Corrosion of Silica-Based Optical Fibers in Various Environments
Abstract
1. Introduction
2. Experimental Methodology
2.1. Test Conditions and Materials
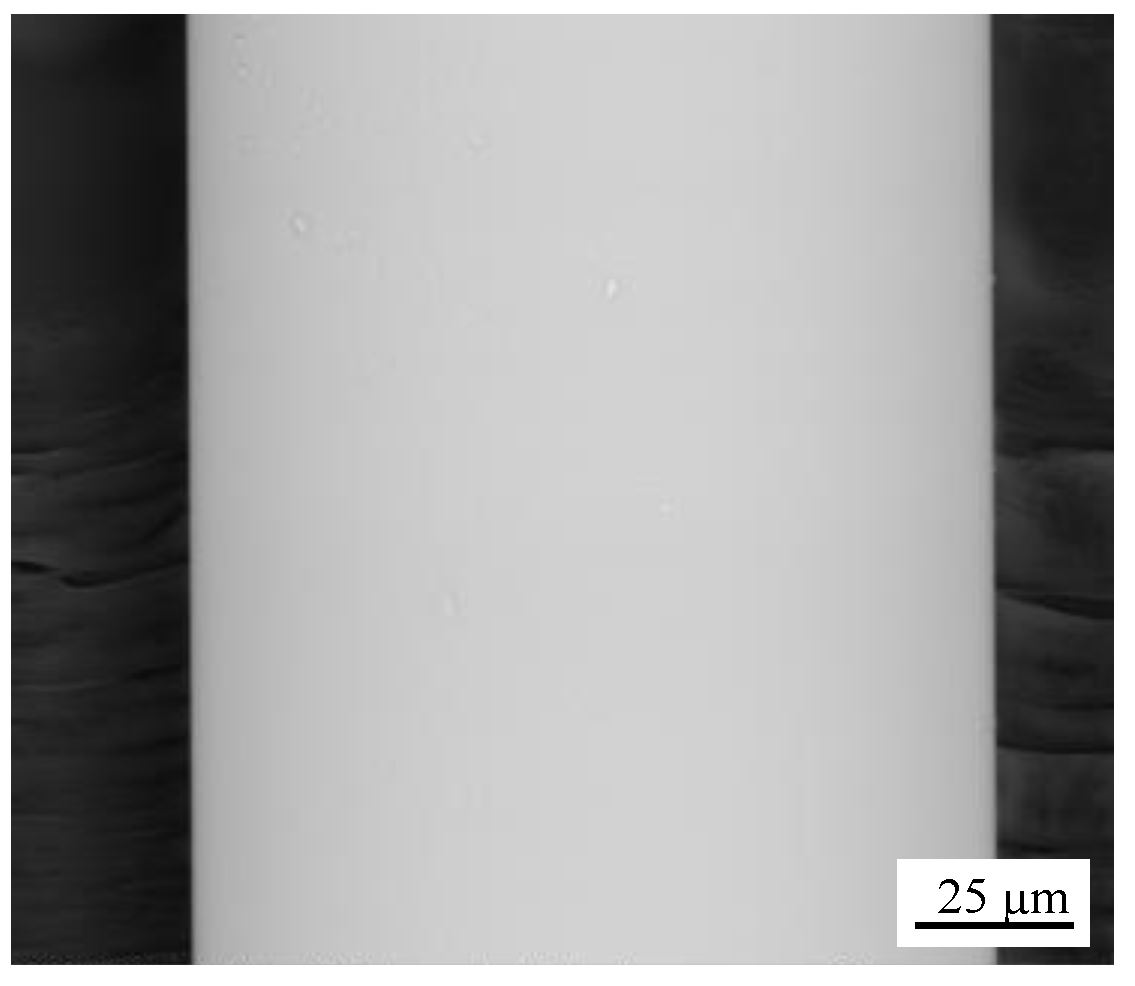
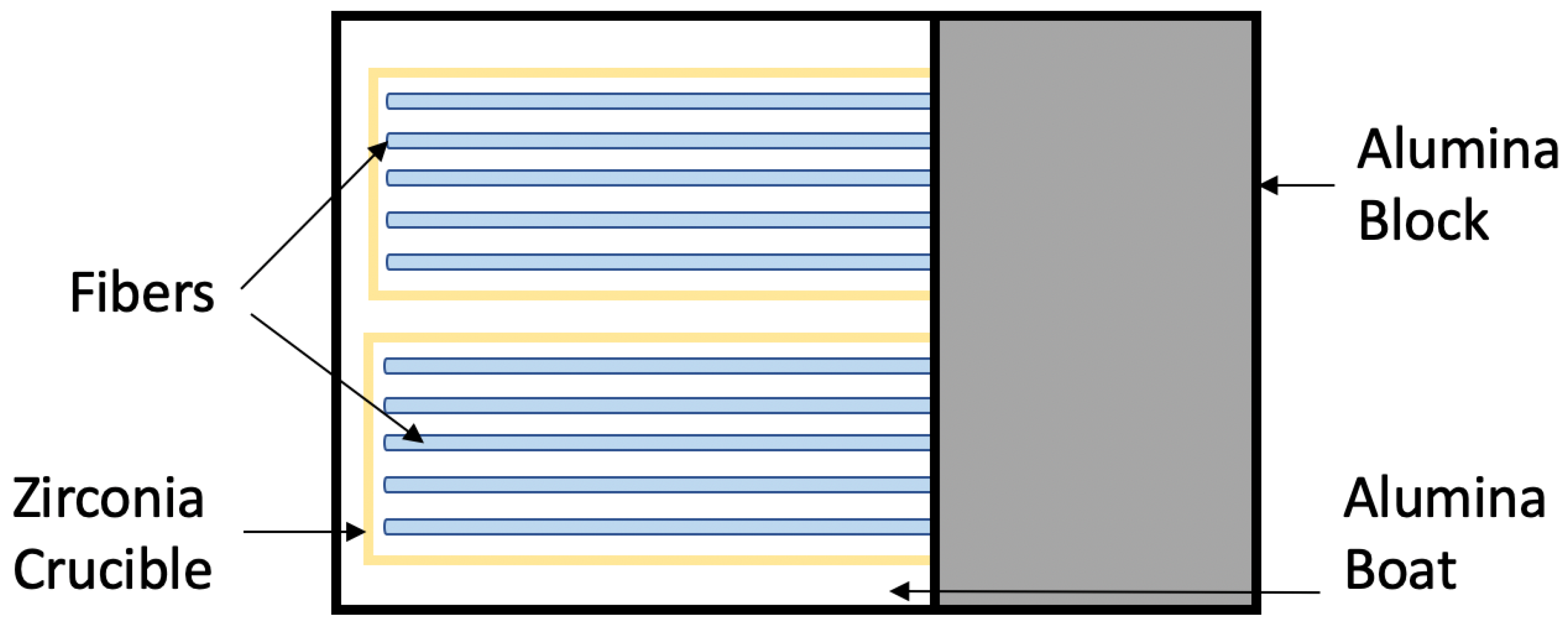
2.2. Test Setup and Post-Test Analysis
3. Results and Discussion
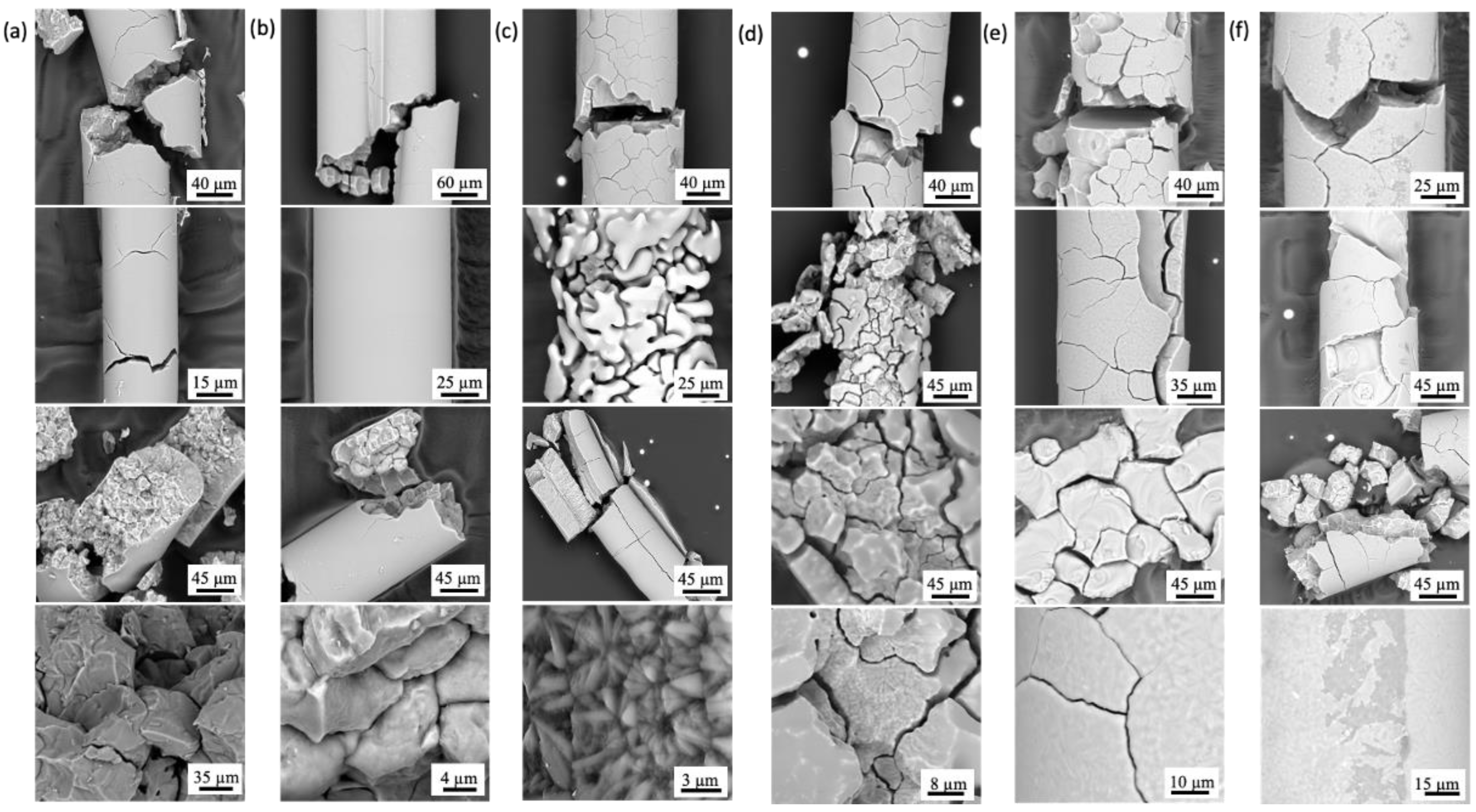
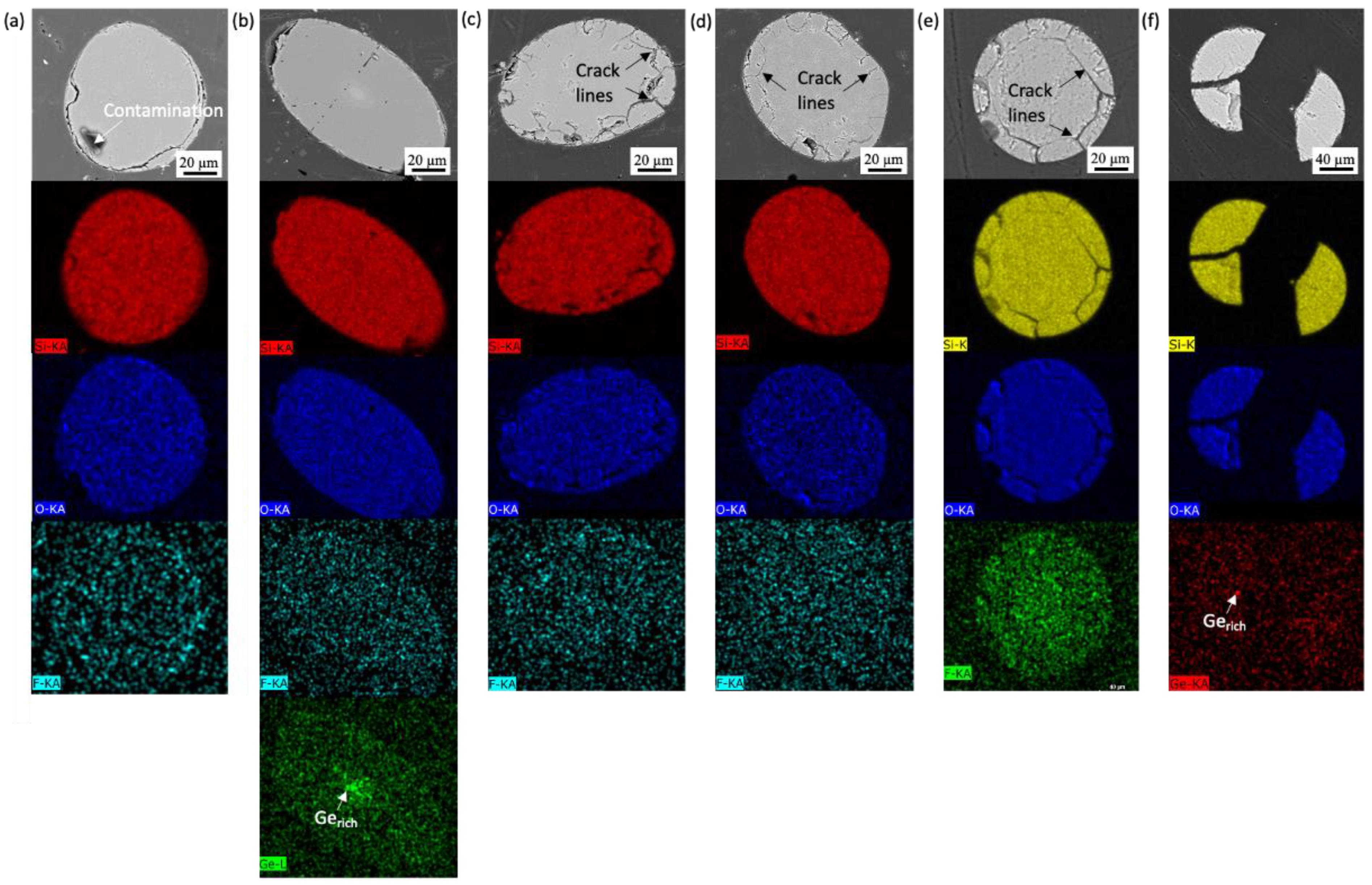
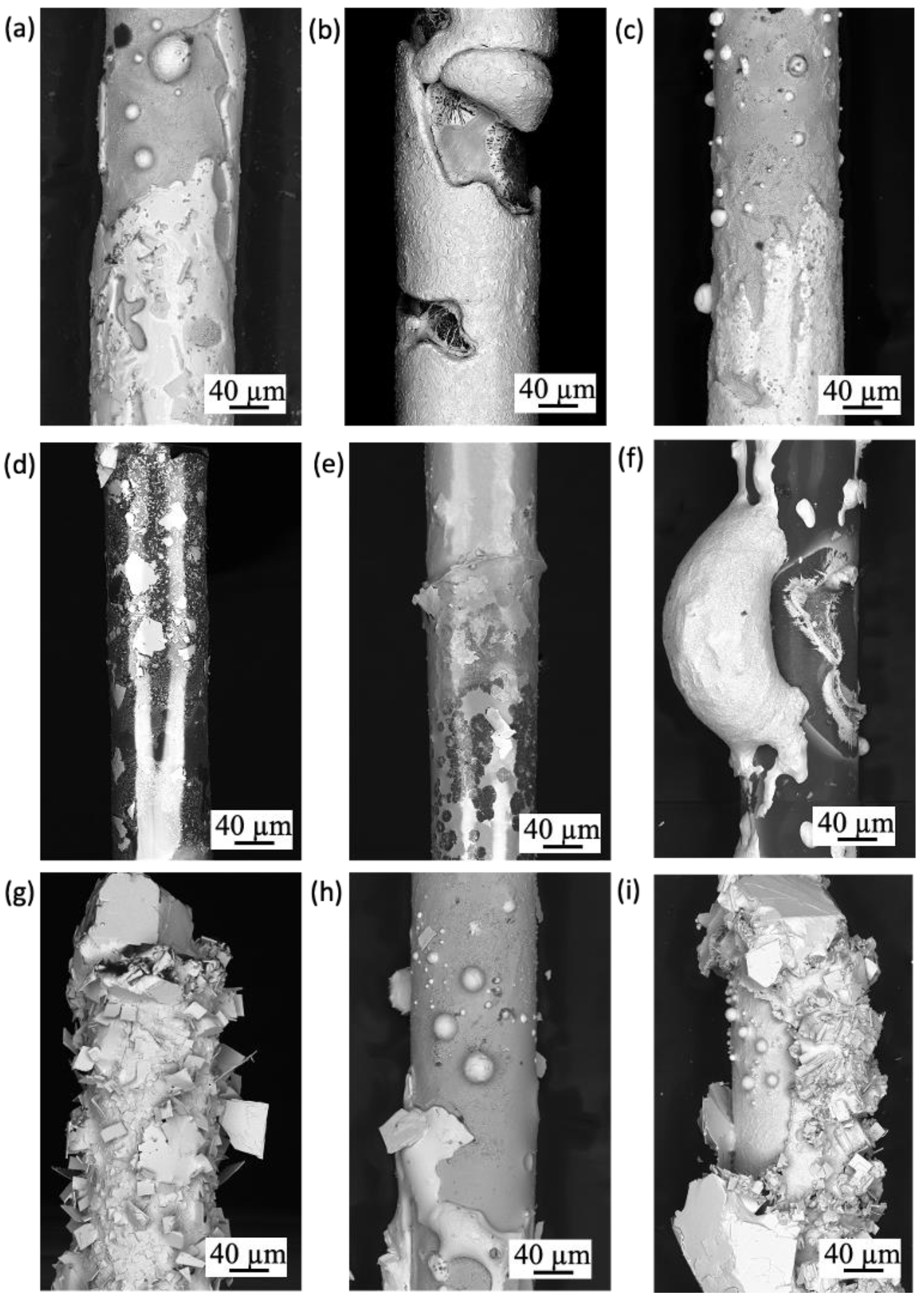
3.1. Uncoated SiO2 Fibers
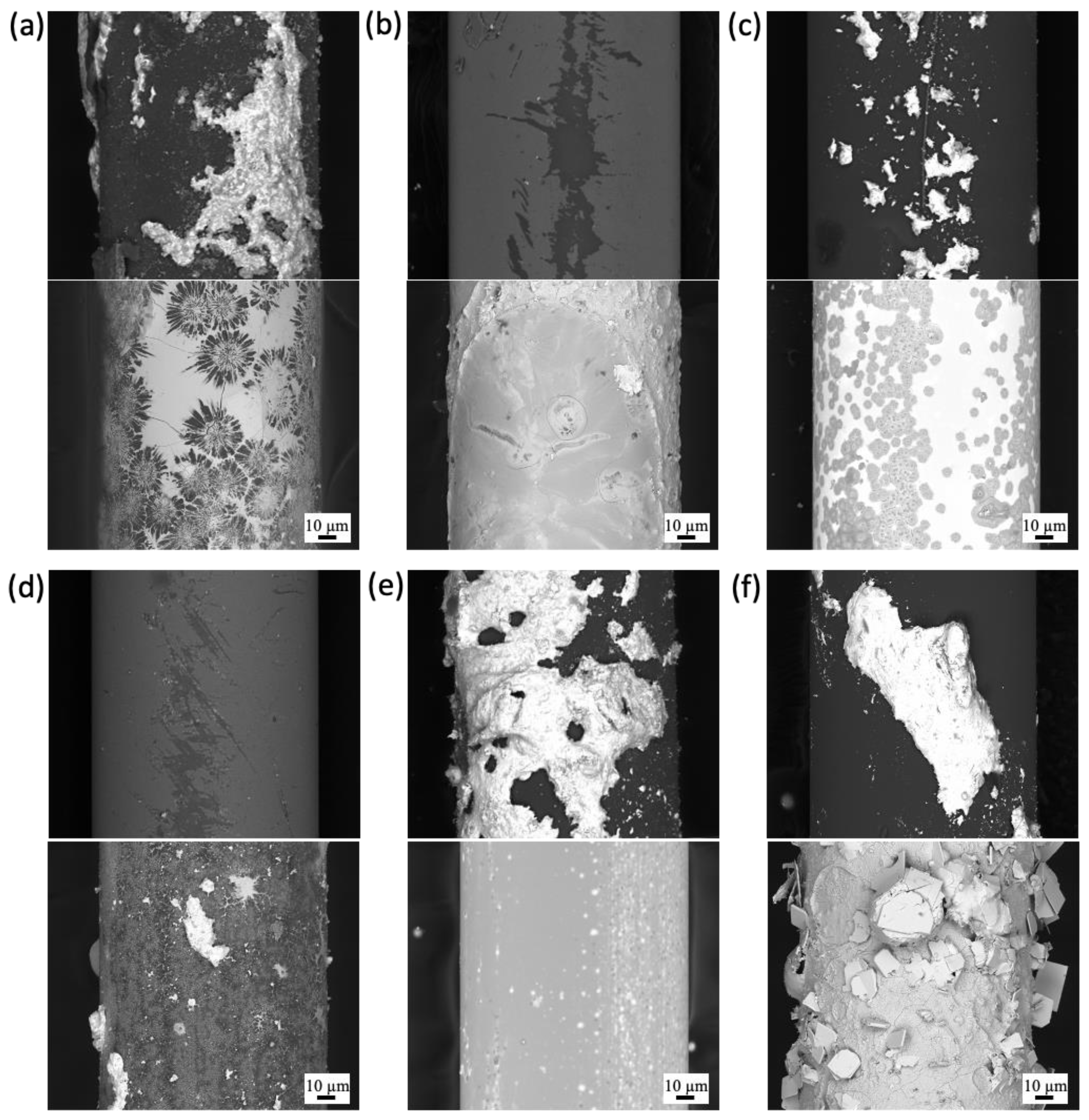
3.1.1. High-Temperature Steam
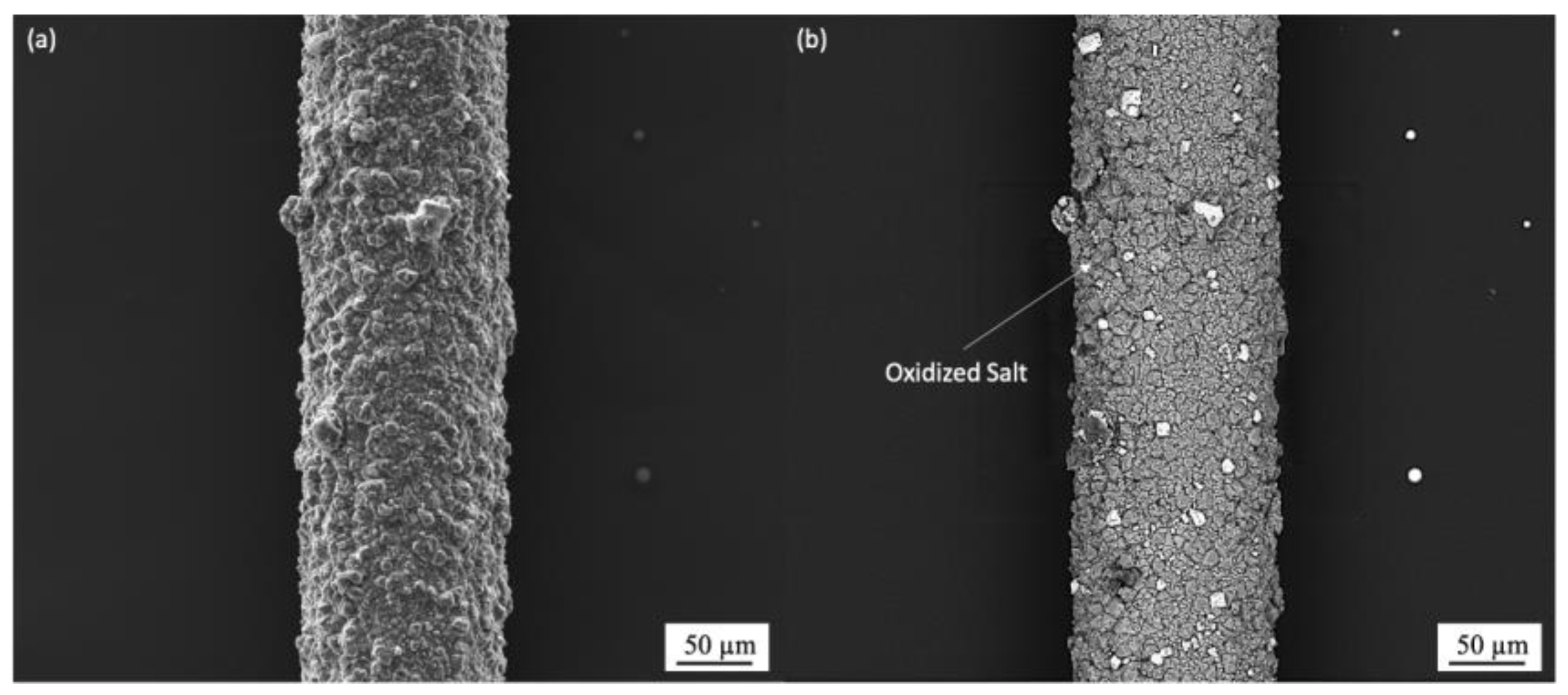
3.1.2. Molten Salt
3.1.3. Lead Bismuth Eutectic
3.1.4. Pressurized Water Reactor (PWR)
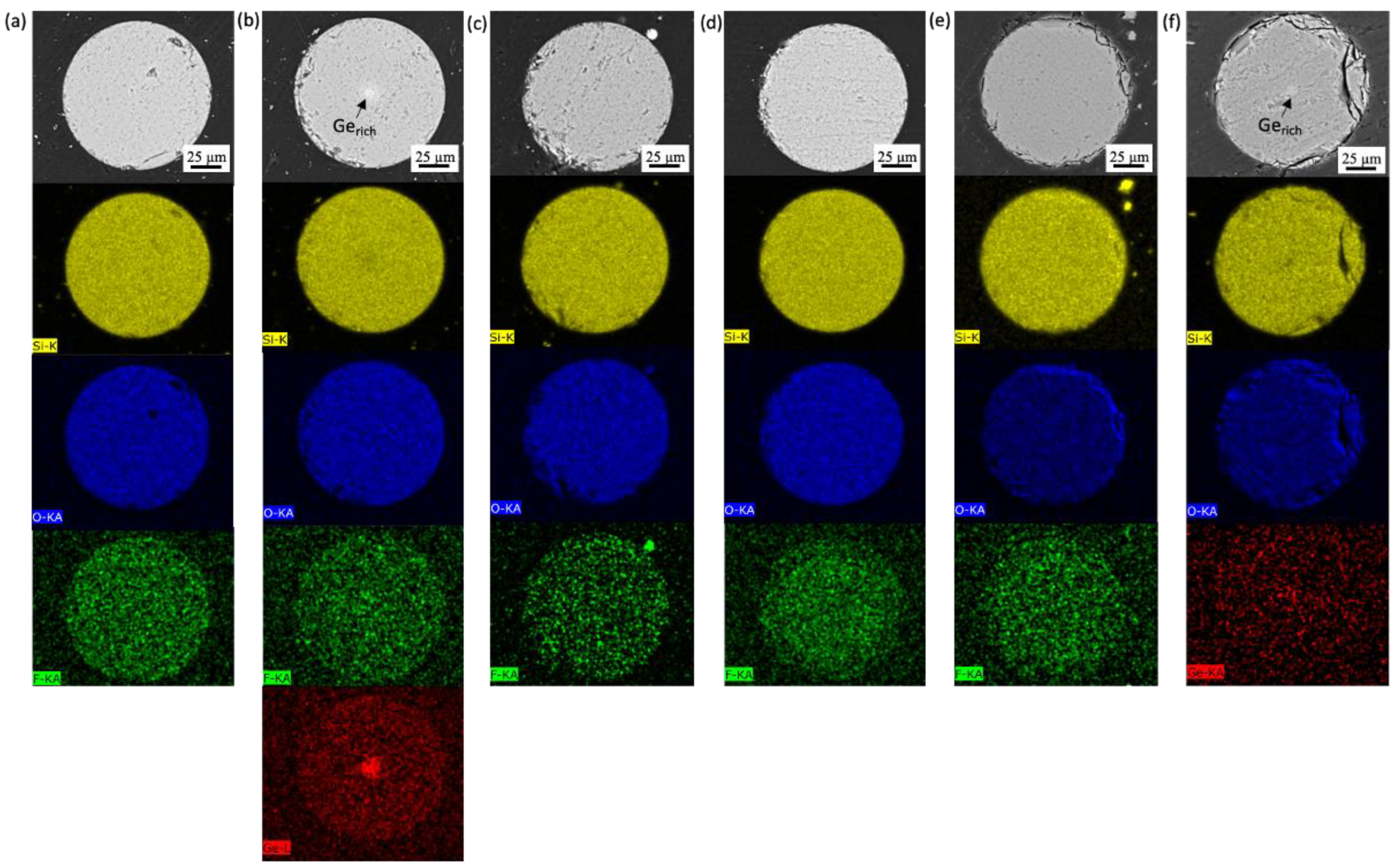
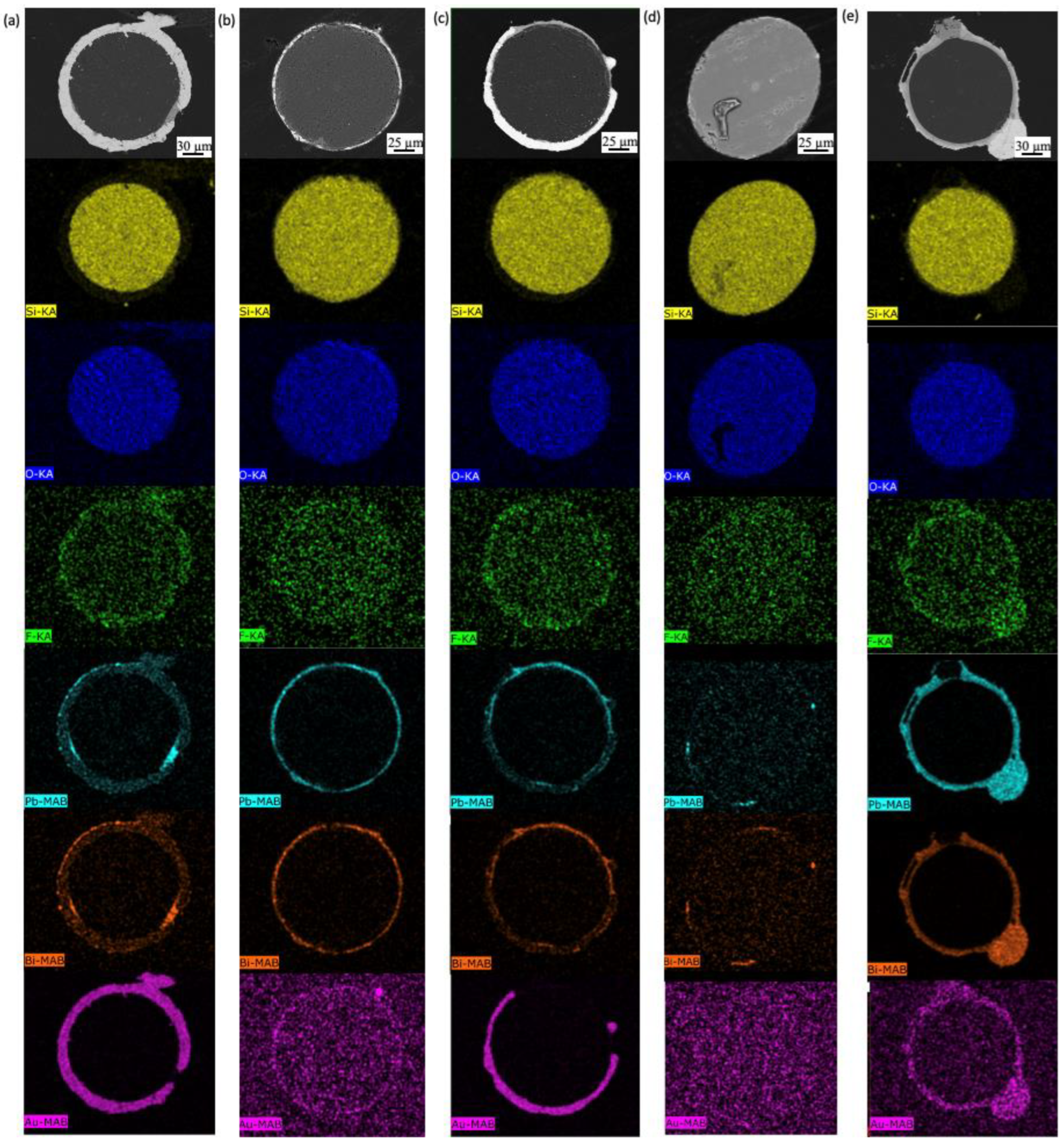
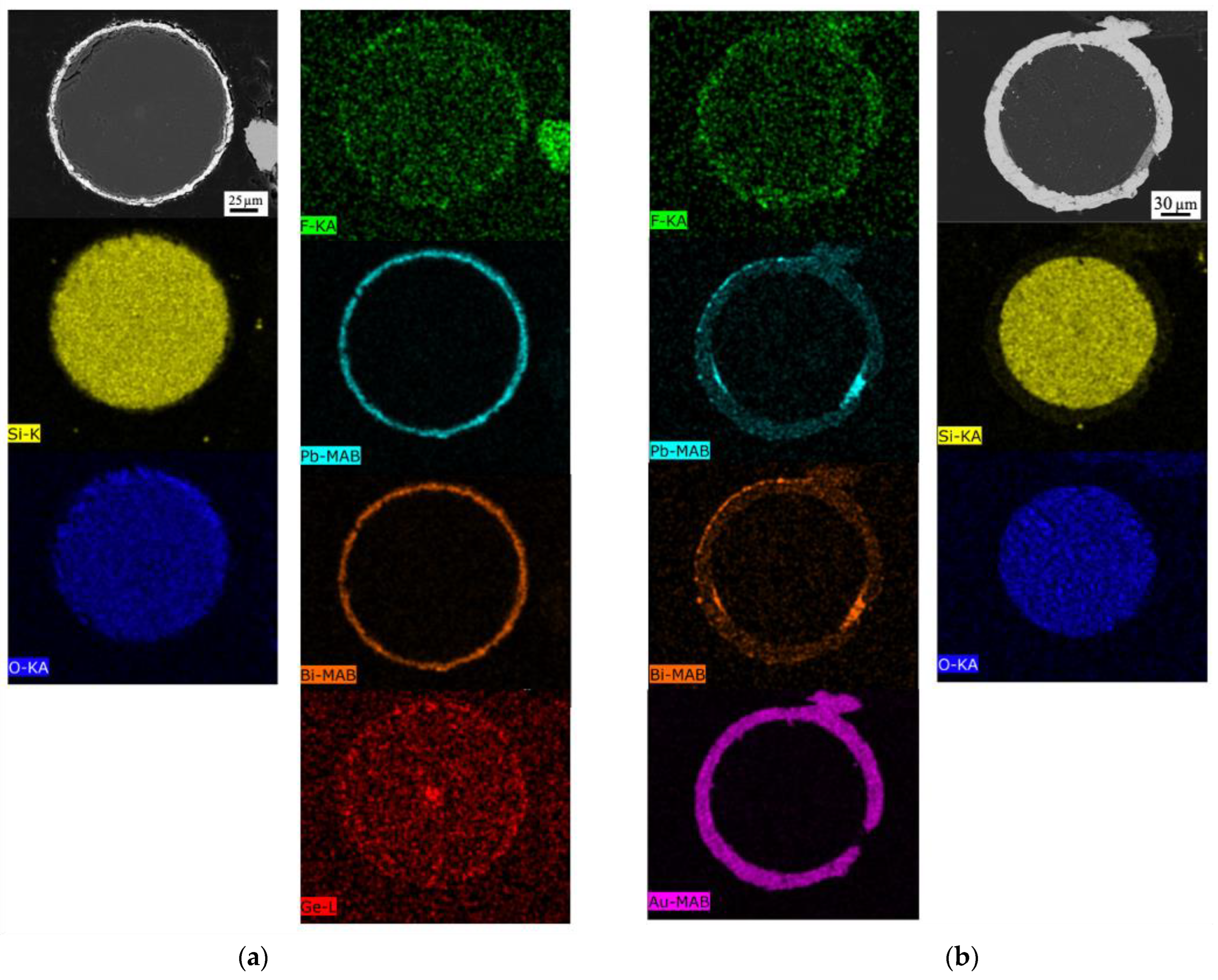
3.2. Coated SiO2 Fibers
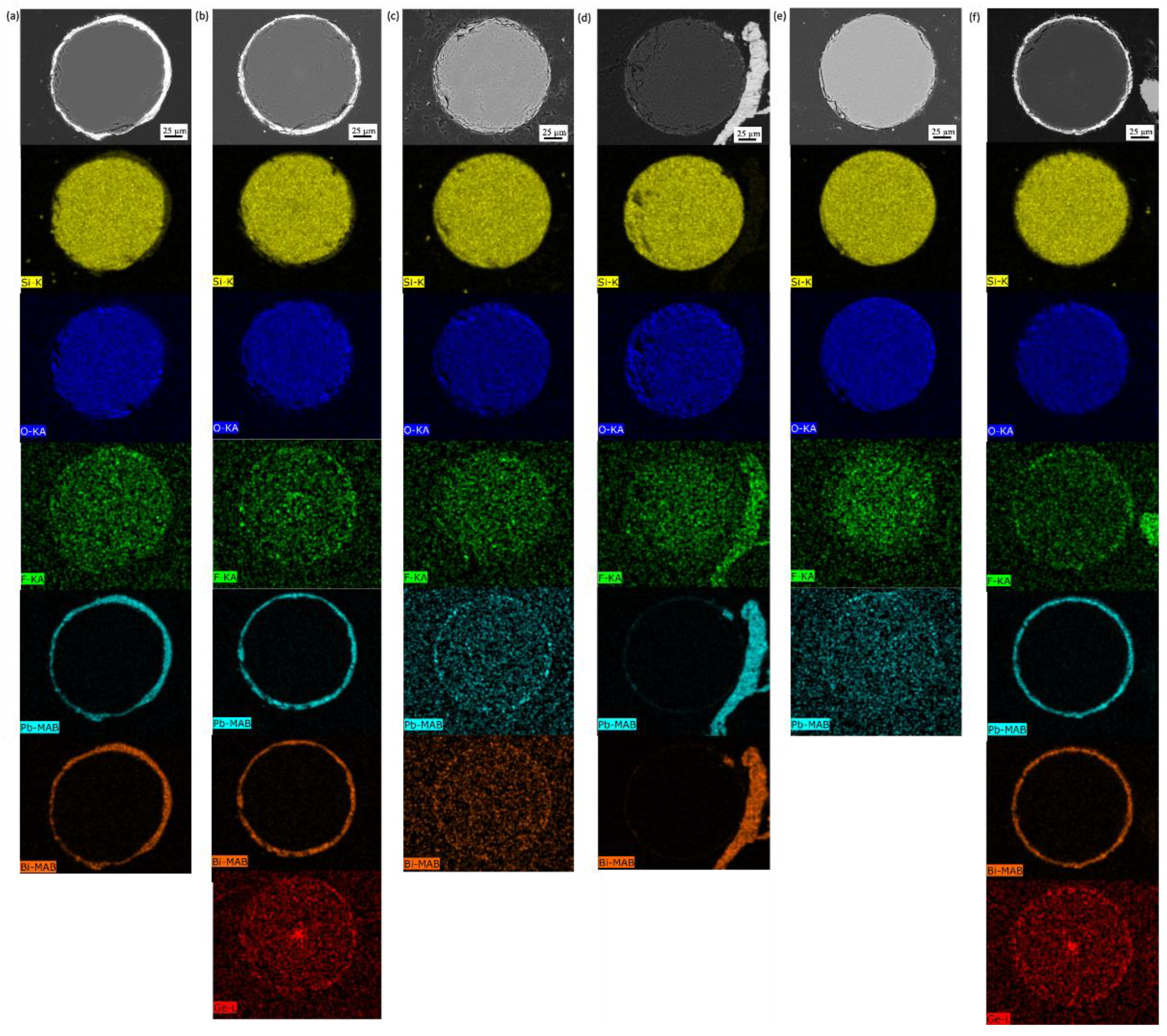
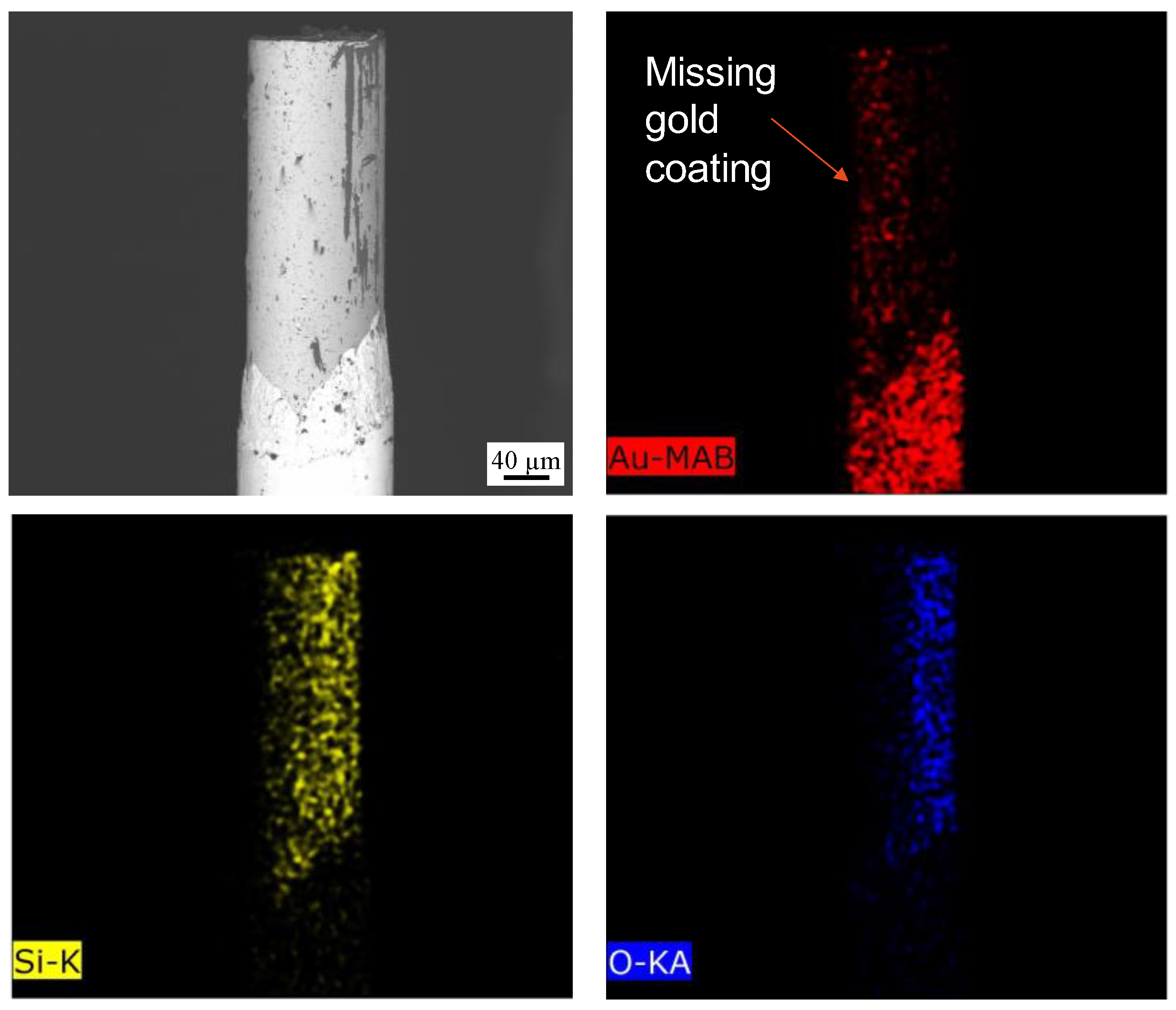
3.2.1. Gold-Coated Fibers Exposed to Molten Salt
3.2.2. Gold-Coated Fibers Exposed to LBE
