Abstract
Iron and iron-nickel alloy electrodeposits synthesized from sulfate-based electroplating baths were applied to a mild carbon steel substrate. Coated specimens were immersed in an oxygen-saturated, weak ammonium hydroxide solution (pH 9.5–10.0), and their corrosion performance was evaluated using electrochemical techniques. Galvanic and general corrosion behaviors were analyzed to assess the sacrificial protection provided by Fe and Fe-Ni coatings relative to uncoated steel. The influence of anode-to-cathode (A/C) surface area ratios (1:1, 10:1, and 100:1) on the occurrence of plating-induced surface cracks was also examined. Surface morphology and elemental composition of the deposits were characterized. Results of the study indicated that increasing the Ni2+/Fe2+ molar ratio of the electroplating bath from 0 to 0.167 led to (1) reduced surface porosity and cracking, (2) decreased galvanic corrosion rates between the electrodeposit and substrate, and (3) a progressive increase in the temperature dependence of the general corrosion rate between 20 °C and 60 °C. The development of Fe and Fe-Ni alloy electrodeposits as protective coatings is of particular interest in water-tube power boiler applications, where production of corrosion products must be controlled. Further research is needed to develop coatings that perform predictably under elevated pressures and temperatures typical of operating boiler environments.
1. Introduction
Standard carbon steel pipe and tube are commonly used in a wide range of industrial fluid transfer systems across a variety of industries, including oil and gas, chemical, paper, and power generation [1]. As an engineering material, carbon steel exhibits an excellent blend of strength, ductility, machinability, and weldability suitable for many diverse applications. As such, a wide variety of cost-effective, standardized carbon steel pipe and tube products are readily available through a well-established production and distribution network. Owing to these factors, subcritical power boilers and recovery boilers are often constructed with seamless carbon steel boiler and superheater tubes produced in accordance with ASME material specification SA-210 [2,3,4]. In these applications, boiler tube materials for water-tube-type boilers are exposed to relatively harsh environments at elevated temperatures and pressures for extended periods of time. Corrosion is generally controlled through a multipronged approach that includes material selection in the design phase, the use of water conditioning equipment such as filters and ion exchange systems, and a variety of available chemical treatments [5,6,7]. The presence of dissolved oxygen readily accelerates corrosion mechanisms in boiler systems, making its control a primary concern for all treatment programs. Both mechanical deaerators and oxygen-scavenging chemicals such as sodium sulfite and hydrazine are commonly used to keep dissolved oxygen levels low (typically less than 0.005 ppm O2). Cathodic protection systems of both the sacrificial anode type and the impressed current type have been used to mitigate corrosion in the wetted components of boilers [5,8]. However, traditional internal cathodic protection of the water-side surfaces of typical power boilers has significant limitations, including the capability of anodes to maintain boiler water purity requirements [5]. Additionally, maintenance access limitations in combination with complex system geometry can make it difficult to achieve the placement of anodes necessary to obtain a uniform current distribution. A variety of different water-side degradation mechanisms, such as pitting corrosion, stress corrosion cracking, and corrosion fatigue, often manifest as tube failures [4]. The resulting unplanned outages reduce plant availability and have substantial financial and societal implications [4,9]. Exacerbating these conditions is an increasing need to thermocycle power-generating plants more frequently to match actual electrical demand [9,10]. The consequent increase in pressure-temperature fluctuations and additional start-up/shut-down cycles amplify degradation mechanisms, causing material damage to further accumulate in vital operating components. Oxygen may enter the boiler system through the makeup water or by air in-leakage, accelerating active corrosion in these applications [5,7]. Deterioration of mild carbon steel material in an oxygen-saturated, alkaline solution of 9.5 pH proceeds according to the simplified chemical reactions shown in Equations (1)–(4) [7,11]. Equation (1) is the anodic dissolution of iron, which is supported by the reduction of oxygen on the steel surface as shown in Equation (2). These electrochemical half-reactions can be combined to produce the overall corrosion reaction shown in Equation (3), which illustrates the reaction of iron, oxygen, and water to produce ferrous hydroxide. Ferrous hydroxide may then further react with oxygen in the presence of water, as shown in Equation (4), to form ferric hydroxide.
This insoluble “iron rust” is also often denoted as a hydrated ferric oxide with variable water content ( [12].
Routine inspection and maintenance activities serve to detect and correct degraded or damaged components in maintaining plant safety, reliability, and availability [10]. Reducing forced outages requires a comprehensive approach emphasizing the use of unit-specific data, strategic inspection, and maintenance prioritization. This research investigates potential electroplating treatments of Fe-Ni alloys that may be applied as part of a routine preventative maintenance regimen to extend the life of mild carbon steel components in typical boiler water applications subjected to high concentrations of dissolved oxygen. The introduction of a slightly more active coating serves to provide a sacrificial anode capable of cathodic protection of the underlying mild carbon steel substrate under typical plant conditions and during water chemistry excursions. Emphasis is placed on producing a sacrificial metallic coating that is only slightly more reactive than the substrate. This allows the desired cathodic protection to be achieved without excessive production of corrosion products that must be purged by the boiler blowdown system. The present study is an initial investigation of Fe and Fe-Ni alloy electrodeposited coatings and will serve to direct future research and investment in higher-temperature experiments with various typical boiler water chemistries.
Electrochemically deposited metallic coatings are an effective and low-cost technology to improve the surface performance of materials [13]. They have been used to enhance resistance to wear [14], corrosion [15,16,17,18], and fatigue [19]. Recent research has emphasized the development of Zn-Fe and Zn-Ni coatings to improve corrosion resistance in general industrial and automotive applications [20,21,22]. By alloying Zn with Fe or Ni, corrosion resistance was improved over that of a Zn-only coating. The development of Fe-Ni coatings has been studied extensively in the literature [23,24]. However, research on the corrosion resistance of Fe-Ni alloys in various environments, particularly when Fe is the predominant constituent, is still lacking. In this work, Fe and Fe-Ni alloy electrochemical deposits with high Fe concentrations were synthesized from sulfate-based electroplating baths and experimentally investigated. Their protective attributes when applied to mild carbon steel and immersed in oxygen-saturated, weak ammonium hydroxide solution (9.5–10.0 pH) were evaluated. Using electrochemical corrosion measurement techniques, each electrodeposit overlay was evaluated for galvanic and general corrosion behavior as compared to the unplated SA-210-A1 mild carbon steel substrate. Galvanic corrosion, also known as dissimilar metal corrosion, occurs due to the coupling of dissimilar metals and results in the preferential localized attack of the more anodic material and protection of the more noble material. This form of corrosion is the basis for cathodic protection systems that use sacrificial anodes for corrosion protection. General corrosion, also known as uniform corrosion, is the uniform thinning of a material across the exposed surface [6]. The protective characteristics of each electroplating bath were compared and discussed. Variations of the anode-to-cathode (A/C) surface area ratio (1:1, 10:1, and 100:1) were also investigated in relation to surface cracks that arise from tensile stress in the plated materials. Surface morphology and elemental composition of the resulting deposits were analyzed using field emission scanning electron microscopy (FE-SEM) and energy-dispersive X-ray spectroscopy (EDS).
2. Materials and Methods
2.1. Materials and Solutions
Galvanostatic electrochemical deposition of Fe alloys with varying nickel composition was conducted in a single cell using a Solartron 1287 potentiostat with CorrWare software version 3.5h released 5 March 2020 (Ametek, Inc., Berwyn, PA, USA). The cell was arranged in the typical three-electrode configuration shown in Figure 1. No stirring of the electrolyte was provided during electroplating. The working electrode was mild carbon steel boiler tube per ASME specification SA-210 Grade A1 (tube). The chemical composition and mechanical properties of the working electrode are provided in Table 1 and Table 2, respectively.
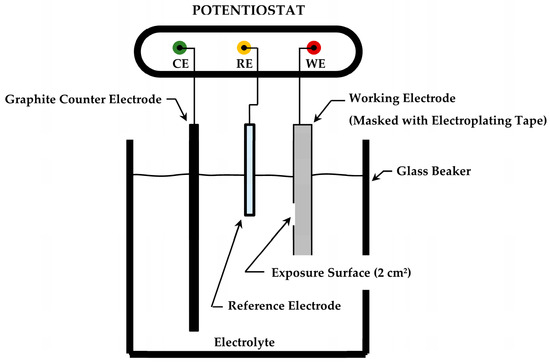
Figure 1.
Electrochemical cell configuration used to conduct electroplating and to estimate the general corrosion rate using linear polarization resistance (LPR).

Table 1.
Chemical composition (wt.%) of ASME SA-210-A1.

Table 2.
Mechanical properties of ASME SA-210-A1.
