Avant-Garde Polymer/Graphene Nanocomposites for Corrosion Protection: Design, Features, and Performance
Abstract
:1. Introduction
2. Polymers in Corrosion Reticence
3. Corrosion Resistance by Polymeric Nanocomposites
4. Polymer/Graphene Nanocomposites Coatings for Corrosion Protection
5. Significance and Challenges of Polymer/Graphene Nanocomposites in Corrosion Protection
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hegde, M.; Kavanagh, Y.; Duffy, B.; Tobin, E.F. Abrasion and Cavitation Erosion Resistance of Multi-Layer Dip Coated Sol-Gel Coatings on AA2024-T3. Corros. Mater. Degrad. 2022, 3, 661–671. [Google Scholar] [CrossRef]
- Atrens, A.; Chen, X.; Shi, Z. Mg Corrosion—Recent Progress. Corros. Mater. Degrad. 2022, 3, 566–597. [Google Scholar] [CrossRef]
- Song, G.-L.; Feng, Z. Modification, degradation and evaluation of a few organic coatings for some marine applications. Corros. Mater. Degrad. 2020, 1, 408–442. [Google Scholar] [CrossRef]
- Andrade, C.; Izquierdo, D. Statistical Treatments of Chloride Threshold and Corrosion Propagation Rate. Corros. Mater. Degrad. 2022, 3, 598–611. [Google Scholar] [CrossRef]
- Kumar, A.M.; Adesina, A.Y.; Veeramani, J.; Rahman, M.M.; Nirmal Ram, J. Hybrid Polyurethane/Polypyrrole Composite Coatings on Passivated 316L SS for Surface Protective Action against Corrosion in Saline Medium. Corros. Mater. Degrad. 2022, 3, 612–627. [Google Scholar] [CrossRef]
- Kausar, A. Corrosion prevention prospects of polymeric nanocomposites: A review. J. Plast. Film Sheeting 2019, 35, 181–202. [Google Scholar] [CrossRef]
- Teijido, R.; Ruiz-Rubio, L.; Echaide, A.G.; Vilas-Vilela, J.L.; Lanceros-Mendez, S.; Zhang, Q. State of the art and current trends on layered inorganic-polymer nanocomposite coatings for anticorrosion and multi-functional applications. Prog. Org. Coat. 2022, 163, 106684. [Google Scholar] [CrossRef]
- Chauhan, D.S.; Quraishi, M.; Ansari, K.; Saleh, T.A. Graphene and graphene oxide as new class of materials for corrosion control and protection: Present status and future scenario. Prog. Org. Coat. 2020, 147, 105741. [Google Scholar] [CrossRef]
- Othman, N.H.; Ismail, M.C.; Mustapha, M.; Sallih, N.; Kee, K.E.; Jaal, R.A. Graphene-based polymer nanocomposites as barrier coatings for corrosion protection. Prog. Org. Coat. 2019, 135, 82–99. [Google Scholar] [CrossRef]
- Cui, G.; Zhang, C.; Wang, A.; Zhou, X.; Xing, X.; Liu, J.; Li, Z.; Chen, Q.; Lu, Q. Research progress on self-healing polymer/graphene anticorrosion coatings. Prog. Org. Coat. 2021, 155, 106231. [Google Scholar] [CrossRef]
- George, J.S.; Paduvilan, J.K.; Salim, N.; Sunarso, J.; Kalarikkal, N.; Hameed, N.; Thomas, S. Advances and future outlook in epoxy/graphene composites for anticorrosive applications. Prog. Org. Coat. 2022, 162, 106571. [Google Scholar] [CrossRef]
- Sunil, S.; Porkodi, P.; Kottiyatil, A.J.; Ghosh, P. Polymer-graphene composites as anticorrosive materials. In Polymer Nanocomposites Containing Graphene; Elsevier: Amsterdam, The Netherlands, 2022; pp. 589–614. [Google Scholar]
- Zelinka, S.L. Corrosion of metals in wood products. In Corrosion of Metals in Wood Products, Developments in Corrosion Protection; Aliofkhazraei, M., Ed.; InTech: London, UK, 2014; pp. 567–592. ISBN 978-953-51-1223-5. [Google Scholar]
- Koli, D.K.; Agnihotri, G.; Purohit, R. Advanced aluminium matrix composites: The critical need of automotive and aerospace engineering fields. Mater. Today Proc. 2015, 2, 3032–3041. [Google Scholar] [CrossRef]
