Influence of H2S and CO2 Partial Pressures and Temperature on the Corrosion of Superduplex S32750 Stainless Steel
Abstract
1. Introduction
2. Materials and Methods
- Upstream oil and gas systems
- Electrolytes in water
- Mixtures involving water, salts, and hydrocarbons
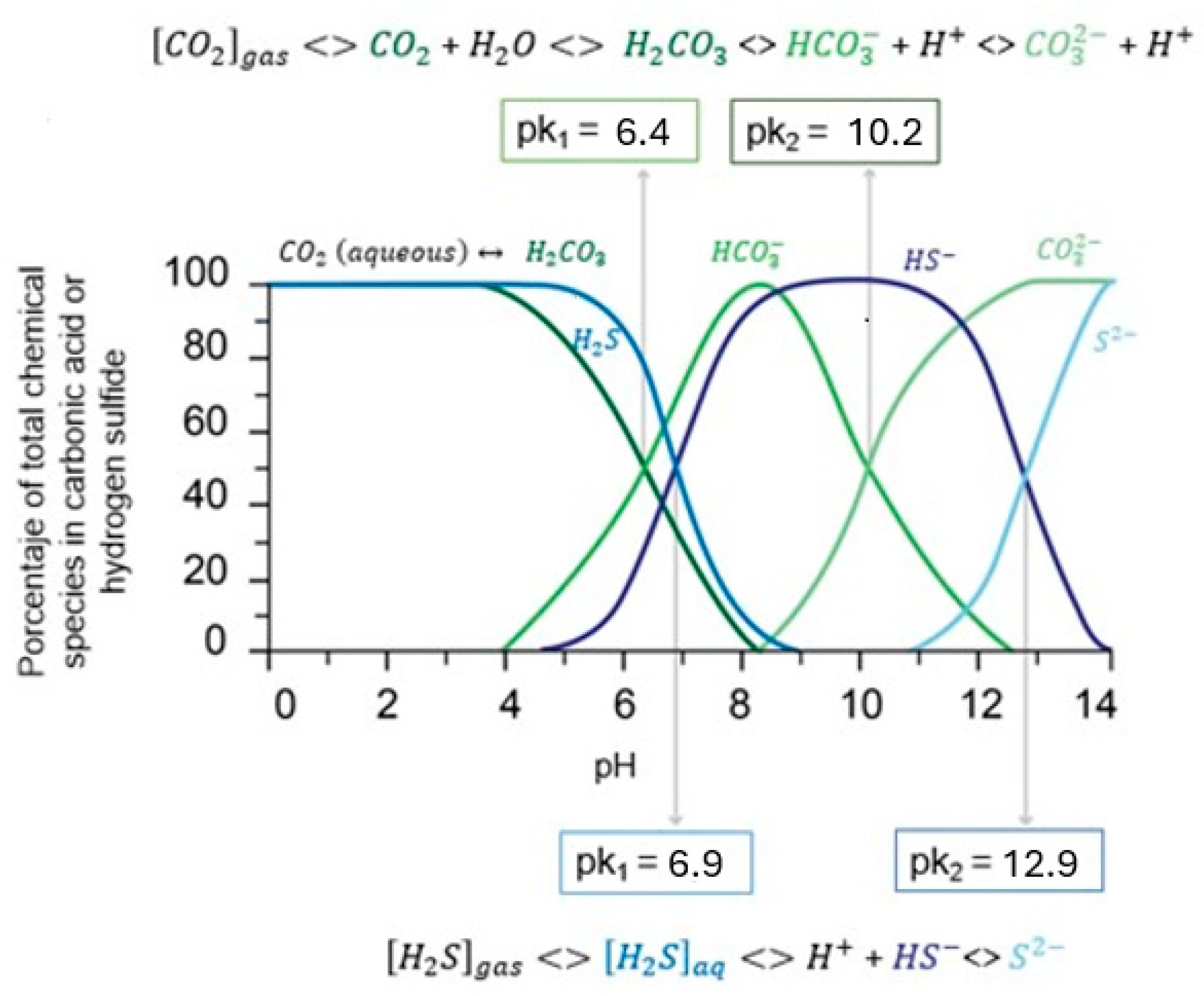
- Hydrocarbons mixed with water, salts, acid gases, and other light components (e.g., light hydrocarbons, CO2, H2S, and N2)
3. Effect of CO2, H2S, and Temperature
3.1. Influence of CO2
3.2. Influence of H2S
3.3. Influence of CO2 and H2S
3.4. Prediction of Corrosion Products and Rates Using OLI Study
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fowler, A.M.; Macreadie, P.I.; Jones, D.O.B.; Booth, D.J. A multi-criteria decision approach to decommissioning of offshore oil and gas infrastructure. Ocean Coast. Manag. 2014, 87, 20–29. [Google Scholar] [CrossRef]
- Canepa, E.; Stifanese, R.; Merotto, L.; Traverso, P. Corrosion behaviour of aluminium alloys in deep-sea environment: A review and the KM3NeT test results. Mar. Struct. 2018, 59, 271–284. [Google Scholar] [CrossRef]
- Chen, S.; Hartt, W.; Wolfson, S. Deep Water Cathodic Protection: Part 2-Field Deployment Results. Corrosion 2003, 59, 721–732. [Google Scholar] [CrossRef]
- Javidi, M.; Horeh, S.B. Investigating the mechanism of stress corrosion cracking in near-neutral and high pH environments for API 5L X52 steel. Corros. Sci. 2014, 80, 213–220. [Google Scholar] [CrossRef]
- Kampmann, P.; Kirchner, F. Towards a fine-manipulation system with tactile feedback for deep-sea environments. Robot. Auton. Syst. 2015, 67, 115–121. [Google Scholar] [CrossRef]
- Kan, B.; Wu, W.; Yang, Z.; Zhang, X.; Li, J. Effects of hydrostatic pressure and pH on the corrosion behavior of 2205 duplex stainless steel. J. Electroanal. Chem. 2021, 886, 115134. [Google Scholar] [CrossRef]
