Evaluation of 4-Year Atmospheric Corrosion of Carbon Steel, Aluminum, Copper and Zinc in a Coastal Military Airport in Greece
Abstract
1. Introduction
2. Materials and Methods
3. Results
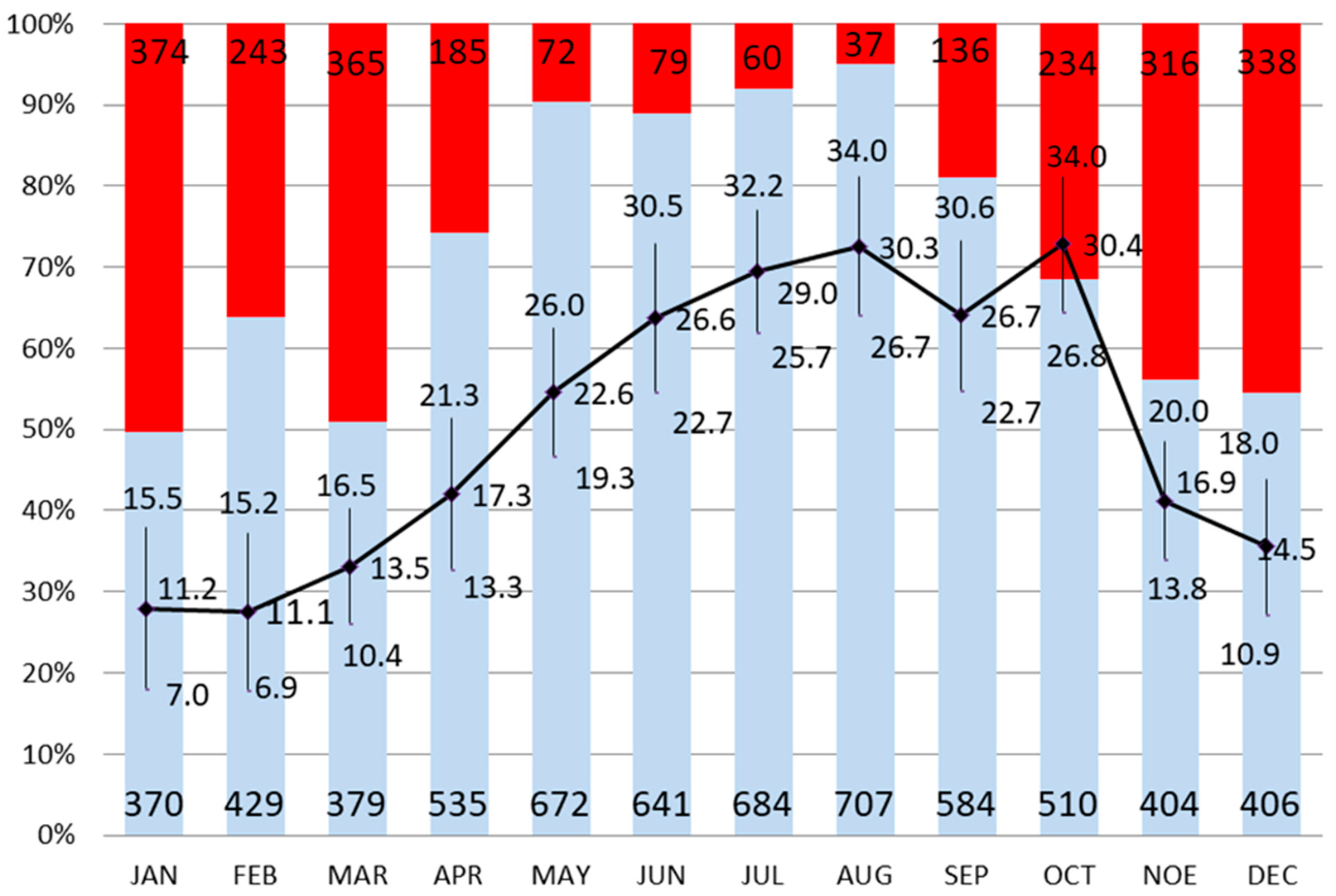
3.1. Regional Environmental Parameters
3.1.1. Pollutants
3.1.2. Meteorological Data
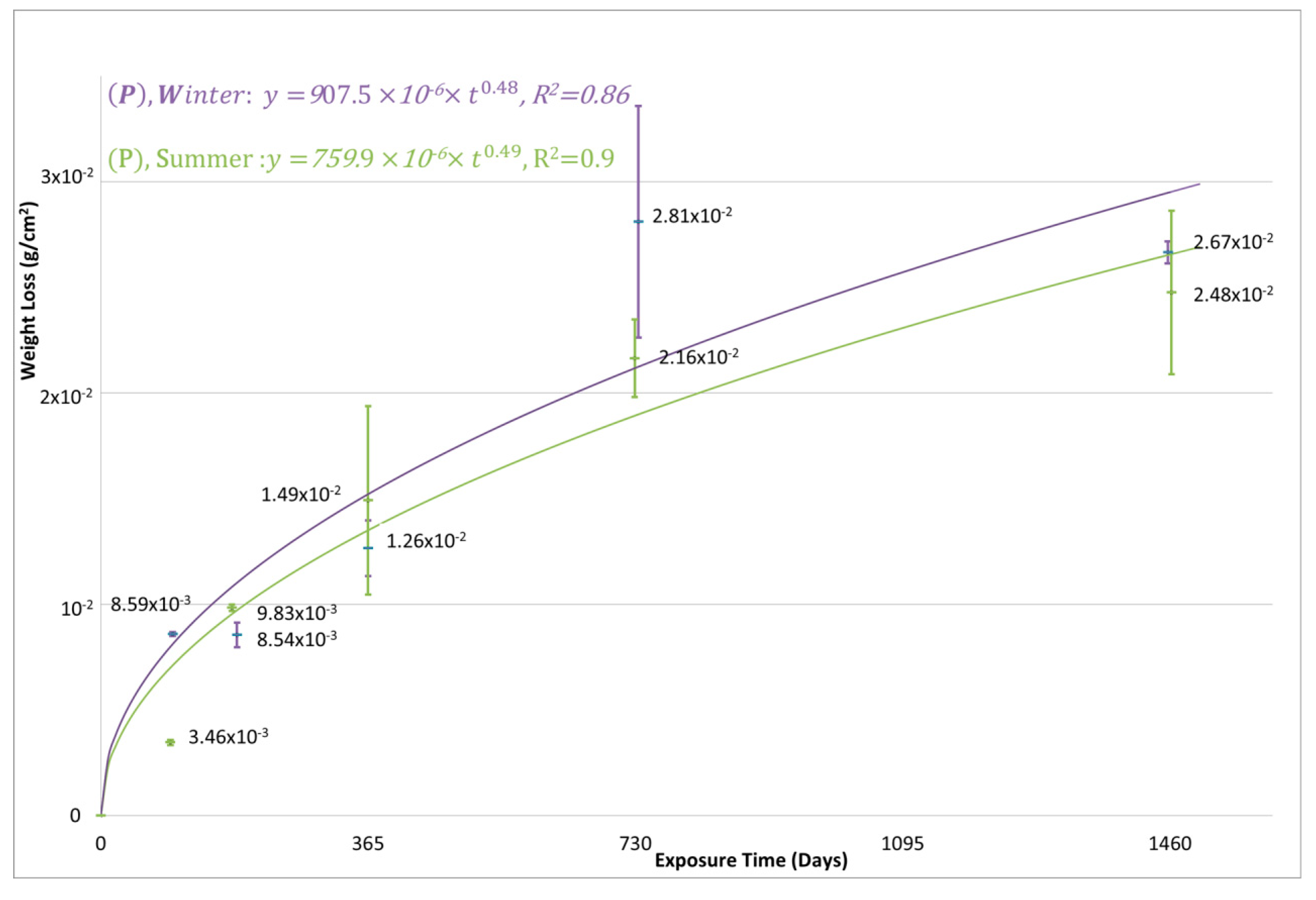