4. Corrosion Mechanism
5. Material Compatibility
6. Conclusions
7. Future Work
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shampo, M.A.; Kyle, R.A.; Steensma, D.P.; Charles, K. Kao—Father of fiber optics. Mayo Clin. Proc. 2011, 86, e45. [Google Scholar] [CrossRef]
- Udd, E. An overview of fiber-optic sensors. Rev. Sci. Instrum. 1995, 66, 4015–4030. [Google Scholar] [CrossRef]
- Epstein, M. Fiber optics in medicine. Crit. Rev. Biomed. Eng. 1982, 7, 79–120. [Google Scholar]
- Mehrvar, M.; Bis, C.; Scharer, J.M.; Moo-Young, M.; Luong, J.H. Fiber-optic biosensors-trends and advances. Anal. Sci. 2000, 16, 677–692. [Google Scholar] [CrossRef]
- Sharma, P.; Pardeshi, S.; Arora, R.K.; Singh, M. A review of the development in the field of fiber optic communication systems. Int. J. Emerg. Technol. Adv. Eng. 2013, 3, 113–119. [Google Scholar]
- Bassil, A.; Chapeleau, X.; Leduc, D.; Abraham, O. Concrete crack monitoring using a novel strain transfer model for distributed fiber optics sensors. Sensors 2020, 20, 2220. [Google Scholar] [CrossRef]
