1. Introduction
It has been known for almost 150 years that interaction with hydrogen can drastically reduce the ductility and strength of structural steels, particularly when they exhibit high strength [
1]. The effect is referred to as hydrogen embrittlement (HE). Numerous studies further showed that the cyclic properties of steels such as the fatigue lifetime and the fatigue limit, i.e., the stress amplitude a material can withstand for a specified number of cycles, may degrade under the influence of hydrogen [
2,
3,
4,
5]. This may be crucial in applications such as hydrogen storage tanks, which experience cyclic loads due to emptying and refilling or pipelines subject to oscillating pressures. However, there are also reports of positive hydrogen effects, e.g., for austenitic steels under bending fatigue loading [
6,
7]. Steels with higher initial strength degrade typically more under hydrogen influence [
8]. Despite considerable research efforts, the mechanisms of hydrogen degradation of steels are still under debate [
9]. For structural materials, which do not form hydrides, the hydrogen-enhanced decohesion theory (HEDE, also known as HID, hydrogen-induced decohesion) and the hydrogen-enhanced local plasticity (HELP) theory are considered as most viable [
10,
11]. The HEDE mechanism assumes that hydrogen reduces the cohesive energy along crystallographic planes, thus facilitating brittle fracture [
12]. The HELP mechanism states that hydrogen promotes dislocation motion and thus plastic strain localization [
13], which in turn reduces the fatigue strength [
14]. Once monoatomic hydrogen has diffused into a metallic material, it may be trapped at open volume defects, e.g., inclusions, cracks, grain boundaries, retained austenite or dislocations [
15,
16,
17,
18,
19]. The traps can be classified into reversible (shallow) traps acting as either sink or source of diffusible hydrogen and irreversible (deep) traps acting only as hydrogen sinks [
15].
The hypereutectic steel SAE 52100 is typically applied in rolling bearings as rolling elements but also in highly loaded shafts. Both rolling elements and shafts may uptake hydrogen during production processes [
20], during service in hydrogen-containing atmospheres, or through hydrogen-containing lubricants [
21]. Karsch et al. and Ogawa et al. have shown that under tension-compression high frequency loading, the fatigue lifetimes as well as the fatigue limit in the high and very high cycle regime of SAE 52100 in martensitic state significantly reduce upon electrolytic hydrogen charging [
8,
22]. Both studies applied axial fatigue loading, where the stress amplitude is homogeneous throughout the volume and cracks initiated mainly subsurface. However, for typical shaft components subjected to rotating bending fatigue under hydrogen influence, the maximum stress amplitude occurs only at the surface, while the volume sees lower stress amplitudes. Since hydrogen diffusion will act also mainly near the surface, the lifetime of such shafts may be rather limited by surface crack initiation. With this study, we report on the influence of hydrogen charging on SAE 52100 under near service rotating bending fatigue. In addition to a standard heat treatment state annealed at 180 °C, we test also a softer state annealed at 400 °C. The soft state is expected to exhibit a lower dislocation density and thus a better resistance against hydrogen degradation. Further, we address the effects of hydrogen effusion on the fatigue resistance. Already, Johnson showed that a hydrogen-degraded material may restore its original properties upon aging at room temperature, which allows the hydrogen to effuse to the atmosphere containing a lower hydrogen partial-pressure [
1]. Murakami et al. showed for a quenched and tempered steel SCM435 that continuous hydrogen effusion through aging at room temperature after charging restores the fatigue lifetime gradually [
23]. Here, we investigate this behavior for SAE51200.
2. Materials, Specimens and Experimental Methods
Hypereutectoid steel SAE 52100 (100Cr6, JIS SUJ2) raw material was supplied in the form of rolled round bars with a diameter of 14 mm.