- Yadav, S.; Gangwar, S.; Singh, S. Micro/nano reinforced filled metal alloy composites: A review over current development in aerospace and automobile applications. Mater. Today Proc. 2017, 4, 5571–5582. [Google Scholar] [CrossRef]
- Floch, V.; Doleyres, Y.; Amand, S.; Aufray, M.; Pébère, N.; Verchère, D. Adherence Measurements and Corrosion Resistance in Primer/Hot-Dip Galvanized Steel Systems. J. Adhes. 2013, 89, 339–357. [Google Scholar] [CrossRef] [Green Version]
- Kumari, S.; Tiyyagura, H.R.; Pottathara, Y.B.; Sadasivuni, K.K.; Ponnamma, D.; Douglas, T.E.; Skirtach, A.G.; Mohan, M. Surface functionalization of chitosan as a coating material for orthopaedic applications: A Comprehensive Review. Carbohydr. Polym. 2020, 255, 117487. [Google Scholar] [CrossRef]
- Schwarzenbach, R.P.; Gschwend, P.M.; Imboden, D.M. Environmental Organic Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Talbot, D.E.; Talbot, J.D. Corrosion Science and Technology; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Umoren, S.A.; Eduok, U.M. Application of carbohydrate polymers as corrosion inhibitors for metal substrates in different media: A review. Carbohydr. Polym. 2016, 140, 314–341. [Google Scholar] [CrossRef]
- Deshpande, P.P.; Jadhav, N.G.; Gelling, V.J.; Sazou, D. Conducting polymers for corrosion protection: A review. J. Coat. Technol. Res. 2014, 11, 473–494. [Google Scholar] [CrossRef]
- Navarchian, A.H.; Joulazadeh, M.; Karimi, F. Investigation of corrosion protection performance of epoxy coatings modified by polyaniline/clay nanocomposites on steel surfaces. Prog. Org. Coat. 2014, 77, 347–353. [Google Scholar] [CrossRef]
- Sazou, D.; Deshpande, P.P. Conducting polyaniline nanocomposite-based paints for corrosion protection of steel. Chem. Pap. 2017, 71, 459–487. [Google Scholar] [CrossRef]
- Auepattana-Aumrung, K.; Phakkeeree, T.; Crespy, D. Polymer-corrosion inhibitor conjugates as additives for anticorrosion application. Prog. Org. Coat. 2022, 163, 106639. [Google Scholar] [CrossRef]
- Tareq, S. Fabrication and Characterisation of Polymeric Nano-Composites. Master’s Thesis, Western Sydney University, Penrith, Australia, 2019. [Google Scholar]
- Bhattacharya, M. Polymer nanocomposites—A comparison between carbon nanotubes, graphene, and clay as nanofillers. Materials 2016, 9, 262. [Google Scholar] [CrossRef] [Green Version]
- Balakrishnan, P.; John, M.J.; Pothen, L.; Sreekala, M.; Thomas, S. Natural fibre and polymer matrix composites and their applications in aerospace engineering. In Advanced Composite Materials for Aerospace Engineering; Elsevier: Amsterdam, The Netherlands, 2016; pp. 365–383. [Google Scholar]
- Leygraf, C.; Wallinder, I.O.; Tidblad, J.; Graedel, T. Atmospheric Corrosion; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Umoren, S.A.; Solomon, M.M. Protective polymeric films for industrial substrates: A critical review on past and recent applications with conducting polymers and polymer composites/nanocomposites. Prog. Mater. Sci. 2019, 104, 380–450. [Google Scholar] [CrossRef]
- Zhu, Z.; Chen, S.; Zhang, Y.; Wang, W. Corrosion resistance of polyvinyl butyral/reduced graphene ox-ide/titanium dioxide composite coatings for stainless steel in different environments. Prog. Org. Coat. 2022, 173, 107226. [Google Scholar] [CrossRef]
- Shukla, S.K.; Quraishi, M.; Prakash, R. A self-doped conducting polymer “polyanthranilic acid”: An efficient corrosion inhibitor for mild steel in acidic solution. Corros. Sci. 2008, 50, 2867–2872. [Google Scholar] [CrossRef]
