Research Progress in Corrosion Mechanism of Reinforced Alkali-Activated Concrete Structures
Abstract
:1. Introduction
2. Carbonation and Chloride Ingress
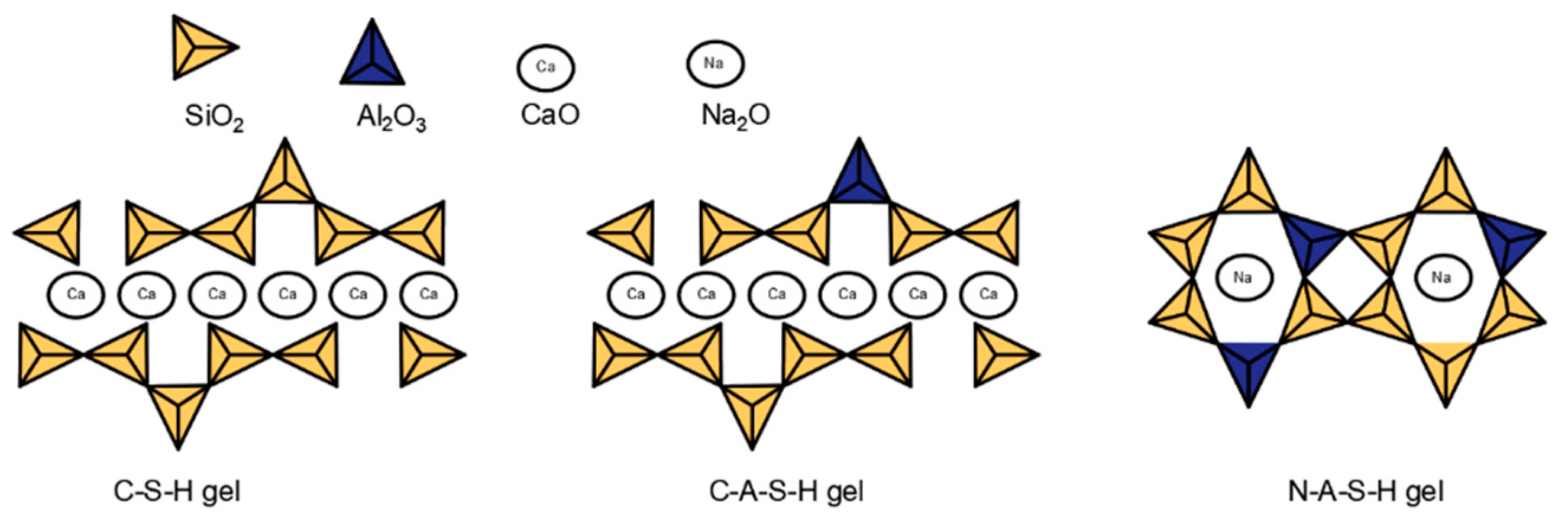
2.1. Composition and Microstructure
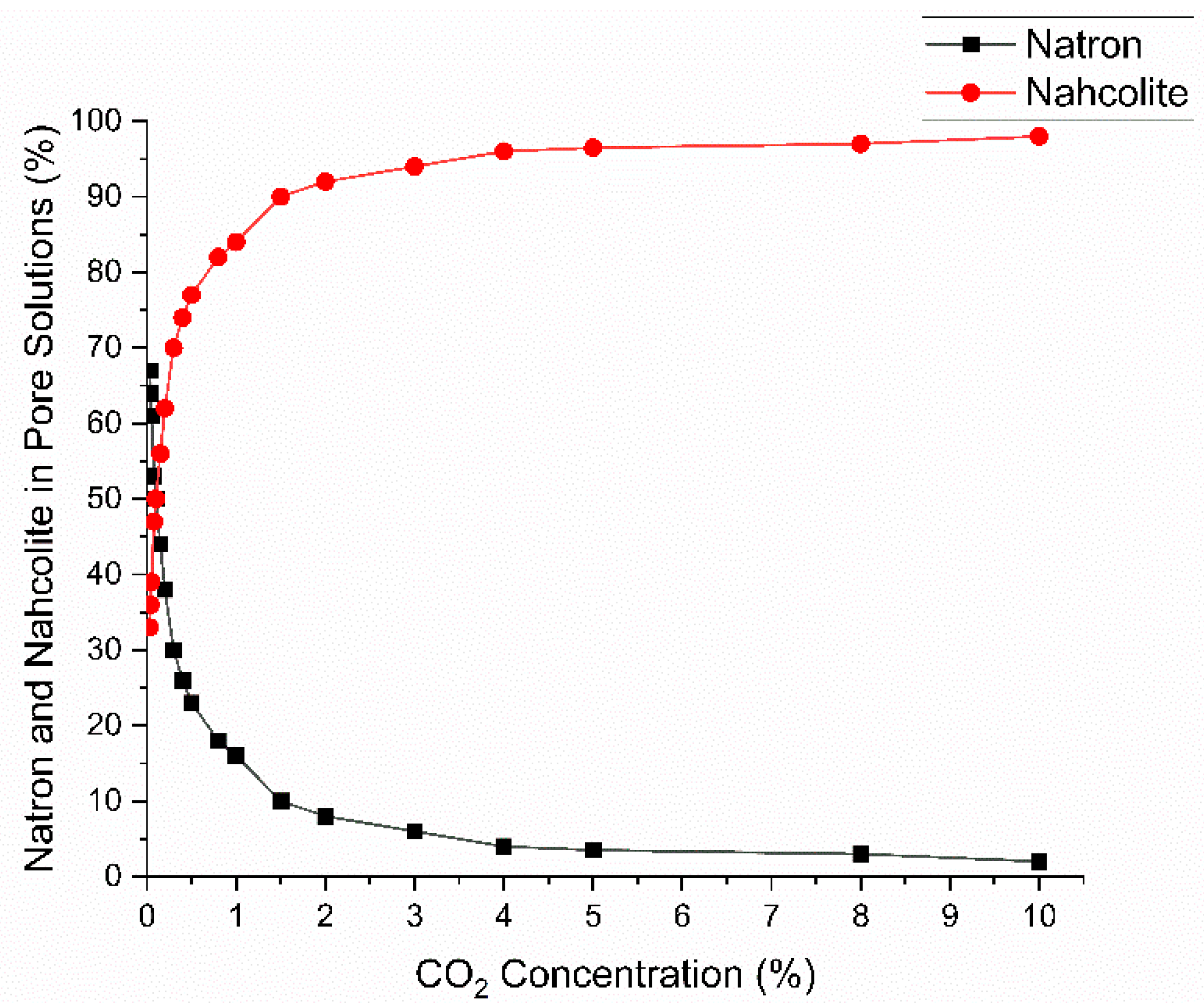
2.2. Carbonation
2.3. Chloride Penetration
2.4. Combined Corrosion Process of Carbonation and Chloride Ingress
3. Steel–AAM Concrete Interface
4. Electrochemical Measurement
4.1. Half-Cell Potential
| Reference | Material | Half-Cell Potential (Ecorr) | |
|---|---|---|---|
| Chloride Contaminated Sample | Non-Chloride Contaminated Sample | ||
| [95] | OPC + Metakaolin | N/A | Around −300 mVSCE |
| [27] | Fly ash | Around −600 mVSCE | −150 mVSCE to −200 mVSCE |
| [73] | Fly ash | N/A | −550 mVSCE to −600 mVSCE |
| [35] | Fly ash + Slag | N/A | −550 mVSCE to −650 mVSCE |
| [30] | Fly ash + Slag | −600 mVSCE to −700 mVSCE | −200 mVSCE to −400 mVSCE |
| [31] | Slag | Around −650 mVAgCl | Around −100 mVAgCl |
| [94] | Fly ash | −400 mVCSE to −600 mVCSE | Around −150 mVCSE |
| [33] | Natural pozzolan | N/A | −300 mVSCE to −600 mVSCE |
| [96] | Slag + Palm oil fuel ash + Rice husk ash Slag + fly ash +Rice husk ash | N/A | −230 mVCSE to −500 mVCSE |
4.2. Linear Polarization Resistance and Tafel Polarization
5. Research Gaps
6. Conclusions
- AAMs exhibit a good chloride resistance property but poorer carbonation resistance, in comparison with OPC. The mechanisms are well understood, especially on the material level, e.g., chloride binding, CH reaction with CO2, formation of new microstructure, etc. However, the long-term performance of the alkali-activated concrete structure under the combined carbonation and chloride ingress, and the underlying mechanism for the combined effect, need to be investigated.
- The interfacial properties of alkali-activated concrete are different from OPC concrete, including the physical and chemical aspects. This results in different corrosion accumulation and evolution. This is much less clear than the carbonation and chloride ingress and warrants further research.
- The current criteria for judging corrosion potential and measuring corrosion rates for OPC concrete cannot be directly used for the alkali-activated concrete structures. In some cases, the results are even controversial. To avoid inaccurate determination of corrosion state, new methods, perhaps, and new experimental data are urgently needed for application to alkali-activated concrete structures.
- In the context of a carbon neutral or net zero target in most countries, using alkali-activated waste materials to fully or partially replace the ordinary concrete seems a promising approach. In terms of known properties and mechanisms, the alkali-activated concrete structures may well be used in various infrastructure applications, including high corrosive marine environments.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ali, M.; Saidur, R.; Hossain, M. A Review on Emission Analysis in Cement Industries. Renew. Sustain. Energy Rev. 2011, 15, 2252–2261. [Google Scholar] [CrossRef]
- Koch, G.; Brongers, M.; Thompson, N.; Virmani, Y.P.; Payer, J. Corrosion Cost and Preventive Strategies in the United States; Federal Highway Administration: Washington, DC, USA, 2001.