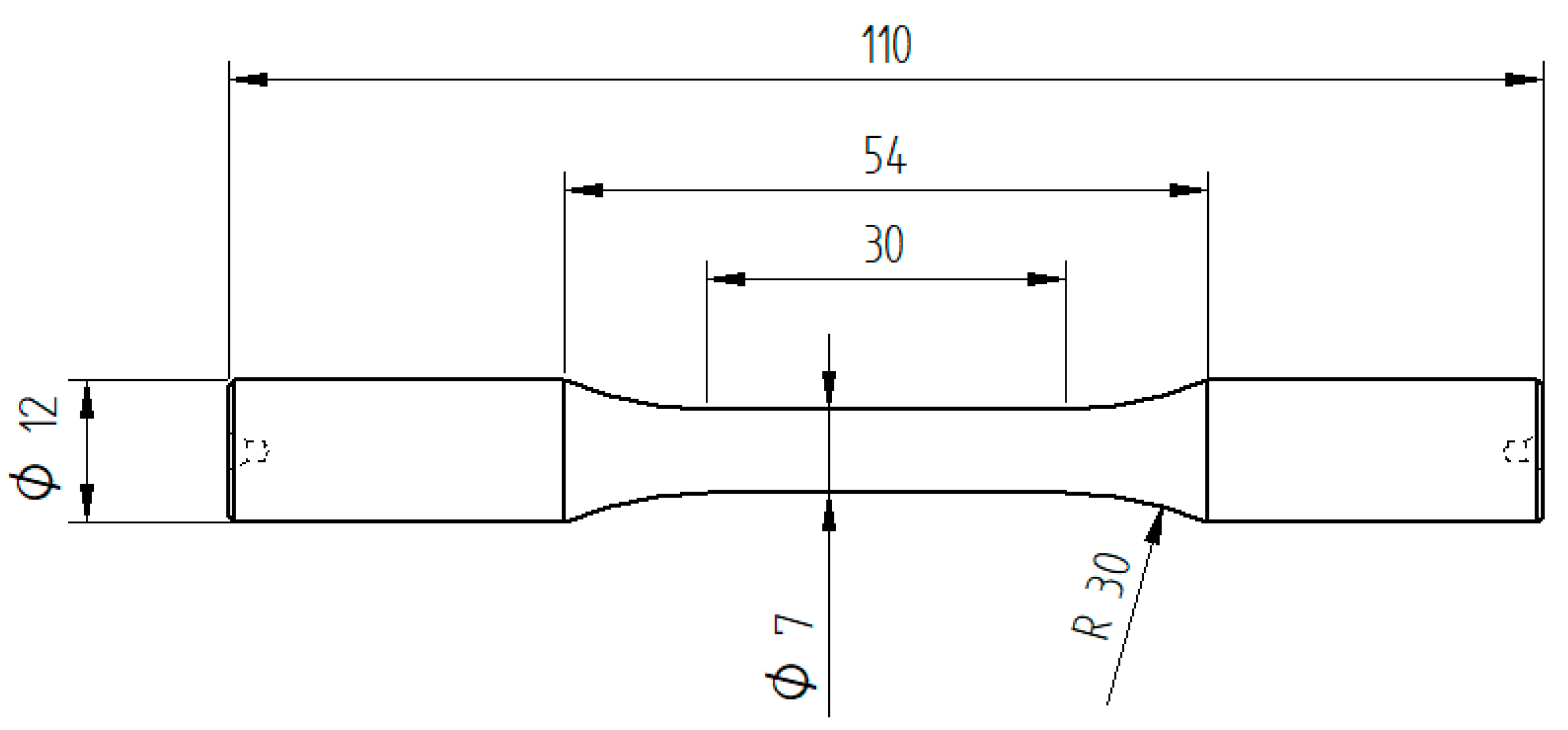
Table 1 shows the chemical composition of the bars measured by optical emission spectroscopy. From the raw material, near-net-shape specimens were machined for the heat treatment, which consisted of austenitization at 850 °C (1123 K) for 20 min, oil-quenching to 60 °C and subsequent tempering for 120 min at either 180 °C (453 K) (treatment A) or 400 °C (673 K) (treatment B). After the heat treatment, the raw specimens were machined to the final dog-bone-shaped rotating bending fatigue specimens with a cylindrical gauge length of 30 mm with 7 mm diameter. The specimen geometry is shown in
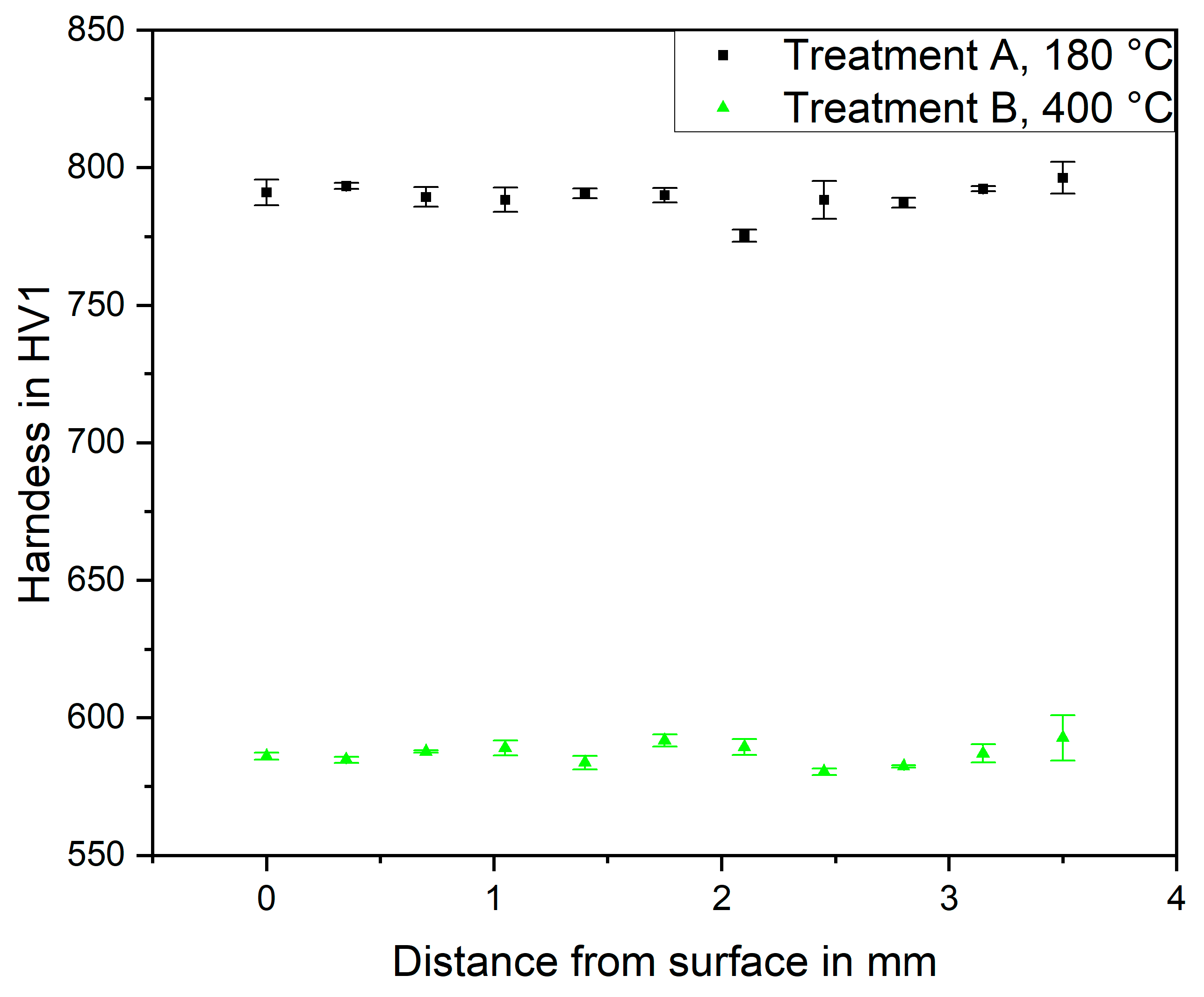
Figure 1. The gauge length surfaces were ground using a sequence of #1000, #2000 and #4000 grit silicon carbide and afterwards cleaned with acetone. After this finish, hardness profiles were measured across specimen cross sections that were prepared from the gauge lengths using a Vickers-type microhardness tester Qness Q10 A+ with an indentation load of 1 kg (HV 1.0). For each heat treatment state, three specimens were measured. The results are shown in
Figure 2. For both treatments, the hardness is homogeneous across the gauge diameter, showing barely scatter, indicating that decarburization has not occurred during the heat treatment. The resulting mean hardness values were 789 ± 5 HV1 for treatment A and 587 ± 4 HV1 for treatment B. While treatment A is a standard for SAE 52100 resulting in a martensitic microstructure, treatment B leads to a tempered microstructure, which was chosen because of the possibly higher resistance to hydrogen-induced degradation of the fatigue resistance due to the lower hardness [
8]. For hydrogen content measurements after charging, 5 × 5 × 5 mm cube specimens were machined from the same bar material and heat-treated using the treatment B parameters. These samples then underwent the same grinding routine as the fatigue specimens.
Hydrogen charging was performed cathodically with a precision current source meter of type 2601 by Keithely. The specimens were charged in an aqueous solution of 0.5-molar H
2SO
4 (sulphuric acid) using a platinum counter-electrode at a current density of 1.3
for 2 h at 20 °C. Details to the charging method can be, e.g., found in [