Specimens of 76.2 mm (3 in) length and 19 mm (0.75 in) width were longitudinally sectioned from 76.2 mm (3 in) OD tubing with 6.35 mm (0.25 in) wall thickness. After machining, specimens were stress relieved at 590 °C (1100 °F) for one hour, followed by glass bead blasting with 80 mesh glass abrasive (CAS 6599-17-3, Black Diamand Abrasive Products, Tinley Park, IL, USA) to remove oxidation products. Each specimen was cleaned with deionized water and ethanol, followed promptly by warm air blow-drying. Electroplating Tape 470 (3M Industrial Adhesives and Tapes Division, St. Paul, MN, USA) was used to mask each specimen, creating a 2 cm2 (0.31 in2) exposure area on the inside surface of the tube section. A graphite counter electrode and a Cu/CuSO4 reference electrode (+0.316 V vs. SHE at 25 °C) were used to complete the electroplating cell. Three electroplating baths containing varying nickel compositions were evaluated. All three electroplating baths contained a 0.6 M (mol/L) concentration of ammonium iron (II) sulfate hexahydrate. The iron concentration was adapted from prior work to provide a high concentration of ions without exceeding the maximum solubility [18,19]. Nickel (II) sulfate hexahydrate was added in concentrations of 0.00 M, 0.05 M, and 0.10 M, constituting the three electroplating bath designs of the study. The nickel concentrations were selected based on providing a slight shift in the corrosion potential to mitigate the production of corrosion products while still achieving the desired cathodic protection of the substrate. These bath chemicals were reagent grade, manufactured by ThermoFisher Scientific, Ward Hill, MA. The electroplating baths used in this study are summarized in Table 3. Electroplating was carried out in 400 mL cells (Figure 1) at a current density of −0.02 A/cm2 for a duration of 1-h (3600 s) at 20 °C.

Table 3.
Electroplating bath compositions.
2.2. Corrosion-Electrochemical Investigations
A series of corrosion experiments were conducted while specimens were immersed in oxygen-saturated, deionized water at 20 °C and 60 °C. Ammonium hydroxide (5 wt% NH4OH) was used to maintain the solution pH between 9.5 and 10.0 during all experiments. Air was sparged for at least 1 h prior to and continuously during all testing to provide a consistently saturated dissolved oxygen environment for comparative evaluation. The open circuit potential (OCP) was monitored for stabilization after immersion and measured against a saturated KCl-filled Ag/AgCl reference electrode (+0.197 V vs. SHE at 25 °C) for 60 s using the Solartron 1287 potentiostat. The electrode configuration used is shown in Figure 1.
The experimental protocol also included investigation of galvanic corrosion behavior between plated and unplated SA-210-A1 mild carbon steel specimens using galvanic potential and galvanic current measurements. A high-resistance voltmeter was used to measure galvanic potential for specimens of each plating bath design with respect to the SA-210-A1 mild carbon steel substrate when immersed in weak ammonium hydroxide solution (9.5–10.0 pH). Measurements of galvanic potential were made immediately upon completing the circuit to mitigate polarization. The zero-resistance ammeter (ZRA) function of the Solartron 1287 potentiostat was used to quantify the galvanic corrosion current for all three plating systems using an anode-to-cathode area ratio of 1:1. For specimens plated with the 0.00 M Ni (and 0.6 M Fe) bath, the ZRA was also used to measure galvanic current for anode-to-cathode area ratios of 10:1 and 100:1. For ZRA measurements, plated and unplated specimens were immersed with their exposure surfaces facing each other and spaced 6.35 mm (0.25 in) apart, as illustrated in Figure 2.
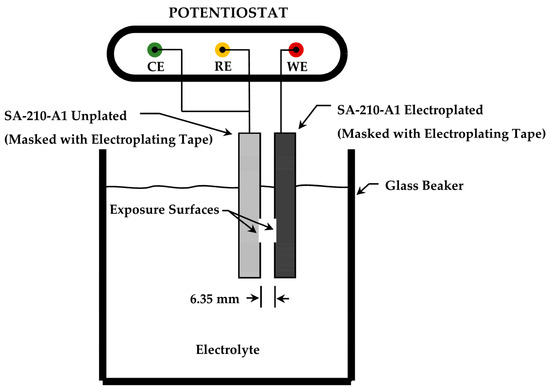
Figure 2.
Electrochemical cell configuration used for determining galvanic corrosion current between electroplated and unplated specimens with the zero-resistance ammeter (ZRA) technique.
The general uniform corrosion behavior of the plated and unplated specimens was characterized by active polarization immediately following OCP measurements using a combination of the Solartron 1287 potentiostat with CorrWare software and a Wavedriver 40DC potentiostat with AfterMath software version 1.6.10523 released 12 April 2022 (Pine Research Instrumentation, Inc., Durham, NC, USA). A three-electrode configuration (Figure 1) was used for these experiments and included a graphite counter electrode and an Ag/AgCl (saturated KCl) reference electrode. Overpotentials of ±10 mV were applied to samples immersed in weak ammonium hydroxide solution (9.5–10.0 pH) using the linear polarization resistance (LPR) technique at a scan rate of 0.1667 mV/s (Solartron 1287). These experiments provided an estimate of general uniform corrosion rates for specimens plated with each of the electroplating baths and for bare SA-210-A1 carbon steel at 20 °C and 60 °C. Additional potentiodynamic polarization scans from −0.250 V to 1.5 V of OCP were conducted at a scan rate of 0.125 mV/s with the WaveDriver 40DC potentiostat at 20 °C using linear sweep voltammetry (LSV) on representative companion specimens plated with each of the three electroplating baths as well as for bare SA-210-A1 carbon steel. Tafel extrapolation was used to establish electrochemical parameters and confirm corrosion rates obtained from LPR. Statistical analysis of the corrosion data followed the methodology of ASTM G16 [25]. A minimum of five samples were used for each study except for potentiodynamic polarization, which was conducted on a single representative specimen from each group. Additional information on sample sizes is included in the statistical analysis results presented in Section 3.
2.3. Morphological Investigations
Surface morphology was investigated using an S-4800 field emission scanning electron microscope (FE-SEM) (Hitachi, Tokyo, Japan) with elemental composition analysis by energy-dispersive X-ray spectroscopy (EDS) with an XFlash 6160 detector and accompanying ESPRIT spectrometry software version 2.1.2.17832 (Bruker Corporation, Billerica, MA, USA) at an accelerating voltage of 20 keV. Specimens were sectioned, ultrasonically cleaned in deionized water, rinsed with ethanol, and blow-dried prior to mounting. Planar (top surface) metallographic specimens were affixed to 15 × 6 mm A1 Hitachi SEM mounts using carbon conductive paint (SPI Supplies, West Chester, PA, USA). Cross-sectional metallographic specimens were epoxy mounted, ground, and polished. Grinding was conducted using 120, 240, 320, and 800 grit silicon carbide papers with the Alpha-Beta Polisher (Buehler, Lake Bluff, IL, USA). Micro-polishing was completed using Buehler polishing cloths and suspensions in the following sequence: (1) UltraPad with 9 µm MetaDi diamond suspension, (2) VerduTex with 3 µm MetaDi diamond suspension, and finally (3) MicroCloth with MasterPrep 0.05 µm alumina suspension. Specimens were rinsed with deionized water and ethanol between each step to remove any particulates from previous stages. Prepared specimens were placed in a mount holder with a nitrogen atmosphere for transport to the imaging center. Image processing was conducted using the ImageJ software package version 1.54d (Wayne Rasband and contributors, National Institutes of Health, Bethesda, MD, USA) [26].
3. Results
3.1. Open Circuit Potential
The OCP was obtained for specimens coated with each of the three electroplating baths and for bare SA-210-A1 carbon steel in oxygen-saturated, weak ammonium hydroxide solution (9.5–10.0 pH). Figure 3 depicts the mean OCP at 20 °C and 60 °C with error bars representing a 90% confidence interval. At 20 °C, specimens with electrodeposits from 0.00 M, 0.05 M, and 0.10 M Ni baths exhibited a shift in OCP of −130 ± 54 mV, −9 ± 35 mV, and +44 ± 53 mV from the uncoated specimen, respectively. Likewise, OCP tests conducted at 60 °C produced similar results with shifts of −152 ± 115 mV, −81 ± 45 mV, and −41 ± 35 mV, respectively. It was observed (Figure 3) that the deposited material became progressively less electrochemically active with increasing nickel composition. In addition, an increasing nickel composition tended to reduce the margin of error in OCP when temperature was increased from 20 °C to 60 °C. This effect is illustrated by the progressively shrinking error bars observed in Figure 3 for OCP at 60 °C as nickel composition was increased.
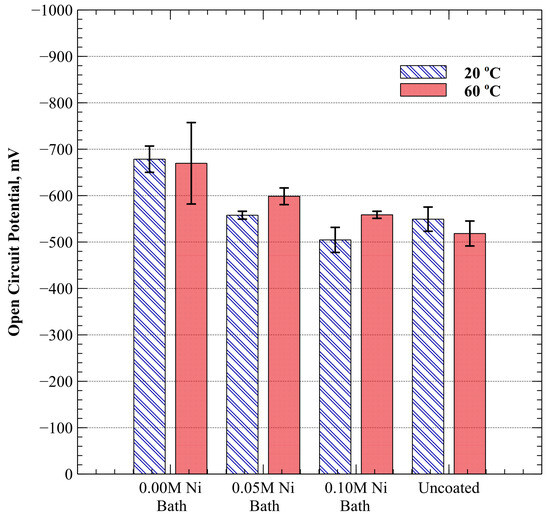
Figure 3.
Average open circuit potential (versus Ag/AgCl) at 20 °C and 60 °C in dilute ammonium hydroxide. Error bars represent a 90% confidence interval.
Independent samples t-tests assuming unequal variance were used to determine statistical significance in differentiating between population means. The results of statistical analysis of OCP are provided in Table 4. While a clear distinction in OCP between specimens electroplated with 0.05 M and 0.10 M Ni baths as compared to the uncoated mild carbon steel at 20 °C was not observed, statistical significance (p-value 0.05) was observed when comparing specimens electroplated with the 0.00 M Ni bath at 20 °C as well as for all three baths at 60 °C.

