Corrosion at the Steel–Medium Interface
Abstract
1. Introduction
2. Classical Models for Corrosion Initiation and Early Development
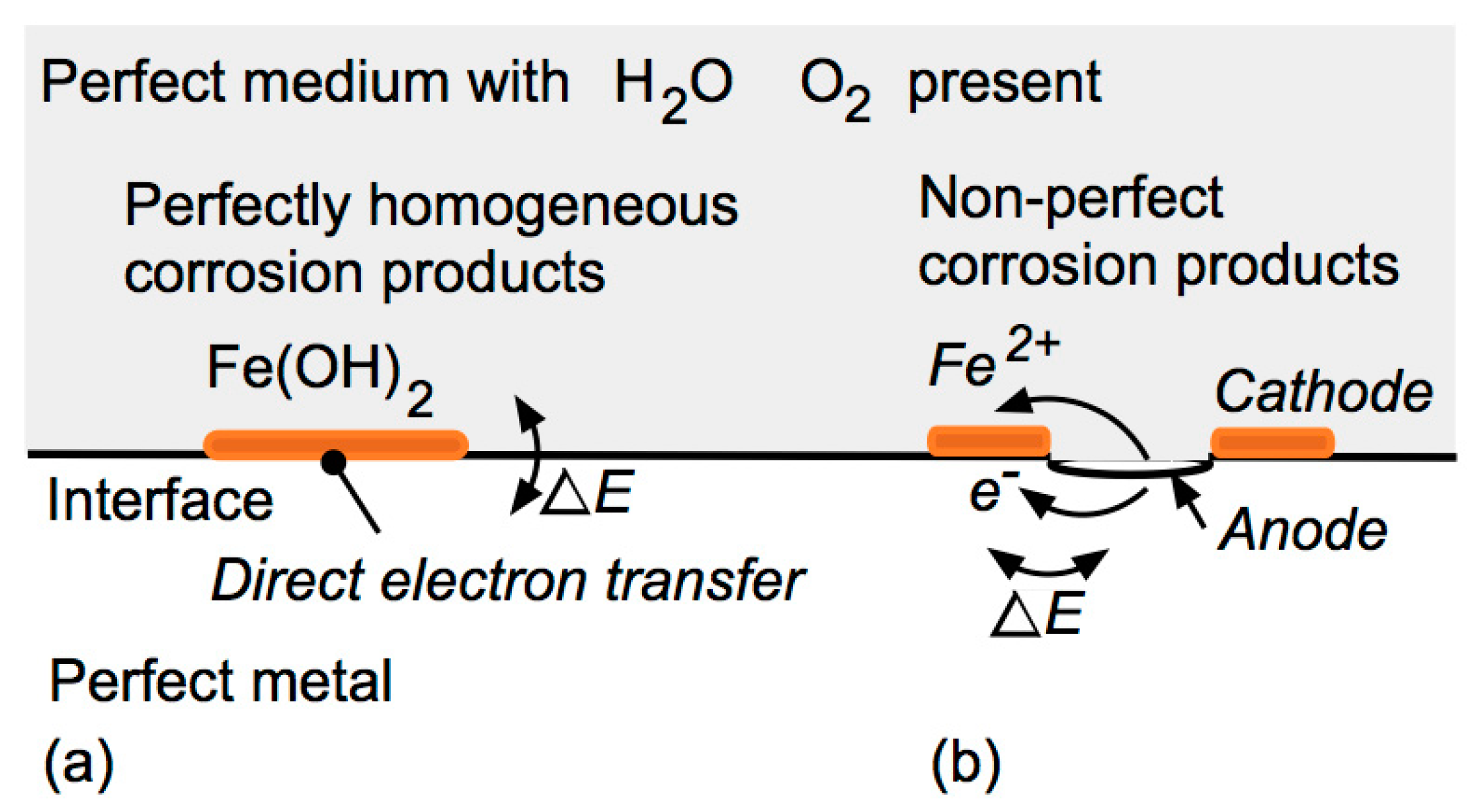
2.1. ‘Pure’ Metal in Contact with a Wet Homogeneous (Pure) Medium
2.2. Practical Metals and a Wet Homogeneous (Pure) Medium