- Umoren, S.; Ogbobe, O.; Igwe, I.; Ebenso, E. Inhibition of mild steel corrosion in acidic medium using synthetic and naturally occurring polymers and synergistic halide additives. Corros. Sci. 2008, 50, 1998–2006. [Google Scholar] [CrossRef]
- Liu, J.; Liu, T.; Guo, Z.; Guo, N.; Lei, Y.; Chang, X.; Yin, Y. Promoting barrier performance and cathodic protection of zinc-rich epoxy primer via single-layer graphene. Polymers 2018, 10, 591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xavier, J.R.; Vinodhini, S.; Beryl, J.R. Effect of silane-functionalized RuO2 nanoparticles on the anticorrosive and mechanical properties of poly (methyl methacrylate) coatings. Metall. Mater. Trans. A 2021, 52, 3896–3909. [Google Scholar] [CrossRef]
- Atta, A.M.; El-Azabawy, O.E.; Ismail, H.; Hegazy, M. Novel dispersed magnetite core–shell nanogel polymers as corrosion inhibitors for carbon steel in acidic medium. Corros. Sci. 2011, 53, 1680–1689. [Google Scholar] [CrossRef]
- Yao, H.; Zhang, X.; Shen, L.; Bao, N. Tribological and anticorrosion properties of polyvinyl butyral (PVB) coating reinforced with phenol formaldehyde resin (PF). Prog. Org. Coat. 2021, 158, 106382. [Google Scholar] [CrossRef]
- Zomorodian, A.; Garcia, M.; e Silva, T.M.; Fernandes, J.; Fernandes, M.; Montemor, M. Corrosion resistance of a composite polymeric coating applied on biodegradable AZ31 magnesium alloy. Acta Biomater. 2013, 9, 8660–8670. [Google Scholar] [CrossRef]
- Khattak, N.S.; Khan, M.S.; Shah, L.A.; Farooq, M.; Khan, A.; Ahmad, S.; Jan, S.U.; Rehman, N. The effect of low weight percent multiwalled carbon nanotubes on the dielectric properties of non-conducting polymer/ceramic nanocomposites for energy storage materials. Z. Für Phys. Chem. 2020, 234, 11–26. [Google Scholar] [CrossRef]
- Kango, S.; Kalia, S.; Celli, A.; Njuguna, J.; Habibi, Y.; Kumar, R. Surface modification of inorganic nanoparticles for development of organic–inorganic nanocomposites—A review. Prog. Polym. Sci. 2013, 38, 1232–1261. [Google Scholar] [CrossRef]
- Mallakpour, S.; Khadem, E. Recent development in the synthesis of polymer nanocomposites based on nano-alumina. Prog. Polym. Sci. 2015, 51, 74–93. [Google Scholar] [CrossRef]
- Radhakrishnan, S.; Siju, C.; Mahanta, D.; Patil, S.; Madras, G. Conducting polyaniline–nano-TiO2 composites for smart corrosion resistant coatings. Electrochim. Acta 2009, 54, 1249–1254. [Google Scholar] [CrossRef]
- Sonker, R.K.; Yadav, B.; Gupta, V.; Tomar, M. Fabrication and characterization of ZnO-TiO2-PANI (ZTP) micro/nanoballs for the detection of flammable and toxic gases. J. Hazard. Mater. 2019, 370, 126–137. [Google Scholar] [CrossRef] [PubMed]
- Nikravesh, B.; Ramezanzadeh, B.; Sarabi, A.; Kasiriha, S. Evaluation of the corrosion resistance of an epoxy-polyamide coating containing different ratios of micaceous iron oxide/Al pigments. Corros. Sci. 2011, 53, 1592–1603. [Google Scholar] [CrossRef]
- Fadl, A.; Abdou, M.; Al-Elaa, S.A.; Hamza, M.; Sadeek, S. Evaluation the anti-corrosion behavior, impact resistance, acids and alkali immovability of nonylphenol ethoxylate/TiO2 hybrid epoxy nanocomposite coating applied on the carbon steel surface. Prog. Org. Coat. 2019, 136, 105263. [Google Scholar] [CrossRef]
- Ramezanzadeh, B.; Niroumandrad, S.; Ahmadi, A.; Mahdavian, M.; Moghadam, M.M. Enhancement of barrier and corrosion protection performance of an epoxy coating through wet transfer of amino functionalized graphene oxide. Corros. Sci. 2016, 103, 283–304. [Google Scholar] [CrossRef]
