Influence of Organic Matter/Bacteria on the Formation and Transformation of Sulfate Green Rust
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preliminary Corrosion Experiments
2.2. Preparation of Green Rust Precipitates
2.3. Bacterial Strains and Culture Conditions
2.4. Numeration of Bacteria
2.5. XRD Analysis of the Precipitates
3. Results
3.1. Characterization of the Corrosion Product Layers Formed in Artificial/Natural Seawater
3.2. Characterization of the Precipitate Obtained in Abiotic Conditions
3.3. Influence of Bacteria
3.4. Influence of Acetate Ions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, W.; Lewandowski, Z.; Nielsen, P.H.; Hamilton, W.A. Role of sulfate-reducing bacteria in corrosion of mild steel: A review. Biofouling 1995, 8, 165–194. [Google Scholar] [CrossRef]
- Melchers, R.E.; Wells, T. Models for the anaerobic phases of marine immersion corrosion. Corros. Sci. 2006, 48, 1791–1811. [Google Scholar] [CrossRef]
- Melchers, R.E.; Jeffrey, R. Corrosion of long vertical steel strips in the marine tidal zone and implications for ALWC. Corros. Sci. 2012, 65, 26–36. [Google Scholar] [CrossRef]
- Beech, I.B.; Zinkevich, V.; Tapper, R.; Gubner, R. The direct involvement of extracellular compounds from a marine sulphate-reducing bacterium in deterioration of steel. Geomicrobiol. J. 1998, 15, 119–132. [Google Scholar] [CrossRef]
- Malard, E.; Kervadec, D.; Gil, O.; Lefevre, Y.; Malard, S. Interactions between steels and sulphide-producing bacteria-Corrosion of carbon steels and low-alloy steels in natural seawater. Electrochim. Acta 2008, 54, 8–13. [Google Scholar] [CrossRef]
- Kumar, A.V.R.; Singh, R.; Nigam, R.K. Mössbauer spectroscopy of corrosion products of mild steel due to microbiologically influenced corrosion. J. Radioanal. Nucl. Chem. 1999, 242, 131–137. [Google Scholar] [CrossRef]