2.3. Effect of Chlorides in a Wet Homogeneous Medium
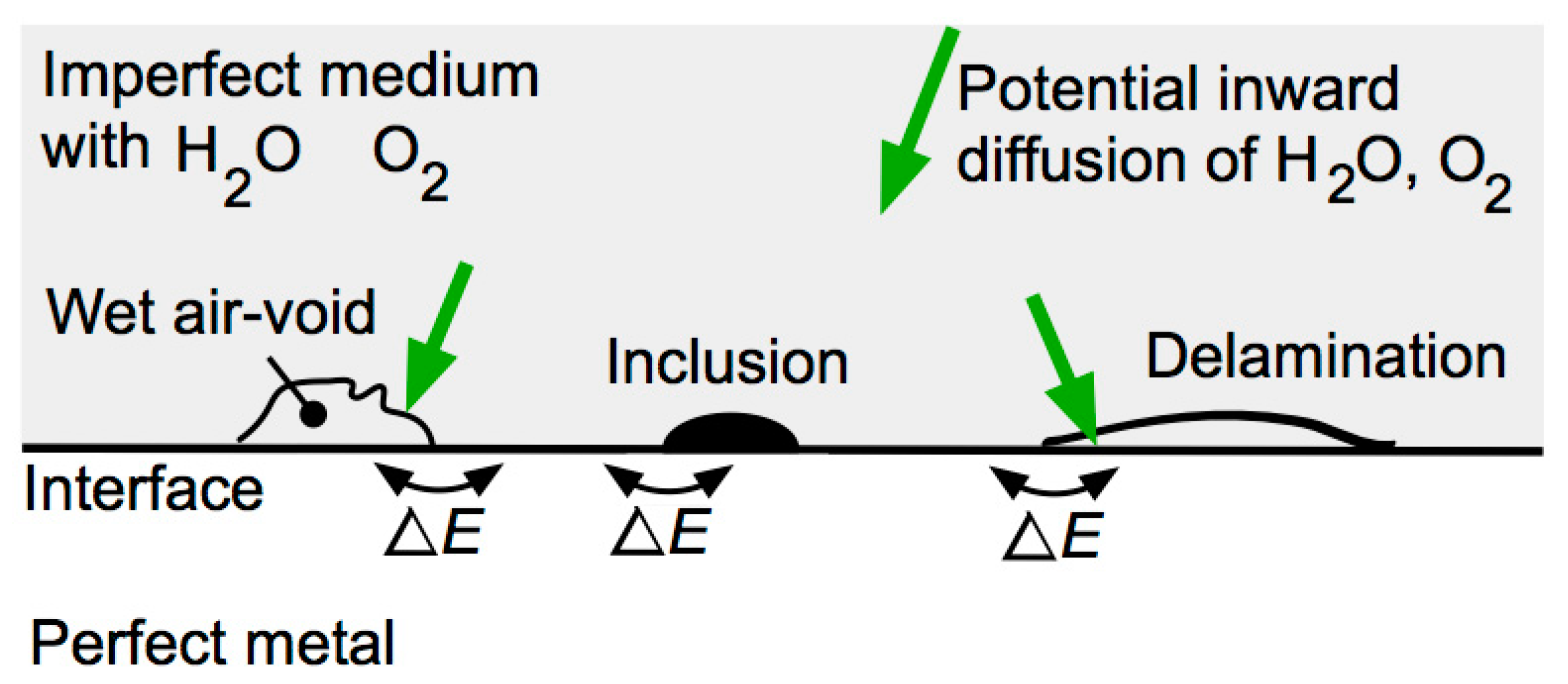
3. Corrosion Initiation for Non-Homogeneous Media
3.1. Overview
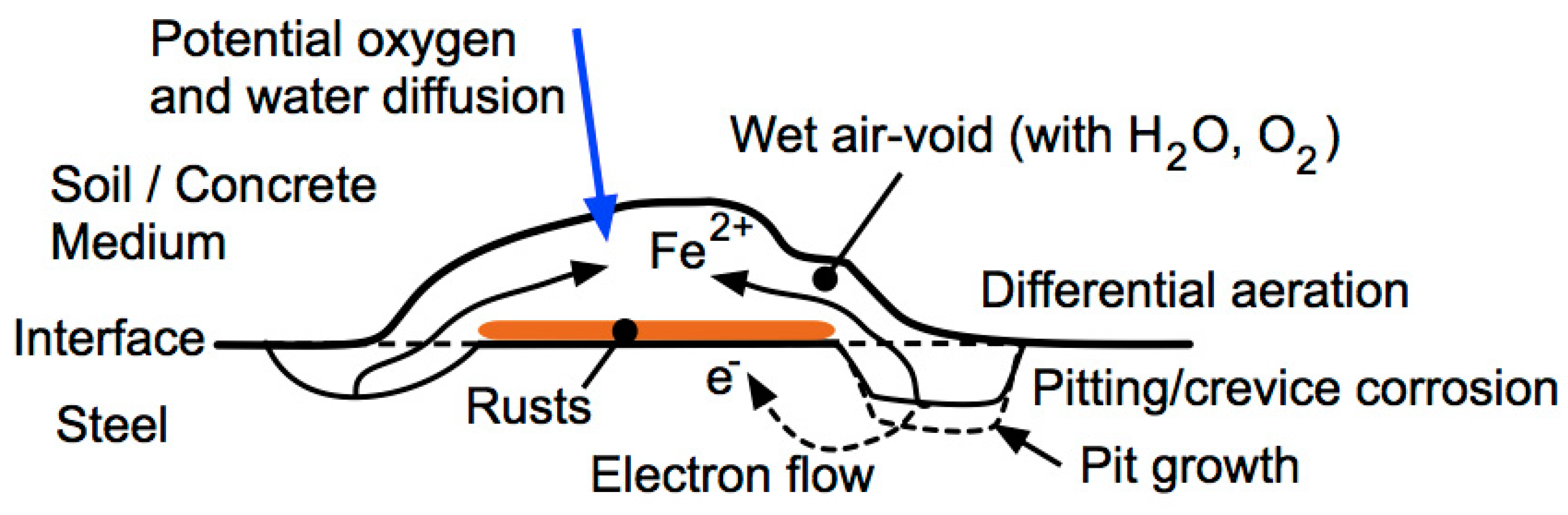
3.2. Corrosion Initiation for Ferrous Metals in Contact with Soils
3.3. Corrosion Initiation for Reinforcing Steel in Concrete