8]. To avoid chemical attack of the specimens by the sulphuric acid solution, the current source was activated before placing the specimens in the electrolyte. In this way, the specimen was protected using impressed current cathodic protection. SEM observation did not show any visible damage on the specimen surfaces after charging. Using the hydrogen diffusion coefficient of hardened SAE 52100 at room temperature of D
eff = 4.9 × 10
−12 m
2/s measured by Kürten et al. [
24], the hydrogen penetration depth l after charging can be estimated by the one-dimensional Fick’s law (
) resulting in l of the order of 250 µm for treatment A specimens. The diffusion coefficient of hydrogen in hardened steel alloyed with about 1 wt. % C increases by about an order of magnitude upon tempering at 400 °C [
25]. Hence, for the treatment B specimens, the estimated hydrogen penetration depth should be of the order of 750 µm. In both cases, the hydrogen penetrates only the surface area of the gauge length, which corresponds to the subsequent rotating bending fatigue testing where the maximum stress occurs at the surface, while the core region sees only lower stresses.
The fatigue tests were conducted on a 100 Nm moment-controlled rotating bending machine at room temperature in laboratory air. The test setup is shown in
Figure 3 and allows for loading with constant bending moments using a servoelectric motor and a force sensor. Owing to the rotating bending test setup, the tests were carried out at a load ratio of R = −1, i.e., with zero mean stress. The testing frequency was 50 Hz, which corresponds to a revolution speed of 3000 rpm. The maximum cycle number was set to 10
7 after which, in case no previous fracture occurred, the cycling was stopped and the specimens considered as runout. For the hydrogen-charged specimens, fatigue testing was started within 15 min after the charging process. Using the above-mentioned diffusion coefficients [
24,
25], the estimated hydrogen diffusion depth during 10
7 fatigue cycles (2 × 10
5 s, 55 h) is about 1.4 mm for state A, while for state B, it is larger than the specimen radius, i.e., full penetration. Further, effusion fatigue tests were conducted, where some specimens were deliberately aged at room temperature for 24, 48 and 72 h between hydrogen charging and fatigue testing.