- Mahanna, M.; Basseguy, R.; Delia, M.-L.; Girbal, L.; Demuez, M.; Bergel, A. New hypotheses for hydrogenase implication in the corrosion of mild steel. Electrochim. Acta 2008, 54, 140–147. [Google Scholar] [CrossRef] [Green Version]
- Pineau, S.; Sabot, R.; Quillet, L.; Jeannin, M.; Caplat, C.; Dupont-Morral, I.; Refait, P. Formation of the Fe(II-III) hydroxysulphate green rust during marine corrosion of steel associated to molecular detection of dissimilatory sulphite-reductase. Corros. Sci. 2008, 50, 1099–1111. [Google Scholar] [CrossRef]
- Lanneluc, I.; Langumier, M.; Sabot, R.; Jeannin, M.; Refait, P.; Sablé, S. On the bacterial communities associated with the corrosion product layer during the early stages of marine corrosion of carbon steel. Int. Biodeterior. Biodegrad. 2015, 99, 55–65. [Google Scholar] [CrossRef]
- Smith, J.S.; Miller, J.D.A. Nature of sulfides and their corrosive effect on ferrous metals: A review. Br. Corros. J. 1975, 10, 136–143. [Google Scholar] [CrossRef]
- Enning, D.; Venzlaff, H.; Garrelfs, J.; Dinh, H.T.; Meyer, V.; Mayrhofer, K.J.J.; Hassel, A.W.; Stratmann, M.; Widdel, F. Marine sulfate-reducing bacteria cause serious corrosion of iron under electroconductive biogenic mineral crust. Environ. Microbiol. 2012, 14, 1772–1787. [Google Scholar] [CrossRef] [Green Version]
- Zegeye, A.; Huguet, L.; Abdelmoula, A.; Carteret, C.; Mullet, M.; Jorand, F. Biogenic hydroxysulfate green rust, a potential electron acceptor for SRB activity. Geochim. Cosmochim. Acta 2007, 71, 5450–5462. [Google Scholar] [CrossRef] [Green Version]
- Langumier, M.; Sabot, R.; Obame-Ndong, R.; Jeannin, M.; Sablé, S.; Refait, P. Formation of Fe(III)-containing mackinawite from hydroxysulphate green rust by sulphate reducing bacteria. Corros. Sci. 2009, 51, 2694–2702. [Google Scholar] [CrossRef]