- Gobara, M.; Baraka, A.; Akid, R.; Zorainy, M. Corrosion protection mechanism of Ce 4+/organic inhibitor for AA2024 in 3.5% NaCl. RSC Adv. 2020, 10, 2227–2240. [Google Scholar] [CrossRef] [Green Version]
- Qiang, Y.; Guo, L.; Li, H.; Lan, X. Fabrication of environmentally friendly Losartan potassium film for corrosion inhibition of mild steel in HCl medium. Chem. Eng. J. 2020, 406, 126863. [Google Scholar] [CrossRef]
- Wang, J.; Li, J.J.; Weng, G.J.; Su, Y. The effects of temperature and alignment state of nanofillers on the thermal conductivity of both metal and nonmetal based graphene nanocomposites. Acta Mater. 2020, 185, 461–473. [Google Scholar] [CrossRef]
- Boppana, S.B.; Dayanand, S.; Kumar, M.A.; Kumar, V.; Aravinda, T. Synthesis and characterization of nano graphene and ZrO2 reinforced Al 6061 metal matrix composites. J. Mater. Res. Technol. 2020, 9, 7354–7362. [Google Scholar] [CrossRef]
- Kausar, A. Applications of polymer/graphene nanocomposite membranes: A review. Mater. Res. Innov. 2019, 23, 276–287. [Google Scholar] [CrossRef]
- Cieślik, M.; Zimowski, S.; Gołda, M.; Engvall, K.; Pan, J.; Rakowski, W.; Kotarba, A. Engineering of bone fixation metal implants biointerface—Application of parylene C as versatile protective coating. Mater. Sci. Eng. C 2012, 32, 2431–2435. [Google Scholar] [CrossRef]
- Wang, X.; Qi, X.; Lin, Z.; Battocchi, D. Graphene Reinforced Composites as Protective Coatings for Oil and Gas Pipelines. Nanomaterials 2018, 8, 1005. [Google Scholar] [CrossRef] [Green Version]
- Calovi, M.; Dirè, S.; Ceccato, R.; Deflorian, F.; Rossi, S. Corrosion protection properties of functionalised graphene–acrylate coatings produced via cataphoretic deposition. Prog. Org. Coat. 2019, 136, 105261. [Google Scholar] [CrossRef]
- Mendez, J.A.C.; Escobedo, V.N.M.; Vong, Y.M.; Bueno, J.d.J.P. A Review on Atmospheric Pressure Plasma Jet and Related Electrochemical Evaluation of Corrosion. Green Mater. 2020, 10, 11–22. [Google Scholar] [CrossRef]
- Mahulikar, P.P.; Jadhav, R.S.; Hundiwale, D.G. Performance of polyaniline/TiO2 nanocomposites in epoxy for corrosion resistant coatings. Iran. Polym. J. 2011, 20, 367–376. [Google Scholar]
- Wahby, M.H.; Atta, A.M.; Moustafa, Y.M.; Ezzat, A.O.; Hashem, A.I. Hydrophobic and Superhydrophobic Bio-Based Nano-Magnetic Epoxy Composites as Organic Coating of Steel. Coatings 2020, 10, 1201. [Google Scholar] [CrossRef]
- Bobby, S.; Samad, M.A. Tribological characterization of epoxy hybrid nanocomposite coatings reinforced with graphene oxide and titania. Wear 2020, 466, 203560. [Google Scholar]
- Feng, Y.; Cui, Y.; Zhang, M.; Li, M.; Li, H. Preparation of Tung Oil-Loaded PU/PANI Microcapsules and Synergetic Anti-Corrosion Properties of Self-Healing Epoxy Coatings. Macromol. Mater. Eng. 2020, 306, 2000581. [Google Scholar] [CrossRef]
- Hughes, A.E.; Varley, R. New Generation Coatings for Metals; MDPI: Basel, Switzerland, 2020. [Google Scholar]
- Njoku, D.I.; Cui, M.; Xiao, H.; Shang, B.; Li, Y. Understanding the anticorrosive protective mechanisms of modified epoxy coatings with improved barrier, active and self-healing functionalities: EIS and spectroscopic techniques. Sci. Rep. 2017, 7, 15597. [Google Scholar] [CrossRef] [PubMed]
- Kozlov, G.; Dolbin, I. Aggregation of Nanofiller in Polymer/Carbon Nanotube Composites. J. Appl. Mech. Tech. Phys. 2020, 61, 263–266. [Google Scholar] [CrossRef]
- Zare, Y. Study of nanoparticles aggregation/agglomeration in polymer particulate nanocomposites by mechanical properties. Compos. Part A Appl. Sci. Manuf. 2016, 84, 158–164. [Google Scholar] [CrossRef]