Table 4.
T-test results for open circuit potential (OCP), including the mean (), standard deviation (SD), sample size (n), t-statistic (t-Stat), degrees of freedom (df), and probability value (p-value) for 0.05 level of significance ().
3.2. Galvanic Potential
The galvanic potential between specimens synthesized from each electroplating bath was measured in oxygen-saturated, weak ammonium hydroxide solution (9.5–10.0 pH) at 20 °C. The galvanic cell was created by electrically connecting each specimen to unplated SA-210-A1 carbon steel using a high-resistance voltmeter. The mean galvanic potential for specimens electroplated from each of the three bath designs is provided in Figure 4.
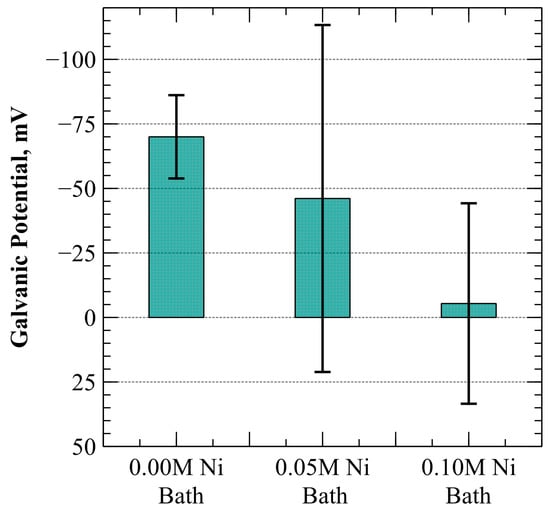
Figure 4.
Average galvanic potential in oxygen-saturated, dilute ammonium hydroxide at 20 °C. Error bars represent a 90% confidence interval.
Error bars representing a 90% confidence interval (CI) about the mean are included in the figure. As can be seen, electroplating with 0.00 M, 0.05 M, and 0.10 M Ni baths produced specimens with average galvanic potentials of −70 mV, −46.1 mV, and −5.4 mV, respectively. The 0.00 M Ni electroplating bath, which did not contain nickel ions, provided a margin of error (for a 90% CI) of ±16.1 mV with measurements ranging from 30 mV to 130 mV more active than the unplated mild carbon steel base metal. The addition of nickel ions into the electroplating bath (0.05 M and 0.10 M Ni) expanded the range of galvanic potential measurements obtained. The 0.05 M Ni electroplating bath exhibited a margin of error of ±67.2 mV, while the margin of error for 0.10 M Ni bath specimens was ±38.8 mV. Additionally, as may also be observed from Figure 4, a degree of polarity reversal was observed for both electroplating baths containing the addition of nickel (0.05 M and 0.10 M Ni).
3.3. Zero-Resistance Ammeter
The zero-resistance-ammeter (ZRA) technique was used to determine galvanic corrosion currents for a 1:1 anode-to-cathode surface area ratio, which were converted to corrosion rates as follows:
where is the corrosion rate in , is a unit conversion constant, is the corrosion current density (), and is the equivalent weight in gram equivalents [27]. Results are shown in Figure 5. As depicted, the galvanic corrosion rates at 20 °C for specimens plated with the 0.00 M, 0.05 M, and 0.10 M Ni baths were 0.09 ± 0.03 mm/yr, 0.03 ± 0.04 mm/yr, and 0.02 ± 0.04 mm/yr, respectively (for a 90% CI). At a temperature of 60 °C, galvanic corrosion rates increased to 0.34 ± 0.12 mm/yr, 0.32 ± 0.17 mm/yr, and 0.21 ± 0.06 mm/yr (for a 90% CI) for specimens plated with the 0.00 M, 0.05 M, and 0.10 M Ni bath, respectively. As can be seen in Figure 5, and in agreement with galvanic potential observations shown in Figure 4, a general numerical trend of decreasing galvanic corrosion rate as nickel composition is increased was noted. For the ambient temperature of 20 °C, reversal of the cathode/anode relationship occurs as the mild carbon steel substrate corroded preferentially for a small subset of samples plated with the 0.05 M and 0.10 M Ni baths. The largest resulting galvanic corrosion rate for the SA-210-A1 mild carbon steel in these cases was 0.05 mm/yr at 20 °C. When the temperature was increased to 60 °C, however, specimens coated with all three electroplating baths were anodic as compared to the unplated SA-210-A1 mild carbon steel substrate with average galvanic corrosion rates of 0.34, 0.32, and 0.21 mm/yr for 0.00 M, 0.05 M, and 0.10 M Ni electroplating baths, respectively.
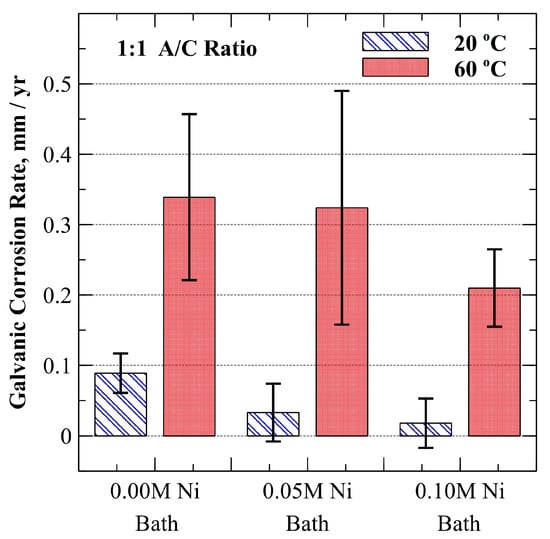
Figure 5.
Average galvanic corrosion rate at 20 °C and 60 °C in oxygen-saturated, dilute ammonium hydroxide (9.5–10.0 pH). Error bars represent a 90% confidence interval.
As noted earlier, some exposure of unplated and plated surfaces is generally anticipated due to surface cracking and porosity that accompanies a given coating application. With this in mind, the anode-to-cathode (A/C) surface area ratio effects on galvanic corrosion were assessed for specimens plated with the 0.00 M Ni bath at 20 °C and 60 °C. The galvanic corrosion rates (mm/yr) for A/C area ratios of 1:1, 10:1, and 100:1 are provided in Figure 6.
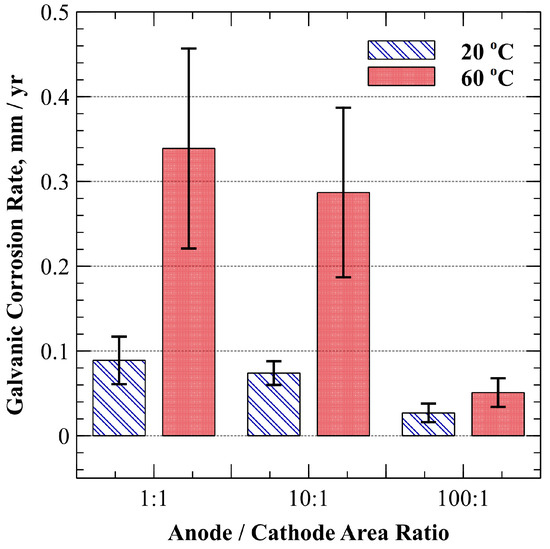
Figure 6.
Average galvanic corrosion rate for 1:1, 1:10, and 1:100 anode-to-cathode area ratios at 20 °C and 60 °C in oxygen-saturated, dilute ammonium hydroxide (9.5–10.0 pH). Error bars represent a 90% confidence interval.
As can be seen, galvanic corrosion rates at 20 °C for 1:1, 10:1, and 100:1 A/C area ratios were 0.09 ± 0.03 mm/yr, 0.07 ± 0.01 mm/yr, and 0.03 ± 0.01 mm/yr, respectively (90% CI). Likewise, galvanic corrosion rates at 60 °C increased to 0.34 ± 0.12 mm/yr, 0.29 ± 0.10 mm/yr, and 0.05 ± 0.02 mm/yr, respectively. As the surface area of the cathode decreased relative to the surface area of the anode, a distinct corresponding reduction in galvanic corrosion rate at both 20 °C and 60 °C was observed.
While independent samples t-tests with unequal variance (Table 5) indicated that a clear distinction could not be established between the 1:1 and 10:1 A/C surface area ratios at either test temperature, a significant difference in galvanic corrosion rate existed between all other A/C area ratios at both test temperatures. In addition, it was observed that while variance increased at elevated temperature, there was no statistically significant difference in galvanic corrosion rate observed between 20 °C and 60 °C when the A/C area ratio was 100:1, as indicated by the values t(7) = 0.882, p = 0.407.

Table 5.
T-test results for anode/cathode (A/C) surface area ratio effects on galvanic corrosion, including the mean (), standard deviation (SD), sample size (n), t-statistic (t-Stat), degrees of freedom (df), and probability value (p-value) for 0.05 level of significance ().
3.4. Linear Polarization Resistance
General corrosion behavior was estimated using the LPR method, as described in ASTM G59, which relates the corrosion current density () to the polarization resistance () through the Stern–Geary constant (B), as shown in Equation (6) [28]. The corresponding corrosion rates were subsequently calculated using Equation (5) above.