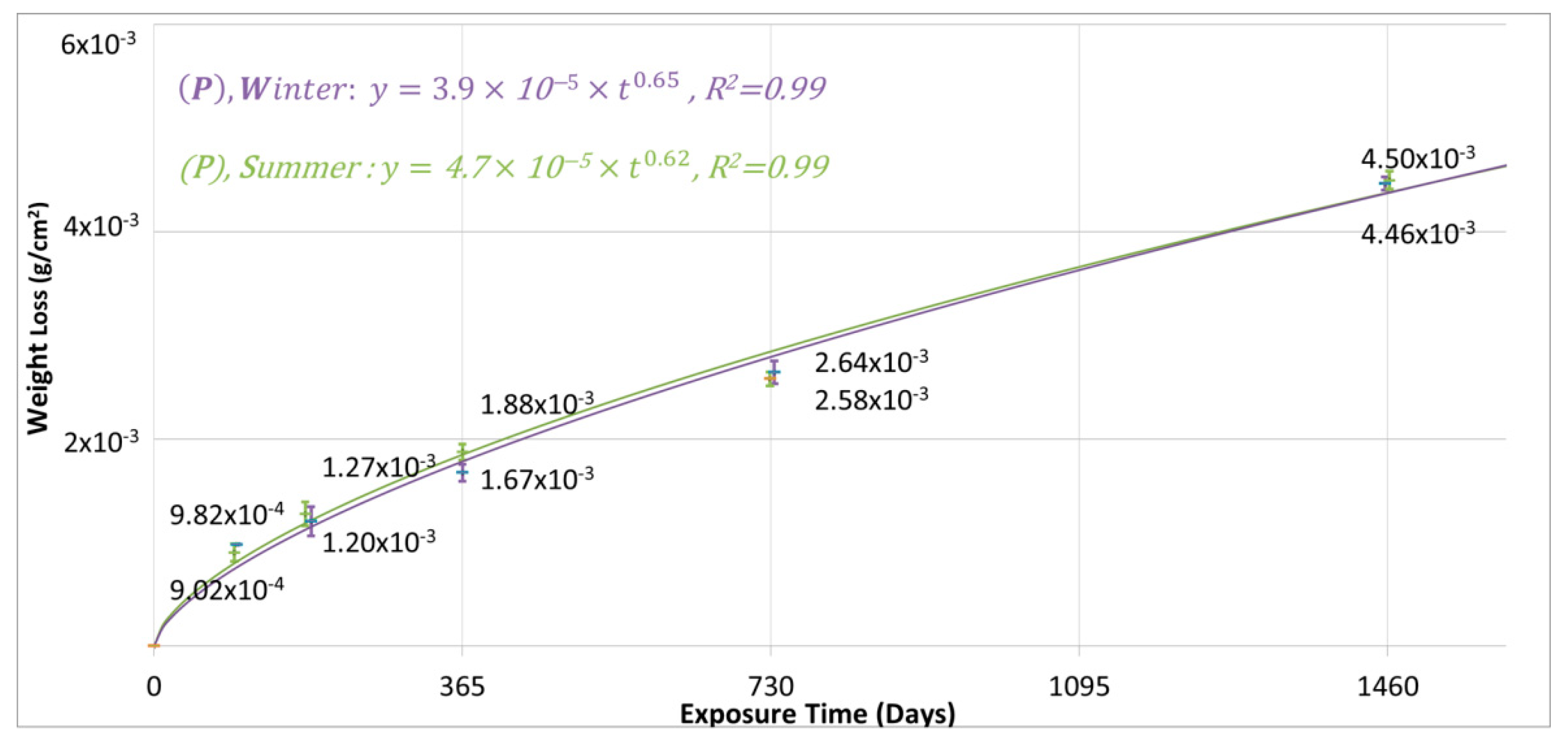
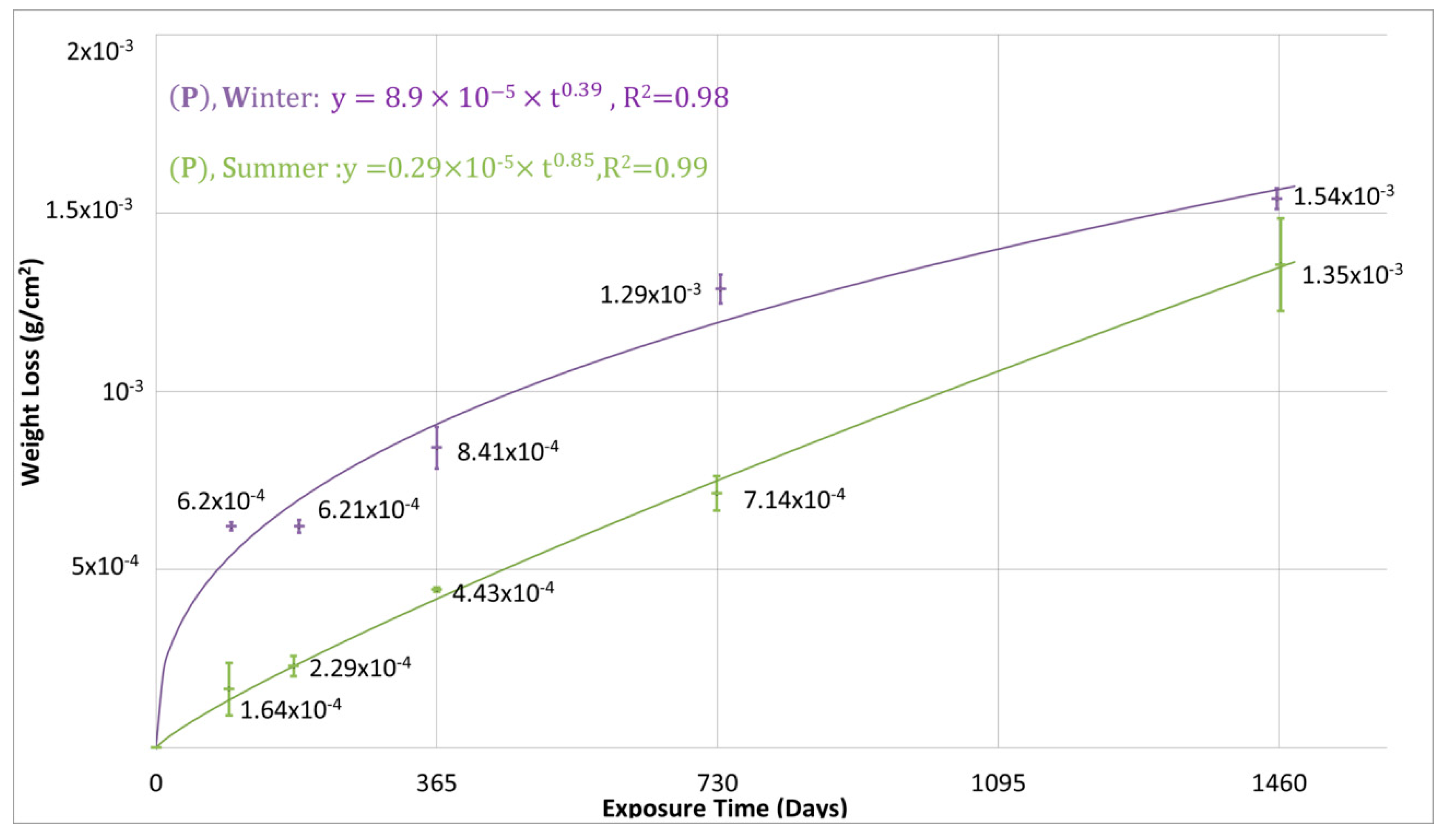
3.2. The 4-Year Corrosion Assessment of the Tested Metals and the Anticipated 30-Year Corrosion Loss