3.4. Corrosion Initiation under Protective Coatings
3.5. Other Cases
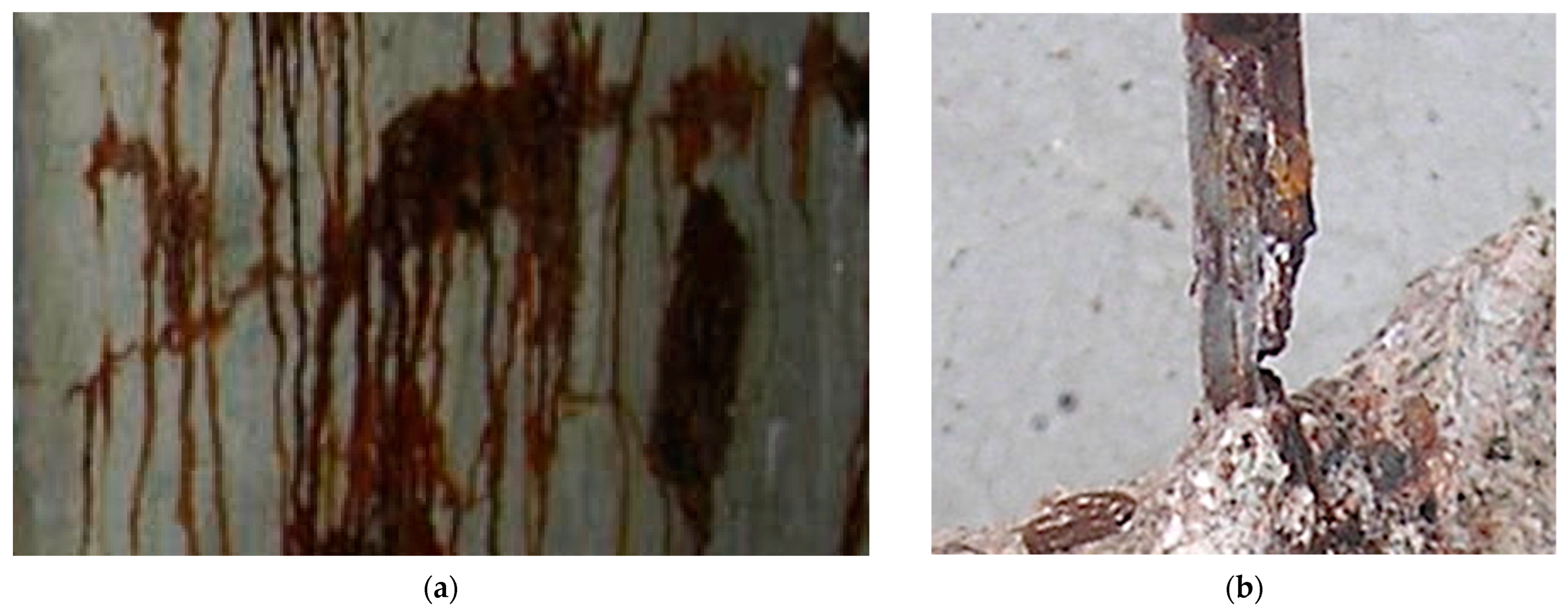
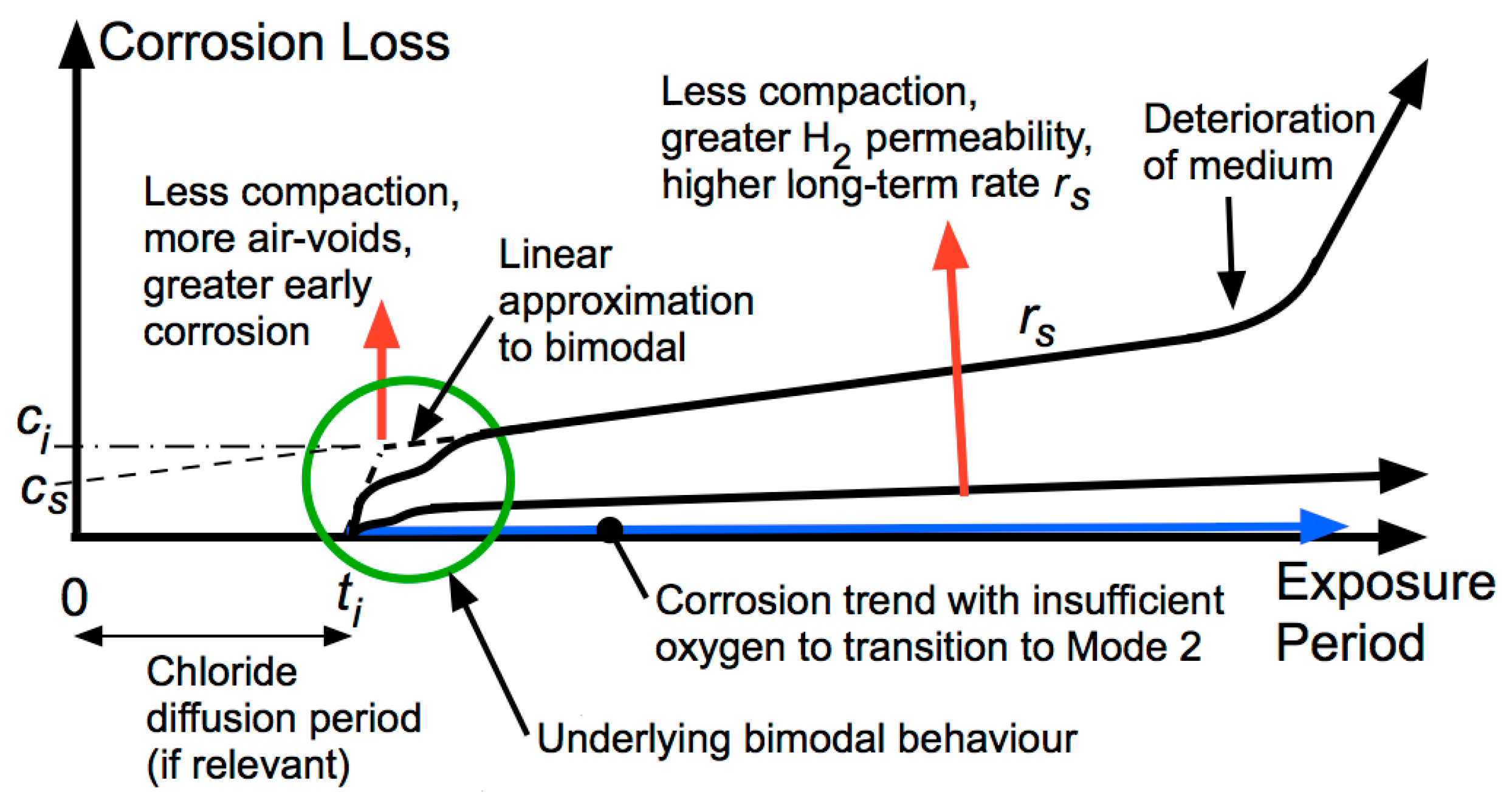
4. Development of Corrosion and Pitting with Continued Exposure
5. Long-Term Corrosion
6. Discussion
7. Conclusions
- Both local differences in electrochemical potential due to inhomogeneities of the steel and inhomogeneities of the surface of the medium interfacing with the steel can be sources of initiation of corrosion for the steel, although, for many practical applications, the inhomogeneities of the medium are of greater importance due to their greater physical scale.