Figure 7 shows the general corrosion rate for specimens from each of the three electroplating baths and for unplated SA-210-A1 mild carbon steel. At 20 °C, specimens plated with the 0.00 M, 0.05 M, and 0.10 M Ni baths exhibited average general corrosion rates of 0.11 ± 0.02 mm/yr, 0.21 ± 0.02 mm/yr, and 0.16 ± 0.01 mm/yr, respectively (for a 90% CI). The general corrosion rate of the unplated mild carbon steel at 20 °C was 0.14 ± 0.02 mm/yr (90% CI). When the temperature was increased to 60 °C, the general corrosion rates increased to 0.17 ± 0.03 mm/yr, 0.28 ± 0.03 mm/yr, and 0.36 ± 0.03 mm/yr for specimens plated with 0.00 M, 0.05 M, and 0.10 M Ni baths, respectively (90% CI). The general corrosion rate of unplated mild carbon steel increased to 0.26 ± 0.04 mm/yr (90% CI) at 60 °C. It was observed that specimens plated with the 0.00 M Ni bath, which did not contain nickel ions, exhibited a less pronounced increase in corrosion rate when the temperature was increased. The average increase in general corrosion rate when the temperature was increased from 20 °C to 60 °C was 0.06 mm/yr for 0.00 M Ni bath specimens, 0.07 mm/yr for 0.05 M Ni bath specimens, and 0.20 mm/yr for specimens produced from 0.10 M Ni bath.
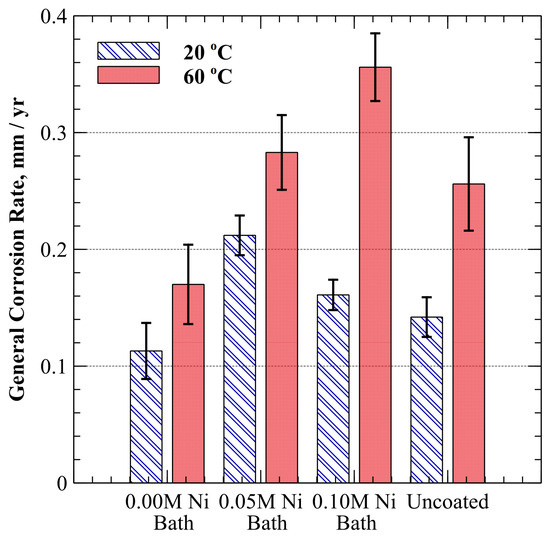
Figure 7.
Average general corrosion rate at 20 °C and 60 °C in oxygen-saturated, dilute ammonium hydroxide (9.5–10.0 pH). Error bars represent a 90% confidence interval.
3.5. Potentiodynamic Polarization and Tafel Extrapolation
Figure 8 provides a series of potentiodynamic polarization curves representing specimens electroplated with each of the three plating baths as well as the unplated SA-210-A1 carbon steel when immersed in oxygen-saturated, weak ammonium hydroxide solution (9.5–10.0 pH) at 20 °C. The arrows in Figure 8 indicate the direction of increasing Ni composition in the coating.
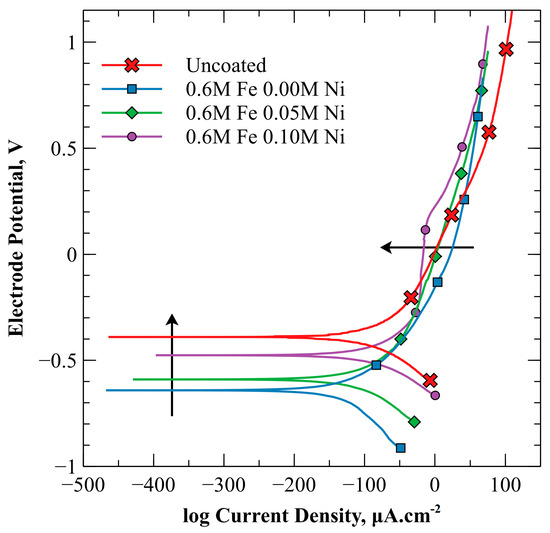
Figure 8.
Potentiodynamic polarization curves in oxygen–saturated, weak ammonium hydroxide (9.5 to 10.0 pH) at 20 °C. Scans were conducted from 0.25 V below OCP to 1.5 V above OCP (versus Ag/AgCl) at a scan rate of 0.125 mV/s. Arrows indicate the direction of increasing Ni in the coating.
The polarization curves illustrate a continuous active corrosion without a clear active-to-passive transition. Also from this figure, an OCP shift in the more active direction was obtained from the three plating baths as compared to the unplated SA-210-A1 steel. However, it was noted that the OCP obtained from the potentiodynamic polarization scan of the representative unplated SA-210-A1 specimen was about −0.4 V relative to the Ag/AgCl (saturated KCl) reference electrode as compared to the average value of −0.549 V reported in Figure 3. This result was beyond the margin of error for a 90% confidence interval but was consistent with the upper end of the range of values obtained for the unplated SA-210-A1 carbon steel. The OCP obtained from the potentiodynamic polarization scans of the three electrodeposits were −0.64 V, −0.59 V, and −0.48 V for the 0.00 M Ni, 0.05 M Ni, and 0.10 M Ni electroplating solutions, respectively. These results were representative of the average OCP values obtained just prior to LPR testing as reported in Figure 3 (−0.679 V, −0.558 V, and −0.505 V, respectively).
Tafel extrapolation was performed on the potentiodynamic polarization curves shown in Figure 8. The corresponding Tafel plots for the unplated specimen and the specimens plated using the 0.00 M, 0.05 M, and 0.10 M Ni baths are displayed in Figure 9a, Figure 9b, Figure 9c, and Figure 9d, respectively.
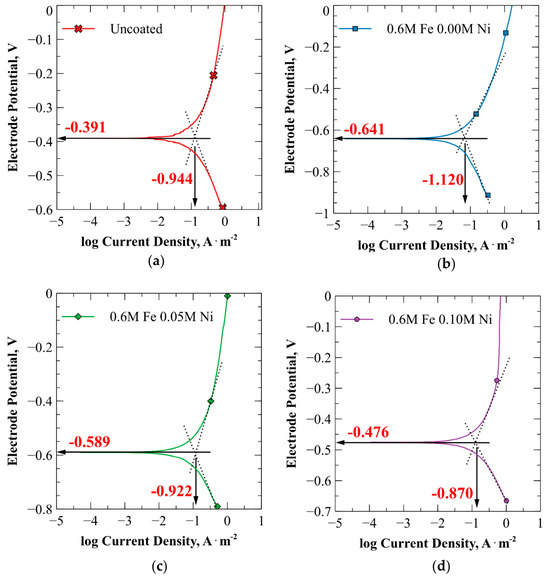
Figure 9.
Tafel extrapolation plots for (a) the uncoated steel, (b) steel plated using the 0.6 M Fe–0.00 M Ni bath, (c) steel plated using the 0.6 M Fe–0.05 M Ni bath, and (d) steel plated using the 0.6 M Fe–0.10 M Ni bath. The scan rate was 0.125 mV/s.
Electrochemical parameters extracted from these polarization curves are summarized in Table 6. The corrosion current densities () obtained from Tafel extrapolation were 11.4 µA/cm2, 7.6 µA/cm2, 12.0 µA/cm2, and 13.5 µA/cm2 for the unplated specimen and those plated in the 0.00 M, 0.05 M, and 0.10 M Ni baths, respectively. The corrosion rates, as shown in Table 6, were computed using Equation (5) based on these current densities and align well with the general corrosion rates measured via LPR testing at 20 °C (Figure 7). Additionally, the anodic and cathodic Tafel slopes ( and ) were used to calculate the Stern–Geary constants (B) and polarization resistance () following Equations (7) and (8).

Table 6.
Electrochemical parameters from the Tafel extrapolation at 20 °C. Refer to Table 3 for complete bath composition.
3.6. Surface Morphology and Composition
Figure 10, Figure 11, Figure 12 and Figure 13 provide FE-SEM micrographs of SA-210-A1 mild carbon steel in the unplated condition and after electroplating with the 0.00 M, 0.05 M, and 0.10 M Ni baths, respectively. No grinding or polishing of the metallographic samples was conducted prior to imaging. As such, a distinctive surface roughness can be observed in Figure 10, which is a result of the seamless tube manufacturing process typical of SA-210-A1 boiler tubes.
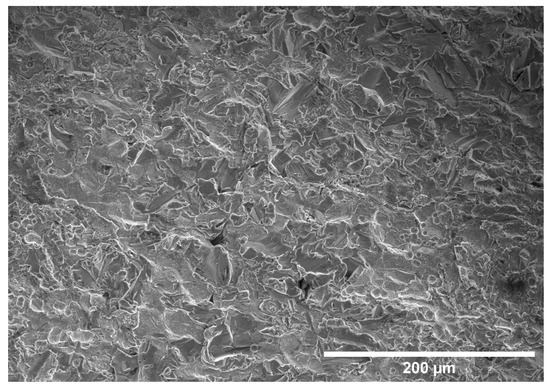
Figure 10.
Micrograph of unplated A210-A1 mild carbon steel at ×250 magnification.
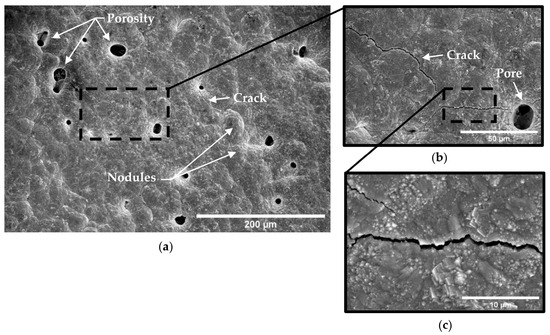
Figure 11.