- Li, M.C.; Cheng, Y.F. Corrosion of the stressed pipe steel in carbonate–bicarbonate solution studied by scanning localized electrochemical impedance spectroscopy. Electrochim. Acta 2008, 53, 2831–2836. [Google Scholar] [CrossRef]
- Song, F.M. Predicting the mechanisms and crack growth rates of pipelines undergoing stress corrosion cracking at high pH. Corros. Sci. 2009, 51, 2657–2674. [Google Scholar] [CrossRef]
- Askari, M.; Aliofkhazraei, M.; Jafari, R.; Hamghalam, P.; Hajizadeh, A. Downhole corrosion inhibitors for oil and gas production —A review. Appl. Surf. Sci. Adv. 2021, 6, 100128. [Google Scholar] [CrossRef]
- Tems, R.D. Downhole corrosion. In Trends in Oil and Gas Corrosion Research and Technologies; El-Sherik, A.M., Ed.; Woodhead Publishing: Cambridge, UK, 2017; pp. 79–94. [Google Scholar] [CrossRef]
- Venkatesan, R.; Venkatasamy, M.A.; Bhaskaran, T.A.; Dwarakadasa, E.S.; Ravindran, M. Corrosion of ferrous alloys in deep sea environments. Br. Corros. J. 2002, 37, 257–266. [Google Scholar] [CrossRef]
- Nesic, S.; Zheng, Y.; Jing, N.; Esmaeely, S.N.; Ma, Z. Freecorptm 2.0. Theoretical Background and Verification; Institute for Corrosion and Multiphase Technology: Athens, OH, USA, 2018; p. 101. [Google Scholar]
- Zhang, W.; Brown, B.; Young, D.; Bota, G.; Nesic, S.; Singer, M. Pitting mechanism of mild steel in marginally sour environments—Part I: A parametric study based on formation of protective layers. Corros. Sci. 2021, 183, 109305. [Google Scholar] [CrossRef]
- ISO 15156-3:2020; Petroleum and Natural Gas Industries—Materials for Use in H2S-Containing Environments in Oil and Gas Production—Part 3: Cracking-Resistant CRAs (Corrosion-Resistant Alloys) and Other Alloys. International Organization for Standardization (ISO): Geneva, Switzerland, 2020.
