The Influence of CaCl2-Blended Acrylic Polymer on Steel Rebar Corrosion and Acid Attack Resistance of Mortar
Abstract
:1. Introduction
2. Experimental Methods
2.1. Materials
2.2. Mixture Proportion and Specimen Preparation
2.3. Accelerated Corrosion Test
2.4. Half-Cell Potential Test
2.5. XRD Analysis of Corrosion Byproduct
2.6. Acid Attack Resistance
3. Results and Discussions
3.1. Accelerated Corrosion Test
3.1.1. Half-Cell Potential
3.1.2. Corrosion Current Density and Crack Time
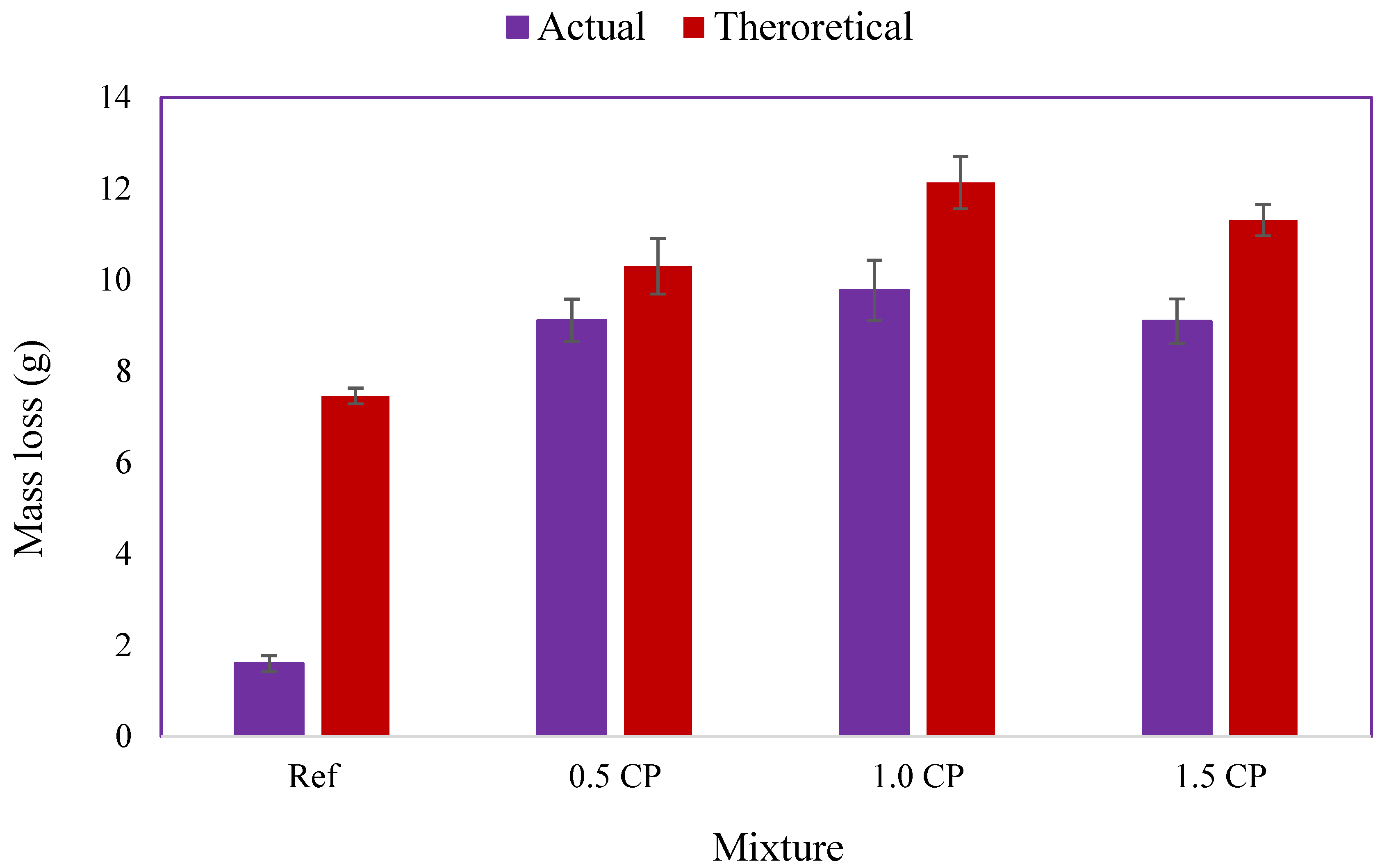
3.1.3. Rebar Mass Loss
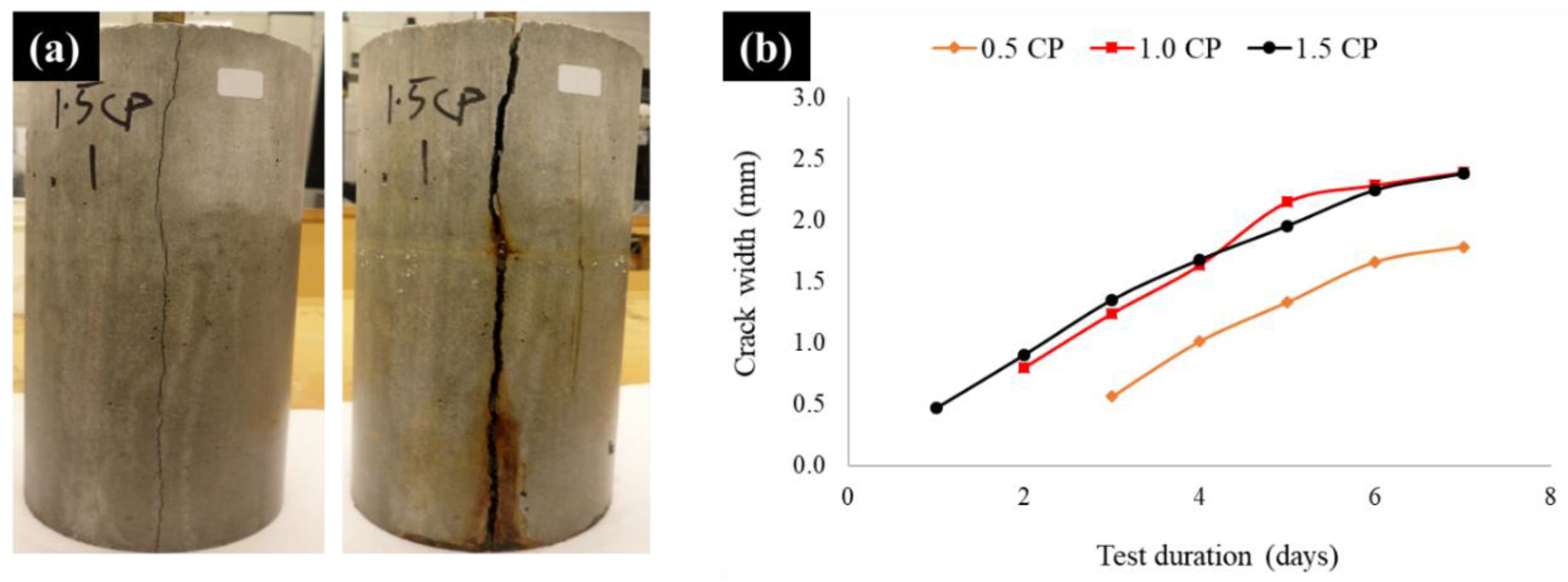
3.1.4. Crack Analyses
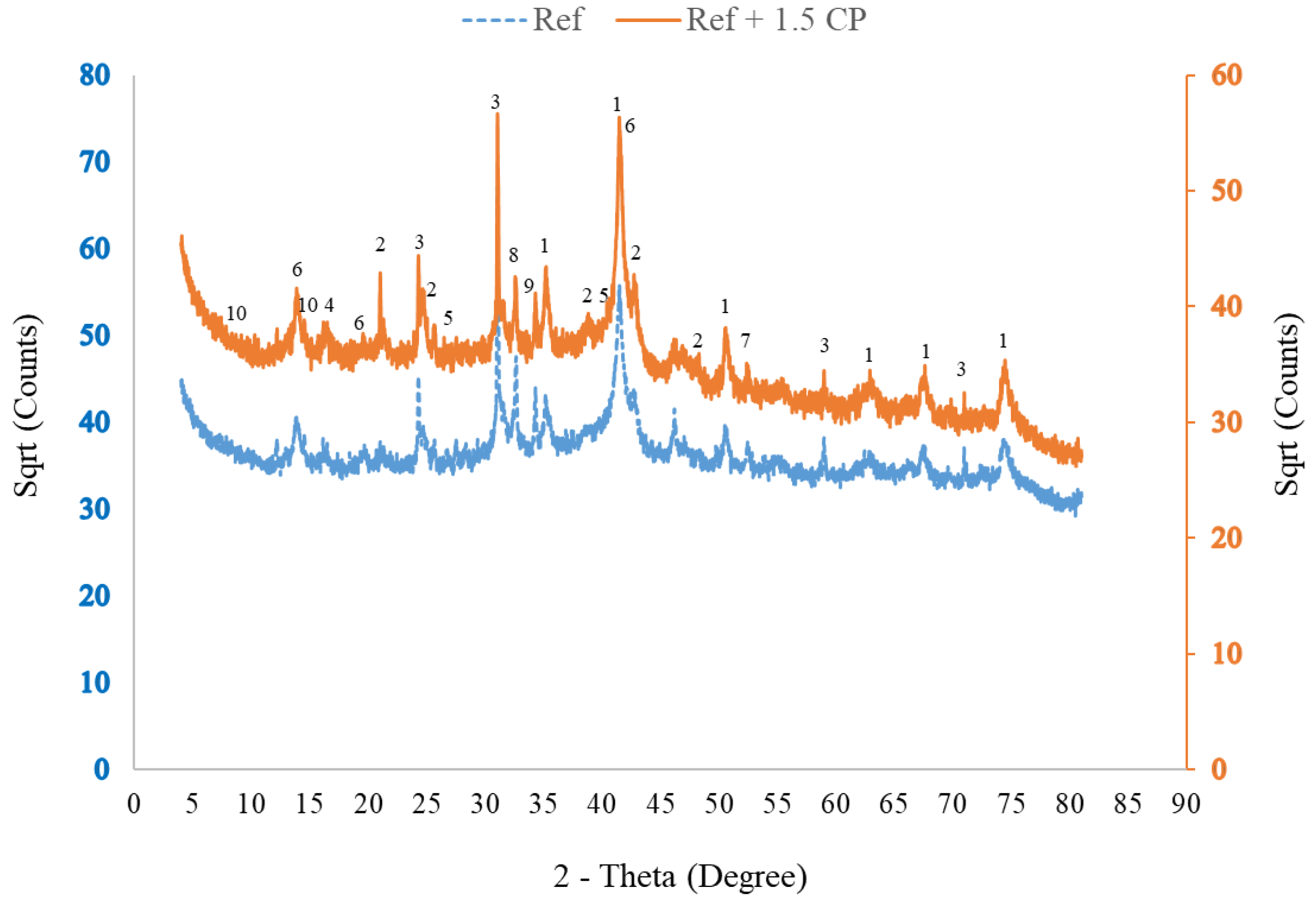
3.1.5. Corrosion Byproduct Phase Composition
3.2. Acid Resistance
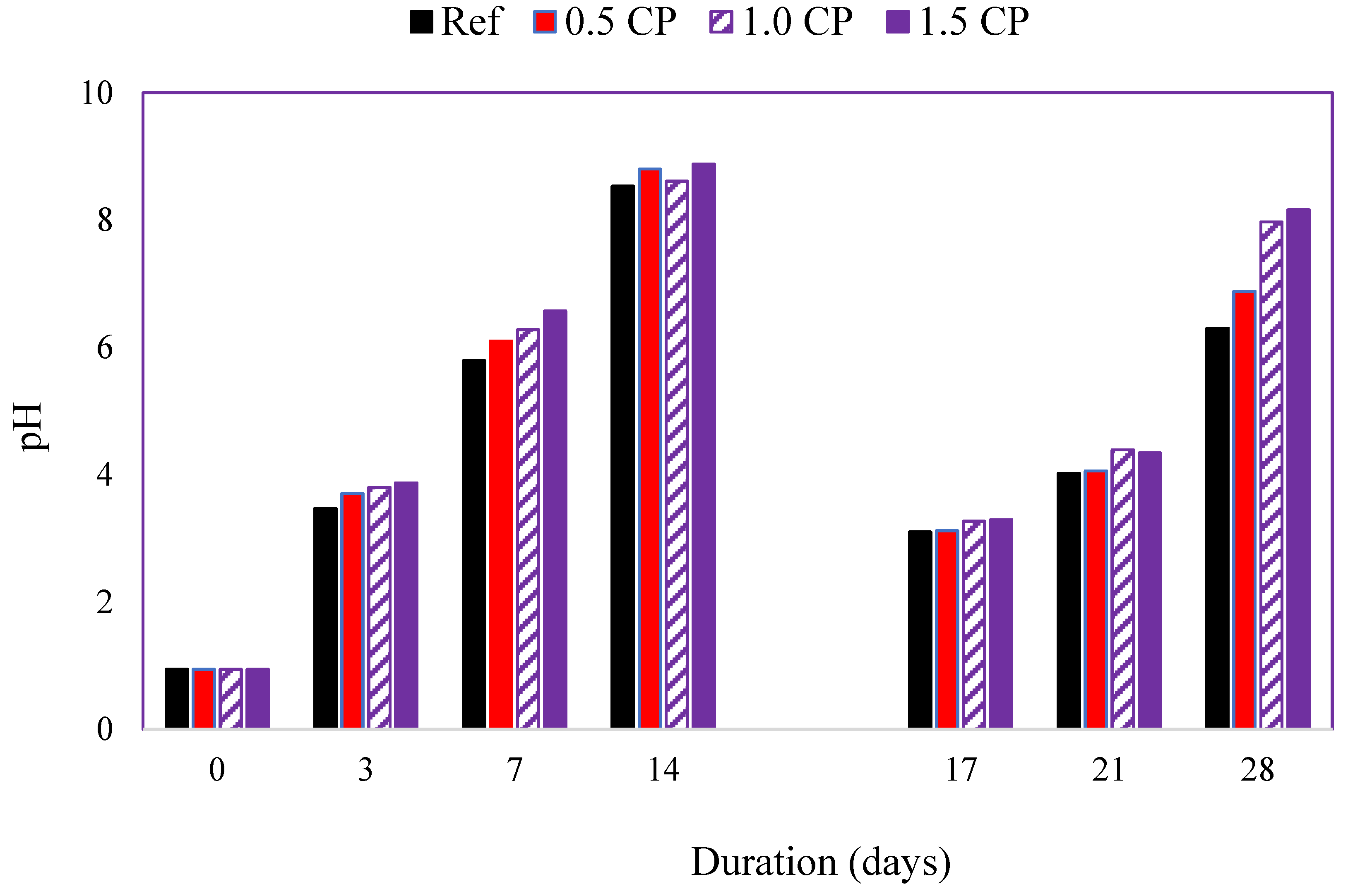
3.2.1. Test Solution pH
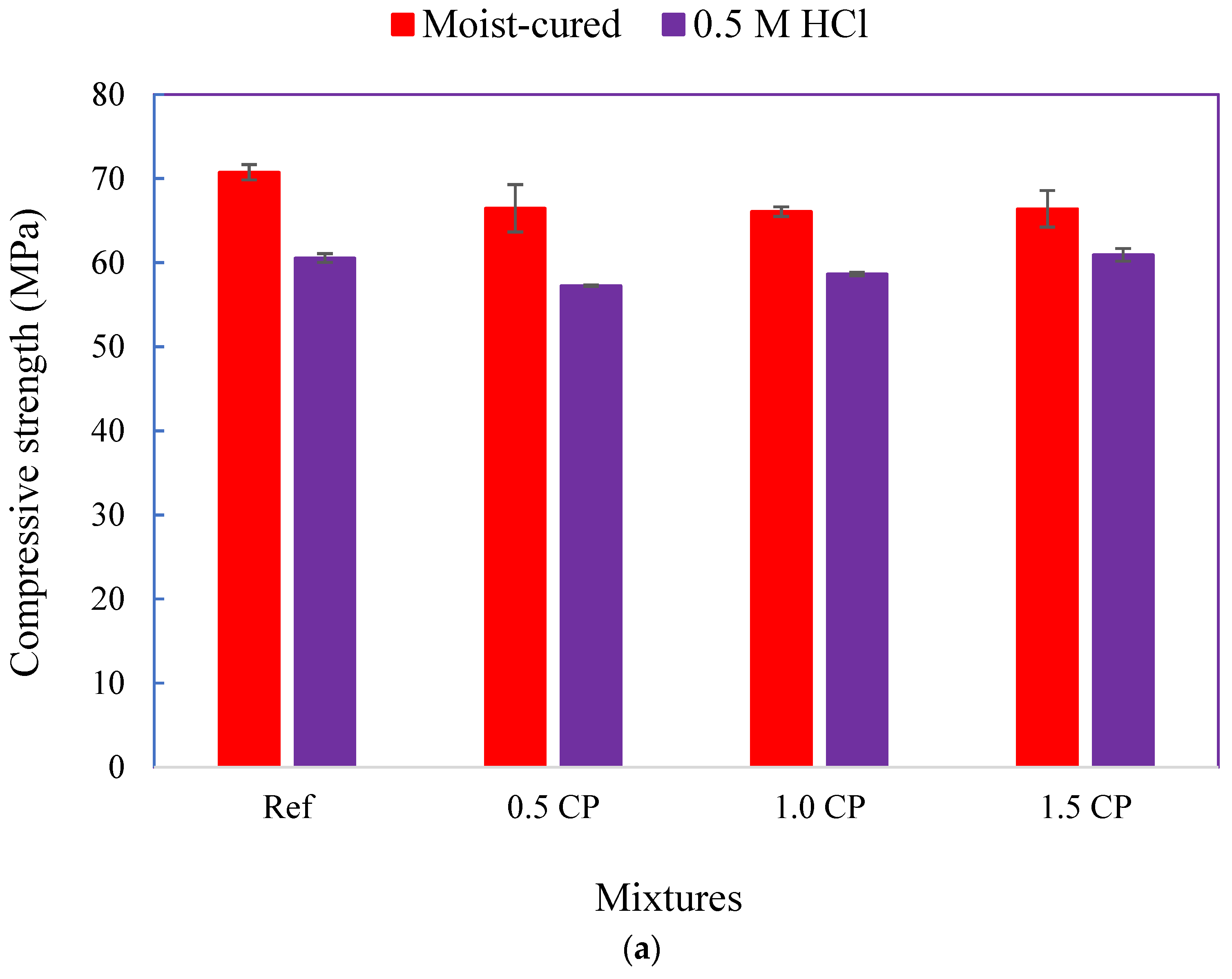
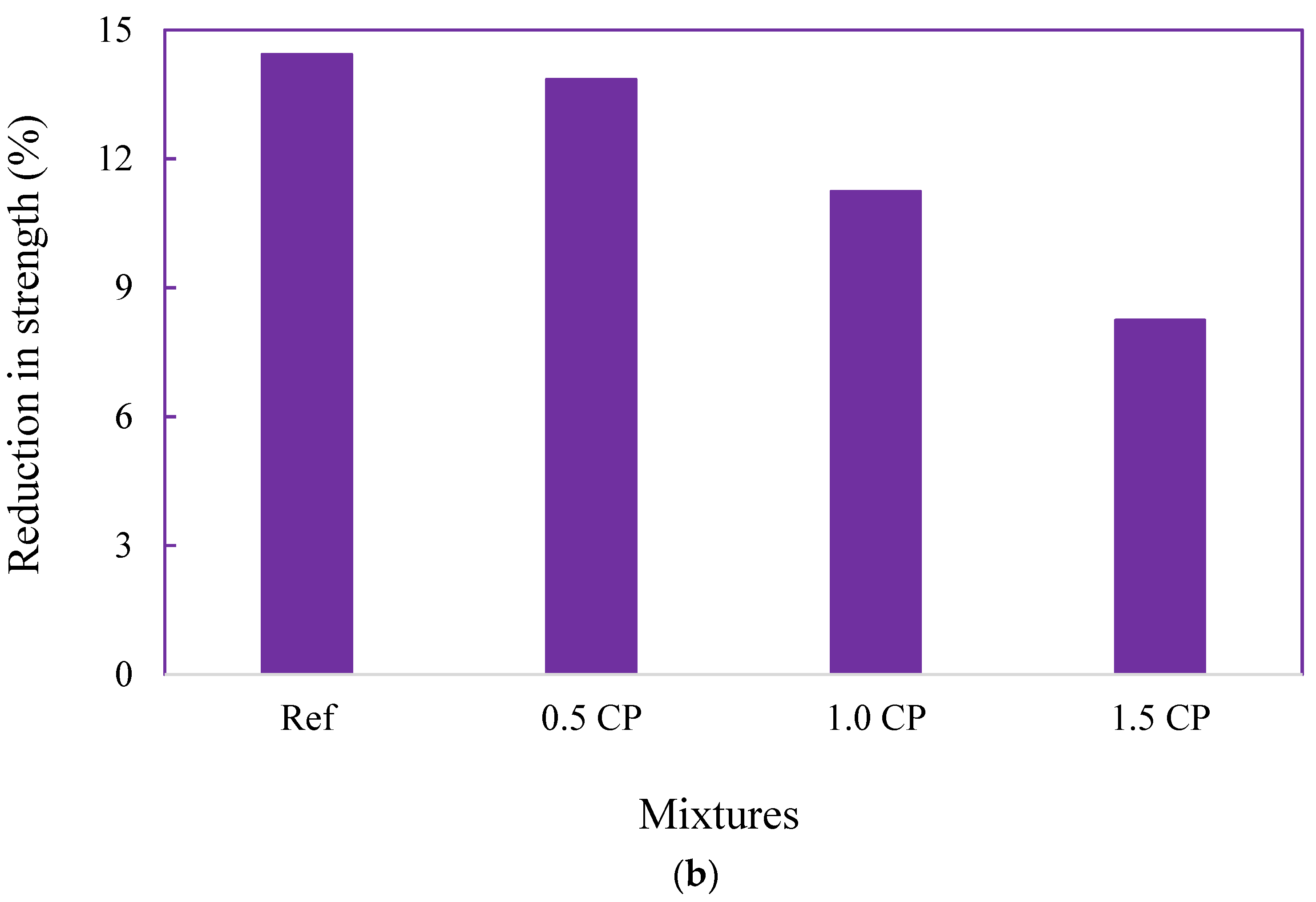
3.2.2. Changes in Compressive Strength
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- British Standard European Norm (BS EN) 206-1; Concrete—Part 1: Specification, Performance, Production and Conformity. European Committee for Standardization (CEN): Brussels, Belgium, 2013.