- Corrosion of steel in water and the atmosphere may be considered specialized cases of corrosion in homogeneous media extending over semi-infinite spaces.
- As corrosion at the interface progresses, the instantaneous corrosion rate is increasingly governed by the properties of the corrosion products at the interface and less by the properties of the steel or the medium, with corrosion progressing eventually to and then through the hydrogen evolution as the principal long-term cathodic reaction in all cases.
- The same mechanisms are likely to hold for other media of types similar to those considered herein when in contact with alloys and metals other than steel and for which the hydrogen evolution reaction is feasible.
- Breakdown of the medium itself may lead to steel corrosion—it is not always the case that the corrosion of the steel causes damage to, and possibly breakdown of, the medium.
Funding
Acknowledgments
Conflicts of Interest
References
- Ackland, B.G.; Dylejko, K.P. Critical questions and answers about cathodic protection. Corros. Eng. Sci. Technol. 2019, 54, 688–697. [Google Scholar] [CrossRef]
- Coogan, C. Marine Corrosion and Cathodic Protection; CRC Press: Boca Raton, FL, USA, 2022. [Google Scholar]
- Jones, D.A. Principles and Prevention of Corrosion, 2nd ed.; Prentice Hall: Upper Saddle River, NJ, USA, 1996. [Google Scholar]
- Garrels, R.M.; Christ, C.L. Solutions, Minerals and Equilibria; Harper & Row: New York, NY, USA, 1965. [Google Scholar]
- Pourbaix, M. Atlas of Electrochemical Equilibria in Aqueous Solutions; NACE Int.: Houston, TX, USA, 1974. [Google Scholar]
- Mercer, A.D.; Lumbard, E.A. Corrosion of mild steel in water. Br. Corros. J. 1995, 30, 43–55. [Google Scholar] [CrossRef]
- Abiko, K.; Nakajima, T.; Harima, N.; Takaki, S. Preparation of 10 kg ingot of ultra-pure iron. Phys. Status Solidi 1998, 167, 347–355. [Google Scholar] [CrossRef]
- Abiko, K. Why do we study ultra-pure base metals? Mater. Trans. JIM 2000, 41, 233–237. [Google Scholar] [CrossRef]
- Khan, L.; Sato, K.; Okuyama, S.; Kobayashi, T.; Ohashi, K.; Hirasaka, K.; Nikawa, T.; Takada, K.; Higashitani, A.; Abiko, K. Ultra-high purity iron is a novel and very compatible biomaterial. J. Mech. Behav. Biomed. Mater. 2020, 106, 103744. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, E.; Olivetti, E.; Fiorillo, F.; Forton, R.; Martino, L.; Rocchino, L. Microstructure and magnetic properties of pure iron for cyclotron electromagnets. J. Alloys Compd. 2014, 615, S291–S295. [Google Scholar] [CrossRef]
- Li, S.; Hihara, L.H. The comparison of the corrosion of ultrapure iron and low-carbon steel under NaCl-electrolyte droplets. Corros. Sci. 2016, 108, 200–204. [Google Scholar] [CrossRef]
- Evans, U.R. The Corrosion and Oxidation of Metals: Scientific Principles and Practical Applications; Edward Arnold: London, UK, 1960. [Google Scholar]
- Atrens, A.; Song, G.-L.; Cao, F.; Shi, Z.; Bowen, P.K. Advances in Mg corrosion and research suggestions. J. Magnes. Alloys 2013, 1, 177–200. [Google Scholar] [CrossRef]
- van der Voort, G.F. Metallurgy: Principles and Practice; McGraw-Hill: New York, NY, USA, 1984. [Google Scholar]
- Wranglen, G. Review article on the influence of sulphide inclusions on the corrodibility of Fe and steel. Corros. Sci. 1969, 9, 585–602. [Google Scholar] [CrossRef]
- Wranglen, G. Pitting and sulphide inclusions in steel. Corros. Sci. 1974, 14, 331–349. [Google Scholar] [CrossRef]
