Finite Element Method for Thin Film Corrosion Modelling: Where We Advanced and Where We would like to Advance?
Abstract
1. Introduction
2. The Current Status of FEM Thin Film Corrosion Modelling: Where We Advanced?
3. The Future Needs and Prospects of FEM Thin Film Corrosion Modelling: Where We would Like to Advance?
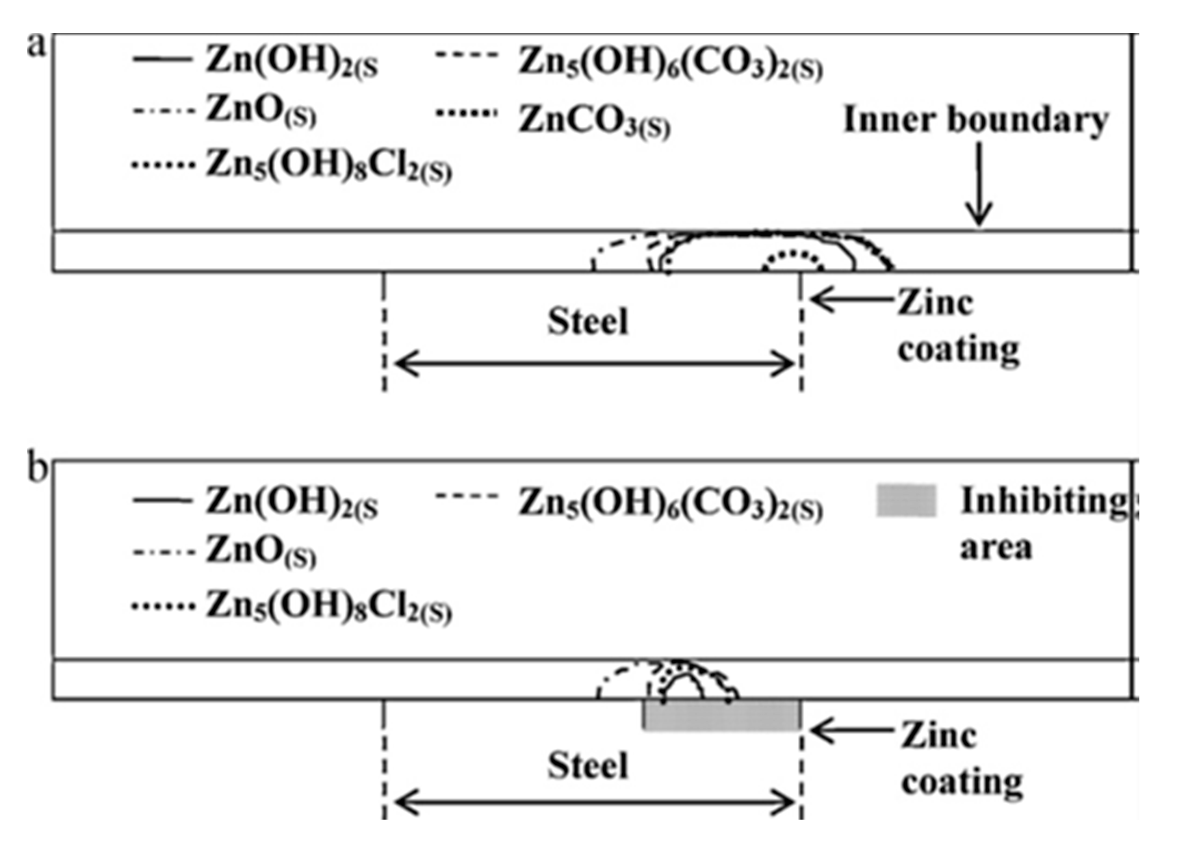
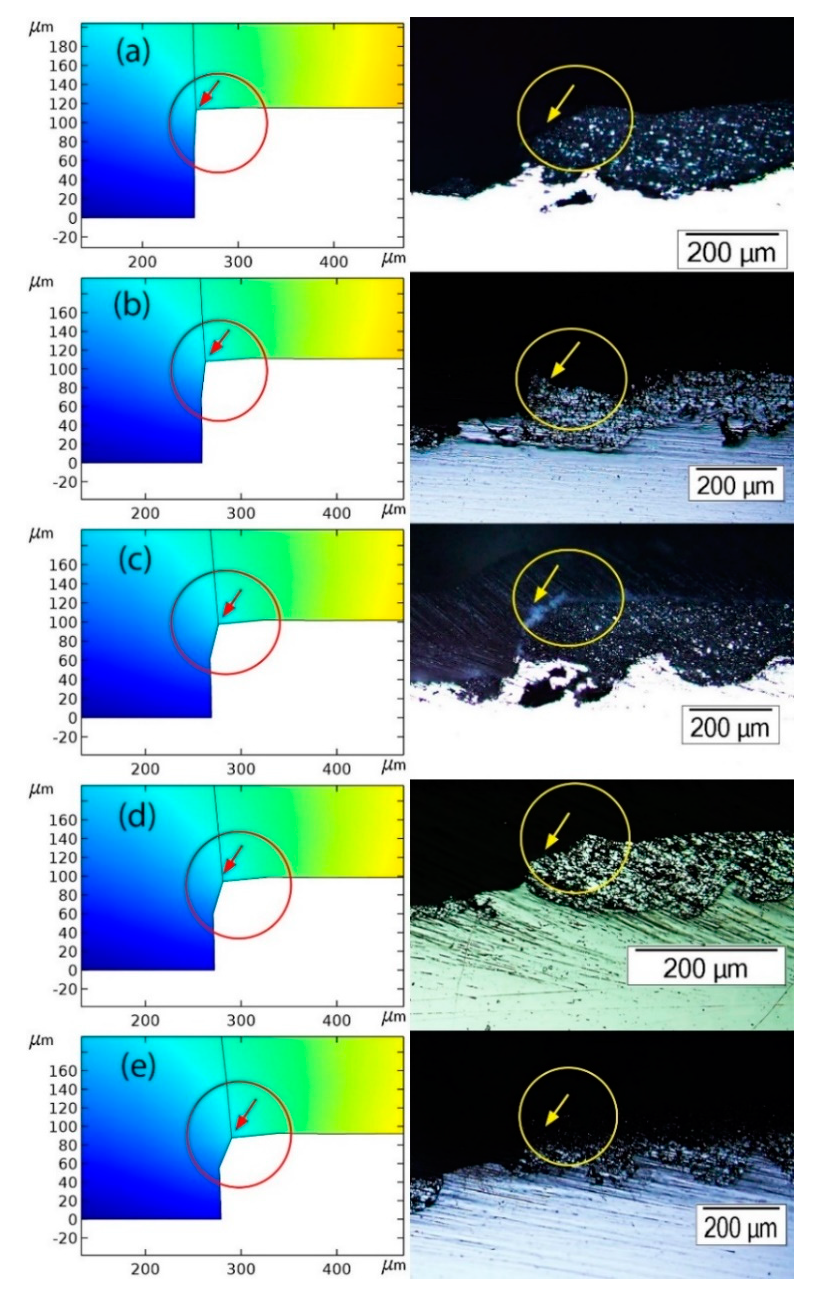
- Electrode blockage due to corrosion products: Thébault et al. [13] included an insulating (inactive) stick in their model to account for the surface deactivation due to corrosion products deposition. Saeedikhani et al. [11] considered that the surfaces of electrodes were covered by porous corrosion products, incorporating into their model the porosity values obtained by image analysis of corroded samples in the early stages of exposure [22]. In contrast, Dolgikh et al. did not consider surface coverage by corrosion products in their model as they had not experimentally observed any blocking action by corrosion products [15,23]. Future thin film models are needed to be developed in a way that both predicts the locations of corrosion-products-blocked sites as well as their porosity, rather than having them as pre-existing parameters. An analysis utilizing the Pilling-Bedworth ratio might be a good place to start for assessing how well the corrosion products adhere to a substrate.
- A possible issue with electroneutrality condition: The electroneutrality equation () that is used in conjunction with the NP equation simply states that the net charge in the electrolyte body is zero. In an electrolyte comprised of n species, (n + 1) variables do exist, with electrolyte potential being the (n + 1)th variable. Therefore, (n + 1) PDEs must be solved on each element of the electrolyte domain. Species 1 to n make a total number of n equations (in the form of NP equation), and the electroneutrality equation makes the equation (n + 1). In this regard, electroneutrality is helpful in solving the simulation problem. However, in modelling approaches, a “reference ion” is selected or a “make-up ion” is added/removed to ensure electroneutrality is achieved. This enforces the system towards electroneutrality, but at the same time may deviate the system from reality.