- Duboscq, J.; Sabot, R.; Jeannin, M.; Refait, P. Localized corrosion of carbon steel in seawater: Processes occurring in cathodic zones. Mater. Corros. 2019, 70, 973–984. [Google Scholar] [CrossRef]
- Duan, J.; Wu, S.; Zhang, X.; Huang, G.; Du, M.; Hou, B. Corrosion of carbon steel influenced by anaerobic biofilm in natural seawater. Electrochim. Acta 2008, 54, 22–28. [Google Scholar] [CrossRef]
- Refait, P.; Nguyen, D.D.; Jeannin, M.; Sablé, S.; Langumier, M.; Sabot, R. Electrochemical formation of green rusts in deaerated seawater-like solutions. Electrochim. Acta 2011, 56, 6481–6488. [Google Scholar] [CrossRef]
- Refait, P.; Grolleau, A.-M.; Jeannin, M.; François, E.; Sabot, R. Localized corrosion of carbon steel in marine media: Galvanic coupling and heterogeneity of the corrosion product layer. Corros. Sci. 2016, 111, 583–595. [Google Scholar] [CrossRef]
- Refait, P.; Grolleau, A.M.; Jeannin, M.; Rémazeilles, C.; Sabot, R. Corrosion of carbon steel in marine environments: Role of the corrosion product layer. Corros. Mater. Degrad. 2020, 1, 198–218. [Google Scholar] [CrossRef]
- Refait, P.; Géhin, A.; Abdelmoula, M.; Génin, J.-M.R. Coprecipitation thermodynamics of iron(II–III) hydroxysulphate green rust from Fe(II) and Fe(III) salts. Corros. Sci. 2003, 45, 656–676. [Google Scholar] [CrossRef]
- Simon, L.; François, M.; Refait, P.; Renaudin, G.; Lelaurain, L.; Génin, J.M. Structure of the Fe(II-III) layered double hydroxysulphate green rust two from Rietveld analysis. Solid State Sci. 2003, 5, 327–334. [Google Scholar] [CrossRef]