- Kasinathan, M.; Sosamma, S.; BabuRao, C.; Murali, N.; Jayakumar, T. Fiber optic sensors for nuclear power plant applications. In Proceedings of the Annual Review of Progress in Quantitative Nondestructive Evaluation, Burlington, VT, USA, 17–22 July 2011; pp. 1013–1020. [Google Scholar]
- Rountree, S.D. Fiber-Optic Sensor for Simultaneous Measurement of Temperature and Pressure. In Proceedings of the 2018 Advanced Sensors and Instrumentation Webinar, Washington, DC, USA, 6 November 2018; Available online: https://www.energy.gov/ne/articles/2018-advanced-sensors-and-instrumentation-webinar (accessed on 19 June 2023).
- Kune, D.F.; Backes, J.; Clark, S.S.; Kramer, D.; Reynolds, M.; Fu, K.; Kim, Y.; Xu, W. Ghost talk: Mitigating EMI signal injection attacks against analog sensors. In Proceedings of the 2013 IEEE Symposium on Security and Privacy, Berkeley, CA, USA, 19–22 May 2013; pp. 145–159. [Google Scholar]
- Hirayama, N.; Sano, Y. Fiber Bragg grating temperature sensor for practical use. ISA Trans. 2000, 39, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wang, M.; Zhao, K.; Huang, S.; Zaghloul, M.A.; Cao, R.; Carpenter, D.; Zheng, G.; Rountree, S.D.; Chen, K.P. Distributed fiber sensors with high spatial resolution in extreme radiation environments in nuclear reactor cores. J. Light. Technol. 2021, 39, 4873–4883. [Google Scholar] [CrossRef]
- Bonopera, M. Fiber-bragg-grating-based displacement sensors: Review of recent advances. Materials 2022, 15, 5561. [Google Scholar] [CrossRef] [PubMed]
- Rountree, S.D.; Ohanian, O.J.; Boulanger, A.; Kominsky, D.; Davis, M.; Wang, M.; Chen, K.; Leong, A.; Zhang, J.; Scurti, F. Multi-parameter fiber optic sensing for harsh nuclear environments. In Proceedings of the Fiber Optic Sensors and Applications XVII, Online, 12–17 April 2021; pp. 63–82. [Google Scholar]