- 3.
- The impact of charged species on electrolyte potential: In dilute electrolytes, the NP approach for simulation might suffice, as the charged species can be assumed not to be interacting with each other. However, for concentrated electrolytes, overlooking the charged species interactions might not be the best approach. In atmospheric corrosion, as the thin film evaporates it becomes highly concentrated and the distribution of charged species impacts the electrolyte potential as suggested by the Poisson equation:where is the permittivity of the electrolyte.
4. Conclusions
- Incorporation of the Nernst Planck approach with multiple corrosive species along with the homogeneous chemical reactions
- Dynamic electrolyte thickness modelling as a function of environmental parameters
- Integration of corrosion products into modelling
- Moving boundary modelling
- Conjunction of dynamic electrolyte thickness modelling (as a function of environmental parameters) and moving boundary modelling
- Prediction of the sites that are blocked by corrosion products during the simulation time
- Modelling the electrolyte potential via the Nernst-Planck-Poisson approach
Author Contributions
Funding
Conflicts of Interest
References
- Leygraf, C.; Wallinder, I.O.; Tidblad, J.; Graedel, T. Atmospheric Corrosion, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2016; pp. 2–3. [Google Scholar]
- Helle, H.P.E. Numerical Determination of Potential Distributions and Current Densities in Multi-Electrode Systems. Corrosion 1981, 37, 522–530. [Google Scholar] [CrossRef]
- Kennell, G.; Evitts, R.; Heppner, K. A critical crevice solution and IR drop crevice corrosion model. Corros. Sci. 2008, 50, 1716–1725. [Google Scholar] [CrossRef]
- Chen, H.; Chen, Y.; Zhang, J. Cellular automaton modeling on the corrosion/oxidation mechanism of steel in liquid metal environment. Prog. Nucl. Energy 2008, 50, 587–593. [Google Scholar] [CrossRef]
- Onishi, Y.; Takiyasu, J.; Amaya, K.; Yakuwa, H.; Hayabusa, K. Numerical method for time-dependent localized corrosion analysis with moving boundaries by combining the finite volume method and voxel method. Corros. Sci. 2012, 63, 210–224. [Google Scholar] [CrossRef]
- Varela, F.; Kurata, Y.; Sanada, N. The influence of temperature on the galvanic corrosion of a cast iron-stainless steel couple (prediction by boundary element method). Corros. Sci. 1997, 39, 775–788. [Google Scholar] [CrossRef]
- Van den Steen, N.; Simillion, H.; Dolgikh, O.; Terryn, H.; Deconinck, J. An integrated modelling approach for atmospheric corrosion in presence of a varying electrolyte film. Electrochim. Acta 2016, 187, 714–723. [Google Scholar] [CrossRef]
- Javidi, M.; Saeedikhani, M.; Omidi, R. Failure Analysis of a Gas Well Tubing due to Corrosion: A Case Study. J. Fail. Anal. Preven 2012, 12, 550–557. [Google Scholar] [CrossRef]
- Liu, C.; Kelly, R.G. A review of the application of finite element method (FEM) to localized corrosion modelling. Corrosion 2019, 75, 1285–1299. [Google Scholar] [CrossRef]
- Saeedikhani, M.; Wijesinghe, S.; Blackwood, D.J. Moving boundary simulation and mechanistic studies of the electrochemical corrosion protection by a damaged zinc coating. Corros. Sci. 2020, 163, 108296. [Google Scholar] [CrossRef]
- Saeedikhani, M.; Van den Steen, N.; Wijesinghe, S.; Vafakhah, S.; Terryn, H.; Blackwood, D.J. Moving Boundary Simulation of Iron-Zinc Sacrificial Corrosion under Dynamic Electrolyte Thickness Based on Real-Time Monitoring Data. J. Electrochem. Soc. 2020, 167, 041503. [Google Scholar] [CrossRef]
- Cole, I.S.; Lau, D.; Paterson, D.A. Holistic model for atmospheric corrosion Part 6—From wet aerosol to salt deposit. Corros. Eng. Sci. Technol. 2004, 39, 209–218. [Google Scholar] [CrossRef]