- American Concrete Institute (ACI) 318; Building Code Requirements for Structural Concrete and Commentary. ACI: Detroit, MI, USA, 2019.
- Andrade, C.; Page, C.L. Pore solution chemistry and corrosion in hydrated cement systems containing chloride salts. A study of cation specific effects. Br. Corros. J. 1986, 21, 49–54. [Google Scholar] [CrossRef]
- Pruckner, F.; Gjørv, O.E. Effect of CaCl2 and NaCl additions on concrete corrosivity. Cem. Concr. Res. 2004, 34, 1209–1217. [Google Scholar] [CrossRef]
- Xu, J.; Jiang, L.; Wang, W.; Jiang, Y. Influence of CaCl2 and NaCl from different sources on chloride threshold value for the corrosion of steel reinforcement in concrete. Constr. Build. Mater. 2011, 25, 663–669. [Google Scholar] [CrossRef]
- Delagrave, A.; Marchand, J.; Ollivier, J.-P.; Julien, S.; Hazrati, K. Chloride binding capacity of various hydrated cement paste systems. Adv. Cem. Based Mater. 1997, 6, 28–35. [Google Scholar] [CrossRef]
- Zhu, Q.; Jiang, L.; Chen, Y.; Xu, J.; Mo, L. Effect of chloride salt type on chloride binding behavior of concrete. Constr. Build. Mater. 2012, 37, 512–517. [Google Scholar] [CrossRef]
- De Weerdt, K.; Colombo, A.; Coppola, L.; Justnes, H.; Geiker, M. Impact of the associated cation on chloride binding of Portland cement paste. Cem. Concr. Res. 2015, 68, 196–202. [Google Scholar] [CrossRef]
- Shi, Z.; Geiker, M.R.; De Weerdt, K.; Østnor, T.A.; Lothenbach, B.; Winnefeld, B.; Skibsted, J. Role of calcium on chloride binding in hydrated Portland cement–metakaolin–limestone blends. Cem. Concr. Res. 2017, 95, 205–216. [Google Scholar] [CrossRef]
- Page, C.L.; Vennesland, O. Pore solution composition and chloride binding capacity of silica-fume cement pastes. Mater. Struct. 1983, 16, 19–25. [Google Scholar] [CrossRef]
- Arya, C.; Buenfeld, N.R.; Newman, J.B. Factors influencing chloride binding in concrete. Cem. Concr. Res. 1990, 20, 291–300. [Google Scholar] [CrossRef]