The cube specimens for hydrogen content measurements were charged using the same parameters as for the fatigue specimens. The measurements were carried out on a G8 GALILEO ONH carrier gas hot extraction device by Bruker immediately either after charging or after different aging periods. Additionally, non-charged samples were tested in an ELEMENTRAC ONH device by Eltra to obtain the base level hydrogen content after manufacturing.
The fracture surfaces of the broken rotating bending fatigue specimens were investigated using a Zeiss Evo 50 scanning electron microscope (SEM) with an acceleration voltage of 20 kV.
3. Results and Discussion
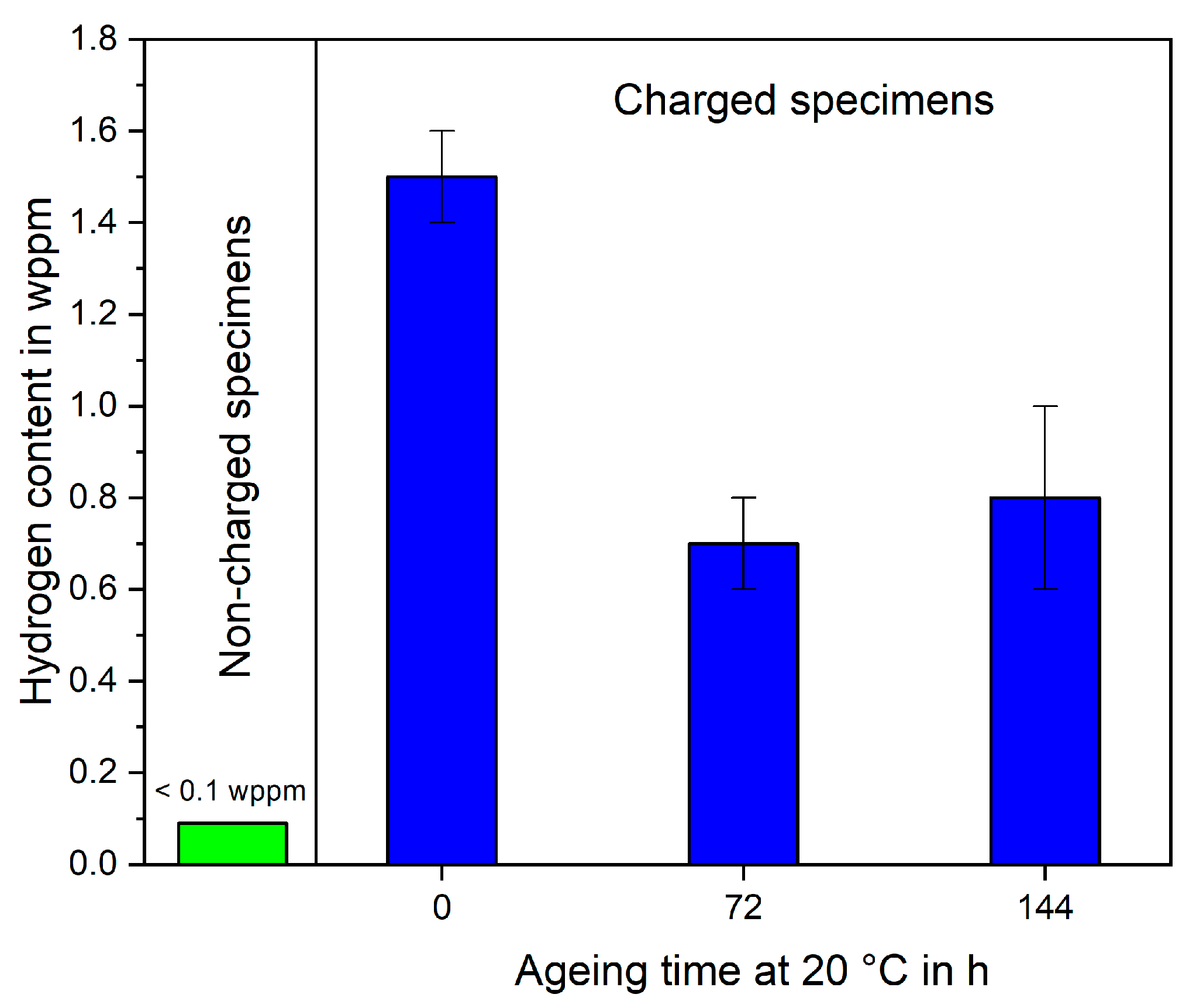
Figure 4 shows the measured hydrogen content of SAE 52100 treatment B cube specimens in the non-charged state as well as hydrogen-charged for 2 h at 20 °C. The non-charged state showed hydrogen contents below 0.1 wppm, thus any further increase in hydrogen content is assumed to be due to the electrolytic hydrogen charging. The non-charged hydrogen content is lower than typical literature values of quenched and tempered SAE 52100, which are in the range of 0.6 to 0.7 wppm [
8,
26]. However, these values were derived for material tempered at temperatures around 200 °C, while the specimens measured here were tempered at 400 °C. Apparently, the hydrogen content from the as-hardened state effuses mostly during tempering at 400 °C, indicating that this content is either freely movable or reversibly trapped. This finding agrees well with thermal desorption measurements [
27,
28]. For the treatment A fatigue specimens, an initial hydrogen content in the range of 0.7 wppm can be assumed.