- Provis, J.L. Geopolymers and other Alkali Activated Materials: Why, How, and What? Mater. Struct. 2013, 47, 11–25. [Google Scholar] [CrossRef]
- Awoyera, P.; Adesina, A. A Critical Review on Application of Alkali Activated Slag as a Sustainable Composite Binder. Case Stud. Constr. Mater. 2019, 11, e00268. [Google Scholar] [CrossRef]
- Pulselli, R.; Simoncini, E.; Ridolfi, R.; Bastianoni, S. Specific Emergy of Cement and Concrete: An Energy-Based Appraisal of Building Materials and their Transport. Ecol. Indic. 2008, 8, 647–656. [Google Scholar] [CrossRef]
- Yang, K.-H.; Song, J.-K.; Song, K.-I. Assessment of CO2 Reduction of Alkali-Activated Concrete. J. Clean. Prod. 2013, 39, 265–272. [Google Scholar] [CrossRef]
- Duxson, P.; Provis, J.; Lukey, G.C.; Van Deventer, J.S. The Role of Inorganic Polymer Technology in the Development of ‘Green Concrete’. Cem. Concr. Res. 2007, 37, 1590–1597. [Google Scholar] [CrossRef]
- Duxson, P.; Provis, J.; Lukey, G.C.; Mallicoat, S.W.; Kriven, W.M.; van Deventer, J.S. Understanding the Relationship between Geopolymer Composition, Microstructure and Mechanical Properties. Colloids Surf. A Physicochem. Eng. Asp. 2005, 269, 47–58. [Google Scholar] [CrossRef]
- Guo, X.; Shi, H.; Dick, W.A. Compressive Strength and Microstructural Characteristics of Class C Fly Ash Geopolymer. Cem. Concr. Compos. 2010, 32, 142–147. [Google Scholar] [CrossRef]
- Mo, K.H.; Alengaram, U.J.; Jumaat, M.Z. Structural Performance of Reinforced Geopolymer Concrete Members: A review. Constr. Build. Mater. 2016, 120, 251–264. [Google Scholar] [CrossRef]
- Sofi, M.; van Deventer, J.; Mendis, P.; Lukey, G. Engineering Properties of Inorganic Polymer Concretes (IPCs). Cem. Concr. Res. 2007, 37, 251–257. [Google Scholar] [CrossRef]
- Aperador, W.; De Gutierrez, R.M.; Bastidas, D.M. Steel Corrosion Behaviour in Carbonated Alkali-Activated Slag Concrete. Corros. Sci. 2009, 51, 2027–2033. [Google Scholar] [CrossRef] [Green Version]
- Bernal, A.S.; Provis, J.; Walkley, B.; Nicolas, R.S.; Gehman, J.D.; Brice, D.G.; Kilcullen, A.R.; Duxson, P.; Van Deventer, J.S. Gel Nanostructure in Alkali-Activated Binders Based on Slag and Fly Ash, and Effects of Accelerated Carbonation. Cem. Concr. Res. 2013, 53, 127–144. [Google Scholar] [CrossRef]
- Zhao, K.; Liang, Y.; Ji, T.; Lu, Y.; Lin, X. Effect of Activator Types and Concentration of CO2 on the Steel Corrosion in the Carbonated Alkali-Activated Slag Concrete. Constr. Build. Mater. 2020, 262, 120044. [Google Scholar] [CrossRef]
- Bakharev, T.; Sanjayan, J.; Cheng, Y.-B. Resistance of Alkali-Activated Slag Concrete to Carbonation. Cem. Concr. Res. 2001, 31, 1277–1283. [Google Scholar] [CrossRef]
- Bernal, S.A.; Nicolas, R.S.; Provis, J.; De Gutierrez, R.M.; Van Deventer, J.S.J. Natural carbonation of aged alkali-activated slag concretes. Mater. Struct. 2014, 47, 693–707. [Google Scholar] [CrossRef]
- Bernal, S.A.; Provis, J.L.; Brice, D.G.; Kilcullen, A.; Duxson, P.; van Deventer, J.S. Accelerated Carbonation Testing of Alkali-Activated Binders Significantly Underestimates Service Life: The Role of Pore Solution Chemistry. Cem. Concr. Res. 2012, 42, 1317–1326. [Google Scholar] [CrossRef]
- Bernal, A.S.; Provis, J.L.; De Gutierrez, R.M.; Van Deventer, J.S.J. Accelerated Carbonation Testing of Alkali-Activated Slag/Metakaolin Blended Concretes: Effect of Exposure Conditions. Mater. Struct. 2015, 48, 653–669. [Google Scholar] [CrossRef] [Green Version]
- Babaee, M.; Khan, M.; Castel, A. Passivity of Embedded Reinforcement in Carbonated Low-Calcium Fly Ash-Based Geopolymer Concrete. Cem. Concr. Compos. 2018, 85, 32–43. [Google Scholar] [CrossRef]
- Salazar, R.A.R.; Aguirre-Guerrero, A.M.; de Gutiérrez, R.M. Carbonation-Induced Corrosion of Alkali-Activated Binary Concrete Based on Natural Volcanic Pozzolan. Constr. Build. Mater. 2020, 232, 117189. [Google Scholar] [CrossRef]