- Detournay, J.; De Miranda, L.; Dérie, R.; Ghodsi, M. The region of stability of green rust II in the electrochemical potential-pH equilibrium diagram of iron in sulphate medium. Corros. Sci. 1975, 15, 295–306. [Google Scholar] [CrossRef]
- Olowe, A.A.; Génin, J.-M.R. The mechanism of oxidation of ferrous hydroxide in sulphated aqueous media: Importance of the initial ratio of reactants. Corros. Sci. 1991, 32, 965–984. [Google Scholar] [CrossRef]
- Refait, P.; Sabot, R.; Jeannin, M. Role of Al(III) and Cr(III) on the formation and oxidation of the Fe(II–III) hydroxysulfate green rust. Colloids Surf. A Phys. Eng. Asp. 2017, 531, 203–212. [Google Scholar] [CrossRef]
- Ona-Nguema, G.; Carteret, C.; Benali, O.; Abdelmoula, M.; Génin, J.-M.R.; Jorand, F. Competitive formation of hydroxycarbonate green rust I vs hydroxysulphate green rust 2 in Shewanella putrefaciens cultures. Geomicrobiol. J. 2004, 21, 79–90. [Google Scholar] [CrossRef]
- Zegeye, A.; Ona-Nguema, G.; Carteret, C.; Huguet, L.; Abdelmoula, M.; Jorand, F. Formation of hydroxysulphate green rust 2 as a single iron(II-III) mineral in microbial culture. Geomicrobiol. J. 2005, 22, 389–399. [Google Scholar] [CrossRef] [Green Version]
- ASTM D1141-98(2013); Standard Practice for the Preparation of Substitute Ocean Water. ASTM International: West Conshohocken, PA, USA, 2013.
- De Faria, D.L.A.; Silva, S.V.; Oliveira, M.T.D. Raman micro spectroscopy study of some iron oxides and oxyhydroxides. J. Raman Spectrosc. 1997, 28, 873–878. [Google Scholar] [CrossRef]
- Shebanova, O.N.; Lazor, P. Raman study of magnetite (Fe3O4): Laser-induced thermal effects and oxidation. J. Raman Spectrosc. 2003, 34, 845–852. [Google Scholar] [CrossRef]
- Refait, P.; Duboscq, J.; Aggoun, K.; Sabot, R.; Jeannin, M. Influence of Mg2+ ions on the formation of green rust compounds in simulated marine environments. Corros. Mater. Degrad. 2021, 2, 46–61. [Google Scholar] [CrossRef]