- Manera, M.; Vennesland, O.; Bertolini, L. Chloride threshold for rebar corrosion in concrete with addition of silica fume. Corros. Sci. 2008, 50, 554–560. [Google Scholar] [CrossRef]
- Hou, J.; Chung, D.D.L. Effect of admixture in concrete on the corrosion resistance of steel reinforced concrete. Corros. Sci. 2000, 42, 1489–1507. [Google Scholar] [CrossRef]
- Dotto, J.M.R.; De Abreu, A.G.; Dal Molin, D.C.C.; Müller, I.L. Influence of silica fume addition on concretes physical properties and on corrosion behaviour of reinforcement bars. Cem. Concr. Compos. 2004, 26, 31–39. [Google Scholar] [CrossRef]
- Kelestemur, O.; Demirel, B. Corrosion behavior of reinforcing steel embedded in concrete produced with finely ground pumice and silica fume. Constr. Build. Mater. 2010, 24, 1898–1905. [Google Scholar] [CrossRef] [Green Version]
- American Society of Concrete Contractors (ASCC). Position Statement #31—Acceptable Use of Calcium Chloride in Concrete. Retrieved on 16 March 2019. 2020. Available online: https://www.ascconline.org/Portals/0/docs/POSITION-STATEMENTS/PS-31-acceptable-use-calcium-chloride-concrete.pdf (accessed on 14 January 2022).