- Khan, M.; Castel, A. Effect of MgO and Na2SiO3 on the Carbonation Resistance of Alkali Activated Slag Concrete. Mag. Concr. Res. 2018, 70, 685–692. [Google Scholar] [CrossRef]
- Santarsiero, G.; Masi, A.; Picciano, V. Durability of Gerber Saddles in RC Bridges: Analyses and Applications (Musmeci Bridge, Italy). Infrastructures 2021, 6, 25. [Google Scholar] [CrossRef]
- Bouteiller, V.; Cremona, C.; Baroghel-Bouny, V.; Maloula, A. Corrosion Initiation of Reinforced Concretes based on Portland or GGBS cements: Chloride Contents and Electrochemical Characterizations Versus Time. Cem. Concr. Res. 2012, 42, 1456–1467. [Google Scholar] [CrossRef]
- Bernal, A.S.; Provis, J.L. Durability of Alkali-Activated Materials: Progress and Perspectives. J. Am. Ceram. Soc. 2014, 97, 997–1008. [Google Scholar] [CrossRef]
- Provis, J.; Palomo, A.; Shi, C. Advances in Understanding Alkali-Activated Materials. Cem. Concr. Res. 2015, 78, 110–125. [Google Scholar] [CrossRef]
- Choi, Y.-S.; Kim, J.-G.; Lee, K.-M. Corrosion Behavior of Steel Bar Embedded in Fly Ash Concrete. Corros. Sci. 2006, 48, 1733–1745. [Google Scholar] [CrossRef]
- Miranda, J.; Fernández-Jiménez, A.; González, J.; Palomo, A. Corrosion Resistance in Activated Fly Ash Mortars. Cem. Concr. Res. 2005, 35, 1210–1217. [Google Scholar] [CrossRef]
- Khan, M.; Kayali, O.; Troitzsch, U. Chloride Binding Capacity of Hydrotalcite and the Competition with Carbonates in Ground Granulated Blast Furnace Slag Concrete. Mater. Struct. 2016, 49, 4609–4619. [Google Scholar] [CrossRef]
- Andrade, C.; Alonso, C. Test Methods for On-Site Corrosion Rate Measurement of Steel Reinforcement in Concrete by Means of the Polarization Resistance Method. Mater. Struct. 2004, 37, 623–643. [Google Scholar] [CrossRef]
- Tennakoon, C.; Shayan, A.; Sanjayan, J.; Xu, A. Chloride Ingress and Steel Corrosion in Geopolymer Concrete Based on Long Term Tests. Mater. Des. 2017, 116, 287–299. [Google Scholar] [CrossRef]
- Criado, M.; Provis, J.L. Alkali Activated Slag Mortars Provide High Resistance to Chloride-Induced Corrosion of Steel. Front. Mater. 2018, 5. [Google Scholar] [CrossRef]
- Mundra, S.; Criado, M.; Bernal, A.S.; Provis, J.L. Chloride-Induced Corrosion of Steel Rebars in Simulated Pore Solutions of Alkali-Activated Concretes. Cem. Concr. Res. 2017, 100, 385–397. [Google Scholar] [CrossRef]
- Ibrahim, M.; Rahman, M.K.; Johari, M.A.M.; Nasir, M.; Oladapo, E.A. Chloride Diffusion and Chloride-Induced Corrosion of Steel Embedded in Natural Pozzolan-Based Alkali Activated Concrete. Constr. Build. Mater. 2020, 262, 120669. [Google Scholar] [CrossRef]
- ASTM C876-15, Standard Test Method for Corrosion Potentials of Uncoated Reinforcing Steel in Concrete; ASTM International: West Conshohocken, PA, USA, 2015. [CrossRef]
- Babaee, M.; Castel, A. Chloride-Induced Corrosion of Reinforcement in Low-Calcium Fly Ash-Based Geopolymer Concrete. Cem. Concr. Res. 2016, 88, 96–107. [Google Scholar] [CrossRef]
- Andrade, C.; Alonso, C. Corrosion Rate Monitoring in the Laboratory and On-Site. Constr. Build. Mater. 1996, 10, 315–328. [Google Scholar] [CrossRef]
- Feng, X.; Tang, Y.; Zuo, Y. Influence of Stress on Passive Behaviour of Steel Bars in Concrete Pore Solution. Corros. Sci. 2011, 53, 1304–1311. [Google Scholar] [CrossRef]
- Ye, H.; Cartwright, C.; Rajabipour, F.; Radlińska, A. Understanding the Drying Shrinkage Performance of Alkali-Activated Slag Mortars. Cem. Concr. Compos. 2017, 76, 13–24. [Google Scholar] [CrossRef] [Green Version]
- Mastali, M.; Kinnunen, P.; Dalvand, A.; Firouz, R.M.; Illikainen, M. Drying Shrinkage in Alkali-Activated Binders—A Critical Review. Constr. Build. Mater. 2018, 190, 533–550. [Google Scholar] [CrossRef]
- Juenger, M.; Winnefeld, F.; Provis, J.; Ideker, J. Advances in Alternative Cementitious Binders. Cem. Concr. Res. 2011, 41, 1232–1243. [Google Scholar] [CrossRef]