- Lillard, R.S.; Kashfipour, M.A.; Niu, W. Pit propagation at the boundary between manganese sulfide inclusions and austenitic stainless steel 303 and the role of copper. J. Electro. Soc. 2016, 163, C440–C451. [Google Scholar] [CrossRef]
- Melchers, R.E.; Chaves, I.A.; Jeffrey, R. A conceptual model for the interaction between carbon content and manganese sulphide inclusions in the short-term seawater corrosion of low carbon steel. Metals 2016, 6, 132. [Google Scholar] [CrossRef]
- King, R.A.; Miller, J.D.A. Corrosion by the sulphate-reducing bacteria. Nature 1971, 233, 491–492. [Google Scholar] [CrossRef] [PubMed]
- Crolet, J.-L. From biology and corrosion to biocorrosion. In Microbial Corrosion; Sequeira, C.A., Tiller, A.K., Eds.; The Institute of Metals: London, UK, 1992; pp. 50–60. [Google Scholar]
- Jia, R.; Unsal, T.; Xu, D.; Lekbach, X.; Gu, T. Microbiologically infleucned corroison and current mitigation strategies: A state of the art review. Int. Biodeterior. Biodegrad. 2019, 137, 42–58. [Google Scholar] [CrossRef]
- Melchers, R.E.; Jeffrey, R. Surface roughness effect on marine immersion corrosion of mild steel. Corrosion 2004, 60, 697–703. [Google Scholar] [CrossRef]
- Melchers, R.E.; Ahammed, M.; Jeffrey, R.J.; Simundic, G. Statistical characterization of corroded steel surfaces. Mar. Struct. 2010, 23, 274–287. [Google Scholar] [CrossRef]
- Phull, B.S.; Pikul, S.J.; Kain, R.M. Seawater corrosivity around the world: Results from five years of testing. In Corrosion Testing in Natural Waters, Second Volume; Kain, R.M., Young, W.T., Eds.; STP1300; American Society for Testing and Materials: West Conshohocken, PA, USA, 1997; pp. 34–73. [Google Scholar]
- Burstein, G.T.; Pistorius, P.C.; Mattin, S.P. The nucleation and growth of corrosion pits on stainless steels. Corros. Sci. 1993, 35, 57–62. [Google Scholar] [CrossRef]
- Foley, R.T. Role of the chloride ion in iron corrosion. Corrosion 1970, 26, 58–70. [Google Scholar] [CrossRef]
- Foley, R.T. Complex ions and corrosion. J. Electrochem. Soc. 1975, 11, 1493–1994. [Google Scholar] [CrossRef]
- Heyn, E.; Bauer, O. Ueber den Angriff des Eisens durch Wasser und wässerige Losungen. Stahl. Eisen 1908, 28, 1564–1573. [Google Scholar]
- Brasher, D. Stability of the oxide film on metals in relation to inhibition of corrosion. I. Mild steel in the presence of aggressive ions. Brit. Corr. J. 1967, 2, 95–103. [Google Scholar] [CrossRef]
- Baylis, J.R. Factors other than dissolved oxygen influencing the corrosion of iron pipes. Ind. Engrg. Chem. 1926, 18, 370–380. [Google Scholar] [CrossRef]
- Little, B.J.; Gerke, T.L.; Lee, J.S. Mini-review: The morphology, mineralogy and microbiology of accumulated iron corrosion products. Biofouling 2014, 30, 941–948. [Google Scholar] [CrossRef]
- Greene, N.D.; Fontana, M.G. A critical analysis of pitting corrosion. Corrosion 1959, 15, 41–47. [Google Scholar] [CrossRef]
- Sharland, S.M.; Tasker, P.W. A mathematical model of crevice and pitting corrosion—I. The physical model. Corros. Sci. 1988, 28, 603–620. [Google Scholar] [CrossRef]
- Butler, G.; Stretton, P.; Beynon, J.G. Initiation and growth of pits on high-purity iron and its alloys with chromium and copper in neutral chloride solutions. Br. Corros. J. 1972, 7, 168–173. [Google Scholar] [CrossRef]
- Pourbaix, M. Significance of protection potential in pitting and intergranular corrosion. Corrosion 1970, 26, 31–438. [Google Scholar] [CrossRef]
- Romanoff, M. Underground Corrosion, National Bureau of Standards Circular 579; US Government Printing Office: Washington, DC, USA, 1957.