- Hansen, H.C.B. Composition, stabilisation, and light absorption of Fe(II)-Fe(III) hydroxycarbonate (green rust). Clay Miner. 1989, 24, 663–669. [Google Scholar] [CrossRef]
- Refait, P.; Grolleau, A.-M.; Jeannin, M.; François, E.; Sabot, R. Corrosion of carbon steel at the mud zone/seawater interface: Mechanisms and kinetics. Corros. Sci. 2018, 130, 76–84. [Google Scholar] [CrossRef]
- Chicot, D.; Mendoza, J.; Zaoui, A.; Louis, G.; Lepingle, V.; Roudet, F.; Lesage, J. Mechanical properties of magnetite (Fe3O4), hematite (α-Fe2O3) and goethite (α-FeO·OH) by instrumented indentation and molecular dynamics analysis. Mater. Chem. Phys. 2011, 129, 862–870. [Google Scholar] [CrossRef]
- Tomic, Z.; Makreski, P.; Gajic, B. Identification and spectra–structure determination of soil minerals: Raman study supported by IR spectroscopy and X-ray powder diffraction. J. Raman Spectrosc. 2010, 41, 582–586. [Google Scholar] [CrossRef]
- Ohfuji, H.; Rickard, D. High resolution transmission electron microscopic study of synthetic nanocrystalline mackinawite. Earth Planet. Sci. Lett. 2006, 241, 227–233. [Google Scholar] [CrossRef]
- Bourdoiseau, J.A.; Jeannin, M.; Sabot, R.; Rémazeilles, C.; Refait, P. Characterisation of mackinawite by Raman spectroscopy: Effects of crystallisation, drying and oxidation. Corros. Sci. 2008, 50, 3247–3255. [Google Scholar] [CrossRef]
- Legrand, L.; Sagon, G.; Lecomte, S.; Chausse, A.; Messina, R. A Raman and infrared study of a new carbonate green rust obtained by electrochemical way. Corros. Sci. 2001, 43, 1739–1749. [Google Scholar] [CrossRef]
- Sabot, R.; Jeannin, M.; Gadouleau, M.; Guo, Q.; Sicre, E.; Refait, P. Influence of lactate ions on the formation of rust. Corros. Sci. 2007, 49, 1610–1624. [Google Scholar] [CrossRef]