- Shi, W.; Najimi, M.; Shafei, B. Reinforcement Corrosion and Transport of Water and Chloride Ions in Shrinkage-Compensating Cement Concretes. Cem. Concr. Res. 2020, 135, 106121. [Google Scholar] [CrossRef]
- ASTM C618-19, Standard Specification for Coal Fly Ash and Raw or Calcined Natural Pozzolan for Use in Concrete; ASTM International: West Conshohocken, PA, USA, 2019. [CrossRef]
- Bernal, A.S.; DE Gutierrez, R.M.; Pedraza, A.L.; Provis, J.; Rodriguez, E.; Delvasto, S. Effect of Binder Content on the Performance of Alkali-Activated Slag Concretes. Cem. Concr. Res. 2011, 41, 1–8. [Google Scholar] [CrossRef]
- Provis, J.L.; Bernal, S.A. Geopolymers and Related Alkali-Activated Materials. Annu. Rev. Mater. Res. 2014, 44, 299–327. [Google Scholar] [CrossRef]
- García-Lodeiro, I.; Fernández-Jiménez, A.; Palomo, A. Variation in Hybrid Cements Over Time. Alkaline Activation of Fly Ash–Portland Cement Blends. Cem. Concr. Res. 2013, 52, 112–122. [Google Scholar] [CrossRef]
- Provis, J.; Myers, R.; White, C.; Rose, V.; van Deventer, J.S. X-ray Microtomography Shows Pore Structure and Tortuosity in Alkali-Activated Binders. Cem. Concr. Res. 2012, 42, 855–864. [Google Scholar] [CrossRef]
- Sindhunata; van Deventer, J.S.J.; Lukey, G.C.; Xu, H. Effect of Curing Temperature and Silicate Concentration on Fly-Ash-Based Geopolymerization. Ind. Eng. Chem. Res. 2006, 45, 3559–3568. [Google Scholar] [CrossRef]
- Hardjito, D.; Wallah, S.E.; Sumajouw, D.M.J.; Rangan, B.V. On the Development of Fly Ash-Based Geopolymer Concrete. ACI Mater. J. 2004, 101, 467–472. [Google Scholar]
- Fang, S.; Lam, E.S.S.; Li, B.; Wu, B. Effect of Alkali Contents, Moduli and Curing Time on Engineering Properties of Alkali Activated Slag. Constr. Build. Mater. 2020, 249, 118799. [Google Scholar] [CrossRef]
- Noushini, A.; Castel, A. The Effect of Heat-Curing on Transport Properties of Low-Calcium Fly Ash-Based Geopolymer Concrete. Constr. Build. Mater. 2016, 112, 464–477. [Google Scholar] [CrossRef]
- Mangat, P.; Ojedokun, O. Influence of Curing on Pore Properties and Strength of Alkali Activated Mortars. Constr. Build. Mater. 2018, 188, 337–348. [Google Scholar] [CrossRef] [Green Version]
- Sun, Z.; Vollpracht, A. Isothermal Calorimetry and In-Situ XRD Study of the Naoh Activated Fly Ash, Metakaolin and Slag. Cem. Concr. Res. 2018, 103, 110–122. [Google Scholar] [CrossRef]
- Ma, Y.; Hu, J.; Ye, G. The Pore Structure and Permeability of Alkali Activated Fly Ash. Fuel 2013, 104, 771–780. [Google Scholar] [CrossRef]
- Bernal, A.S.; DE Gutierrez, R.M.; Provis, J.; Rose, V. Effect of Silicate Modulus and Metakaolin Incorporation on the Carbonation of Alkali Silicate-Activated Slags. Cem. Concr. Res. 2010, 40, 898–907. [Google Scholar] [CrossRef]
- Shi, J.; Ming, J. Influence of Defects at the Steel-Mortar Interface on the Corrosion Behavior of Steel. Constr. Build. Mater. 2017, 136, 118–125. [Google Scholar] [CrossRef]
- Saetta, A.; Schrefler, B.A.; Vitaliani, R.V. The Carbonation of Concrete and the Mechanism of Moisture, Heat and Carbon Dioxide Flow through Porous Materials. Cem. Concr. Res. 1993, 23, 761–772. [Google Scholar] [CrossRef]
- Shi, J.; Wu, M.; Ming, J. Long-Term Corrosion Resistance of Reinforcing Steel in Alkali-Activated Slag Mortar after Exposure to Marine Environments. Corros. Sci. 2021, 179, 109175. [Google Scholar] [CrossRef]
- Leemann, A.; Moro, F. Carbonation of Concrete: The Role of CO2 Concentration, Relative Humidity and CO2 Buffer Capacity. Mater. Struct. 2017, 50, 1–14. [Google Scholar] [CrossRef]
- Galan, I.; Andrade, C.; Castellote, M. Natural and Accelerated CO2 Binding Kinetics in Cement Paste at Different Relative Humidities. Cem. Concr. Res. 2013, 49, 21–28. [Google Scholar] [CrossRef]