- Rajani, B.; Kleiner, Y. Comprehensive review of structural deterioration of water mains: Physically based models. Urban. Water 2001, 3, 151–164. [Google Scholar] [CrossRef]
- Cole, I.S.; Marney, D. The science of pipe corrosion: A review of the literature on the corrosion of ferrous metals in soils. Corros. Sci. 2012, 56, 5–16. [Google Scholar] [CrossRef]
- Kleiner, Y.; Rajani, B.; Krys, D. Performance of ductile iron pipes. I: Characterization of exterior corrosion patterns. J. Infrastruct. Syst. ASCE 2013, 19, 108–119. [Google Scholar] [CrossRef][Green Version]
- von Wolzogen Kuhr, C.A.H.; van der Vlugt, L.S. Graphitization of cast iron as an electrochemical process in anaerobic soils, Water (den Haag), 18: 147–165 (in Dutch). Transl. Corros. 1971 1934, 17, 293–299. [Google Scholar]
- Ricker, R.E. Analysis of pipeline steel corrosion data from NBS (NIST) studies conducted between 1922–1940 and relevance to pipeline management. J. Res. Nat. Inst. Stand. Technol. 2011, 115, 373–392. [Google Scholar] [CrossRef]
- Melchers, R.E. Models for prediction of long-term corrosion of cast iron water mains. Corrosion 2020, 76, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Wichers, C.M. Korrosion asphaltierter eiserner Rohre. Das. Gas. Wasserfach 1934, 77, 131–132. [Google Scholar]
- Liengen, T.; Feron, D.; Basseguy, R.; Beech, I.B. (Eds.) Understanding Biocorrosion, European Federation of Corrosion Publications; Number 66; Woodhead Publishing in Materials: Cambridge, UK, 2014. [Google Scholar]
- Skovhus, T.L.; Eckert, R.B. (Eds.) Failure Analysis of Microbiologically Influenced Corrosion; CRC Press: Boca Raton, FL, USA, 2021. [Google Scholar]
- Melchers, R.E.; Chaves, I.A. A comparative study of chlorides and longer-term reinforcement corrosion. Mater. Corros. 2017, 68, 613–621. [Google Scholar] [CrossRef]
- Burns, M.; Salley, D.J. Particle size as a factor in the corrosion of lead by soils. Ind. Eng. Chem. 1930, 22, 293–297. [Google Scholar] [CrossRef]
- Petersen, R.B.; Melchers, R.E. Pitting corrosion of mild steel in long-term contact with particulate media in seawater. Corrosion 2023, 79, 1040–1061. [Google Scholar] [CrossRef] [PubMed]
- Richardson, M.G. Fundamentals of Durable Reinforced Concrete; Spon Press: London, UK, 2002. [Google Scholar]
- Angst, U.M.; Geiker, M.R.; Michel, A.; Gehlen, C.; Wong, H.; Isgor, O.B.; Elsener, B.; Hansson, C.M.; François, R.; Hornbostel, K.; et al. The steel-concrete interface. Mater. Struct. 2017, 50, 1–24. [Google Scholar] [CrossRef]
- Wakeman, C.M.; Dockweiler, E.V.; Stover, H.E.; Whiteneck, L.L. Use of concrete in marine environments. Proc. ACI 1958, 54, 841–856. [Google Scholar]
- Melchers, R.E.; Li, C.Q. Reinforcement corrosion initiation and activation times in concrete structures exposed to severe marine environments. Cem. Concr. Res. 2009, 39, 1068–1076. [Google Scholar] [CrossRef]
- Angst, U.M.; Elsener, B.; Larsen, C.K.; Vennesland, O. Critical chloride content in reinforced concrete—A review. Cem. Concr. Res. 2009, 39, 1122–1138. [Google Scholar] [CrossRef]
- Nawy, E.G. Concrete Construction Engineering Handbook; CRC Press: Boca Raton, FL, USA, 2008; pp. 30–57. [Google Scholar]
- Page, C.L. Mechanism of corrosion protection in reinforced concrete marine structures. Nature 1975, 258, 514–515. [Google Scholar] [CrossRef]
- Lea, F.M.; Hewlett, P.C. Lea’s Chemistry of Cement and Concrete, 4th ed.; Butterworth Heinemann: London, UK, 1997. [Google Scholar]
- Johnston, J.; Grove, C. The solubility of calcium hydroxide in aqueous salt solutions. J. Am. Chem. Soc. 1931, 53, 3976–3991. [Google Scholar] [CrossRef]
- Cornell, R.M.; Schwertmann, U. The Iron Oxides: Structure, Properties, Reactions, Occurences and Uses, 2nd ed.; VCH Publishers: Weinheim, Germany, 1996. [Google Scholar]