- Gao, S.; Jin, P.; Brown, B.; Young, D.; Nesic, S.; Singer, M. Corrosion behavior of mild steel in sour environments at elevated temperatures. Corrosion 2017, 73, 915–926. [Google Scholar] [CrossRef]
- Genchev, G.; Erbe, A. Raman spectroscopy of mackinawite FeS in anodic iron sulfide corrosion products. J. Electrochem. Soc. 2016, 163, C333. [Google Scholar] [CrossRef]
- Ning, J.; Zheng, Y.; Brown, B.; Young, D.; Nesic, S. The role of iron sulfide polymorphism in localized H2S corrosion of mild steel. Corrosion 2016, 73, 155–168. [Google Scholar] [CrossRef]
- Rickard, D.; Luther, G.W. Chemistry of iron sulfides. Chem. Rev. 2007, 107, 514–562. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Nešić, S.; Yang, Y.; Brown, B. Spontaneous passivation observations during scale formation on mild steel in CO2 brines. Electrochim. Acta 2011, 56, 5396–5404. [Google Scholar] [CrossRef]
- Banaś, J.; Mazurkiewicz, B.; Lelek-Borkowska, U.; Krawiec, H.; Solarski, W.; Kowalski, K. The effect of CO2 and H2S on the passivation of chromium and stainless steels in aqueous solutions at elevated temperature and under high pressure. In Passivation of Metals and Semiconductors, and Properties of Thin Oxide Layers; Marcus, P., Maurice, V., Eds.; Elsevier Science: Amsterdam, The Netherlands, 2006; pp. 417–423. [Google Scholar] [CrossRef]
- Bale, C.W.; Bélisle, E.; Chartrand, P.; Decterov, S.A.; Eriksson, G.; Hack, K.; Jung, I.-H.; Kang, Y.-B.; Melançon, J.; Pelton, A.; et al. FactSage thermochemical software and databases—Recent developments. Calphad 2009, 33, 295–311. [Google Scholar] [CrossRef]
- Tayactac, R.G.; Basilia, B. Corrosion in the Geothermal Systems: A Review of Corrosion Resistance Alloy (CRA) Weld Overlay Cladding Application. IOP Conf. Ser. Earth Environ. Sci. 2022, 1008, 12018. [Google Scholar] [CrossRef]
- Waseda, Y.; Suzuki, S. (Eds.) Characterization of Corrosion Products on Steel Surfaces; Springer: Berlin, Germany, 2006. [Google Scholar] [CrossRef]
- Wilbraham, A.; Staley, D.; Waterman, E.; Matta, M. Pearson Chemistry, Florida; Pearson: London, UK, 2012. [Google Scholar]
- Haghi, R.K.; Chapoy, A.; Peirera, L.M.C.; Yang, J.; Tohidi, B. pH of CO2 saturated water and CO2 saturated brines: Experimental measurements and modelling. Int. J. Greenh. Gas Control 2017, 66, 190–203. [Google Scholar] [CrossRef]
- Peng, C.; Crawshaw, J.P.; Maitland, G.C.; Martin Trusler, J.P.; Vega-Maza, D. The pH of CO2-saturated water at temperatures between 308K and 423K at pressures up to 15MPa. J. Supercrit. Fluids 2013, 82, 129–137. [Google Scholar] [CrossRef]
- Engel, T.; Bastida Pascual, A.; Hehre, W.; Reid, P.; Requena Rodríguez, A.; Zuñiga Román, J. Química Física; Pearson Addison Wesley: Madrid, Spain, 2006. [Google Scholar]
- Revie, R.W.; Uhlig, H.H. Corrosion and Corrosion Control: An Introduction to Corrosion Science and Engineering, 4th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2010. [Google Scholar]
- Pedeferri, P. Corrosion Science and Engineering; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Furukawa, T.; Inagaki, Y.; Aritomi, M. Corrosion behavior of FBR structural materials in high temperature supercritical carbon dioxide. J. Power Energy Syst. 2010, 4, 252–261. [Google Scholar] [CrossRef]