- Shaker, F.A.; El-Dieb, A.S.; Reda, M.M. Durability of styrene butadiene latex modified concrete. Cem. Concr. Res. 1997, 27, 711–720. [Google Scholar] [CrossRef]
- Rossignolo, J.A.; Agnesini, M.V. Durability of polymer-modified lightweight aggregate concrete. Cem. Concr. Compos. 2004, 26, 375–380. [Google Scholar] [CrossRef]
- Sokolova, I. Corrosion resistance of steel reinforcement in polymer silicate shyngizite concrete. E3S Web Conf. 2020, 164, 02032. [Google Scholar] [CrossRef]
- Monteny, J.; Belie, N.; Vincke, E.; Verstraete, W.; Taerwe, L. Chemical and microbiological tests to simulate sulphuric acid corrosion of polymer-modified concrete. Cem. Concr. Res. 2001, 31, 1359–1365. [Google Scholar] [CrossRef]
- Pacheco-Torgal, F.; Jalali, S. Sulphuric acid resistance of plain, polymer modified, and fly ash cement concretes. Constr. Build. Mater. 2009, 23, 3485–3491. [Google Scholar] [CrossRef] [Green Version]
- Onuaguluchi, O.; Ratu, R.; Banthia, N. The effects of CaCl2-blended acrylic polymer emulsion on the properties of cement mortar. Mater. Struct. 2018, 51, 50. [Google Scholar] [CrossRef]
- Onuaguluchi, O.; Banthia, N. Alkali-silica reaction resistance of cementitious material containing CaCl2-blended acrylic polymer emulsion. J. Mater. Civil. Eng. 2020, 33, 04019378. [Google Scholar] [CrossRef]
- American Society of Testing and Materials (ASTM) C305; Standard Practice for Mechanical Mixing of Hydraulic Cement Pastes and Mortars of Plastic Consistency. ASTM International: West Conshohocken, PA, USA, 2014.
- ASTM A276; Standard Specifications for Stainless Steel Bars and Shapes. ASTM International: West Conshohocken, PA, USA, 2017.
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- ASTM G1-03; Standard Practice for Preparing, Cleaning and Evaluating Corrosion Test Specimens. ASTM International: West Conshohocken, PA, USA, 2017.
- ASTM C876; Standard Test Method for Corrosion Potentials of Uncoated Reinforcing Steel in Concrete. ASTM International: West Conshohocken, PA, USA, 2015.