- Corazza, C.; Rosseel, K.; Leysen, W.; Gladinez, K.; Marino, A.; Lim, J.; Aerts, A. Optical fibre void fraction detection for liquid metal fast neutron reactors. Exp. Therm. Fluid Sci. 2020, 113, 109865. [Google Scholar] [CrossRef]
- Petrie, C.M. Fiber Optic Sensor for Corrosion Monitoring in Molten Salt Irradiation Experiments; Oak Ridge National Lab. (ORNL): Oak Ridge, TN, USA, 2019. [Google Scholar]
- Wang, X.-D.; Wolfbeis, O.S. Fiber-optic chemical sensors and biosensors (2015–2019). Anal. Chem. 2019, 92, 397–430. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.; Andrade, G.F.; Brolo, A.G. A review on recent advances in the applications of surface-enhanced Raman scattering in analytical chemistry. Anal. Chim. Acta 2020, 1097, 1–29. [Google Scholar] [CrossRef]
- Nakanishi, R.; Saeki, M.; Wakaida, I.; Ohba, H. Detection of gadolinium in surrogate nuclear fuel debris using fiber-optic laser-induced breakdown spectroscopy under gamma irradiation. Appl. Sci. 2020, 10, 8985. [Google Scholar] [CrossRef]
- Seitz, W.R. Chemical sensors based on fiber optics. Anal. Chem. 1984, 56, 16A–34A. [Google Scholar] [CrossRef]
- Tan, W.; Shi, Z.Y.; Kopelman, R. Development of submicron chemical fiber optic sensors. Anal. Chem. 1992, 64, 2985–2990. [Google Scholar] [CrossRef]
- Freeman, T.M.; Seitz, W.R. Chemiluminescence fiber optic probe for hydrogen peroxide based on the luminol reaction. Anal. Chem. 1978, 50, 1242–1246. [Google Scholar] [CrossRef]
- Yu, Q.; Zhou, X. Pressure sensor based on the fiber-optic extrinsic Fabry-Perot interferometer. Photonic Sens. 2011, 1, 72–83. [Google Scholar] [CrossRef]
- Bremer, K.; Lewis, E.; Leen, G.; Moss, B.; Lochmann, S.; Mueller, I.A. Feedback stabilized interrogation technique for EFPI/FBG hybrid fiber-optic pressure and temperature sensors. IEEE Sens. J. 2011, 12, 133–138. [Google Scholar] [CrossRef]