- Refait, P.; Abdelmoula, M.; Génin, J.-M.R. Mechanisms of formation and structure of green rust one in aqueous corrosion of iron in the presence of chloride ions. Corros. Sci. 1998, 40, 1547–1560. [Google Scholar] [CrossRef]
- McGill, J.R.; McEnaney, B.; Smith, D.C. Crystal structure of green rust formed by corrosion of cast iron. Nature 1976, 259, 200–201. [Google Scholar] [CrossRef]
- Miyata, S. Anion-exchange properties of hydrotalcite-like compounds. Clays Clay Miner. 1983, 31, 305–311. [Google Scholar] [CrossRef]
- Mendiboure, A.; Schöllhorn, R. Formation and anion exchange reactions of layered transition metal hydroxides [Ni1-xMx](OH)2(CO3)x/2(H2O)z (M=Fe,Co). Rev. Chim. Miner. 1986, 23, 819–827. [Google Scholar] [CrossRef]
- Benali, O.; Abdelmoula, M.; Refait, P.; Génin, J.-M.R. Effect of orthophosphate on the oxidation products of Fe(II)-Fe(III) hydroxycarbonate; the transformation of green rust to ferrihydrite. Geochim. Cosmochim. Acta 2001, 65, 1715–1726. [Google Scholar] [CrossRef]
- Refait, P.; Reffass, M.; Landoulsi, J.; Sabot, R.; Jeannin, M. Role of nitrite species during the formation and transformation of the Fe(II-III) hydroxycarbonate Green Rust. Colloids Surf. A Phys. Eng. Asp. 2014, 459, 225–232. [Google Scholar] [CrossRef]
- Bocher, F.; Géhin, A.; Ruby, C.; Ghanbaja, J.; Abdelmoula, M.; Génin, J.-M.R. Coprecipitation of Fe(II-III) hydroxycarbonate green rust stabilised by phosphate adsorption. Solid State Sci. 2004, 6, 117–124. [Google Scholar] [CrossRef]
- Jorand, F.P.A.; Sergent, A.-S.; Rémy, P.-P.; Bihannic, I.; Ghanbaja, J.; Lartiges, B.; Hanna, K.; Zegeye, A. Contribution of anionic vs. neutral polymers to the formation of green rust 1 from γ-FeOOH bioreduction. Geomicrobiol. J. 2013, 30, 600–615. [Google Scholar] [CrossRef]
