cham, Switzerland, 2024; pp. 161–178. [Google Scholar]
- Ruammaitree, A.; Phokharatkul, D.; Nuntawong, N.; Wisitsoraat, A. Improvement in Corrosion Resistance of Stainless Steel Foil by Graphene Coating Using Thermal Chemical Vapor Deposition. Surf. Rev. Lett. 2018, 25, 1840003. [Google Scholar] [CrossRef]
- Schriver, M.; Regan, W.; Gannett, W.J.; Zaniewski, A.M.; Crommie, M.F.; Zettl, A. Graphene as a long-term metal oxidation barrier: Worse than nothing. ACS Nano 2013, 7, 5763–5768. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Patlolla, V.R.; Chiao, D.; Kalla, D.K.; Misak, H.; Asmatulu, R. Galvanic corrosion of Al/Cu meshes with carbon fibers and graphene and ITO-based nanocomposite coatings as alternative approaches for lightning strikes. Int. J. Adv. Manuf. Technol. 2013, 67, 1317–1323. [Google Scholar] [CrossRef]
- Roberge, P.R. Recognizing the Forms of Corrosion. In Corrosion Engineering Principles and Practice; McGraw Hill: New York, NY, USA, 2008; p. 177. [Google Scholar]
- Roberge, P.R. Handbook of Corrosion Engineering, 3rd ed.; McGraw-Hill: New York, NY, USA, 2019; p. 132. [Google Scholar]
- Yin, Q.; Wang, X.W.; Liu, S.; Wang, X.-Z.; Fu, X.Z.; Luo, J.L. High corrosion resistance of reduced graphene oxide coated 316L stainless steel bipolar plate for proton exchange membrane fuel cell prepared by a facile method. Mater. Chem. Phys. 2022, 290, 126663. [Google Scholar] [CrossRef]
- Xiao, J.; Zhai, P.; Wei, Y.; Zhang, X.; Yang, W.; Cui, S.; Jin, C.; Liu, W.; Wang, X.; Jiang, H.; et al. In-situ formed protecting layer from organic/inorganic concrete for dendrite-free lithium metal anodes. Nano Lett. 2020, 20, 3911–3917. [Google Scholar] [CrossRef] [PubMed]
- Bai, M.; Chen, Z.; Zhang, K.; Yuan, K.; Hong, B.; Lai, Y. Designable ultra-stable electrode surface engineering by the electrophoretic deposition of modified graphene oxide for rechargeable batteries. Appl. Surf. Sci. 2022, 605, 154704. [Google Scholar] [CrossRef]
- Das, A.; Sahu, D.K.; Das, S.; Mallik, A. Electrophoresed Graphene Coatings for Corrosion Prevention: A Review. Nano 2022, 17, 2230004. [Google Scholar] [CrossRef]
- Kushwaha, A.; Sharma, A.; Bhatt, B.B.; Mukhopadhyay, A.; Gupta, D. Inkjet-Printed Graphene-Modified Aluminum Current Collector for High-Voltage Lithium-Ion Battery. ACS Appl. Energy Mater. 2023, 6, 4168–4178. [Google Scholar] [CrossRef]
- Zhang, X.Q.; Wang, X.M.; Li, B.Q.; Shi, P.; Huang, J.Q.; Chen, A.; Zhang, Q. Crosstalk shielding of transition metal ions for long cycling lithium-metal batteries. J. Mater. Chem. A 2020, 8, 4283–4289. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, J.; Zhang, Y.; Wang, Z.; Chen, Z.; Gao, A.; Zhang, J.; Wang, Y.; Zhao, R. Electrostatic adsorption facilitating dual-layer coating for stabilized cathode-electrolyte interphases and boosted lithium intercalation thereof in LiNi0.8Co0.1Mn0.1O2 cathode. Appl. Surf. Sci. 2022, 577, 151716. [Google Scholar] [CrossRef]
- Ma, Q.; Hu, M.; Yuan, Y.; Pan, Y.; Chen, M.; Zhang, Y.; Long, D. Colloidal dispersion of Nb2O5/reduced graphene oxide nanocomposites as functional coating layer for polysulfide shuttle suppression and lithium anode protection of Li-S battery. J. Coll. Interf. Sci. 2020, 566, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yang, X.; Ye, J.; Xia, G.; Fu, Z.; Hu, C. A trifunctional modified separator based on Fe tetraaminophthalocyanine@rGO for lithium-sulfur batteries. Chem. Eng. J. 2021, 405, 126947. [Google Scholar] [CrossRef]
- Wang, M.; Tang, M.; Chen, S.; Ci, H.; Wang, K.; Shi, L.; Lin, L.; Ren, H.; Shan, J.; Gao, P.; et al. Graphene-Armored Aluminum Foil with Enhanced Anticorrosion Performance as Current Collectors for Lithium-Ion Battery. Adv. Mater. 2017, 29, 1703882. [Google Scholar] [CrossRef] [PubMed]