- Zhang, J.; Kong, G.; Li, S.; Le, Y.; Che, C.; Zhang, S.; Lai, D.; Liao, X. Graphene-reinforced epoxy powder coating to achieve high performance wear and corrosion resistance. J. Mater. Res. Technol. 2022, 20, 4148–4160. [Google Scholar] [CrossRef]
- Hosseinpour, A.; Abadchi, M.R.; Mirzaee, M.; Tabar, F.A.; Ramezanzadeh, B. Recent advances and future perspectives for carbon nanostructures reinforced organic coating for anti-corrosion application. Surf. Interfaces 2021, 23, 100994. [Google Scholar] [CrossRef]
- Khezerlou, H.; Hosseini, M.G.; Şenel, M.C.; Gürbüz, M. The Corrosion Behavior of Graphene-Reinforced Al Matrix Composites in 3.5 wt.% NaCl Solution. J. Mater. Eng. Perform. 2022, 1–10. [Google Scholar] [CrossRef]
- Wu, T.; Yang, Y.; Sun, W.; Yang, Z.; Wang, L.; Wang, J.; Liu, G. Unfolding graphene nanosheets towards high barrier performance of epoxy/graphene nanocomposite coating. Compos. Part A Appl. Sci. Manuf. 2022, 153, 106732. [Google Scholar] [CrossRef]
- Yoshida, H.; Bocquet, L. Labyrinthine water flow across multilayer graphene-based membranes: Molecular dynamics versus continuum predictions. J. Chem. Phys. 2016, 144, 234701. [Google Scholar] [CrossRef] [Green Version]
- Emamgholi, K.; Dehaghi, S.M.; Ranjbar, Z. Amine functionalization of graphene oxide (AFGO) and corrosion behavior of epoxy-AFGO nanocomposites. Mater. Chem. Phys. 2022, 290, 126339. [Google Scholar] [CrossRef]
- Ren, S.; Cui, M.; Liu, C.; Wang, L. A comprehensive review on ultrathin, multi-functionalized, and smart graphene and graphene-based composite protective coatings. Corros. Sci. 2022, 212, 110939. [Google Scholar] [CrossRef]
- Jiang, F.; Zhao, W.; Wu, Y.; Dong, J.; Zhou, K.; Lu, G.; Pu, J. Anti-corrosion behaviors of epoxy composite coatings enhanced via graphene oxide with different aspect ratios. Prog. Org. Coat. 2019, 127, 70–79. [Google Scholar] [CrossRef]
- Jena, G.; Philip, J. A review on recent advances in graphene oxide-based composite coatings for anticorrosion applications. Prog. Org. Coat. 2022, 173, 107208. [Google Scholar] [CrossRef]
- Tsai, P.-Y.; Chen, T.-E.; Lee, Y.-L. Development and characterization of anticorrosion and antifriction properties for high performance polyurethane/graphene composite coatings. Coatings 2018, 8, 250. [Google Scholar] [CrossRef] [Green Version]
- Bai, T.; Lv, L.; Du, W.; Fang, W.; Wang, Y. Improving the tribological and anticorrosion performance of waterborne polyurethane coating by the synergistic effect between modified graphene oxide and polytetrafluoroethylene. Nanomaterials 2020, 10, 137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, B.P.; Jena, B.K.; Bhattacharjee, S.; Besra, L. Development of oxidation and corrosion resistance hydrophobic graphene oxide-polymer composite coating on copper. Surf. Coat. Technol. 2013, 232, 475–481. [Google Scholar] [CrossRef] [Green Version]
- Qi, K.; Sun, Y.; Duan, H.; Guo, X. A corrosion-protective coating based on a solution-processable polymer-grafted graphene oxide nanocomposite. Corros. Sci. 2015, 98, 500–506. [Google Scholar] [CrossRef]
- Mohamed, A.M.; Abdullah, A.M.; Younan, N.A. Corrosion behavior of superhydrophobic surfaces: A review. Arab. J. Chem. 2015, 8, 749–765. [Google Scholar] [CrossRef] [Green Version]
- Harb, S.V.; Pulcinelli, S.H.; Santilli, C.V.; Knowles, K.M.; Hammer, P. A comparative study on graphene oxide and carbon nanotube reinforcement of PMMA-siloxane-silica anticorrosive coatings. ACS Appl. Mater. Interfaces 2016, 8, 16339–16350. [Google Scholar] [CrossRef] [Green Version]