- Rémy, P.-P.; Etique, M.; Hazotte, A.A.; Sergent, A.-S.; Estrade, N.; Cloquet, C.; Hanna, K.; Jorand, F.P.A. Pseudo-first-order reaction of chemically and biologically formed green rusts with HgII and C15H15N3O2: Effects of pH and stabilizing agents (phosphate, silicate, polyacrylic acid, and bacterial cells). Water Res. 2015, 70, 266–278. [Google Scholar] [CrossRef] [PubMed]
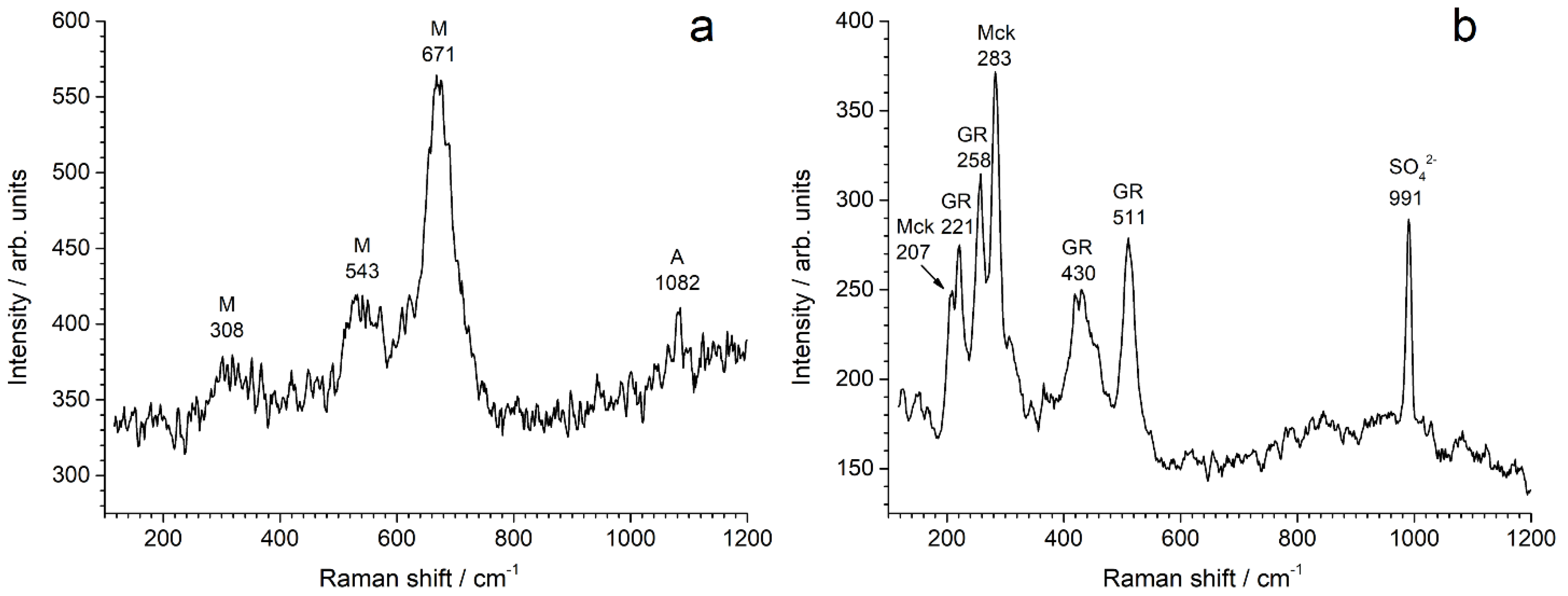

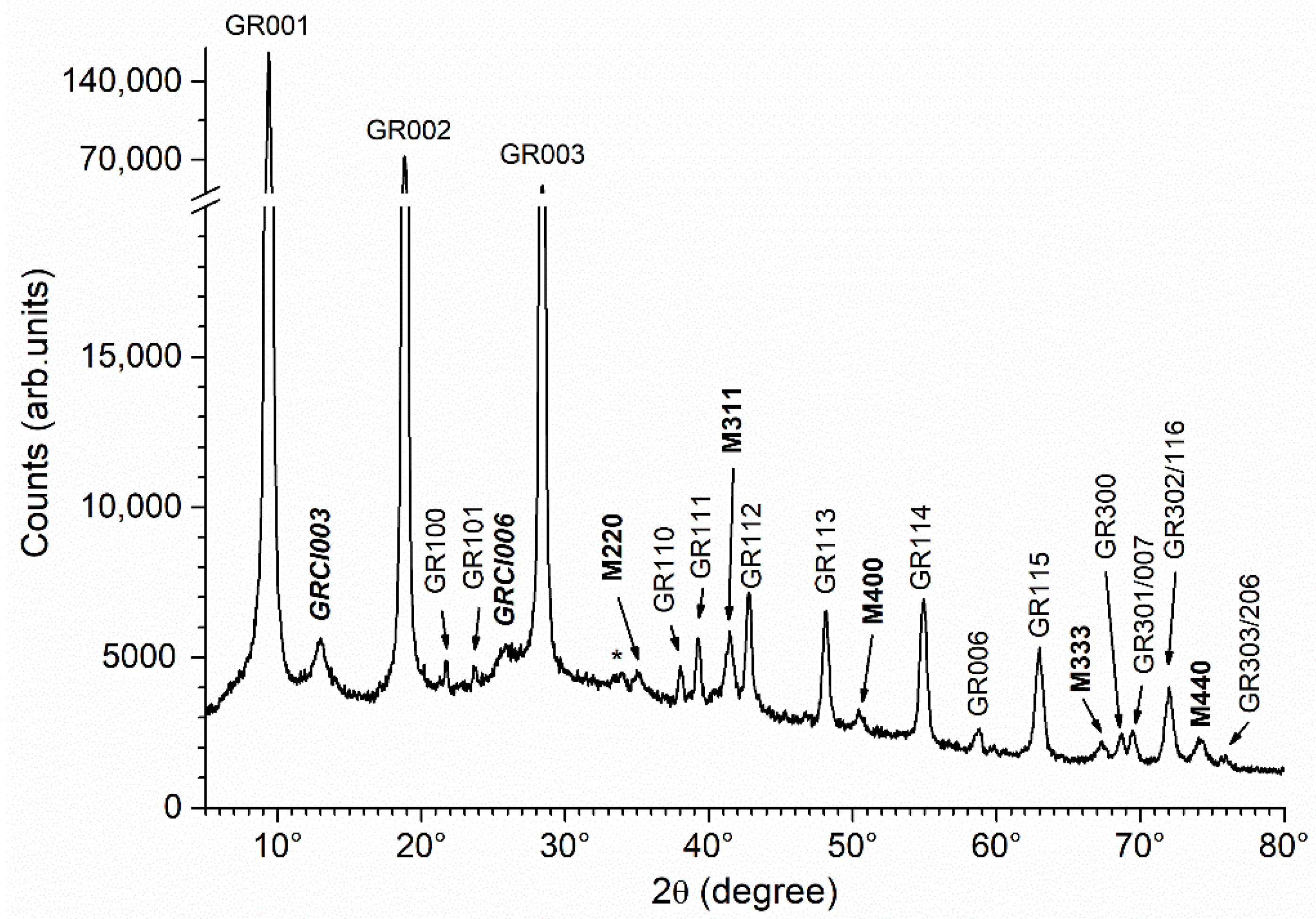

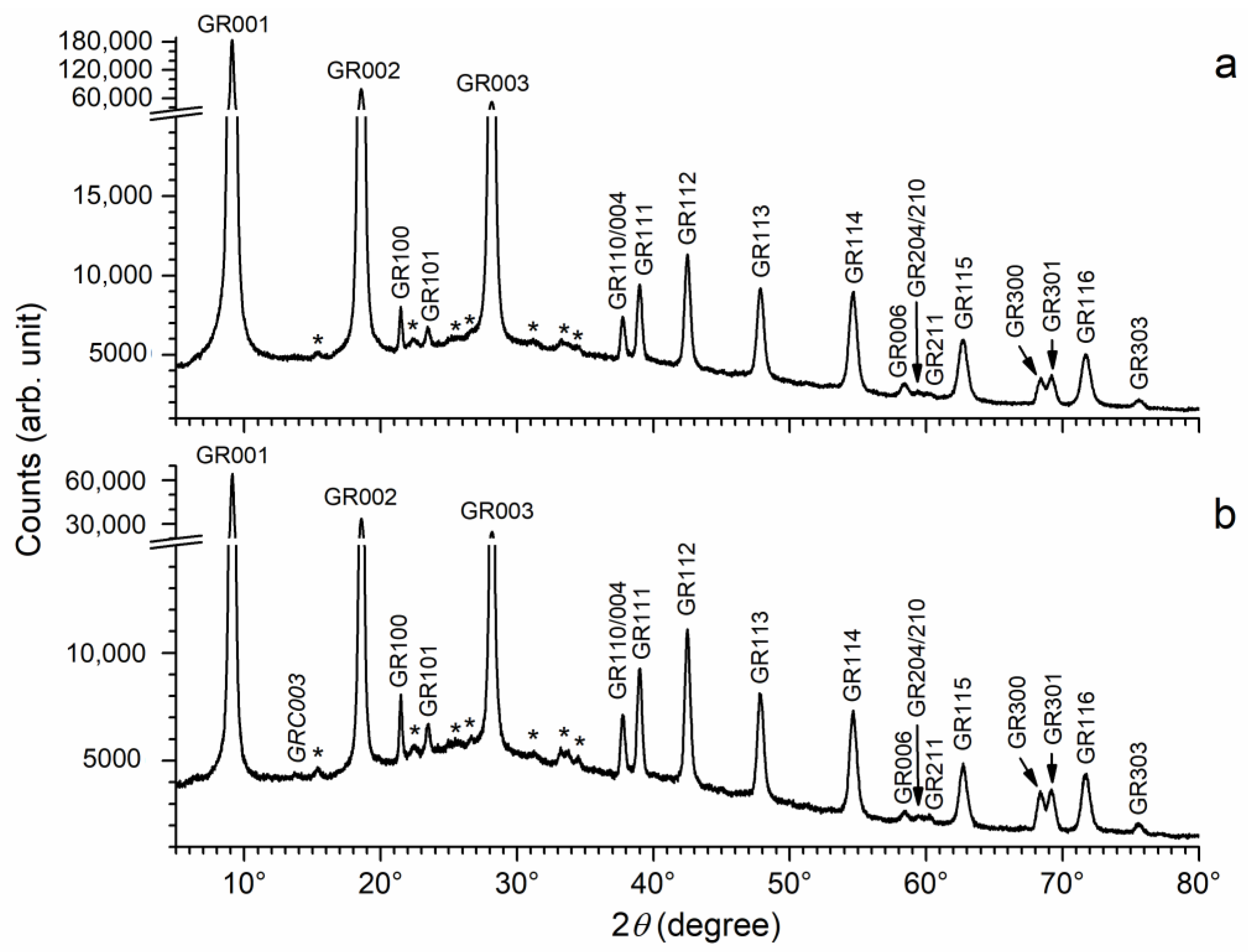
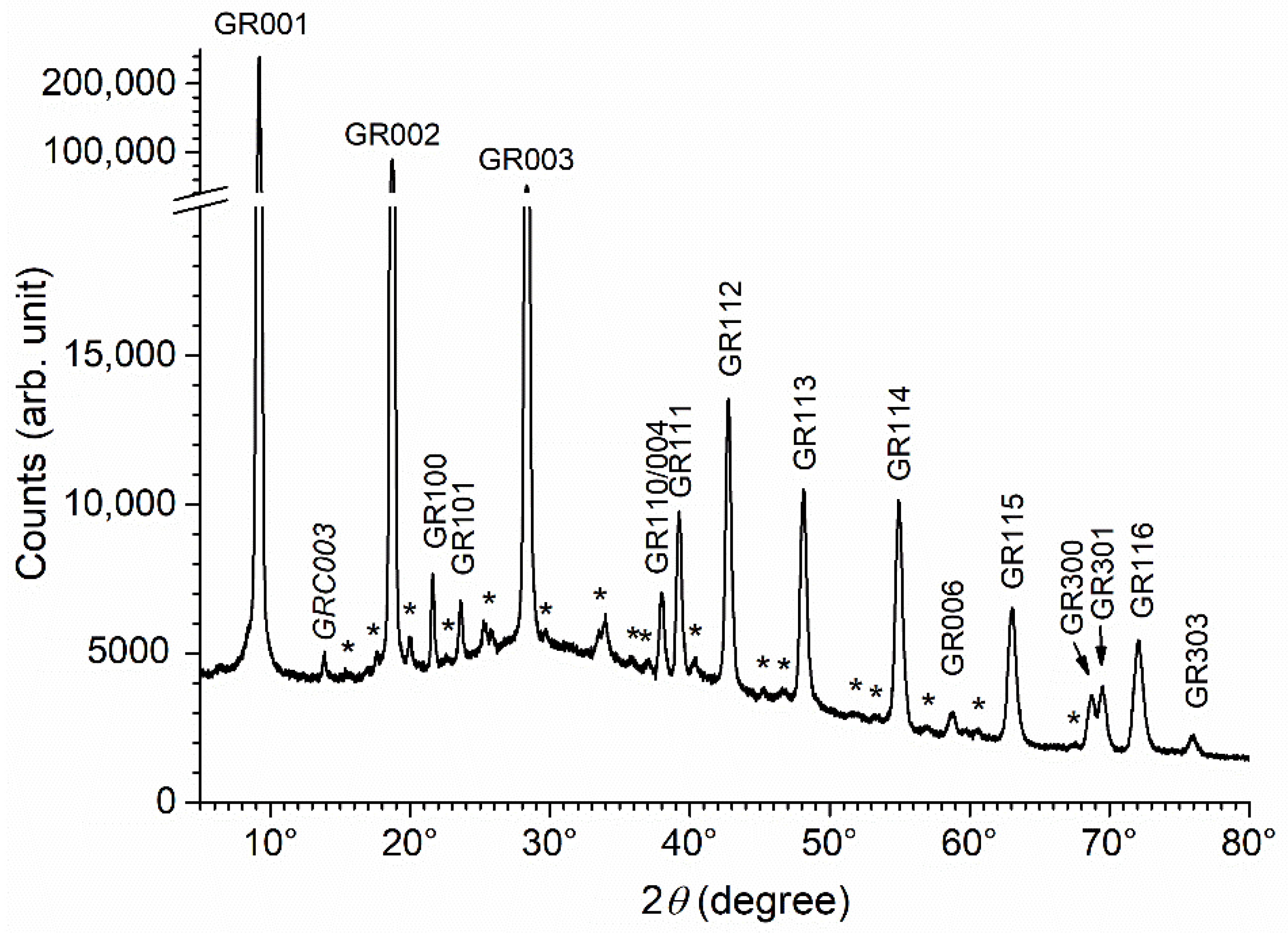
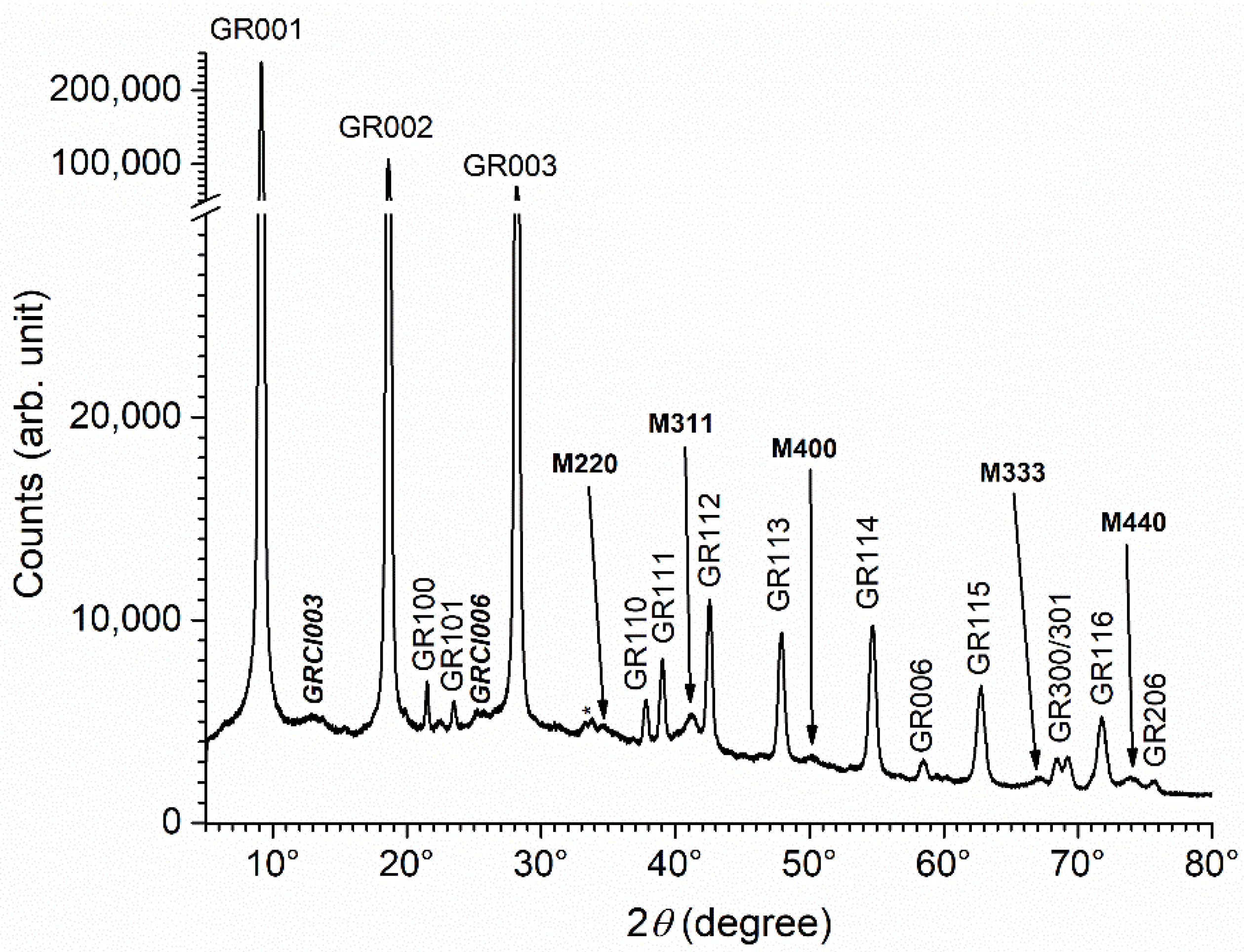
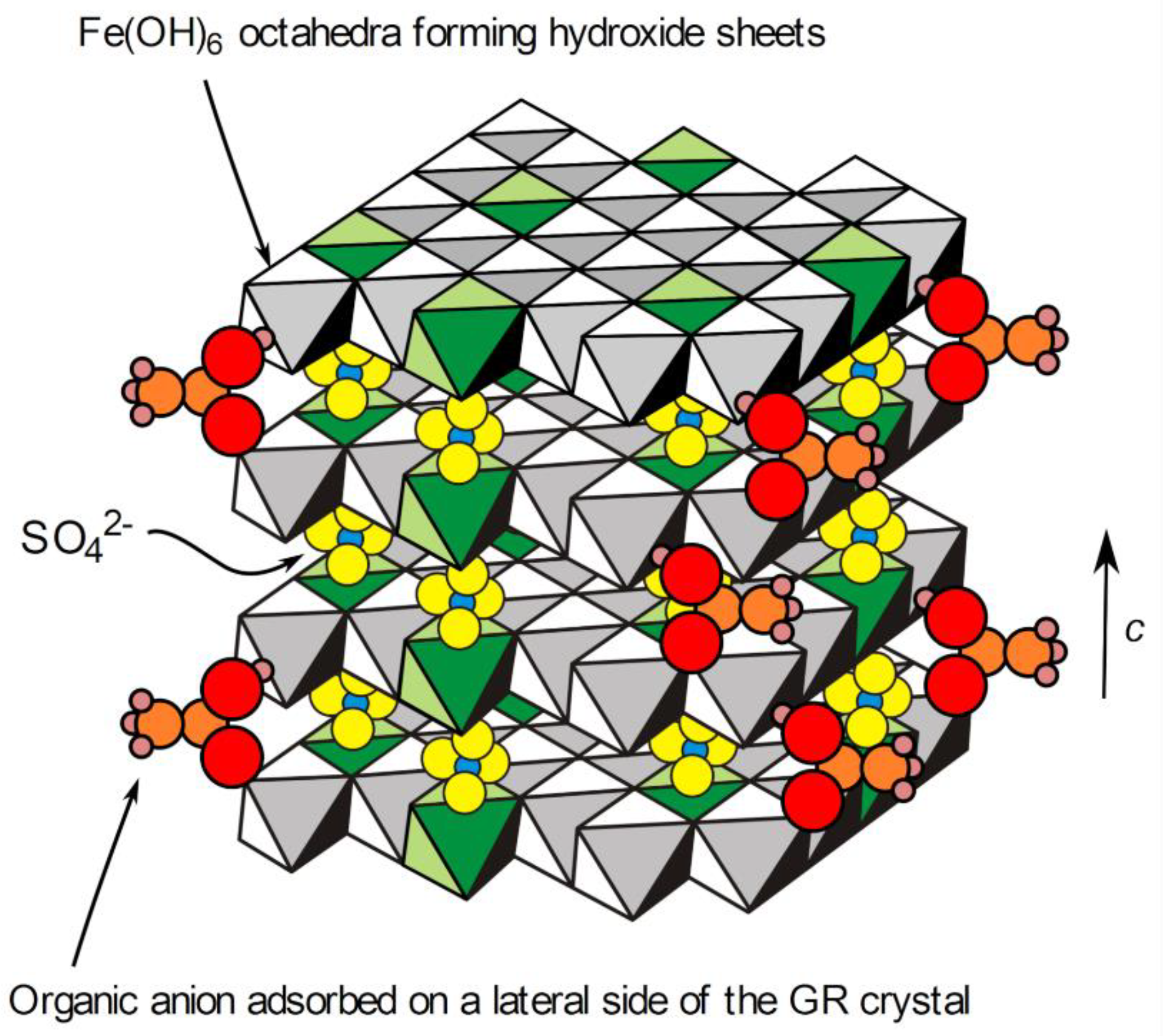
| Reactants | Solution 1 | Solution 2 |
|---|---|---|
| NaOH | 0.24 | - |
| NaCl | - | 0.27 |
| Na2SO4·10H2O | - | 0.03 |
| FeCl2·4H2O | - | 0.04 |
| FeCl3·6H2O | - | 0.08 |
| Bacteria 1 | No or Pseudoalteromonas IIIA004 or Micrococcus IVA008 or Bacillus IVA016 | - |
| NaCH3COO·3H2O | 0 or 0.06 | - |
| Sample | Main Components | Frequently Identified Components | Minor Components |
|---|---|---|---|
| Steel coupon in artificial seawater | Magnetite | Aragonite, Lepidocrocite | Chukanovite, Ferrihydrite, Green Rust |
| Steel coupon in natural seawater 1 | Magnetite, Mackinawite | Green Rust | Ferrihydrite |
| Diffraction Peak | 2θ | d | FWHM | I |
|---|---|---|---|---|
| GR001 | 9.33 ± 0.01 | 11.00 ± 0.01 | 0.27 ± 0.01 | 100 |
| GR112 | 42.73 ± 0.01 | 2.455 ± 0.001 | 0.45 ± 0.02 | 3.2 ± 0.2 |
| GRCl003 | 12.94 ± 0.01 | 7.94 ± 0.01 | 1.45 ± 0.02 | 7.0 ± 0.4 |
| M311 | 41.38 ± 0.01 | 2.532 ± 0.001 | 0.65 ± 0.02 | 4.6 ± 0.3 |
| Bacteria | Culture of 24 h in Marine Broth | Initial Concentrated Suspensions | After Precipitation | After 1 Week of Ageing: Supernatant/Precipitate |