- García-Alonso, M.C.; Escudero, M.L.; Miranda, J.M.; Vega, M.I.; Capilla, F.; Correia, M.J.; Salta, M.; Bennani, A.; González, J.A. Corrosion behaviour of new stainless steels reinforcing bars embedded in concrete. Cem. Concr. Res. 2007, 37, 1463–1471. [Google Scholar] [CrossRef] [Green Version]
- Uthaman, S.; George, R.P.; Vishwakarma, V.; Harilal, M.; Philip, J. Enhanced seawater corrosion resistance of reinforcement in nanophase modified fly ash concrete. Constr. Build. Mater. 2019, 221, 232–243. [Google Scholar] [CrossRef]
- Addari, D.; Elsener, B.; Rossi, A. Electrochemistry and surface chemistry of stainless steels in alkaline media simulating concrete pore solutions. Electrochim. Acta 2008, 53, 8078–8086. [Google Scholar] [CrossRef]
- Ouglova, A.; Raharinaivo, A.; Berthaud, Y.; Petre-Lazar, I.; Boukhenhouf, H. Numerical simulation of potential distribution to the corrosion of reinforcement in concrete structures. Mater. Struct. 2005, 38, 711–719. [Google Scholar] [CrossRef]
- Gonzales, J.A.; Otero, E.; Feliu, S.; Lopez, W. Initial steps of corrosion in the steel/Ca(OH)2 + Cl− system: The role of heterogeneities on the steel surface and oxygen supply. Cem. Concr. Res. 1993, 23, 33–40. [Google Scholar] [CrossRef]
- Idriss, A.F.; Negi, S.C.; Jofriet, J.C.; Hayward, G.I. Effect of hydrogen sulphide emissions on cement mortar specimens. Can. Biosyst. Eng. 2001, 43, 5.23–5.28. [Google Scholar]
- Hong, S.; Zheng, F.; Shi, G.; Li, J.; Luo, X.; Xing, F.; Tang, L.; Dong, B. Determination of impressed current efficiency during accelerated corrosion of reinforcement. Cem. Concr. Compos. 2020, 108, 103536. [Google Scholar] [CrossRef]
- Goñi, S.; Andrade, C. Synthetic concrete pore solution chemistry and rebar corrosion rate in the presence of chlorides. Cem. Concr. Res. 1990, 20, 525–539. [Google Scholar] [CrossRef]
- Enevoldsen, J.N.; Hansson, C.M.; Hope, B.B. The influence of internal relative humidity on the rate of corrosion of steel embedded in concrete and mortar. Cem. Concr. Res. 1994, 24, 1373–1382. [Google Scholar] [CrossRef]
- Kim, J.-H.; Robertson, R.E. Effects of polyvinyl alcohol on aggregate-paste bond strength and the interfacial transition zone. Adv. Cem. Based Mater. 1998, 8, 66–76. [Google Scholar] [CrossRef]
- Caré, S.; Raharinaivo, A. Influence of impressed current on the initiation of damage in reinforced mortar due to the corrosion of embedded steel. Cem. Concr. Res. 2007, 37, 1598–1612. [Google Scholar] [CrossRef]
- Tuutti, K. Corrosion of Steel in Concrete; Swedish Cement and Concrete Research Institute: Stockholm, Sweden, 1982. [Google Scholar]
- Alonso, C.; Andrade, C.; Rodriguez, J.; Diez, J.M. Factors Controlling Cracking of Concrete Affected by Reinforcement Corrosion. Mater. Struct. 1998, 31, 435–441. [Google Scholar] [CrossRef]
- El Maaddawy, T.A.; Soudki, K.A. Effectiveness of Impressed Current Technique to Simulate Corrosion of Steel Reinforcement in Concrete. J. Mater. Civ. Eng. 2003, 15, 41–47. [Google Scholar] [CrossRef]
- Jang, B.S.; Oh, B.H. Effects of non-uniform corrosion on the cracking and service life of reinforced concrete structures. Cem. Concr. Res. 2010, 40, 1441–1450. [Google Scholar] [CrossRef]
- Fedosov, S.V.; Rumyantseva, V.E.; Krasilnikov, I.V.; Konovalova, V.S.; Evsyakov, A.S. Mathematical modeling of the colmatation of concrete pores during corrosion. Mag. Civ. Eng. 2018, 83, 198–207. [Google Scholar] [CrossRef]
- Wang, X.; Song, X.; Zhang, M.; Du, X. Experimental comparison of corrosion unevenness and expansive cracking between accelerated corrosion methods used in laboratory research. Constr. Build. Mater. 2017, 153, 36–43. [Google Scholar] [CrossRef]
- Chen, F.; Li, C.; Baji, H.; Ma, B. Quantification of non-uniform distribution and growth of corrosion products at steel-concrete interface. Constr. Build. Mater. 2020, 237, 117610. [Google Scholar] [CrossRef]
- Austin, S.A.; Lyons, R.; Ing, M.J. Electrochemical Behaviour of Steel Reinforced Concrete during Accelerated Corrosion Testing. Corrosion 2004, 60, 203–212. [Google Scholar] [CrossRef]
- Onuaguluchi, O.; Banthia, N.; Gourlay KMinhas, G. Moisture transport and steel rebar corrosion in repair composites incorporating Nano-Fibrillated Cellulose (NFC). Constr. Build Mater. 2021, 309, 125154. [Google Scholar] [CrossRef]