Considering that the estimated penetration depth directly after charging is only about 250 µm for treatment A and 750 µm for treatment B, the effective local hydrogen content in the penetrated volume amounts to about 5.5 wppm and 2.25 wppm, respectively. After 72 h of aging, the estimated hydrogen penetration depth to the sample interior will cover more than 90% of the cube volume even for treatment A. Hence, for the charged and aged specimens, the measured and effective hydrogen content are nearly identical. The evolution of the hydrogen content during aging indicates a rather rapid effusion directly after charging followed by a stable state. A similar evolution was already reported by Karsch et al. 2014 for the same material annealed at 180 °C (treatment A) [
8]. Contrary to this, Murakami and Nagata observed a continuous decrease in hydrogen content for the quenched and tempered steel SCM435 [
23]. Notably, the stable state hydrogen content in charged specimens after aging measured here is similar to that reported for non-charged SAE 52100 when annealed at temperatures around 200 °C [
8,
26]. This indicates that for a heat treatment at around 200 °C, the (at this temperature) sessile hydrogen content remains while all the mobile hydrogen is released.
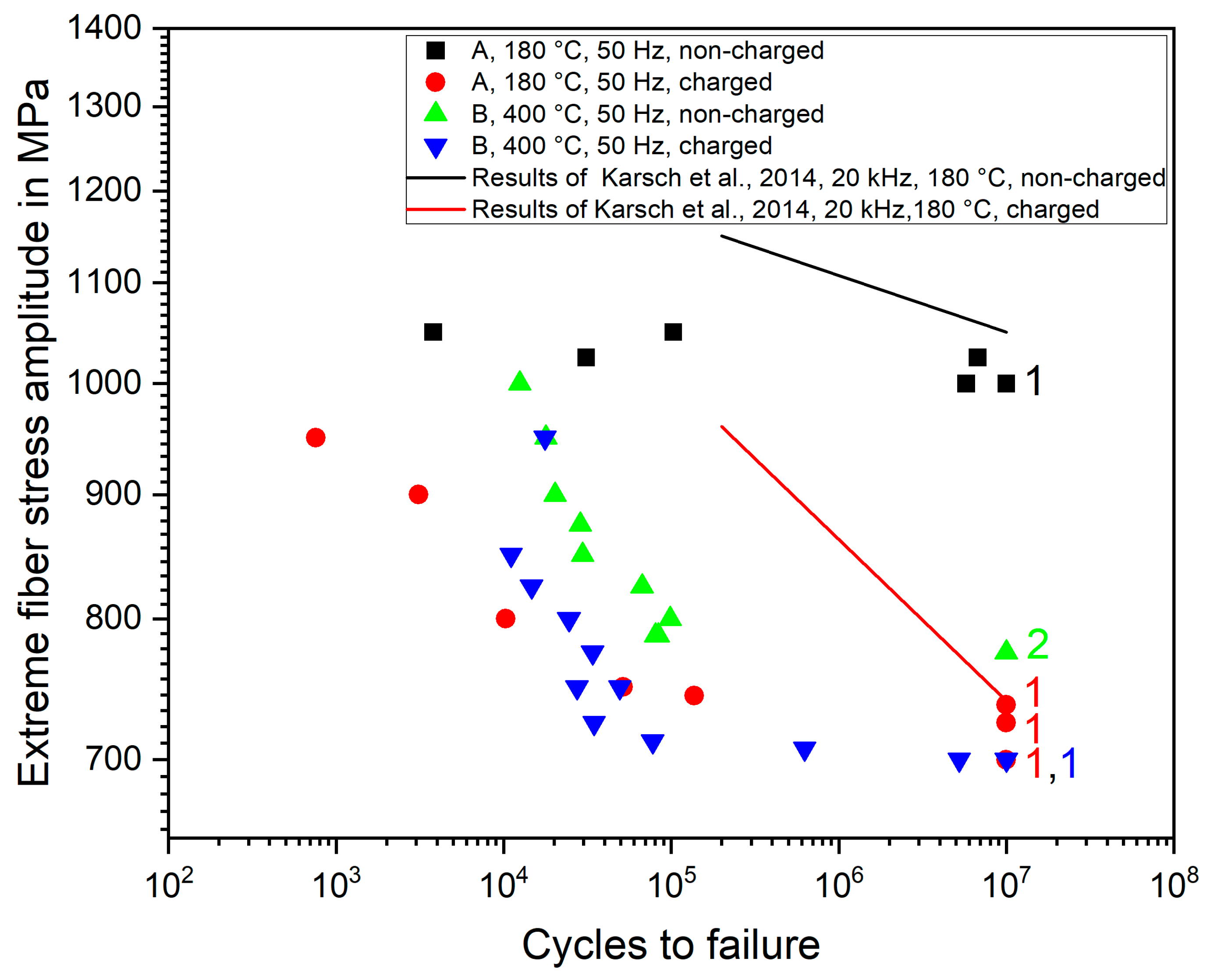
Figure 5 shows the fatigue lifetimes dependent on stress amplitude for treatment A and treatment B specimens in non-charged and charged state, respectively. The numbers next to the specimens indicate the number of runouts at a given load amplitude. For comparison, the life curves obtained by Karsch et al. [
8] for the same material in treatment A state are also given. As expected, hydrogen charging reduces both lifetime and fatigue limit for both annealing states. While for treatment A specimens, the fatigue limit reduces by nearly 30%, treatment B specimens exhibit a fatigue limit drop of about 10%. When comparing both treatments in the charged state, the fatigue limit of treatment A specimens is slightly higher, while the fatigue lifetimes fall into a common scatter band. Hence, under hydrogen-influenced cyclic loading, both material states show nearly equivalent fatigue resistance and the higher initial strength of treatment A specimens degrades almost completely due to hydrogen. Notably, for treatment A specimens, hydrogen charging increases the slope of the life curve significantly. This goes along with a transition of crack initiation from the surface for non-charged specimens to cracking from near-surface oxidic agglomerates containing high amounts of Al, Ca, Si and Mg. Karsch et al. observed a similar increase in cracking from oxidic agglomerates upon hydrogen charging under push–pull loading at 20 kHz [
8]. Apparently, hydrogen particularly weakens the fatigue resistance around such inclusions [