- Richard Prabakar, S.J.; Hwang, Y.H.; Bae, E.G.; Lee, D.K.; Pyo, M. Graphene oxide as a corrosion inhibitor for the aluminum current collector in lithium ion batteries. Carbon 2013, 52, 128–136. [Google Scholar] [CrossRef]
- Shi, X.; Zhang, Y.; Zhang, H.; Zhao, Z.; Liu, B.; Liu, J.; Liu, X.; Li, L. Graphene Enables Aluminum Current Collectors of 5 V Class Battery. Nano Lett. 2024, 24, 12398–12405. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, J.; Xu, Z.; Dong, Q.; Ao, H.; Hou, Z.; Qian, Y. Aqueous electrolyte with moderate concentration enables high-energy aqueous rechargeable lithium ion battery for large scale energy storage. Energy Storage Mater. 2022, 46, 147–154. [Google Scholar] [CrossRef]
- Palaniappan, N.; Cole, I.S.; Kuznetsov, A.; Thomas, K.R.J.; Balasubramanian, K.; Manickam, S. Experimental and DFT studies of gadolinium decorated graphene oxide materials for their redox properties and as a corrosion inhibition barrier layer on Mg AZ13 alloy in a 3.5% NaCl environment. RSC Adv. 2021, 11, 22095–22105. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Guo, J.; Chen, X.; Wang, T.; Huang, Y.; Gao, S.; Wang, T.; Wu, D.; Liu, K. A zincophilic molecular brush for a dendrite-free, corrosion-resistant, zinc metal anode with a long life cycle. Nano Res. 2024, 17, 390–396. [Google Scholar] [CrossRef]
- Wang, M.; Wang, Q.; Yao, H.; Su, F.; Shan, Z.; Shen, H.; Liu, T.; Zhao, J.; Ding, C. Interfacial regulation and protection by conductive graphene coating induces highly reversible zinc behavior for durable aqueous zinc-ion batteries. J. Alloys Compds. 2023, 947, 169678. [Google Scholar] [CrossRef]
- Gull, S.; Weng, C.C.; Chen, H.Y. Carbon armor for zinc anodes: Mitigating dendrite formations and unwanted side reactions in zinc-ion batteries. J. Taiwan Inst. Chem. Eng. 2024, 154, 104977. [Google Scholar] [CrossRef]
- Liu, Y.Q.; Song, Y.H.; Yang, G.D.; Zhang, J.Y.; Shen, X.Y.; Wu, X.L.; Sun, H.Z. Construction of hydrophilic and hydrophobic hybrid interface to achieve controlled zinc deposition for aqueous Zn-ion batteries. Energy Storage Mater. 2024, 72, 103761. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, A.; Hu, X.; Hu, Z.; Zhang, F.; Huang, Y.; Li, L.; Wu, F.; Chen, R. Bifunctional Dynamic Adaptive Interphase Reconfiguration for Zinc Deposition Modulation and Side Reaction Suppression in Aqueous Zinc Ion Batteries. ACS Nano 2023, 17, 11946–11956. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Li, N.; Zhang, T.L.; Feng, Q.P.; Du, Q.; Wu, X.H.; Huang, G.W. Reduced Graphene Oxide Coating with Anticorrosion and Electrochemical Property-Enhancing Effects Applied in Hydrogen Storage System. ACS Appl. Mater. Interfaces 2017, 9, 28980–28989. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Hu, H.; Lei, F.; Hu, J.; Wu, M.; Ho, D. Concurrently Realizing Geometric Confined Growth and Doping of Transition Metals Within Graphene Hosts for Bifunctional Electrocatalysts toward a Solid-State Rechargeable Micro-Zn-Air Battery. ACS Appl. Mater. Interfaces 2020, 12, 38031–38044. [Google Scholar] [CrossRef] [PubMed]
- Sarawutanukul, S.; Phattharasupakun, N.; Wutthiprom, J.; Sawangphruk, M. Oxidative chemical vapour deposition of a graphene oxide carbocatalyst on 3D nickel foam as a collaborative electrocatalyst towards the hydrogen evolution reaction in acidic electrolyte. Sustain. Energy Fuels 2018, 2, 1305–1311. [Google Scholar] [CrossRef]
- Pu, N.W.; Shi, G.N.; Liu, Y.M.; Sun, X.; Chang, J.K.; Sun, C.L.; Ger, M.D.; Chen, C.Y.; Wang, P.C.; Peng, Y.Y.; et al. Graphene grown on stainless steel as a high-performance and ecofriendly anti-corrosion coating for polymer electrolyte membrane fuel cell bipolar plates. J. Power Sources 2015, 282, 248–256. [Google Scholar] [CrossRef]
- Cui, J.; Xu, J.; Xiu, H.; Wang, H.; Li, J.; Yang, J. Graphene-Dominated Hybrid Coatings with Highly Compacted Structure on Stainless Steel Bipolar Plates. ACS Appl. Mater. Interfaces 2022, 14, 37059–37067. [Google Scholar] [CrossRef] [PubMed]