- Wysokiński, K.; Stańczyk, T.; Gibała, K.; Tenderenda, T.; Ziołowicz, A.; Słowikowski, M.; Broczkowska, M.; Nasiłowski, T. New methods of enhancing the thermal durability of silica optical fibers. Materials 2014, 7, 6947–6964. [Google Scholar] [CrossRef] [PubMed]
- Urata, S.; Nakamura, N.; Aiba, K.; Tada, T.; Hosono, H. How fluorine minimizes density fluctuations of silica glass: Molecular dynamics study with machine-learning assisted force-matching potential. Mater. Des. 2021, 197, 109210. [Google Scholar] [CrossRef]
- Liu, Y.; Blokland, W.; Long, C.D.; Riemer, B.W.; Wendel, M.W.; Winder, D.E. Strain measurement in the spallation target using high-radiation-tolerant fiber sensors. IEEE Sens. J. 2018, 18, 3645–3653. [Google Scholar] [CrossRef]
- Sui, K.; Feng, X.; Hou, Y.; Zhang, Q.; Qi, S.; Wang, Y.; Wang, P. Glass-clad semiconductor germanium fiber for high-speed photodetecting applications. Opt. Mater. Express 2017, 7, 1211–1219. [Google Scholar] [CrossRef]
- ASTM D1193; Standard Specification for Reagent Water. ASTM: West Conshohocken, PA, USA, 2003.