29]. However, Murakami et al. found the opposite transition for 0.7C-13Cr martensitic steel, i.e., non-charged specimens initiated cracks from non-metallic inclusions while hydrogen-charged specimens showed crack initiation from the surface [
4]. This suggests varying influences of hydrogen on the fatigue resistance of the surface and of inclusions depending on the inclusion type as well as on the matrix state. For SAE 51200, Karsch et al. found mainly cracking from Cr-carbides for the non-charged material [
8], while we observed only surface cracking. This may either be related to the inhomogeneous rotating bending fatigue stress state featuring the highest stresses directly at the surface or to the much lower loading frequency case leading to higher plasticity in our case [
30,
31], particularly near the surface. Notably, the here found lifetimes of treatment A specimens in both non-charged and charged states, are significantly shorter than those reported by Karsch et al., particularly at higher stress amplitudes [
8]. We assume that this is again due to the 400-fold lower test frequency in our case which gives dislocations more time to slip leading to higher plastic strain amplitudes at a given stress amplitude [
32]. At lower stress amplitudes, the plastic deformation reduces and the influence of test frequency diminishes resulting in almost similar values of the fatigue limit at 10
7 cycles in both studies. For charged specimens, the lower testing frequency and thus higher plastic deformation in our case may further allow for intensified interaction between slipping dislocations and diffusing hydrogen atoms according to the HELP mechanism. Consequently, localized plasticity may increase, particularly at defects such as oxidic agglomerates or fatigue crack tips, which reduces the cycles to crack initiation and increases the fatigue crack growth rate. Interestingly, the lifetimes of charged specimens obtained by Karsch et al. scatter significantly, spanning about three orders of magnitude [
8], while our results fall into a rather narrow scatter band. We assume that the higher plasticity in our experiments in combination with the HELP mechanism [
25] provides the necessary plastic strain localization to induce early fatigue cracks at near-surface oxidic agglomerates of several sizes eliminating most of the scatter. Conversely, for testing at 20 kHz, plasticity and thus plastic strain localization is less pronounced. In this case, fatigue cracks can only form at the largest oxidic agglomerates leading to considerable lifetime scatter. Consequently, fatigue lifetimes under hydrogen influence may be significantly overestimated when the prediction is based on high-frequency fatigue experiments.
For treatment B specimens, all observed cracks initiated from the surface in both charged and non-charged states. This explains the similar slope of the life curves and indicates a higher resistance against cracking from inclusions for the softer material state upon hydrogen charging.