Micrograph of A210-A1 mild carbon steel electroplated with 0.6 M Fe–0.00 M Ni solution at (a) ×250, (b) ×1000, and (c) ×5000 magnification.
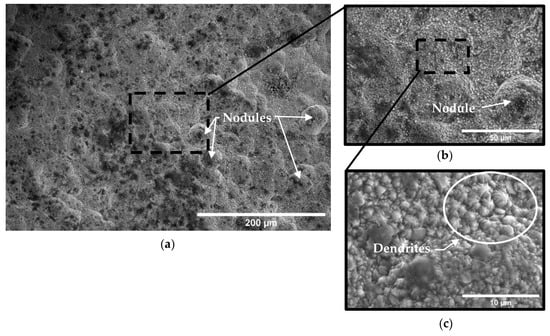
Figure 12.
Micrograph of A210-A1 mild carbon steel electroplated with 0.6 M Fe–0.05 M Ni solution at (a) ×250, (b) ×1000, and (c) ×5000 magnification.
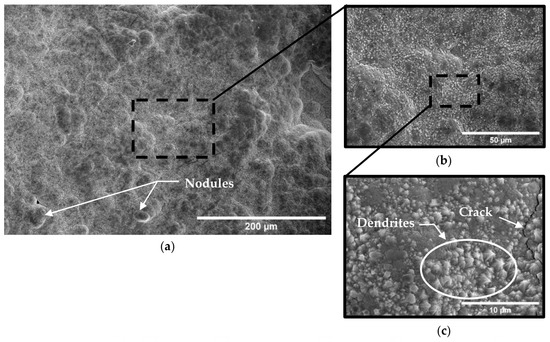
Figure 13.
Micrograph of A210-A1 mild carbon steel electroplated with 0.6 M Fe–0.10 M Ni solution at (a) ×250, (b) ×1000, and (c) ×5000 magnification.
By comparison, specimens coated with all three electroplating baths tended to exhibit a relatively smooth and continuous surface with uniform rounded nodules, as can be seen in Figure 11, Figure 12 and Figure 13. Porosity consistent with hydrogen evolution during the electroplating process was observed in specimens electroplated with the Fe-only bath (Figure 11), while almost no porosity was observed in electrodeposits produced from the nickel ion-containing baths (0.05 M and 0.10 M Ni) (Figure 12 and Figure 13). Cracks were observed in all electrodeposited surfaces; however, crack length density and crack widths associated with the 0.00 M Ni electroplating bath were noticeably higher than those of specimens electroplated with 0.05 M and 0.10 M Ni baths. Crack length densities were estimated from SE-FEM images using image processing software [26] to measure pore surface diameters, crack lengths, and crack widths. Crack width was measured approximately every 10 microns of crack length. These measurements were used to estimate the average anode-to-cathode area ratios resulting from porosity and cracking in each of the electrodeposited surfaces. A summary of these measurements and computed results can be seen in Table 7. The resulting anode-to-cathode (A/C) surface area ratios were 66:1, 128:1, and 363:1 for deposits electroplated from the 0.00 M, 0.05 M, and 0.10 M Ni baths, respectively [23].

Table 7.
Electrodeposit surface feature characteristics.
Figure 14, Figure 15, Figure 16 and Figure 17 show results of EDS analysis of SA-210-A1 carbon steel in the unplated condition and after having been electroplated with the 0.00 M, 0.05 M, and 0.10 M Ni baths, respectively. Each of these four figures includes a secondary electron image of the surface under evaluation and an EDS spectrum identifying elements present on the surface of the sample. In addition to elemental oxygen, the EDS spectrum for unplated SA-210-A1 carbon steel shown in Figure 14b indicated the presence of iron, carbon, silicon, and manganese, consistent with the general chemical composition of SA-210-A1 carbon steel as shown in Table 1. The elemental composition of the electrodeposited surfaces produced from the 0.00 M, 0.05 M, and 0.10 M Ni baths are shown in Figure 15b, Figure 16b, and Figure 17b, respectively. All three baths produced specimens containing high concentrations of iron. For specimens synthesized from the 0.05 M Ni bath (Figure 16) and the 0.10 M Ni bath (Figure 17), the presence of nickel was also detected on the deposit surface.
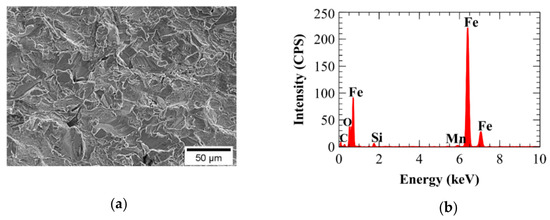
Figure 14.
Electron dispersive spectroscopy (EDS) of bare A210-A1 mild carbon steel showing (a) sample area and (b) spectrum.
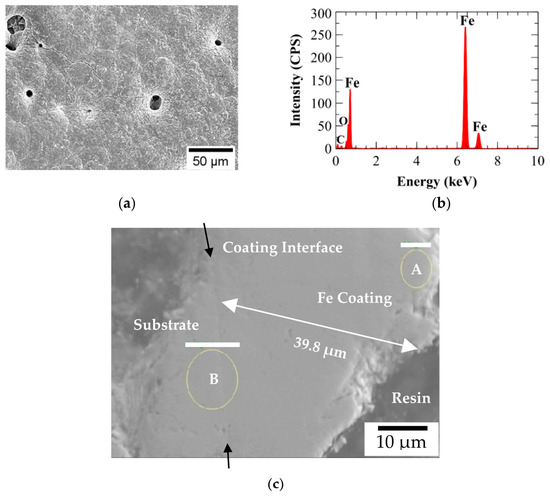
Figure 15.
Electron dispersive spectroscopy (EDS) of A210-A1 mild carbon steel electroplated with the 0.6 M Fe and 0.00 M Ni bath: (a) sample area, (b) spectrum, and (c) polished cross-section of electrodeposited coating indicating thickness and EDS analysis locations A and B.
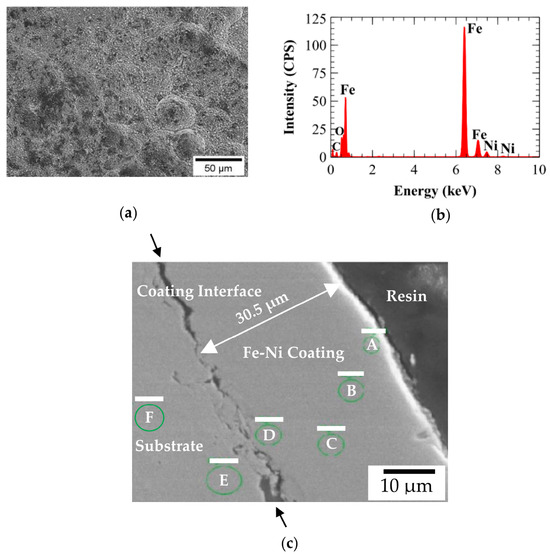
Figure 16.
Electron dispersive spectroscopy (EDS) of A210-A1 mild carbon steel electroplated with the 0.6 M Fe and 0.05 M Ni bath: (a) sample area, (b) spectrum, and (c) polished cross-section of electrodeposited coating indicating thickness and EDS analysis locations A, B, C, D, E, and F.
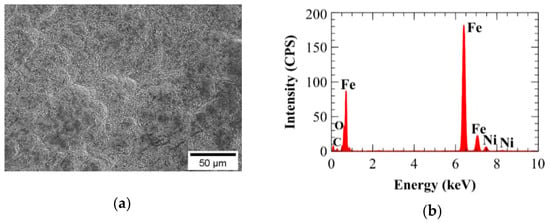
Figure 17.
Electron dispersive spectroscopy (EDS) mapping of A210-A1 mild carbon steel electroplated with the 0.6 M Fe and 0.10 M Ni bath (a) sample area and (b) spectrum.
Cross-sectional metallographic specimens produced from the 0.00 M, 0.05 M, and 0.10 M Ni baths were epoxy mounted, ground, and polished for EDS analysis. EDS was used to quantify elemental composition across the plating thickness. The results, which are shown in Table 8, showed a relatively uniform distribution of Ni through the plating thickness for specimens synthesized from 0.05 M and 0.10 M Ni electroplating baths. In addition, the Ni content observed for 0.10 M Ni specimens was approximately twice that of 0.05 M Ni specimens. Figure 15c and Figure 16c display examples of the resulting secondary electron images with the locations of EDS analysis identified. The complete results of the EDS analysis for each cross-sectionally mounted and polished specimen can be seen in Table 8. All coatings exhibited reasonable uniformity, with thicknesses ranging from approximately 30 to 40 µm. A comparison of Figure 15c with Figure 16c suggests that the coating–substrate interface may be more strongly adhered with the Fe-only (0.00 M Ni) coating, while slight delamination was observed in the 0.05 M Ni specimen shown in Figure 16c.