- Nedeljković, M.; Ghiassi, B.; van der Laan, S.; Li, Z.; Ye, G. Effect of Curing Conditions on the Pore Solution and Carbonation Resistance of Alkali-Activated Fly Ash and Slag Pastes. Cem. Concr. Res. 2019, 116, 146–158. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Provis, J.L.; van Deventer, J.S.J.; Krivenko, P.V. Characterization of Aged Slag Concretes. ACI Mater. J. 2008, 105, 131–139. [Google Scholar] [CrossRef]
- Pasupathy, K.; Berndt, M.; Castel, A.; Sanjayan, J.; Pathmanathan, R. Carbonation of a Blended Slag-Fly Ash Geopolymer Concrete in Field Conditions After 8 Years. Constr. Build. Mater. 2016, 125, 661–669. [Google Scholar] [CrossRef]
- Bernal, A.S.; Nicolas, R.S.; Myers, R.; De Gutierrez, R.M.; Puertas, F.; van Deventer, J.S.; Provis, J. MgO content of Slag Controls Phase Evolution and Structural Changes Induced by Accelerated Carbonation in Alkali-Activated Binders. Cem. Concr. Res. 2014, 57, 33–43. [Google Scholar] [CrossRef] [Green Version]
- Mo, L.; Panesar, D.K. Accelerated Carbonation—A Potential Approach to Sequester CO2 in Cement Paste Containing Slag and Reactive MgO. Cem. Concr. Compos. 2013, 43, 69–77. [Google Scholar] [CrossRef]
- Shi, Z.; Shi, C.; Wan, S.; Li, N.; Zhang, Z. Effect of Alkali Dosage and Silicate Modulus on Carbonation of Alkali-Activated Slag Mortars. Cem. Concr. Res. 2018, 113, 55–64. [Google Scholar] [CrossRef]
- Ismail, I.; Bernal, A.S.; Provis, J.; Nicolas, R.S.; Brice, D.G.; Kilcullen, A.R.; Hamdan, S.; van Deventer, J.S. Influence of Fly Ash on the Water and Chloride Permeability of Alkali-Activated Slag Mortars and Concretes. Constr. Build. Mater. 2013, 48, 1187–1201. [Google Scholar] [CrossRef]
- Babaee, M.; Castel, A. Chloride Diffusivity, Chloride Threshold, and Corrosion Initiation in Reinforced Alkali-Activated Mortars: Role of Calcium, Alkali, And Silicate Content. Cem. Concr. Res. 2018, 111, 56–71. [Google Scholar] [CrossRef]
- Wang, S.-D.; Scrivener, K.L. Hydration Products of Alkali Activated Slag Cement. Cem. Concr. Res. 1995, 25, 561–571. [Google Scholar] [CrossRef]
- Ben Haha, M.; Lothenbach, B.; LE Saout, G.; Winnefeld, F. Influence of Slag Chemistry on the Hydration of Alkali-Activated Blast-Furnace Slag—Part I: Effect of MgO. Cem. Concr. Res. 2011, 41, 955–963. [Google Scholar] [CrossRef]
- Ye, H.; Huang, L.; Chen, Z. Influence of Activator Composition on The Chloride Binding Capacity of Alkali-Activated Slag. Cem. Concr. Compos. 2019, 104. [Google Scholar] [CrossRef]
- Ben Haha, M.; Lothenbach, B.; LE Saout, G.; Winnefeld, F. Influence of Slag Chemistry on the Hydration of Alkali-Activated Blast-Furnace Slag—Part II: Effect of Al2O3. Cem. Concr. Res. 2012, 42, 74–83. [Google Scholar] [CrossRef]
- Kupwade-Patil, K.; Allouche, E.N. Examination of Chloride-Induced Corrosion in Reinforced Geopolymer Concretes. J. Mater. Civ. Eng. 2013, 25, 1465–1476. [Google Scholar] [CrossRef]
- Monticelli, C.; Natali, M.; Balbo, A.; Chiavari, C.; Zanotto, F.; Manzi, S.; Bignozzi, M. Corrosion Behavior of Steel in Alkali-Activated Fly Ash Mortars in the Light of their Microstructural, Mechanical and Chemical Characterization. Cem. Concr. Res. 2016, 80, 60–68. [Google Scholar] [CrossRef]
- Monticelli, C.; Natali, M.; Balbo, A.; Chiavari, C.; Zanotto, F.; Manzi, S.; Bignozzi, M. A Study on the Corrosion of Reinforcing Bars in Alkali-Activated Fly Ash Mortars under Wet and Dry Exposures to Chloride Solutions. Cem. Concr. Res. 2016, 87, 53–63. [Google Scholar] [CrossRef]
- Noushini, A.; Castel, A.; Aldred, J.; Rawal, A. Chloride Diffusion Resistance and Chloride Binding Capacity of Fly Ash-Based Geopolymer Concrete. Cem. Concr. Compos. 2020, 105, 103290. [Google Scholar] [CrossRef]
- Palomo, A.; Grutzeck, M.; Blanco-Varela, M.T. Alkali-Activated Fly Ashes: A Cement for the Future. Cem. Concr. Res. 1999, 29, 1323–1329. [Google Scholar] [CrossRef]
- Chindaprasirt, P.; Chalee, W. Effect of Sodium Hydroxide Concentration on Chloride Penetration and Steel Corrosion of Fly Ash-Based Geopolymer Concrete under Marine Site. Constr. Build. Mater. 2014, 63, 303–310. [Google Scholar] [CrossRef]