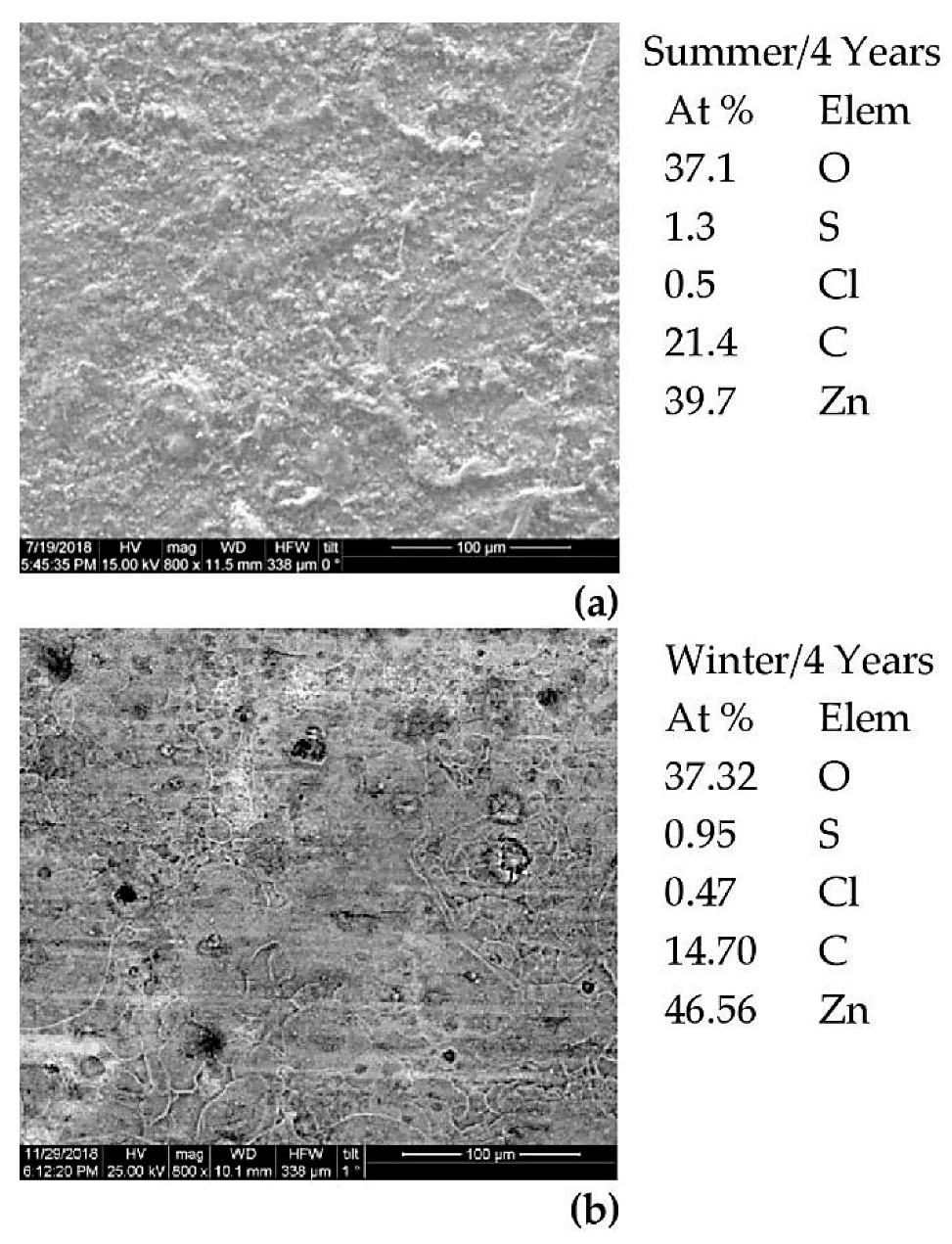
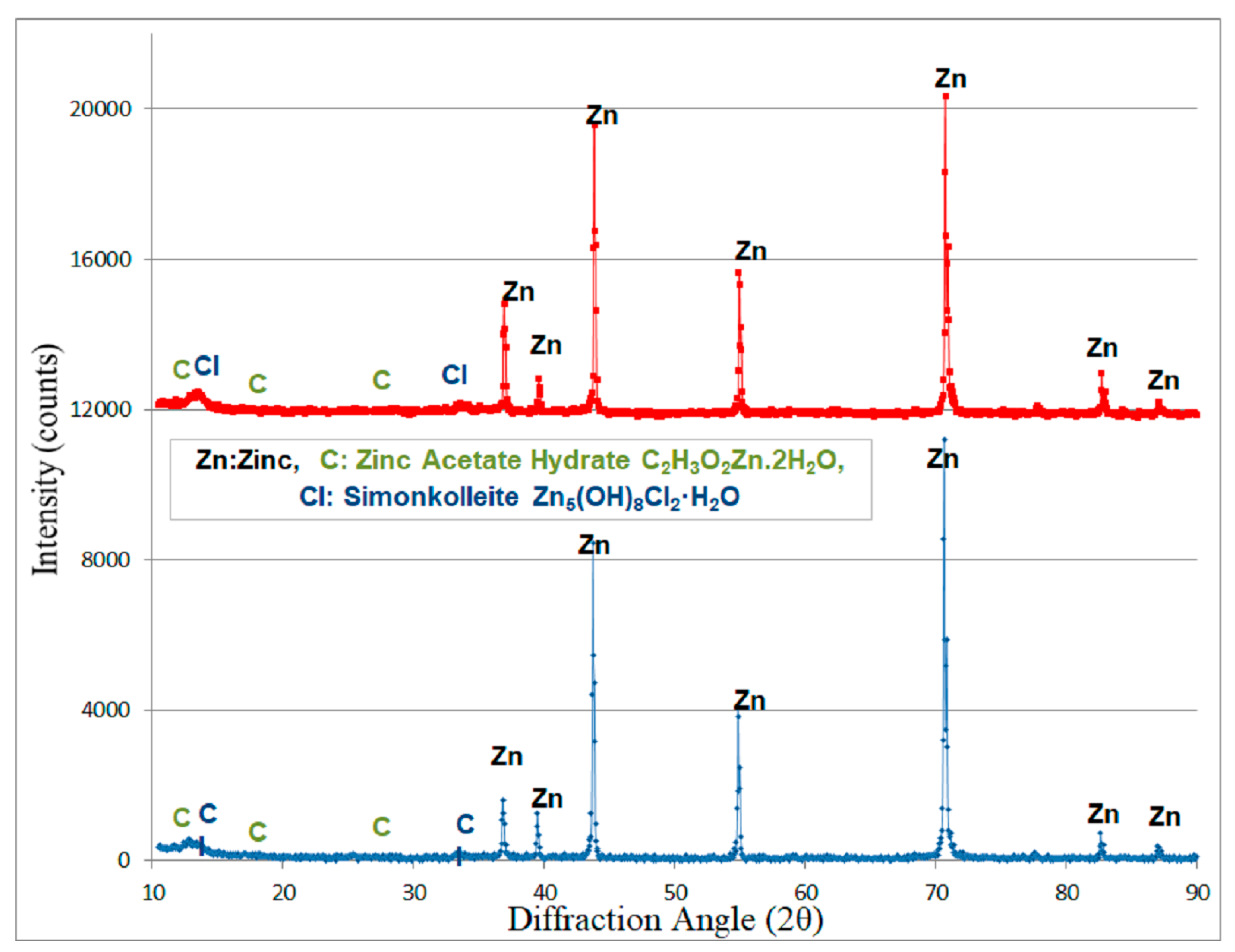
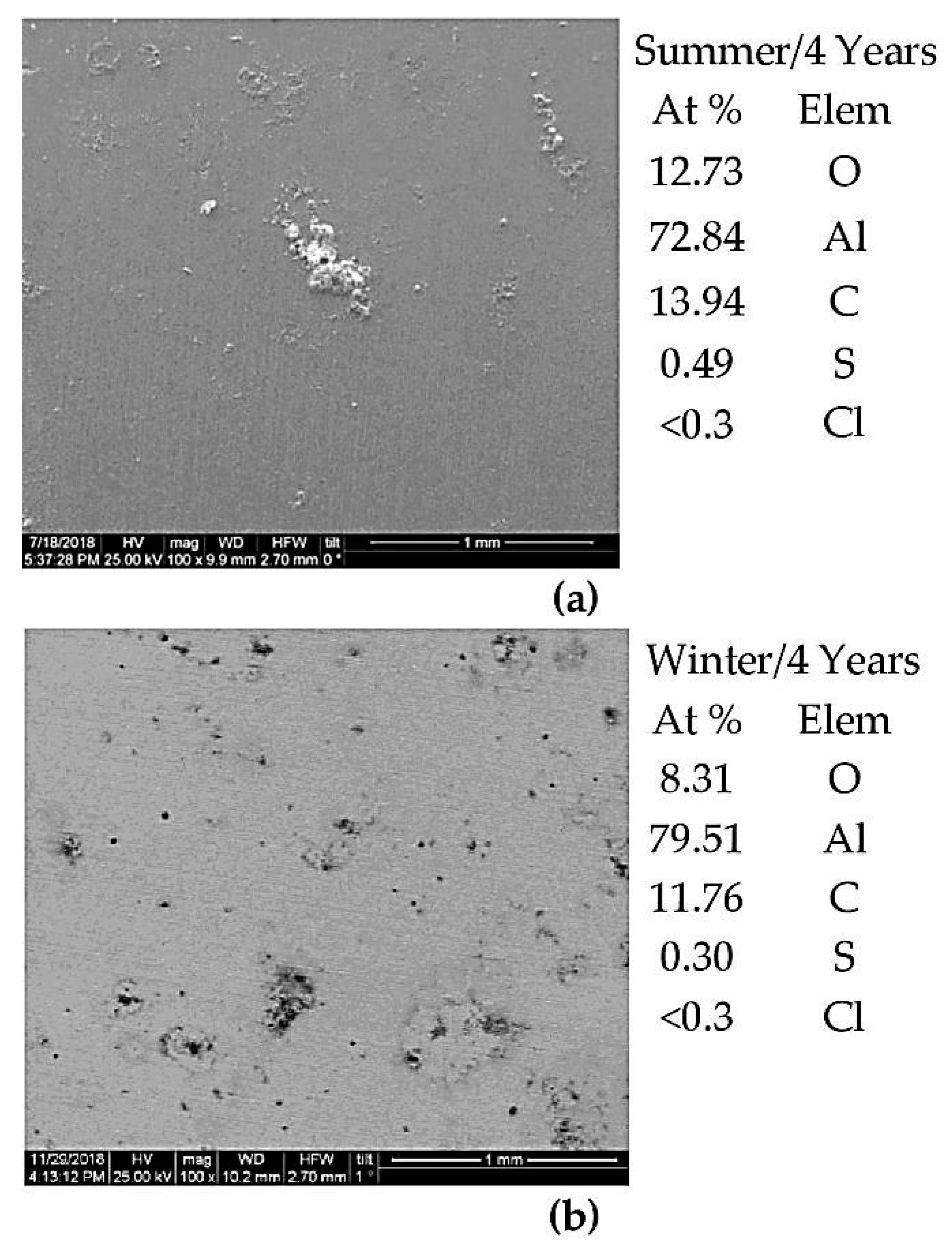
3.3. Characterization of the Metals’ Surfaces After Four Years of Exposure