4. Discussion
The results of experiments conducted in this work provide useful data on the corrosion behavior of Fe and Fe-Ni alloy electrochemical deposits when used as a sacrificial anode for cathodic protection of mild carbon steel in oxygen-saturated, weak ammonium hydroxide solution (9.5–10.0 pH). Emphasis was placed on achieving small shifts in corrosion potential such that the protective coating would be effective for cathodic protection but with a slow rate of degradation to extend coating life and minimize debris buildup. Under the conditions of this study, the Fe-only coating exhibited the most negative galvanic potential with respect to the substrate (Figure 4) and the lowest general corrosion rate (Figure 7). These results suggest that the Fe-only coating may be a promising candidate for cathodic protection while also minimizing the formation of corrosion products at low temperatures. However, it also exhibited the highest galvanic corrosion rate. In contrast, the addition of Ni progressively reduced the galvanic corrosion rate (Figure 5), despite increasing the general corrosion rate of the coating (Figure 7). The higher anodic dissolution from the general corrosion of the Ni-containing coatings may have contributed to the formation of corrosion products, which could have acted as a barrier, limiting galvanic interactions and possibly reducing galvanic corrosion rates. The selection of an optimal coating composition will depend on the application, as it is uncertain whether general corrosion or galvanic corrosion will be the dominant process governing long-term performance. Given that the coating is expected to be sporadically applied on the inside surface of boiler tubes, localized effects may play a more significant role in determining its effectiveness.
The electrodeposition of Fe-Ni alloys has been studied extensively and is well known to exhibit anomalous codeposition, which is characterized by the preferential deposition of the less noble material over the more noble material [29]. In iron-group alloys this phenomenon is most often associated with the formation and adsorption of reaction intermediates (e.g., Ni(I)ads, Fe(I)ads, FeNi(II)ads) on the cathode surface that both inhibits deposition of the more noble metal (i.e., nickel) and favors the deposition of the less noble (i.e., iron) [30,31,32,33,34,35]. Despite the occurrence of anomalous codeposition in electrodeposited Fe-Ni alloys, it has been shown that increasing the mass percentage of in a sulfate-based electroplating solution had the effect of increasing the mass percentage of nickel content in the resulting deposit [36,37]. In the present work, altering the molar ratio in the plating solution was used to modify the alloy composition and adjust the electrochemical properties of the deposit. The open circuit potential (Figure 3), corrosion rates (Figure 5 and Figure 7), and potentiodynamic polarization curves (Figure 8) produced in this work seem to show a distinguishable variation in the corrosion behavior of electroplated specimens as compared to the unplated mild carbon steel boiler tube. Further, the selected concentrations of ions (0.00 M, 0.05 M, and 0.10 M) in the electroplating solution appeared to influence the chemical properties and resulting corrosion behavior of the deposited material in a predictable manner, suggesting tunability.
Increasing the molar ratio in the electroplating bath had several notable implications for the prescribed application of cathodic protection of boiler tubes. The standard reduction potential for nickel () is more noble than that of iron (). As such, increasing the composition ratio would be expected to shift the electrode potential of the alloy in the more noble direction [7]. In competition with this effect of relative nobility, localized lattice strains may result from atomic size mismatch in alloyed deposits and can shift electrode potential in the more active direction due to localized tensile stresses [38,39]. Since the atomic radii of nickel and iron are of similar size (77-Fe2+ vs. 70-Ni2+) [40], lattice strains from alloying iron with nickel appear less influential on corrosion potential than the relative nobility of the two elements (Figure 3). As such, the progressive increase in molar ratio within the electroplating solution correlated strongly with a corrosion potential shift in the more noble direction (from −130 mV to +44 mV at 20 °C and from −152 mV to −41 mV at 60 °C), as can be seen in both Figure 3 and Figure 8. As the nickel composition increased, the potentiodynamic polarization curves (Figure 8) showed a decrease in corrosion current density within the region where passivation typically occurs, suggesting a slight enhancement of the protective oxide layer with higher nickel content.
The galvanic corrosion rate of electroplated specimens when coupled to the unplated carbon steel progressively declined as nickel composition was increased (Figure 5). Specifically, electrodeposits synthesized from the 0.05 M Ni bath and the 0.10 M Ni bath had essentially a null galvanic corrosion effect when coupled to mild carbon steel at 20 °C with an average galvanic corrosion rate of 0.03 mm/yr (± 0.04 mm/yr) and 0.02 mm/yr (± 0.04 mm/yr), respectively (Figure 5). At 60 °C, however, these electrodeposits consistently provided cathodic protection of the carbon steel substrate with an average loss of protective coating of 0.32 mm/yr (±0.17 mm/yr) and 0.21 mm/yr (±0.06 mm/yr), respectively. The temperature dependence of the galvanic corrosion rate in this case (Figure 5) being largely a function of the molar ratio of the electroplating solution suggests that a desired intensity of cathodic protection may be selected for specific temperature ranges.
According to the mechanochemical interaction theory proposed by Gutman [41,42], an applied stress can influence the thermodynamic activity of a material, resulting in a change in equilibrium potential. Under an applied load, the shift in equilibrium potential due to elastic strain () and plastic strain () can be computed according to Equation (9) and Equation (10), respectively.
where is the absolute value of the hydrostatic part of the stress tensor (, is the molar volume of the material (, is the charge number, is Faraday’s constant (96,500 ), is an orientation-dependent factor, is a constant coefficient between 109 and 1011 describing mobile dislocation density dependence on strain, is absolute temperature, is the universal gas constant (8.314 J mol−1 K−1), is the initial dislocation density prior to plastic deformation, and is the plastic strain. The linear coefficient of thermal expansion for nickel () is greater than that of iron (). As nickel composition is increased in the alloy, the linear coefficient of thermal expansion further diverges from that of the mild carbon steel substrate () [38]. For a thin film coating applied to a relatively thicker substrate, the resulting mismatch stress due to thermal expansion () within the elastic region can be defined by [43]
where is the elastic modulus of the substrate material ()., is the thermal expansion mismatch between substrate and coating (), is the change in temperature from the zero-stress reference temperature , and is Poisson’s ratio of the substrate material. From Equation (11), it is evident that the electrodeposited coating is subjected to a compressive stress at elevated temperatures that will tend to close surface cracks and pores, thereby reducing infiltration to the substrate and creating a more protective structure [44]. The minimum and maximum thermal expansion mismatch stress () in going from 20 °C to 60 °C for Fe-Ni electrodeposited coatings applied to a carbon steel substrate can be estimated from Equation (11). The minimum compressive stress as determined for the case of a pure iron coating and the maximum from a pure nickel coating are computed as −1.1 MPa and −18.3 MPa, respectively. These values are well within the elastic range of the coating material (50 MPa). Since no additional applied loads were induced on the specimens, only a linear-elastic stress state resulted, and no potential shift due to plastic strain was anticipated (Equation (10)). In addition, Equation (9) predicts that the potential shift due to elastic strain in the electrodeposited coating was negligible at much less than a millivolt. Hence, the mechanochemical interaction effect was negligible for this work. However, it could be a more important consideration for coatings applied to pressurized boiler tubes at elevated operating temperatures where plastic strain in the coating material may result due to its lower yield strength (50 MPa) as compared to the mild carbon steel tube (255 MPa). As such, further exploration of mechanochemical influences on the corrosion behavior of electrodeposited coatings applied to the water-side of boiler tubes at typical plant operating conditions is needed.
The electrochemical cell potential can be written as the difference between cathodic and anodic reaction potentials, as seen in Equation (12).
From a thermodynamics perspective, the variation in electrochemical cell potential () with temperature () and its relation to the change in free energy () of a voltaic cell are provided by the Nernst equation [12,45] (Equation (13)) and the Gibb’s equation [12] (Equation (14)), respectively.
where is the standard electrode potential and is the reaction quotient of products to reactants. Based on these relations, as the electrochemical potential of the oxidation reaction becomes increasingly negative, the overall cell potential becomes more positive, resulting in an increase in free energy. As can be seen in Figure 3, the mean OCP shift in this work as temperature was increased from 20 °C to 60 °C was −9 mV, +41 mV, and +54 mV for the 0.00 M, 0.05 M, and 0.10 M Ni baths, respectively. As such, increasing the nickel composition in the electroplating solution tended to enhance the thermodynamic favorability of the oxidation reaction at elevated temperature, demonstrating a degree of tuneability in the electrochemical properties of the coating as a function of alloy composition. In addition, Figure 7 indicated a corresponding increase in reaction kinetics as the uniform corrosion rate increased by 0.06, 0.07, and 0.20 mm/yr for the 0.00 M, 0.05 M, and 0.10 M Ni baths, respectively. While the increase in temperature from 20 °C to 60 °C produced the anticipated corresponding increase in reaction rate across all specimens, a distinctive temperature-dependent acceleration in reaction rate was observed and may also be linked to an increasing nickel composition in the alloy deposit. The temperature-dependent amplification of corrosion thermodynamics and kinetics observed in this research suggests that the protective characteristics of Fe-Ni alloy deposits may be reasonably tunable to specific temperature conditions by adjusting elemental composition to achieve an optimal cathodic protection scheme.
Electrodeposits produced from the 0.00 M Ni bath tended to exhibit significantly more porosity (Figure 11a) than electrodeposits produced from baths containing nickel ions (Figure 12a and Figure 13a). The resulting porosity not only tended to expose the substrate to process fluid directly but also promoted the generation of cracks between pores. As a result, both the total surface length of cracks and their average width in electrodeposits from the iron-only bath (0.00 M Ni) were more prevalent than those found in electrodeposits containing nickel (0.05 M and 0.10 M Ni baths). This trend in the electrodeposit surface feature characteristics may be seen in Table 7. Despite nickel generally being a better catalyst than iron for the hydrogen evolution reaction in an acidic electrolyte [46], the addition of nickel to the electroplating solution may have had an inhibiting effect on the evolution of hydrogen gas during the electroplating process. The surface mechanism for hydrogen evolution consists of three possible reaction steps [47]. They include the Volmer step shown in Equation (15), followed by either the Tafel step (Equation (16)) or the Heyrovsky step (Equation (17)).