- Chang, H. Chloride Binding Capacity of Pastes Influenced by Carbonation under Three Conditions. Cem. Concr. Compos. 2017, 84, 1–9. [Google Scholar] [CrossRef]
- Ke, X.; Bernal, S.A.; Provis, J.L. Uptake of Chloride and Carbonate by Mg-Al and Ca-Al Layered Double Hydroxides In Simulated Pore Solutions of Alkali-Activated Slag Cement. Cem. Concr. Res. 2017, 100, 1–13. [Google Scholar] [CrossRef]
- Zheng, Y.; Russell, M.; Davis, G.; McPolin, D.; Yang, K.; Basheer, P.; Nanukuttan, S. Influence of Carbonation on the Bound Chloride Concentration in Different Cementitious Systems. Constr. Build. Mater. 2021, 302, 124171. [Google Scholar] [CrossRef]
- Sun, M.; Sun, C.; Zhang, P.; Liu, N.; Li, Y.; Duan, J.; Hou, B. Influence of Carbonation on Chloride Binding of Mortars Made with Simulated Marine Sand. Constr. Build. Mater. 2021, 303, 124455. [Google Scholar] [CrossRef]
- Shi, J.; Ming, J.; Sun, W. Electrochemical Behaviour of a Novel Alloy Steel in Alkali-Activated Slag Mortars. Cem. Concr. Compos. 2018, 92, 110–124. [Google Scholar] [CrossRef]
- Shi, J.; Ming, J.; Sun, W. Electrochemical Performance of Reinforcing Steel in Alkali-Activated Slag Extract in the Presence of Chlorides. Corros. Sci. 2018, 133, 288–299. [Google Scholar] [CrossRef]
- Shi, J.; Ming, J.; Wu, M. Passivation and Corrosion Behavior of 2304 Duplex Stainless Steel in Alkali-Activated Slag Materials. Cem. Concr. Compos. 2020, 108, 103532. [Google Scholar] [CrossRef]
- Angst, U.M.; Geiker, M.R.; Alonso, M.C.; Polder, R.; Isgor, O.B.; Elsener, B.; Wong, H.; Michel, A.; Hornbostel, K.; Gehlen, C.; et al. The Effect of the Steel–Concrete Interface on Chloride-Induced Corrosion Initiation in Concrete: A Critical Review by RILEM TC 262-SCI. Mater. Struct. 2019, 52, 1–25. [Google Scholar] [CrossRef]
- Chen, R.; Hu, J.; Ma, Y.; Guo, W.; Huang, H.; Wei, J.; Yin, S.; Yu, Q. Characterization of the Passive Film Formed on the Reinforcement Surface in Alkali Activated Fly Ash: Surface Analysis and Electrochemical Evaluation. Corros. Sci. 2020, 165, 108393. [Google Scholar] [CrossRef]
- You, N.; Shi, J.; Zhang, Y. Corrosion Behaviour of Low-Carbon Steel Reinforcement in Alkali-Activated Slag-Steel Slag and Portland Cement-Based Mortars under Simulated Marine Environment. Corros. Sci. 2020, 175, 108874. [Google Scholar] [CrossRef]
- Scott, A.; Alexander, M. Effect of Supplementary Cementitious Materials (Binder Type) on the Pore Solution Chemistry and the Corrosion of Steel in Alkaline Environments. Cem. Concr. Res. 2016, 89, 45–55. [Google Scholar] [CrossRef]
- Mangat, P.; Ojedokun, O.O.; Lambert, P. Chloride-Initiated Corrosion in Alkali Activated Reinforced Concrete. Cem. Concr. Compos. 2021, 115, 103823. [Google Scholar] [CrossRef]
- Elsener, B.; Andrade, C.; Gulikers, J.; Polder, R.; Raupach, M. Half-Cell Potential Measurements—Potential Mapping on Reinforced Concrete Structures. Mater. Struct. 2003, 36, 461–471. [Google Scholar] [CrossRef]
- Lv, W.; Sun, Z.; Su, Z. Study of Seawater Mixed One-Part Alkali Activated GGBFS-Fly Ash. Cem. Concr. Compos. 2020, 106. [Google Scholar] [CrossRef]
- Elsener, B. Half-Cell Potential Mapping to Assess Repair Work on RC structures. Constr. Build. Mater. 2001, 15, 133–139. [Google Scholar] [CrossRef]
- Criado, M.; Bernal, S.A.; Trinanes, P.G.; Provis, J.L. Influence of Slag Composition on the Stability of Steel in Alkali-Activated Cementitious Materials. J. Mater. Sci. 2018, 53, 5016–5035. [Google Scholar] [CrossRef] [Green Version]
- Gunasekara, C.; Law, D.; Bhuiyan, S.; Setunge, S.; Ward, L. Chloride Induced Corrosion in Different Fly Ash Based Geopolymer Concretes. Constr. Build. Mater. 2018, 200, 502–513. [Google Scholar] [CrossRef]