- Moradi, M.; Rezaei, M. Corrosion protection mechanisms of graphene oxide-reinforced polypropylene coating for mild carbon steel by advanced molecular dynamics simulations with emphasis on resistance to water and chloride ion penetration. J. Mol. Liq. 2022, 367, 120389. [Google Scholar] [CrossRef]
- Yuan, R.; Ju, P.; Wu, Y.; Ji, L.; Li, H.; Chen, L.; Zhou, H.; Chen, J. Silane-grafted graphene oxide improves wear and corrosion resistance of polyimide matrix: Molecular dynamics simulation and experimental analysis. J. Mater. Sci. 2019, 54, 11069–11083. [Google Scholar] [CrossRef]
- Gupta, R.K.; Malviya, M.; Ansari, K.; Lgaz, H.; Chauhan, D.; Quraishi, M. Functionalized graphene oxide as a new generation corrosion inhibitor for industrial pickling process: DFT and experimental approach. Mater. Chem. Phys. 2019, 236, 121727. [Google Scholar] [CrossRef]
- Akbarzadeh, S.; Ramezanzadeh, M.; Ramezanzadeh, B.; Mahdavian, M.; Naderi, R. Fabrication of highly effective polyaniline grafted carbon nanotubes to induce active protective functioning in a silane coating. Ind. Eng. Chem. Res. 2019, 58, 20309–20322. [Google Scholar] [CrossRef]
- Bertuoli, P.T.; Baldissera, A.F.; Zattera, A.J.; Ferreira, C.A.; Alemán, C.; Armelin, E. Polyaniline coated core-shell polyacrylates: Control of film formation and coating application for corrosion protection. Prog. Org. Coat. 2019, 128, 40–51. [Google Scholar] [CrossRef]
- Kraljić, M.; Mandić, Z.; Duić, L. Inhibition of steel corrosion by polyaniline coatings. Corros. Sci. 2003, 45, 181–198. [Google Scholar] [CrossRef]
- Ng, F.; Couture, G.; Philippe, C.; Boutevin, B.; Caillol, S. Bio-based aromatic epoxy monomers for thermoset materials. Molecules 2017, 22, 149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, W.; Wang, L.; Wu, T.; Pan, Y.; Liu, G. Synthesis of low-electrical-conductivity graphene/pernigraniline composites and their application in corrosion protection. Carbon 2014, 79, 605–614. [Google Scholar] [CrossRef]
- Chang, C.-H.; Huang, T.-C.; Peng, C.-W.; Yeh, T.-C.; Lu, H.-I.; Hung, W.-I.; Weng, C.-J.; Yang, T.-I.; Yeh, J.-M. Novel anticorrosion coatings prepared from polyaniline/graphene composites. Carbon 2012, 50, 5044–5051. [Google Scholar] [CrossRef]
- Lin, Y.-T.; Don, T.-M.; Wong, C.-J.; Meng, F.-C.; Lin, Y.-J.; Lee, S.-Y.; Lee, C.-F.; Chiu, W.-Y. Improvement of mechanical properties and anticorrosion performance of epoxy coatings by the introduction of polyaniline/graphene composite. Surf. Coat. Technol. 2019, 374, 1128–1138. [Google Scholar] [CrossRef]
- Vinothkumar, K.; Sethuraman, M. A robust method of enhancement of corrosion inhibitive ability of electrodeposited poly-3-amino-5-mercapto-1, 2, 4-triazole films over copper surface using graphene oxide. J. Adhes. Sci. Technol. 2019, 34, 651–669. [Google Scholar] [CrossRef]
- Kausar, A. Graphene nanomesh and polymeric material at cutting edge. Polym.-Plast. Technol. Mater. 2019, 58, 803–820. [Google Scholar] [CrossRef]
- Bo, P.; Yunbin, X.; Jiabao, G.; Zijun, C.; Yanhuang, T.; Gang, Z.; Huanxiang, X. Research progress in preparation and properties of polymer/graphene composites. China Plast. 2022, 36, 190. [Google Scholar]
- Pavase, T.R.; Lin, H.; Hussain, S.; Li, Z.; Ahmed, I.; Lv, L.; Sun, L.; Shah, S.B.H.; Kalhoro, M.T. Recent advances of conjugated polymer (CP) nanocomposite-based chemical sensors and their applications in food spoilage detection: A comprehensive review. Sens. Actuators B Chem. 2018, 273, 1113–1138. [Google Scholar] [CrossRef]