- Stoot, A.C.; Camilli, L.; Spiegelhauer, S.A.; Yu, F.; Bøggild, P. Multilayer graphene for long-term corrosion protection of stainless steel bipolar plates for polymer electrolyte membrane fuel cell. J. Power Sources 2015, 293, 846–851. [Google Scholar] [CrossRef]
- Sanjid, A.; Anisur, M.R.; Singh, R.K. Raman Durable degradation resistance of graphene coated nickel and Monel-400 as bi-polar plates for proton exchange membrane fuel cell. Carbon 2019, 151, 68–75. [Google Scholar] [CrossRef]
- Jiang, L.; Syed, J.A.; Zhang, G.; Ma, Y.; Ma, J.; Lu, H.; Meng, X. Enhanced anticorrosion performance of PPY-graphene oxide/PPY-camphorsulfonic acid composite coating for 304SS bipolar plates in proton exchange membrane fuel cell. J. Ind. Eng. Chem. 2019, 80, 497–507. [Google Scholar] [CrossRef]
- Jiang, L.; Syed, J.A.; Lu, H.; Meng, X. In-situ electrodeposition of conductive polypyrrole-graphene oxide composite coating for corrosion protection of 304SS bipolar plates. J. Alloys Compds. 2019, 770, 35–47. [Google Scholar] [CrossRef]
- Mohamed, M.E.; Abd-El-Nabey, B.A. Superhydrophobic Cobalt-Graphene Composite for the Corrosion Protection of Copper Bipolar Plates in Proton Exchange Membrane Fuel Cells. J. Electrochem. Energy Conv. Stor. 2022, 19, 031007. [Google Scholar] [CrossRef]
- Sadeghian, Z.; Hadidi, M.R.; Salehzadeh, D.; Nemati, A. Hydrophobic octadecylamine-functionalized graphene/TiO2 hybrid coating for corrosion protection of copper bipolar plates in simulated proton exchange membrane fuel cell environment. Int. J. Hydrogen Energy 2020, 45, 15380–15389. [Google Scholar] [CrossRef]
- Liu, Y.; Min, L.; Zhang, W.; Wang, Y. High-performance graphene coating on titanium bipolar plates in fuel cells via cathodic electrophoretic deposition. Coatings 2021, 11, 437. [Google Scholar] [CrossRef]
- Lee, Y.H.; Li, S.M.; Tseng, C.J.; Su, C.Y.; Lin, S.C.; Jhuang, J.W. Graphene as corrosion protection for metal foam flow distributor in proton exchange membrane fuel cells. Int. J. Hydrogen Energy 2017, 42, 22201–22207. [Google Scholar] [CrossRef]
- Sun, C.; Zuo, Q.; Hu, G.; Xia, Y. Graphene composite coating for enhanced corrosion resistance of Ni foam flow field in PEMFC. J. Alloys Compds. 2025, 1010, 177251. [Google Scholar] [CrossRef]
- Sun, C.; Hu, G.; Cao, L.; Pan, T.; Guo, C.; Xia, Y. Ni/Graphene Coating for Enhanced Corrosion Resistance of Metal Foam Flow Field in Simulated PEMFC Cathode Environment. ACS Omega 2024, 9, 29797–29804. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Sotillo, A.; Ferreira-Aparicio, P. Durable corrosion-resistant coating based in graphene oxide for cost-effective fuel cells components. iScience 2023, 26, 106569. [Google Scholar] [CrossRef] [PubMed]
- Malini, S.; Anantharaju, K.S. Nanomaterials for fuel cell and corrosion inhibition: A comprehensive review. Curr. Nanosci. 2021, 17, 591–611. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, D.; Xie, X.; Chen, X.; Holze, R. Graphenes for Corrosion Protection in Electrochemical Energy Technology. Corros. Mater. Degrad. 2025, 6, 33. https://doi.org/10.3390/cmd6030033
Liu D, Xie X, Chen X, Holze R. Graphenes for Corrosion Protection in Electrochemical Energy Technology. Corrosion and Materials Degradation. 2025; 6(3):33. https://doi.org/10.3390/cmd6030033
Chicago/Turabian StyleLiu, Dan, Xuan Xie, Xuecheng Chen, and Rudolf Holze. 2025. "Graphenes for Corrosion Protection in Electrochemical Energy Technology" Corrosion and Materials Degradation 6, no. 3: 33. https://doi.org/10.3390/cmd6030033
APA StyleLiu, D., Xie, X., Chen, X., & Holze, R. (2025). Graphenes for Corrosion Protection in Electrochemical Energy Technology. Corrosion and Materials Degradation, 6(3), 33. https://doi.org/10.3390/cmd6030033