- Batis, G.; Pantazopoulou, P.; Tsivilis, S.; Badogiannis, E. The Effect of Metakaolin on the Corrosion Behavior of Cement Mortars. Cem. Concr. Compos. 2005, 27, 125–130. [Google Scholar] [CrossRef]
- Hossain, M.; Karim, M.; Elahi, M.; Islam, M.; Zain, M. Long-Term Durability Properties of Alkali-Activated Binders Containing Slag, Fly Ash, Palm Oil Fuel Ash and Rice Husk Ash. Constr. Build. Mater. 2020, 251, 119094. [Google Scholar] [CrossRef]
- Buchanan, R.A.; Stansbury, E.E. Electrochemical Corrosion. In Handbook of Environmental Degradation of Materials, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 87–125. [Google Scholar]
- Andrade, F.D.C.; González, J.A. Quantitative Measurements of Corrosion Rate of Reinforcing Steels Embedded in Concrete Using Polarization Resistance Measurements. Mater. Corros. 1978, 29, 515–519. [Google Scholar] [CrossRef]
- Elsener, B. Corrosion Rate of Steel in Concrete—Measurements Beyond the Tafel Law. Corros. Sci. 2005, 47, 3019–3033. [Google Scholar] [CrossRef]
- Ge, J.; Isgor, O. Effects of Tafel Slope, Exchange Current Density and Electrode Potential on the Corrosion of Steel in Concrete. Mater. Corros. 2007, 58, 573–582. [Google Scholar] [CrossRef]
- Chang, Z.-T.; Cherry, B.; Marosszeky, M. Polarisation Behaviour of Steel Bar Samples in Concrete in Seawater. Part 1: Experimental Measurement of Polarisation Curves of Steel in Concrete. Corros. Sci. 2008, 50, 357–364. [Google Scholar] [CrossRef]
- Pour-Ghaz, M.; Isgor, B.; Ghods, P. The Effect of Temperature on the Corrosion of Steel in Concrete. Part 1: Simulated Polarization Resistance Tests and Model Development. Corros. Sci. 2009, 51, 415–425. [Google Scholar] [CrossRef]
- Valencia-Saavedra, W.G.; Aguirre-Guerrero, A.M.; De Gutiérrez, R.M. Alkali-Activated Concretes Based on High Unburned Carbon Content Fly Ash: Carbonation and Corrosion Performance. Eur. J. Environ. Civ. Eng. 2020, 1–21. [Google Scholar] [CrossRef]
- Aguirre-Guerrero, A.M.; Robayo-Salazar, R.A.; de Gutiérrez, R.M. Corrosion Resistance of Alkali-Activated Binary Reinforced Concrete Based on Natural Volcanic Pozzolan Exposed to Chlorides. J. Build. Eng. 2021, 33, 101593. [Google Scholar] [CrossRef]




| Class F Fly Ash (wt.%) | Class C Fly Ash (wt.%) | Slag (wt.%) | OPC (wt.%) | ||
|---|---|---|---|---|---|
| Source | Babaee and Castel [35] | Shi et al. [41] | Babaee and Castel [35] | Bouteiller et al. [23] | Bouteiller et al. [23] |
| SiO2 | 66.56 | 42.46 | 31.52 | 27.6 | 20.6 |
| Al2O3 | 22.47 | 19.46 | 12.22 | 8.6 | 4.9 |
| CaO | 1.64 | 21.54 | 44.53 | 50.10 | 61.3 |
| MgO | 0.65 | 4.67 | 4.62 | 5.7 | 4.5 |
| SO3 | 0.10 | 1.20 | 3.24 | 3.15 | 3.10 |
| (mV vs. CSE) | (mV vs. SCE) | (mV vs. Ag/AgCl, 3 M KCL) | Corrosion Probability |
|---|---|---|---|
| Ecorr > −200 | Ecorr > −127 | Ecorr > −96 | Low probability (10% corrosion risk) |
| −200 < Ecorr < −350 | −127 < Ecorr < −277 | −96 < Ecorr < −246 | Intermediate condition |
| Ecorr < −350 | Ecorr < −277 | Ecorr < −246 | High probability (90% corrosion risk) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, F.; Xi, X.; Yang, S. Research Progress in Corrosion Mechanism of Reinforced Alkali-Activated Concrete Structures. Corros. Mater. Degrad. 2021, 2, 641-656. https://doi.org/10.3390/cmd2040034
Zhang F, Xi X, Yang S. Research Progress in Corrosion Mechanism of Reinforced Alkali-Activated Concrete Structures. Corrosion and Materials Degradation. 2021; 2(4):641-656. https://doi.org/10.3390/cmd2040034
Chicago/Turabian StyleZhang, Feng, Xun Xi, and Shangtong Yang. 2021. "Research Progress in Corrosion Mechanism of Reinforced Alkali-Activated Concrete Structures" Corrosion and Materials Degradation 2, no. 4: 641-656. https://doi.org/10.3390/cmd2040034
APA StyleZhang, F., Xi, X., & Yang, S. (2021). Research Progress in Corrosion Mechanism of Reinforced Alkali-Activated Concrete Structures. Corrosion and Materials Degradation, 2(4), 641-656. https://doi.org/10.3390/cmd2040034