|---|---|---|---|---|
| Pseudoalteromonas IIIA004 | 2.2 × 109 | >3 × 1011 | No growth | No growth/No growth |
| Micrococcus IVA008 | 3.3 × 109 | >3 × 1011 | 1.8 × 107 | No growth/3.0 × 108 |
| Bacillus IVA016 | 1.7 × 109 | >3 × 1011 | 1.3 × 105 | No growth/3.6 × 105 |
| Diffraction Peak | 2θ | d | FWHM | I |
|---|---|---|---|---|
| GR001 | 9.27 ± 0.01 | 11.07 ± 0.015 | 0.24 ± 0.01 | 100 |
| GR112 | 42.66 ± 0.01 | 2.459 ± 0.001 | 0.47 ± 0.02 | 5.9 ± 0.04 |
| GRCl003 | 12.91 ± 0.03 | 7.96 ± 0.02 | 1.6 ± 0.05 | 1.8 ± 0.01 |
| M311 | 41.38 ± 0.01 | 2.532 ± 0.001 | 0.99 ± 0.02 | 2.0 ± 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duboscq, J.; Vincent, J.; Jeannin, M.; Sabot, R.; Lanneluc, I.; Sablé, S.; Refait, P. Influence of Organic Matter/Bacteria on the Formation and Transformation of Sulfate Green Rust. Corros. Mater. Degrad. 2022, 3, 1-16. https://doi.org/10.3390/cmd3010001
Duboscq J, Vincent J, Jeannin M, Sabot R, Lanneluc I, Sablé S, Refait P. Influence of Organic Matter/Bacteria on the Formation and Transformation of Sulfate Green Rust. Corrosion and Materials Degradation. 2022; 3(1):1-16. https://doi.org/10.3390/cmd3010001
Chicago/Turabian StyleDuboscq, Julien, Julia Vincent, Marc Jeannin, René Sabot, Isabelle Lanneluc, Sophie Sablé, and Philippe Refait. 2022. "Influence of Organic Matter/Bacteria on the Formation and Transformation of Sulfate Green Rust" Corrosion and Materials Degradation 3, no. 1: 1-16. https://doi.org/10.3390/cmd3010001
APA StyleDuboscq, J., Vincent, J., Jeannin, M., Sabot, R., Lanneluc, I., Sablé, S., & Refait, P. (2022). Influence of Organic Matter/Bacteria on the Formation and Transformation of Sulfate Green Rust. Corrosion and Materials Degradation, 3(1), 1-16. https://doi.org/10.3390/cmd3010001