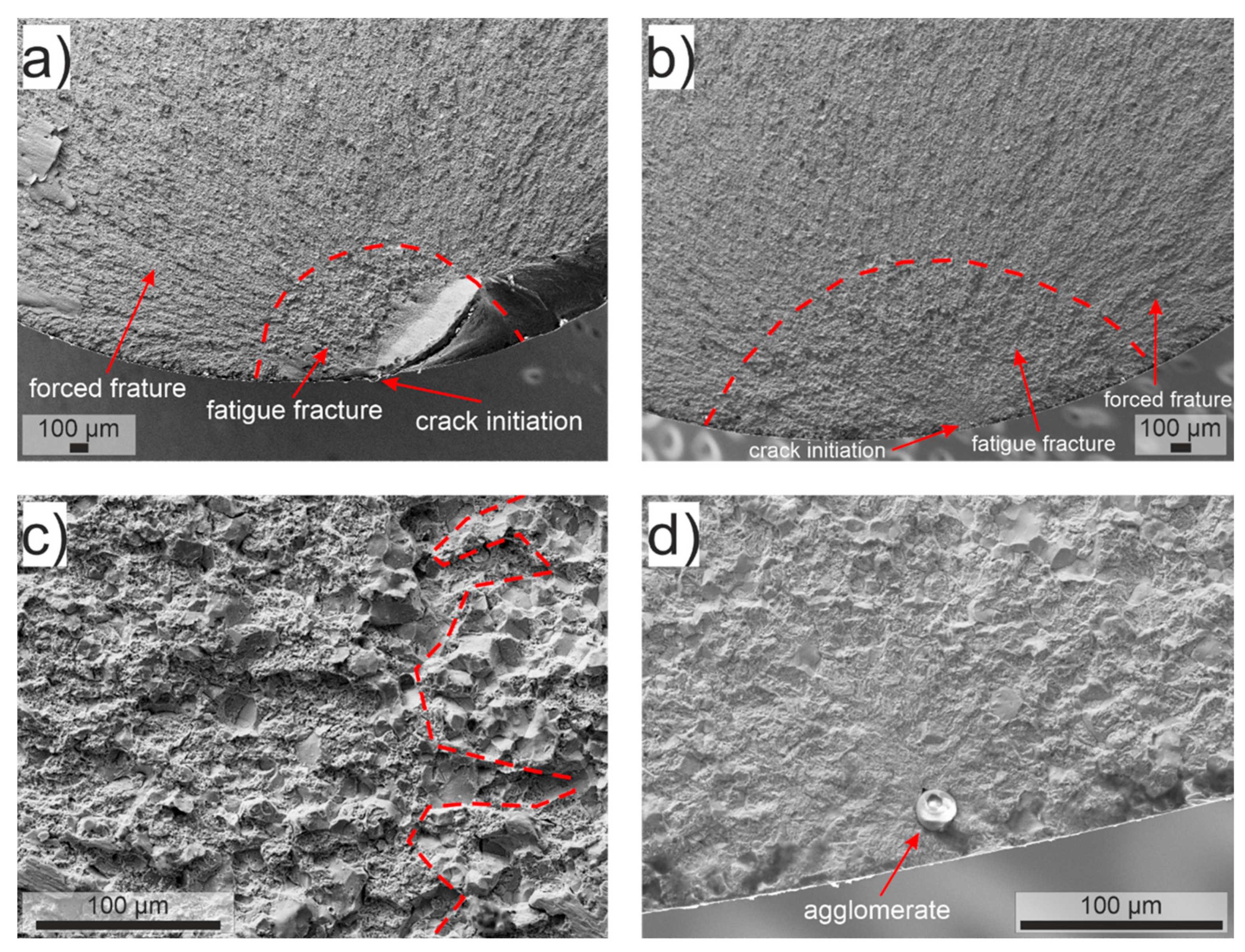
Figure 6 shows representative pictures of fracture surfaces of fatigue-tested specimens. The transition from fatigue fracture to forced fracture is always well visible. As can be seen from
Figure 6a,b, the form of the fatigue fracture surface of treatment A specimens changes from circular to elliptic upon hydrogen charging. A similar but less pronounced trend was observed for treatment B specimens. Considering that for the typically short lifetimes of charged specimens, only the outer areas of the specimens are penetrated by hydrogen, the shape transition of the fatigue fracture surface presumably reflects the higher fatigue crack propagation rate in the hydrogen-affected near-surface volume [
4,
22,
31]. Treatment A specimens showed in both charged and non-charged states a mixed intergranular and transgranular fatigue fracture mode, indicating that hydrogen charging did not promote grain boundary cracking (
Figure 6c,d). As already discussed above,
Figure 6d shows that for the hydrogen-charged treatment A state, fatigue cracks originated mainly from near-surface non-metallic agglomerates. For treatment B specimens, the dominant fatigue fracture mode was transgranular, which did not change upon hydrogen charging.
In summary, hydrogen charging did not affect the fatigue fracture mode; however, it increased the fatigue crack propagation rate in the hydrogen-affected near-surface volume. In the case of treatment A specimens, it promoted crack initiation from near-surface non-metallic agglomerates such as the mentioned oxidic particles.