- Melchers, R.E.; Li, C.Q.; Davison, M.A. Observations and analysis of a 63-year old reinforced concrete promenade railing exposed to the North Sea. Mag. Concr. Res. 2009, 61, 233–243. [Google Scholar] [CrossRef]
- Berensen, A.M. Marine Painting Manual; Springer-Science & Business Media BV: Dordrecht, The Netherlands, 1989. [Google Scholar]
- Hare, C.H. Corrosion Control of Steel by Organic Coatings; Revie, R.W., Ed.; Wiley: New York, NY, USA, 2011; pp. 971–983. [Google Scholar] [CrossRef]
- TSCF. Condition Evaluation and Maintenance of Tanker Structures; Tanker Structures Cooperative Forum, Witherby & Co.: London, UK, 1992; p. 182. [Google Scholar]
- Melchers, R.E. A review of trends for corrosion loss and pit depth in longer-term exposures. Corros. Mater. Degrad. 2018, 1, 4. [Google Scholar] [CrossRef]
- Refait, P.H.; Grolleau, A.-M.; Jeannin, M.; Rémazeilles, C.; Sabot, R. Corrosion of carbon steel in marine environments: Role of the corrosionproduct layer. Corros. Mater. Degrad. 2020, 1, 198–218. [Google Scholar] [CrossRef]
- Melchers, R.E.; Jeffrey, R. The transition from short- to long-term marine corrosion of mild steel—1. experimental observations—2. parameterization and modeling. Corrosion 2022, 78, 415–436. [Google Scholar] [CrossRef]
- Stratmann, M.; Bohnenkamp, K.; Engell, H.J. An electrochemical study of phase-transitions in rust layers. Corros. Sci. 1983, 23, 969–985. [Google Scholar] [CrossRef]
- Melchers, R.E. Long-term immersion corrosion of steels in seawaters with elevated nutrient concentration. Corros. Sci. 2014, 81, 110–116. [Google Scholar] [CrossRef]
- Melchers, R.E. Long-term durability of marine reinforced concrete structures. J. Mar. Sci. Eng. 2020, 8, 290. [Google Scholar] [CrossRef]
- Morley, J. The corrosion and protection of steel-piled structures. Struct. Surv. 1993, 7, 138–151. [Google Scholar] [CrossRef]
- Melchers, R.E.; Chaves, I.A. Reinforcement corrosion in marine concretes—2. Long-term effects. ACI Mater. J. 2020, 117, 217–228. [Google Scholar]
- Melchers, R.E.; Howlett, C.M. Reinforcement corrosion of the Phoenix caissons after 75 years of marine exposure. Proc. Inst. Civ. Eng.-Marit. Eng. 2020, 174, 19–30. [Google Scholar] [CrossRef]
- Melchers, R.E.; Humphrey, H. Concrete alkali-aggregate reactivity and initiation of steel reinforcement corrosion. Corros. Mater. Degrad. 2023, 4, 428–444. [Google Scholar] [CrossRef]
- Melchers, R.E. Mechanisms in long-term marine corrosion of steel reinforcement in concrete. Corrosion 2023, 79, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Southwell, C.R.; Bultman, J.D.; Alexander, A.L. Corrosion of metals in Tropical environments—Final report of 16 years exposures. Mater. Perform. 1976, 15, 9–25. [Google Scholar]
- Melchers, R.E.; Chaves, I.A. Reinforcement corrosion in marine concretes—1: Initiation. ACI Mater. J. 2019, 116, 57–66. [Google Scholar] [CrossRef]
- Popper, K.R. The Growth of Scientific Knowledge; Basic Books: New York, NY, USA, 1963. [Google Scholar]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melchers, R.E. Corrosion at the Steel–Medium Interface. Corros. Mater. Degrad. 2024, 5, 52-72. https://doi.org/10.3390/cmd5010003
Melchers RE. Corrosion at the Steel–Medium Interface. Corrosion and Materials Degradation. 2024; 5(1):52-72. https://doi.org/10.3390/cmd5010003
Chicago/Turabian StyleMelchers, Robert E. 2024. "Corrosion at the Steel–Medium Interface" Corrosion and Materials Degradation 5, no. 1: 52-72. https://doi.org/10.3390/cmd5010003
APA StyleMelchers, R. E. (2024). Corrosion at the Steel–Medium Interface. Corrosion and Materials Degradation, 5(1), 52-72. https://doi.org/10.3390/cmd5010003