Due to the relatively slow charge transfer coefficient (≈0.5) for iron and nickel electrodes, the Volmer step (Equation (15)) is rate-determining for the hydrogen evolution reaction in acidic media. The introduction of ions, and any resulting adsorbed reaction intermediates would tend to increase competition for reaction sites on the electrode surface. Since the production of adsorbed hydrogen atoms on the surface (Equation (15)) is the rate-determining step, less hydrogen gas may have been evolved as proportionally more current was used for metal ion reduction. With less hydrogen evolved, the extent of porosity and cracking would tend to be diminished for higher Ni contents, as observed in the present results (Figure 11, Figure 12 and Figure 13). Based on these observations, for Fe-Ni alloys at low nickel percentages, it appears that increasing the nickel composition tended to lessen the rate of hydrogen evolution, which progressively enhanced the coating quality (less porosity and cracking). As a result, the amount of substrate surface area exposed to the corrosive environment was lessened.
Since the addition of nickel into the plating bath reduced the degree of porosity and cracking of the electrodeposit surface, the anode-to-cathode (A/C) surface area ratio was likely increased as nickel composition was increased (Table 7). With less surface porosity and cracking, fewer sites were available for the reduction of oxygen to occur on the cathode surface (the substrate). Since cathodic reactions tend to control corrosion rates, the average galvanic corrosion rates (as observed in Figure 6) lessen with increasing A/C surface area ratio. Interestingly, while variance in galvanic corrosion rate increased at elevated temperature, there was no statistically significant difference in galvanic corrosion rate between 20 °C and 60 °C when the A/C surface area ratio was 100:1 (Figure 6). This suggests that the galvanic corrosion rate may have been inhibited due to insufficient availability of reaction sites (less cathode surface area) for hosting of the oxygen reduction reaction (Equation (2)) at this high A/C area ratio. This circumstance can benefit durability by extending the service life of the deposit. Based on these observations, anodic reactions (including metal dissolution) were restricted for the higher A/C surface area ratios, thus extending the useful life of the sacrificial electrodeposit coating.
The morphology of the electrodeposit surface was relatively smooth with few localized facets when only iron (0.00 Ni bath) was plated onto the carbon steel substrate (Figure 11). However, with the addition of nickel to the electroplating bath, uniformly distributed dendrite-shaped facets emerged, as can be seen in Figure 12 and Figure 13. The formation of these structures suggests that the addition of ions to the electroplating solution affected the diffusion rate and growth kinetics of the electrodeposit. No agitation of the electrolyte was provided during the plating process. The lack of agitation would tend to increase the diffusion layer thickness, thereby further contributing to a diffusion-controlled growth process as opposed to one more influenced by convection. This relatively stagnant layer would tend to permit more time for ions to segregate into phases that are thermodynamically favored. The resulting uneven grain growth may have promoted variations in current density and local ion concentration as metal ions preferentially reduced at the outermost available surface protrusions [48,49]. The dendritic Fe-Ni phases grew perpendicularly from the substrate surface with characteristic branch-like formations spanning about 2 in size (Figure 12c and Figure 13c).
As was previously discussed, cathodic protection of the mild carbon steel substrate was weakened at ambient temperature and enhanced at elevated temperatures as the molar ratio was increased in the electroplating solution, as shown in Figure 3 and Figure 5. Understanding the influence of this temperature-dependent activation, in combination with composition-dependent surface structure and morphology, may prove beneficial in tailoring the elemental composition of a protective coating for a variety of potential applications, including power boiler systems. In shutdown or low-temperature operations, a weak level of cathodic protection of the boiler tubes, such as may be seen in Figure 5 at 20 °C, serves to minimize galvanic corrosion of the sacrificial coating, thereby extending its useful life. As boiler temperatures increase, the protective coating becomes activated to provide a higher degree of cathodic protection to the tube material during boiler operation. This trend may be seen in Figure 5 galvanic corrosion rates at 60 °C. It is noted, however, that additional testing is needed at yet higher temperatures closer to actual boiler operating conditions. The results of this study suggest the possibility of an optimal Fe-Ni composition that may be tailored to boiler unit-specific operating parameters to provide cathodic protection between maintenance cycles as part of a plant’s strategic boiler management plan.
The electrochemical deposition of alloys allows for tuning of material properties to suit specific applications. However, codeposition of two or more metals is often considerably more complex than that of single metal deposits. Additional study of the process-structure-property-performance relationships for electrodeposited alloys used as protective coatings in boiler systems is needed. Since subcritical power boilers operate at elevated pressures and temperatures up to 22.06 MPa (3200 psi) and 374 °C (705 °F), it is recommended that additional investigation of the potentials and rate of corrosion reactions for Fe-Ni alloy electrodeposited coatings be studied under conditions commensurate with typical plant operating conditions.
5. Conclusions
The corrosion behavior, surface morphology, and elemental composition of Fe-Ni alloy electrodeposits immersed in oxygen-saturated, weak ammonium hydroxide solution have been experimentally investigated. From this investigation, the following conclusions were made:
- Fe and Fe-Ni alloy coatings were successfully applied as sacrificial anodes for the cathodic protection of mild carbon steel boiler tubes. A distinguishable variation in the corrosion behavior of electroplated specimens was observed. The selected concentrations of ions (0.00 M, 0.05 M, and 0.10 M) in the electroplating solution appeared to influence the chemical properties and resulting corrosion behavior of the deposits in a predictable manner, suggesting tuneability. Overall, the findings highlight a trade-off between galvanic and general corrosion performance, indicating that the optimal Fe-Ni coating composition will depend on the specific application and which corrosion mechanism dominates over the long term. Since the coating is expected to be sporadically applied on the interior surface of boiler tubes, localized effects may play a more significant role in determining its effectiveness.
- The progressive increase in molar ratio within the electroplating solution correlated strongly with a corrosion potential shift in the more noble direction. Additionally, a reduction in corrosion current density was observed within the region where passivation typically occurs, suggesting a slight enhancement of the protective oxide layer with higher nickel content.
- The temperature-dependent amplification of corrosion thermodynamics and kinetics observed in this research suggests that the protective characteristics of Fe-Ni alloy electrodeposits may be reasonably tunable to specific temperature conditions by adjusting elemental composition to achieve an optimal cathodic protection scheme.
- Increasing the nickel composition appeared to reduce porosity and cracking of the deposit surface, leading to a reduction in the amount of substrate surface area exposed to the environment and improving the protective capability of the coating.
- The addition of ions to the electroplating solution affected the diffusion rate and growth kinetics of the resulting electrodeposit as dendrite structures immerged and grew perpendicularly to the substrate surface with the characteristic branch-like formation spanning about 2 µm in size. The resulting deposits contained a relatively uniform distribution of Ni through the 30–40 µm plating thickness. The 0.05 M Ni bath yielded an average Ni content of 5.15 wt%, while the 0.10 M Ni bath produced an average of 10.51 wt%.