- Leong, A.; Yang, Q.; McAlpine, S.W.; Short, M.P.; Zhang, J. Oxidation behavior of Fe-Cr-2Si alloys in high temperature steam. Corros. Sci. 2021, 179, 109114. [Google Scholar] [CrossRef]
- Opila, E.J. Oxidation and volatilization of silica formers in water vapor. J. Am. Ceram. Soc. 2003, 86, 1238–1248. [Google Scholar] [CrossRef]
- Morey, G.; Hesselgesser, J. The solubility of quartz and some other substances in superheated steam at high pressures. Trans. Am. Soc. Mech. Eng. 1951, 73, 865–872. [Google Scholar] [CrossRef]
- Zhou, X.; Xie, H.; He, X.; Zhao, Z.; Ma, Q.; Cai, M.; Yin, H. Annihilating the formation of silicon carbide: Molten salt electrolysis of carbon–silica composite to prepare the carbon–silicon hybrid for lithium-ion battery anode. Energy Environ. Mater. 2020, 3, 166–176. [Google Scholar] [CrossRef]
- Liu, J.; Shi, Q.; Luan, H.; Yan, W.; Sha, W.; Wang, W.; Shan, Y.; Yang, K. Lead–bismuth eutectic corrosion behaviors of ferritic/martensitic steels in low oxygen concentration environment. Oxid. Met. 2015, 84, 383–395. [Google Scholar] [CrossRef]
- Abraham, D.; Leibowitz, L.; Maroni, V.; McDEAVITT, S.; Raraz, A. Corrosion of Structural Materials by Lead-Based Reactor Coolants; Argonne National Lab.: Lemont, IL, USA, 2000. [Google Scholar]
- Martın, F.; Soler, L.; Hernández, F.; Gomez-Briceno, D. Oxide layer stability in lead–bismuth at high temperature. J. Nucl. Mater. 2004, 335, 194–198. [Google Scholar] [CrossRef]
- Li, J.; He, X.; Xu, B.; Tang, Z.; Fang, C.; Yang, G. Effect of silicon on dynamic/static corrosion resistance of T91 in lead–bismuth eutectic at 550 C. Materials 2022, 15, 2862. [Google Scholar] [CrossRef]
- Fournier, R.O.; Rowe, J.J. The solubility of amorphous silica in water at high temperatures and high pressures. Am. Mineral. 1977, 62, 1052–1056. [Google Scholar]
- Sivonxay, E.; Aykol, M.; Persson, K.A. The lithiation process and Li diffusion in amorphous SiO2 and Si from first-principles. Electrochim. Acta 2020, 331, 135344. [Google Scholar] [CrossRef]
- Wang, H.-P.; Dai, J.-G.; Wang, X.-Z. Improved temperature compensation of fiber Bragg grating-based sensors applied to structures under different loading conditions. Opt. Fiber Technol. 2021, 63, 102506. [Google Scholar] [CrossRef]
- Lee, Z.-K.; Bonopera, M.; Hsu, C.-C.; Lee, B.-H.; Yeh, F.-Y. Long-term deflection monitoring of a box girder bridge with an optical-fiber, liquid-level system. Structures 2022, 44, 904–919. [Google Scholar] [CrossRef]






| Environment | Temperature (°C) | Test Duration (h) |
|---|---|---|
| Steam | 1200 | 24 |
| FLiNaK | 750 | 24, 168 |
| MgCl₂-NaCl-KCl | 700 | 24 |
| LBE | 600 | 24, 168 |
| PWR | 300 | 24 |
| This Work ID | Supplier | Weight Percentage (wt%) | ||||||
|---|---|---|---|---|---|---|---|---|
| Name | ID | SiO2 | F | Ge (Core) | ||||
| F 101 | Fujikura | RRSMFB | 97.806 | - | 2.194 | - | - | - |
| F 102 | Draka | BB-elite 150C HTA | 99.911 | ±0.008 | 0.063 | ±0.001 | 0.026 | ±0.001 |
| F 103 | Fujikura | RRSMFA | 98.606 | - | 1.394 | - | - | - |
| F 105-1 | Draka | SRH SM DLPC9 | 99.646 | ±0.02 | 0.354 | ±0.02 | - | - |
| F 105-2 | Draka | Super RadHard SM 15PC:12/00 | 99.646 | ±0.02 | 0.354 | ±0.02 | - | - |
| F 106 | Draka | SMF G652 DLPC9 PC:269/00 | 99.974 | ±0.001 | 0.000 | - | 0.026 | ±0.001 |
| Supplier | AMS Technologies |
| Supplier ID | ASI9.0/125/155G |
| Core Material | GeO2 doped SiO2 |
| Core Diameter (µm) | 9.0 ± 0.5 |
| Cladding Material | SiO2 |
| Cladding Diameter (µm) | 125 + 1/−3 |
| Coating Material | Gold |
| With Coating Diameter (µm) | 155 ± 16 |
| Description | Concentration (ppb) |
|---|---|
| Pre-test Si | 723.8 ± 36 |
| Post-test Si | 4301.2 ± 215 |
| Difference between Post-test and Pre-test | 3577.4 ± 179 |
| Expected Si concentration if fiber completely dissolved | 6245 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leong, A.; Rountree, S.D.; Zhang, J. Corrosion of Silica-Based Optical Fibers in Various Environments. Corros. Mater. Degrad. 2023, 4, 445-465. https://doi.org/10.3390/cmd4030023
Leong A, Rountree SD, Zhang J. Corrosion of Silica-Based Optical Fibers in Various Environments. Corrosion and Materials Degradation. 2023; 4(3):445-465. https://doi.org/10.3390/cmd4030023
Chicago/Turabian StyleLeong, Amanda, Steven Derek Rountree, and Jinsuo Zhang. 2023. "Corrosion of Silica-Based Optical Fibers in Various Environments" Corrosion and Materials Degradation 4, no. 3: 445-465. https://doi.org/10.3390/cmd4030023
APA StyleLeong, A., Rountree, S. D., & Zhang, J. (2023). Corrosion of Silica-Based Optical Fibers in Various Environments. Corrosion and Materials Degradation, 4(3), 445-465. https://doi.org/10.3390/cmd4030023