- Selim, M.S.; Shenashen, M.; El-Safty, S.A.; Higazy, S.; Selim, M.M.; Isago, H.; Elmarakbi, A. Recent progress in marine foul-release polymeric nanocomposite coatings. Prog. Mater. Sci. 2017, 87, 1–32. [Google Scholar] [CrossRef]
- Knudsen, O.Ø.; Forsgren, A. Corrosion Control through Organic Coatings; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Su, L.; Zhou, Z.; Shen, P. Ni/C hierarchical nanostructures with Ni nanoparticles highly dispersed in N-containing carbon nanosheets: Origin of Li storage capacity. J. Phys. Chem. C 2012, 116, 23974–23980. [Google Scholar] [CrossRef]
- Teo, W.-E.; Ramakrishna, S. Electrospun nanofibers as a platform for multifunctional, hierarchically organized nanocomposite. Compos. Sci. Technol. 2009, 69, 1804–1817. [Google Scholar] [CrossRef]
- Martin, D.C.; Abidian, M.R. Conducting Polymer Nanotube Actuators for Precisely Controlled Release of Medicine and Bioactive Molecules. U.S. Patent 8,936,794, 20 January 2015. [Google Scholar]
- Shahzad, F.; Alhabeb, M.; Hatter, C.B.; Anasori, B.; Hong, S.M.; Koo, C.M.; Gogotsi, Y. Electromagnetic interference shielding with 2D transition metal carbides (MXenes). Science 2016, 353, 1137–1140. [Google Scholar] [CrossRef] [Green Version]
- Ji, H.; Zhao, R.; Zhang, N.; Jin, C.; Lu, X.; Wang, C. Lightweight and flexible electrospun polymer nanofiber/metal nanoparticle hybrid membrane for high-performance electromagnetic interference shielding. NPG Asia Mater. 2018, 10, 749–760. [Google Scholar] [CrossRef]
- Wasfi, A.; Ismael, H. Characterization of Polyaniline/Single-Walled Carbon Nanotube Composite Films Prepared by Plasma Polymerization. Acta Phys. Pol. A 2019, 135, 578–582. [Google Scholar] [CrossRef]
- Babu, V.J.; Vempati, S.; Sundarrajan, S.; Sireesha, M.; Ramakrishna, S. Effective nanostructred morphologies for efficient hybrid solar cells. Sol. Energy 2014, 106, 1–22. [Google Scholar] [CrossRef]
- Yang, N.; Yang, T.; Wang, W.; Chen, H.; Li, W. Polydopamine modified polyaniline-graphene oxide composite for enhancement of corrosion resistance. J. Hazard. Mater. 2019, 377, 142–151. [Google Scholar] [CrossRef]
- Kong, D.; Li, J.; Guo, A.; Xiao, X. High temperature electromagnetic shielding shape memory polymer composite. Chem. Eng. J. 2021, 408, 127365. [Google Scholar] [CrossRef]
- Toprakci, H.A.; Kalanadhabhatla, S.K.; Spontak, R.J.; Ghosh, T.K. Polymer nanocomposites containing carbon nanofibers as soft printable sensors exhibiting strain-reversible piezoresistivity. Adv. Funct. Mater. 2013, 23, 5536–5542. [Google Scholar] [CrossRef]
- Dan, Y.; Cao, Y.; Mallouk, T.E.; Evoy, S.; Johnson, A.C. Gas sensing properties of single conducting polymer nanowires and the effect of temperature. Nanotechnology 2009, 20, 434014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, L.; Wu, H.; Li, J.; Zhao, H.; Wang, L. Polydopamine modified ultrathin hydroxyapatite nanosheets for anti-corrosion reinforcement in polymeric coatings. Corros. Sci. 2021, 178, 109064. [Google Scholar] [CrossRef]
- Venugopal, J.; Low, S.; Choon, A.T.; Ramakrishna, S. Interaction of cells and nanofiber scaffolds in tissue engineering. J. Biomed. Mater. Res. Part B Appl. Biomater. 2008, 84, 34–48. [Google Scholar] [CrossRef] [PubMed]
- Idumah, C.I.; Odera, S. Recent advancement in self-healing graphene polymer nanocomposites, shape memory, and coating materials. Polym.-Plast. Technol. Mater. 2020, 59, 1167–1190. [Google Scholar] [CrossRef]