- Marcotte, T.D. Characterization of Chloride-Induced Corrosion Products that form in Steel-Reinforced Cementitious Materials. Ph.D. Thesis, University of Waterloo, Waterloo, ON, Canada, 2001. [Google Scholar]
- Duffo, G.S.; Morris, W.; Raspini, I.; Saragovi, C. A study of steel rebars embedded in concrete during 65 years. Corros. Sci. 2004, 46, 2143–2157. [Google Scholar] [CrossRef]
- Chitty, W.-J.; Dillmann, P.; L’Hostis, V.; Lombard, C. Long-term corrosion resistance of metallic reinforcements in concrete—A study of corrosion mechanisms based on archaeological artefacts. Corros. Sci. 2005, 47, 1555–1581. [Google Scholar] [CrossRef]
- Paul, S.C.; Babafemi, A.J.; Conradie, K.; van Zijl, G.P.A.G. Applied voltage on corrosion mass loss and cracking behavior of steel-reinforced shcc and mortar specimens. J. Mater. Civ. Eng. 2016, 29, 04016272. [Google Scholar] [CrossRef]
- Israel, D.; Macphee, D.; Lachowski, E. Acid attack on pore-reduced cements. J. Mater. Sci. 1997, 32, 4109–4116. [Google Scholar] [CrossRef]
- Beddoe, R.E.; Dorner, H.W. Modelling acid attack on concrete: Part I. The essential mechanisms. Cem. Concr. Res. 2005, 35, 2333–2339. [Google Scholar] [CrossRef]
- De Ceukelaire, L. The effects of hydrochloric acid on mortar. Cem. Concr. Res. 1992, 22, 903–914. [Google Scholar] [CrossRef]
- Larbi, J.A.; Bijen, J.M.J.M. Interaction of polymers with Portland cement during hydration: A study of the chemistry of the pore solution of polymer-modified cement systems. Cem. Concr. Res. 1990, 20, 139–147. [Google Scholar] [CrossRef]
- Jenni, A.; Zurbriggen, R.; Holzer, L.; Herwegh, M. Changes in microstructures and physical properties of polymer-modified mortars during wet storage. Cem. Concr. Res. 2006, 36, 79–90. [Google Scholar] [CrossRef]
- Beenldens, A.; Monteny, J.; Vincke, E.; De Belie, N.; van Gemert, D.; Taerwe, L. Resistance to biogenic sulphuric acid corrosion of polymer-modified mortars. Cem. Concr. Res. 2001, 23, 47–56. [Google Scholar] [CrossRef]
- Rossignolo, J.A. Effect of silica fume and SBR latex on the paste aggregate interfacial transition zone. Mat. Res. 2007, 10, 83–86. [Google Scholar] [CrossRef] [Green Version]




| Item | Composition (%) |
|---|---|
| Calcium chloride, hydrated | 10–30 |
| Acrylic copolymer latex | 10–30 |
| Water | 30–60 |
| Other | <1 |
| pH | 6–8 |
| Element | Composition (%) | Element | Composition (%) |
|---|---|---|---|
| Carbon | 0.28 | Chromium | 0.17 |
| Manganese | 1.10 | Molybdenum | 0.02 |
| Phosphorous | 0.015 | Copper | 0.40 |
| Sulfur | 0.044 | Vanadium | 0.02 |
| Silicon | 0.18 | Niobium | 0.002 |
| Nickel | 0.09 | Carbon equivalent | 0.49 |
| Mix | Splitting Tensile Strength (MPa) | Sorptivity (mm/s1/2) |
|---|---|---|
| Ref | 3.4 ± 0.3 | 0.0438 ± 0.0004 |
| 0.5 CP | 3.9 ± 0.2 | 0.0413 ± 0.0009 |
| 1.0 CP | 4.0 ± 0.1 | 0.0394 ± 0.0010 |
| 1.5 CP | 3.9 ± 0.2 | 0.0336 ± 0.0004 |
| Iron Phase | Ref | Ref + 1.5 CP |
|---|---|---|
| Akaganeite (β-FeOOH) | 15.0 | 14.0 |
| Goethite (α-FeOOH) | 18.0 | 19.0 |
| Hematite (α-Fe2O3) | 1.0 | 1.0 |
| Lepidocrocite (γ-FeOOH) | 2.0 | 2.0 |
| Magnetite (Fe3O4) | 30.0 | 32.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Onuaguluchi, O.; Banthia, N. The Influence of CaCl2-Blended Acrylic Polymer on Steel Rebar Corrosion and Acid Attack Resistance of Mortar. Corros. Mater. Degrad. 2022, 3, 160-177. https://doi.org/10.3390/cmd3010009
Onuaguluchi O, Banthia N. The Influence of CaCl2-Blended Acrylic Polymer on Steel Rebar Corrosion and Acid Attack Resistance of Mortar. Corrosion and Materials Degradation. 2022; 3(1):160-177. https://doi.org/10.3390/cmd3010009
Chicago/Turabian StyleOnuaguluchi, Obinna, and Nemkumar Banthia. 2022. "The Influence of CaCl2-Blended Acrylic Polymer on Steel Rebar Corrosion and Acid Attack Resistance of Mortar" Corrosion and Materials Degradation 3, no. 1: 160-177. https://doi.org/10.3390/cmd3010009
APA StyleOnuaguluchi, O., & Banthia, N. (2022). The Influence of CaCl2-Blended Acrylic Polymer on Steel Rebar Corrosion and Acid Attack Resistance of Mortar. Corrosion and Materials Degradation, 3(1), 160-177. https://doi.org/10.3390/cmd3010009