- Cardoso, J.L.; Mandel, M.; Krüger, L.; Herculano, L.F.G.; Lima Neto, P.D.; Silva, M.J.G.D. Corrosion behavior of austenitic stainless steels in CO2 saturated synthetic oil field formation water. Mater. Res. 2019, 22, e20180334. [Google Scholar] [CrossRef]
- Costa EVda Mesquita, T.J.; Ferreira, A.; Nogueira, R.P.; Bastos, I.N. Effect of carbon dioxide and temperature on passive film parameters of superduplex stainless steel. Mater. Res. 2013, 16, 929–936. [Google Scholar] [CrossRef]
- Liu, H.; Hua, Y.; Shi, S.; Lin, X.; Neville, A.; Wang, Y.; Sun, J. Stability of passive film and pitting susceptibility of 2205 duplex stainless steel in CO2/H2S-containing geothermal environment. Corros. Sci. 2023, 210, 110832. [Google Scholar] [CrossRef]
- De Waard, C.; Lotz, U.; Milliams, D.E. Predictive model for CO2 corrosion engineering in wet natural gas pipelines. Corrosion 1991, 47, 976–985. [Google Scholar] [CrossRef]
- Sedriks, J. Corrosion of Stainless Steels; John Wiley & Sons: New York, NY, USA, 1996. [Google Scholar]
- Revie, R.W.; Uhlig, H.H. Corrosion and Corrosion Control: An Introduction to Corrosion Science and Engineering, 3rd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Zhang, G.; Jin, J.; Wang, Z.; Ren, Y.; Zhang, L. Corrosion behavior of 2507 super duplex stainless steel in H2S-free and H2S-containing acidic environments. J. Mater. Eng. Perform. 2023, 32, 1185–1195. [Google Scholar] [CrossRef]
- Zhu, P.; Zou, H.; Wang, Q.; Yang, J.; Yu, Y. Local Corrosion Behavior of 5 Stainless Steels in Medium Containing H2S, CO2 Oil and Water. In Proceedings of the AMPP Corrosion, San Antonio, TX, USA, 6–10 March 2022. [Google Scholar]
- Wang, Z.; Zhang, L.; Tang, X.; Cui, Z.Y.; Xue, J.P.; Lu, M.X. Investigation of the deterioration of passive films in H2S-containing solutions. Int. J. Miner. Metall. Mater. 2017, 24, 943–953. [Google Scholar] [CrossRef]
- Dugstad, A.; Chojak Halseid, M.; Morlang, B. Testing of CO2 Specifications With Respect to Corrosion and Bulk Phase Reactions. Energy Procedia 2014, 63, 2547–2556. [Google Scholar] [CrossRef]




| Temperature (°C) | 30 | 50 | 70 | 90 |
|---|---|---|---|---|
| · | 6.7 × 10−5 | 4.9 × 10−6 | 3.8 × 10−7 | 3.1 × 10−8 |
| 6.9 × 10−2 | 5.0 × 10−3 | 4.0 × 10−4 | 3.4 × 10−5 | |
| H2S/CO2 ratio | 1.04 × 10−3 | 1.01 × 10−3 | 1.04 × 10−3 | 1.08 × 10−3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iglesias, N.; Díaz, E. Influence of H2S and CO2 Partial Pressures and Temperature on the Corrosion of Superduplex S32750 Stainless Steel. Corros. Mater. Degrad. 2025, 6, 20. https://doi.org/10.3390/cmd6020020
Iglesias N, Díaz E. Influence of H2S and CO2 Partial Pressures and Temperature on the Corrosion of Superduplex S32750 Stainless Steel. Corrosion and Materials Degradation. 2025; 6(2):20. https://doi.org/10.3390/cmd6020020
Chicago/Turabian StyleIglesias, Naroa, and Esperanza Díaz. 2025. "Influence of H2S and CO2 Partial Pressures and Temperature on the Corrosion of Superduplex S32750 Stainless Steel" Corrosion and Materials Degradation 6, no. 2: 20. https://doi.org/10.3390/cmd6020020
APA StyleIglesias, N., & Díaz, E. (2025). Influence of H2S and CO2 Partial Pressures and Temperature on the Corrosion of Superduplex S32750 Stainless Steel. Corrosion and Materials Degradation, 6(2), 20. https://doi.org/10.3390/cmd6020020