Author Contributions
Conceptualization, J.A.H. and H.E.C.; methodology, J.A.H. and H.E.C.; formal analysis, J.A.H. and H.E.C.; data curation, J.A.H.; writing—original draft preparation, J.A.H.; writing—review and editing, J.A.H. and H.E.C.; visualization, J.A.H.; supervision, H.E.C.; funding acquisition, H.E.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Louisiana Board of Regents Support Fund R&D Program under Industrial Ties Research Subprogram (ITRS) reference number 20130013610.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors would like to thank Sven Eklund of the Chemistry Department at Louisiana Tech University for helpful discussions of electrochemical characterization techniques. The authors gratefully acknowledge the endorsement and financial support provided for this work by Southwestern Electric Power Co., an American Electric Power company, and LA New Product Development Team, LLC.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- ASM International. ASM Handbook Volume 13C: Corrosion: Environments and Industries; Cramer, S.D., Covino, B.S., Jr., Eds.; ASM International: Materials Park, OH, USA, 2006; ISBN 978-0-87170-709-3. [Google Scholar]
- Malik, A.; Meroufel, A.; Al-Fozan, S. Boiler Tubes Failures: A Compendium of Case Studies. J. Fail. Anal. Prev. 2015, 15, 246–250. [Google Scholar] [CrossRef]
- Duarte, C.A.; Espejo, E.; Martinez, J.C. Failure Analysis of the Wall Tubes of a Water-Tube Boiler. Eng. Fail. Anal. 2017, 79, 704–713. [Google Scholar] [CrossRef]
- Dinesh, S.; Jose Anandh Vino, V. Analysis of Corrosive Degradation and Failure of Water Wall Tubes. Mater. Today Proc. 2022, 62, 2168–2172. [Google Scholar] [CrossRef]
- Frayne, C. Boiler Water Treatment: Principles and Practice; Chemical Publishing Co.: New York, NY, USA, 2002. [Google Scholar]
- Revie, R.W.; Uhlig, H.H. Corrosion and Corrosion Control: An Introduction to Corrosion Science and Engineering, 4th ed.; Wiley: Hoboken, NJ, USA, 2008. [Google Scholar]
- Ahmad, Z. Principles of Corrosion Engineering and Corrosion Control; Elsevier: Burlington, MA, USA, 2006. [Google Scholar]
- von Baeckmann, W.; Schwenk, W.; Prinz, W. Handbook of Cathodic Protection: Theory and Practice of Electrochemical Protection Processes, 3rd ed.; Gulf Professional Publishing: Houston, TX, USA, 1997. [Google Scholar]
- Electric Power Research Institute (EPRI). Typical Boiler Tube Damage from Flexible Operation or Cycling; Report No. 3002002086; Electric Power Research Institute (EPRI): Palo Alto, CA, USA, 2013. [Google Scholar]
- Hamblin-Smoske, P. Improve Boiler Reliability with Unit Specific Strategic Planning. In Proceedings of the ASME Power Conference, Baltimore, MD, USA, 28–31 July 2014; American Society of Mechanical Engineers: Baltimore, MD, USA, 2014; Volume 1. [Google Scholar]
- Bornak, W.E. Chemistry of Iron and Its Corrosion Products in Boiler Systems. Corrosion 1988, 44, 154–158. [Google Scholar] [CrossRef]
- Hill, J.W.; Petrucci, R.H. Electrochemistry. In General Chemistry: An Integrated Approach; Prentice-Hall: Saddle River, NJ, USA, 1999; pp. 766–816. [Google Scholar]
- Giurlani, W.; Zangari, G.; Gambinossi, F.; Passaponti, M.; Salvietti, E.; Di Benedetto, F.; Caporali, S.; Innocenti, M. Electroplating for Decorative Applications: Recent Trends in Research and Development. Coatings 2018, 8, 260. [Google Scholar] [CrossRef]
- Inwood, B.C.; Garwood, A.E. Electroplated Coatings for Wear Resistance. Tribol. Int. 1978, 11, 113–119. [Google Scholar] [CrossRef]
- Rybakova, N.; Souto, M.; Martinz, H.-P.; Andriyko, Y.; Artner, W.; Godinho, J.; Nauer, G.E. Stability of Electroplated Titanium Diboride Coatings in High-Temperature Corrosive Media. Corros. Sci. 2009, 51, 1315–1321. [Google Scholar] [CrossRef]
- Ma, L.; Zhou, K.; Li, Z.; Wei, Q.; Zhang, L. Hot Corrosion of a Novel NiO/NiFe2O4 Composite Coating Thermally Converted from the Electroplated Ni–Fe Alloy. Corros. Sci. 2011, 53, 3712–3724. [Google Scholar] [CrossRef]
- Myung, N.V.; Nobe, K. Electrodeposited Iron Group Thin-Film Alloys: Structure-Property Relationships. J. Electrochem. Soc. 2001, 148, C136. [Google Scholar] [CrossRef]
- Cardenas, H.E.; Shrestha, A. Protective Characteristics of Electroplated Iron Applied to Low Carbon Steel. AIP Conf. Proc. 2018, 2022, 020018. [Google Scholar]
- Huang, J.; Cardenas, H. Fatigue Crack Arrest in Mild Steel via Iron Electroplating. Mater. Sci. Appl. 2021, 12, 484–503. [Google Scholar] [CrossRef]
- Bhat, R.S.; Nagaraj, P.; Priyadarshini, S. Zn–Ni Compositionally Modulated Multilayered Alloy Coatings for Improved Corrosion Resistance. Surf. Eng. 2021, 37, 755–763. [Google Scholar] [CrossRef]
- Bhat, R.S.; Shetty, S.M.; Kumar, N.A. Electroplating of Zn-Ni Alloy Coating on Mild Steel and Its Electrochemical Studies. J. Mater. Eng. Perform. 2021, 30, 8188–8195. [Google Scholar] [CrossRef]
- Bhat, R.S.; Balakrishna, M.K.; Parthasarathy, P.; Hegde, A.C. Structural Properties of Zn-Fe Alloy Coatings and Their Corrosion Resistance. Coatings 2023, 13, 772. [Google Scholar] [CrossRef]
- Torabinejad, V.; Aliofkhazraei, M.; Assareh, S.; Allahyarzadeh, M.H.; Rouhaghdam, A.S. Electrodeposition of Ni-Fe Alloys, Composites, and Nano Coatings–A Review. J. Alloys Compd. 2017, 691, 841–859. [Google Scholar] [CrossRef]
- Chaudhari, A.K.; Singh, V.B. A Review of Fundamental Aspects, Characterization and Applications of Electrodeposited Nanocrystalline Iron Group Metals, Ni-Fe Alloy and Oxide Ceramics Reinforced Nanocomposite Coatings. J. Alloys Compd. 2018, 751, 194–214. [Google Scholar] [CrossRef]
- ASTM G16-95; Standard Guide for Applying Statistics to Analysis of Corrosion Data. ASTM International: West Conshohocken, PA, USA, 2010.
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- ASTM G102-89; Standard Practice for Calculation of Corrosion Rates and Related Information from Electrochemical Measurements. ASTM International: West Conshohocken, PA, USA, 2015.
- ASTM G 59-97; Standard Test Method for Conducting Potentiodynamic Polarization Resistance Measurements. ASTM International: West Conshohocken, PA, USA, 2014.
- Brenner, A. Electrodeposition of the Mutual Alloys of the Iron-Group Metals. In Electrodeposition of Alloys, Principles and Practice; Academic Press: New York, NY, USA, 1963; Volume II. [Google Scholar]
- Tilak, B.V.; Gendron, A.S.; Mosoiu, M.A. Borate Buffer Equilibria in Nickel Refining Electrolytes. J. Appl. Electrochem. 1977, 7, 495–500. [Google Scholar] [CrossRef]
- Popov, B.N.; Yin, K.; White, R.E. Galvanostatic Pulse and Pulse Reverse Plating of Nickel-Iron Alloys from Electrolytes Containing Organic Compounds on a Rotating Disk Electrode. J. Electrochem. Soc. 1993, 140, 1321–1330. [Google Scholar] [CrossRef]
- Matulis, J.; Sližys, R. On Some Characteristics of Cathodic Processes in Nickel Electrodeposition. Electrochim. Acta 1964, 9, 1177–1188. [Google Scholar] [CrossRef]
- Hessami, S.; Tobias, C.W. A Mathematical Model for Anomalous Codeposition of Nickel-Iron on a Rotating Disk Electrode. J. Electrochem. Soc. 1989, 136, 3611–3616. [Google Scholar] [CrossRef]
- Grimmett, D.; Schwartz, M.; Nobe, K. Pulsed Electrodeposition of Iron-Nickel Alloys. J. Electrochem. Soc. 1990, 137, 3414–3418. [Google Scholar] [CrossRef]
- Zech, N.; Podlaha, E.J.; Landolt, D. Anomalous Codeposition of Iron Group Metals: II. Mathematical Model. J. Electrochem. Soc. 1999, 146, 2892–2900. [Google Scholar] [CrossRef]
- Abdel-Karim, R.; Reda, Y.; Muhammed, M.; El-Raghy, S.; Shoeib, M.; Ahmed, H. Electrodeposition and Characterization of Nanocrystalline Ni-Fe Alloys. J. Nanomater. 2011, 2011, 1–8. [Google Scholar] [CrossRef]
- Bedir, M.; Bakkaloğlu, O.F.; Karahan, I.H.; Öztaş, M. A Study on Electrodeposited Ni x Fe1−x Alloy Films. Pramana 2006, 66, 1093–1104. [Google Scholar] [CrossRef]
- Callister, W.D.; Rethwisch, D.G. Materials Science and Engineering: An Introduction, 8th ed.; Wiley: Hoboken, NJ, USA, 2009. [Google Scholar]
- Dini, J.W. Electrodeposition: The Materials Science of Coatings and Substrates; Noyes Publications: Park Ridge, NJ, USA, 1993. [Google Scholar]
- Petrucci, R.; Herring, G.; Madura, J.; Bissonnette, C. General Chemistry: Principles and Modern Applications, 10th ed.; Pearson: Toronto, ON, Canada, 2011. [Google Scholar]
- Gutman, E.M. Mechanochemistry of Materials; Cambridge International Science Publishing: London, UK, 1998. [Google Scholar]
- Gutman, E.M. Mechanochemistry of Solid Surfaces; World Scientific Publishing: Singapore, 1994. [Google Scholar]
- Evans, A.G.; Hutchinson, J.W. The Thermomechanical Integrity of Thin Films and Multilayers. Acta Metall. Mater. 1995, 43, 2507–2530. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, L.; Sun, J.; Cheng, Y.F. Mechano-Electrochemical Interaction for Pipeline Corrosion: A Review. J. Pipeline Sci. Eng. 2021, 1, 1–16. [Google Scholar] [CrossRef]
- Bratsch, S.G. Standard Electrode Potentials and Temperature Coefficients in Water at 298.15 K. J. Phys. Chem. Ref. Data 1989, 18, 1–21. [Google Scholar] [CrossRef]
- Trasatti, S. Work Function, Electronegativity, and Electrochemical Behaviour of Metals. J. Electroanal. Chem. Interfacial Electrochem. 1972, 39, 163–184. [Google Scholar] [CrossRef]
- Compton, R.G.; Banks, C.E. Understanding Voltammetry, 3rd ed.; World Scientific: Singapore, 2018. [Google Scholar]
- Ning, Z.; He, Y.; Gao, W. Mechanical Attrition Enhanced Ni Electroplating. Surf. Coat. Technol. 2008, 202, 2139–2146. [Google Scholar] [CrossRef]
- Landolt, D. Electrochemical and Materials Science Aspects of Alloy Deposition. Electrochem. Acta 1994, 39, 1075–1090. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).