| Polymer | Symbol | Structure | Ref. |
|---|---|---|---|
| Polyaniline (Conducting polymer) | PANI |  | [25] |
| Polypyrrole (Conducting polymer) | PPy |  | [21] |
| Poly(ethylene glycol) (Non-conducting polymer) | PEG |  | [26] |
| Epoxy (Non-conducting polymer) | Bisphenol A diglycidyl ether (DGEBA) |  | [27] |
| Poly(methyl methacrylate) (Non-conducting polymer) | PMMA |  | [28] |
| Poly(acrylamide) (Non-conducting polymer) | PAM |  | [29] |
| Polyvinylbutyral (Non-conducting polymer) | PVB |  | [30] |
| Poly(ether imide) (Non-conducting polymer) | PEI |  | [31] |
| Sample | EP | G0.2%/EP | G0.4%/EP | G0.6%/EP | G0.8%/EP |
|---|---|---|---|---|---|
| Icorr (Acm−2) | 4.11 × 10−6 | 7.26 × 10−8 | 8.08 × 10−9 | 7.96 × 10−8 | 2.75 × 10−7 |
| Ecorr (V) | −0.78 | −0.62 | −0.49 | −0.71 | −0.70 |
| Polymer | Graphene Content | Metal Substrate | Dispersion | Anti-Corrosion Features | Ref |
|---|---|---|---|---|---|
| Epoxy | Graphene 0.2–0.8 wt.% | Steel plates; good adhesion | Fine graphene dispersion; barrier effects | Corrosion current density 8.08 × 10−9 Acm−2; corrosion potential −0.49 at 0.4 wt.% nanofiller | [63] |
| Epoxy | 1 wt.% Graphene oxide prepared with different aspect ration | Q235 steel | Better graphene dispersion; no defects and pores | Coatings tested in 3.5 wt.% NaCl; Bode and Nyquist plots | [70] |
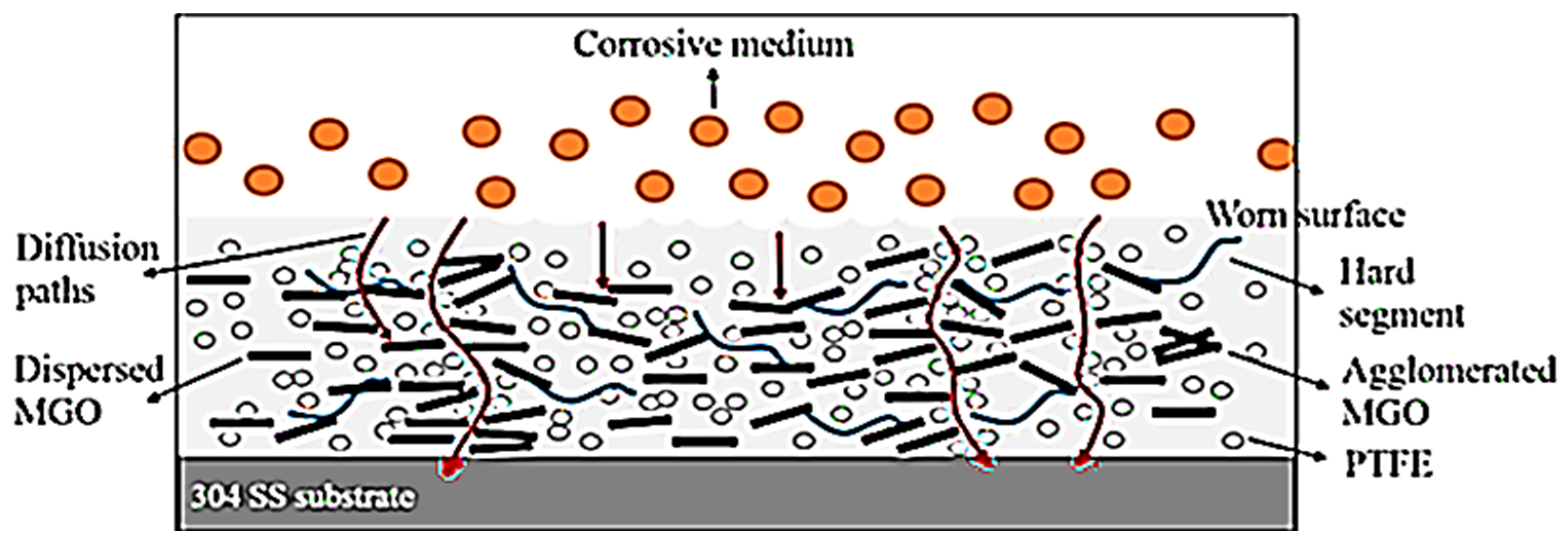
| Waterborne polyurethane and polytetrafluoroethylene | 0.5 wt.% graphene | Stainless steel | Zigzag graphene dispersion to block diffusion path of corrosive medium through coating to reach metal/coating | EIS studies | [73] |
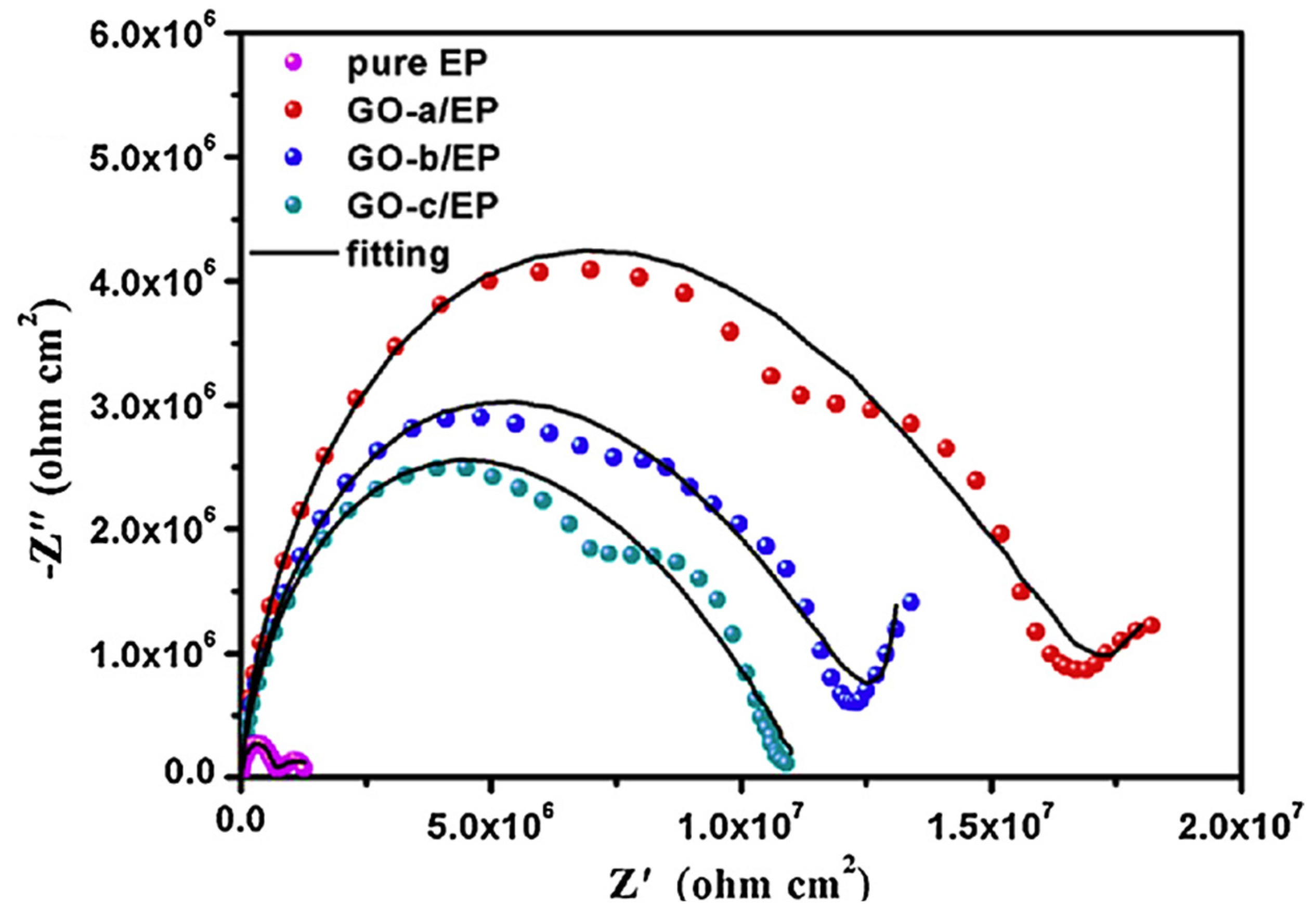
| Isocyanate crosslinked with hydroxy functional acrylic adhesive matrix | Hydrophobic graphene oxide; 0.4 mg | Copper | Electrophoretic deposition | Electrochemical studies; potentiodynamic polarization; EIS studies; electrochemical degradation; Tafel plots; corrosion current density 3.49 μA/cm2 | [74] |
| Polymer | Graphene oxide | Fe surface | High contact area of protective inhibitor film | MD simulation | [80] |
| Polyaniline | Graphene 0.1–0.5 wt.% | Steel | Graphene nanosheets dispersion; lengthened diffusion pathway for percolating corrosive species | Barrier properties against O2 and H2O molecules | [86] |
| Epoxy/poly(styrenesulfonate)- polyaniline | Reduced graphene oxide up to 0.5 wt.% | Carbon steel | Better passivation layer/tortuosity; interfacial matrix–nanofiller adhesion | Anti-corrosion mechanism | [87] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kausar, A.; Ahmad, I.; Eisa, M.H.; Maaza, M. Avant-Garde Polymer/Graphene Nanocomposites for Corrosion Protection: Design, Features, and Performance. Corros. Mater. Degrad. 2023, 4, 33-53. https://doi.org/10.3390/cmd4010004
Kausar A, Ahmad I, Eisa MH, Maaza M. Avant-Garde Polymer/Graphene Nanocomposites for Corrosion Protection: Design, Features, and Performance. Corrosion and Materials Degradation. 2023; 4(1):33-53. https://doi.org/10.3390/cmd4010004
Chicago/Turabian StyleKausar, Ayesha, Ishaq Ahmad, M. H. Eisa, and Malik Maaza. 2023. "Avant-Garde Polymer/Graphene Nanocomposites for Corrosion Protection: Design, Features, and Performance" Corrosion and Materials Degradation 4, no. 1: 33-53. https://doi.org/10.3390/cmd4010004
APA StyleKausar, A., Ahmad, I., Eisa, M. H., & Maaza, M. (2023). Avant-Garde Polymer/Graphene Nanocomposites for Corrosion Protection: Design, Features, and Performance. Corrosion and Materials Degradation, 4(1), 33-53. https://doi.org/10.3390/cmd4010004