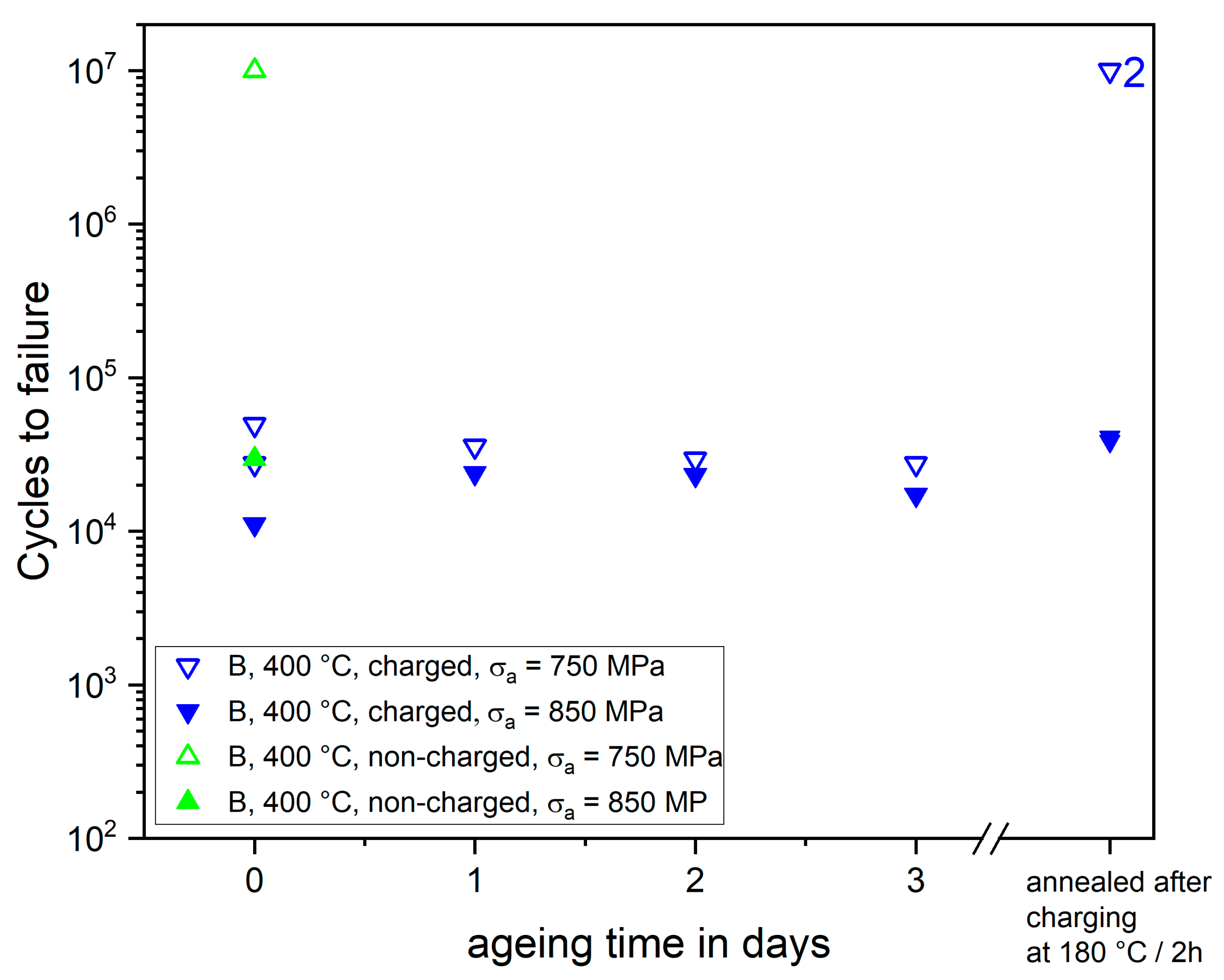
Figure 7 shows how aging at room temperature and annealing at 180 °C for 2 h influences the fatigue lifetimes of hydrogen-charged treatment B specimens. Although the effective hydrogen content in the high-stressed volume decreases significantly during room temperature (see
Figure 4) and reaches a level, which is similar to that of non-charged quenched and annealed material [
8,
25], the fatigue lifetimes remain in the scatter band of charged specimens. This is contrary to observations of Murakami and Nagata [
23], who found that the fatigue lifetimes of hydrogen-charged quenched and tempered steel SCM435 recover gradually upon hydrogen effusion. Interestingly, annealing at 180 °C for 2 h restores the fatigue life of charged specimens to typical values of non-charged specimens. This implies that the hydrogen-induced material degradation is fully reversible. Hence, the charging itself does not cause damage in the form of cracks as it was found by Tiegel et al. [
29], who used much more severe charging conditions. The reversibility of the hydrogen-induced lifetime reduction suggests that the damaging effect of hydrogen is based on small amounts of hydrogen in shallow traps, while the overall hydrogen content may decrease due to effusion. In the case of the treatment B material, which initiates fatigue cracks mainly from the surface, the critical shallow traps are presumably dislocation cores. When trapped at these, hydrogen atoms can promote plasticity according to the HELP mechanisms facilitating slip band formation and fatigue crack initiation [
14]. We assume that the annealing at 180 °C provides the necessary activation energy to release the hydrogen atoms from the dislocation cores and allow them to diffuse out. Consequently, the HELP mechanism does not operate anymore and the fatigue lifetime is restored to that of non-charged specimens. Szost et al. also suggested that the HELP mechanism governs HE in SAE52100 steel [
26]. The fact that fatigue fracture mode does not change upon hydrogen charging supports that the HEDE mechanism does not play a decisive role here. In summary, once SAE52100 has been contaminated with hydrogen, aging and hydrogen effusion at room temperature does not improve fatigue resistance. However, an annealing treatment may restore the original fatigue resistance, as long as no damage, e.g., in the form of cracks has formed.