- Thébault, F.; Vuillemin, B.; Oltra, R.; Ogle, K.; Allely, C. Investigation of self-healing mechanism on galvanized steels cut edges by coupling SVET and numerical modelling. Electrochim. Acta 2008, 53, 5226–5234. [Google Scholar] [CrossRef]
- Thébault, F.; Vuillemin, B.; Oltra, R.; Allely, C.; Ogle, K. Protective mechanisms occurring on zinc coated steel cut-edges in immersion conditions. Electrochim. Acta 2011, 56, 8347–8357. [Google Scholar] [CrossRef]
- Dolgikh, O.; Simillion, H.; Lamaka, S.; Xue, H.B.; Taryba, M.G.; De Strycker, J.; Deconinck, J.; Bastos, A.C.; Oliveira, A.R.; Van Den Bergh, K. Corrosion protection of steel cut-edges by hot-dip galvanized Al(Zn,Mg) coatings in 1 wt% NaCl: Part II. Numerical simulations. Mater. Corros. 2018, 70, 780–792. [Google Scholar] [CrossRef]
- Thébault, F.; Vuillemin, B.; Oltra, R.; Allely, C.; Ogle, K. Modelling bimetallic corrosion under thin electrolyte films. Corros. Sci. 2011, 53, 201–207. [Google Scholar] [CrossRef]
- Dolgikh, O.; Bastos, A.C.; Oliveira, A.; Dan, C.; Deconinck, J. Influence of the electrolyte film thickness and NaCl concentration on the oxygen reduction current on platinum. Corros. Sci. 2016, 102, 338–347. [Google Scholar] [CrossRef]
- Simillion, H.; Van den Steen, N.; Terryn, H.; Deconinck, J. Geometry influence on corrosion in dynamic thin film electrolytes. Electrochim. Acta 2016, 209, 149–158. [Google Scholar] [CrossRef]
- Liu, C.; Srinivasan, J.; Kelly, R.G. Electrolyte Film Thickness Effects on the Cathodic Current Availability in a Galvanic Couple. J. Electrochem. Soc. 2017, 164, C845–C855. [Google Scholar] [CrossRef]
- Liu, C.; Kubacki, G.W.; Kelly, R.G. Application of Laplace equation-based modelling into corrosion damage prediction for galvanic coupling between a zinc plate and stainless steel rods under a thin film electrolyte. Corrosion 2019, 75, 465–473. [Google Scholar] [CrossRef]
- Saeedikhani, M.; Wijesinghe, S.L.; Blackwood, D.J. Barrier and Sacrificial Protection Mechanisms of Zinc Rich Primers. Eng. J. 2019, 23, 223–233. [Google Scholar] [CrossRef]
- Saeedikhani, M.; Wijesinghe, S.L.; Blackwood, D.J. Revisiting Corrosion Protection Mechanisms of Steel Surface by Damaged Zinc Rich Paints. Corrosion 2019, 75, 756–770. [Google Scholar] [CrossRef]
- Dolgikh, O.; Xue, H.B.; Simillion, H.; Lamaka, S.; Bastos, A.C.; Taryba, M.G.; Oliveira, A.R.; Allély, C.; Van Den Bergh, K.; De Strycker, J.; et al. Corrosion protection of steel cut-edges by hot-dip galvanized Al(Zn,Mg) coatings in 1 wt% NaCl: Part I. Experimental study. Mater. Corros. 2018, 70, 768–779. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saeedikhani, M.; Blackwood, D.J. Finite Element Method for Thin Film Corrosion Modelling: Where We Advanced and Where We would like to Advance? Corros. Mater. Degrad. 2020, 1, 273-281. https://doi.org/10.3390/cmd1020013
Saeedikhani M, Blackwood DJ. Finite Element Method for Thin Film Corrosion Modelling: Where We Advanced and Where We would like to Advance? Corrosion and Materials Degradation. 2020; 1(2):273-281. https://doi.org/10.3390/cmd1020013
Chicago/Turabian StyleSaeedikhani, Mohsen, and Daniel John Blackwood. 2020. "Finite Element Method for Thin Film Corrosion Modelling: Where We Advanced and Where We would like to Advance?" Corrosion and Materials Degradation 1, no. 2: 273-281. https://doi.org/10.3390/cmd1020013
APA StyleSaeedikhani, M., & Blackwood, D. J. (2020). Finite Element Method for Thin Film Corrosion Modelling: Where We Advanced and Where We would like to Advance? Corrosion and Materials Degradation, 1(2), 273-281. https://doi.org/10.3390/cmd1020013