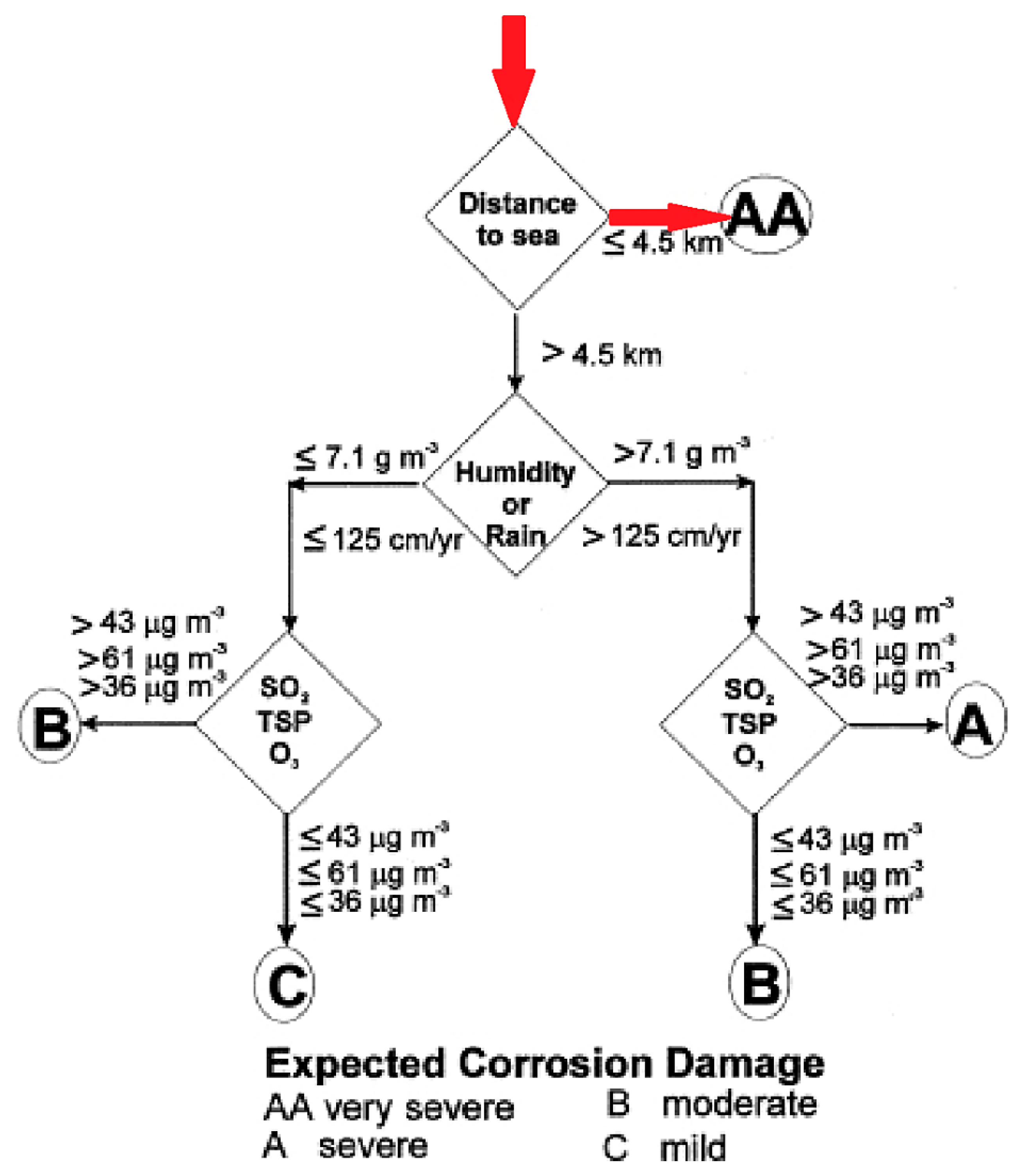
3.4. Classification of the Corrosivity of the Pachi Airport Atmosphere
4. Discussion
4.1. Corrosion Patterns Related to Each Metal Type
- (i)
- The high relative humidity, the atmospheric precipitation (rain) and the temperature play the primary role.
- (ii)
- Salinity and particulate matters play a secondary role to the corrosion rate.
- (iii)
- Finally, the synergy of the above factors with the air pollutants (aircraft emissions, etc.).
- (i)
- In the summer months, under the influence of airborne salinity and particulate matters, the corrosion product layer on Al surface is less protective than its counterpart in the winter months, where higher TOW and rainfall prevail.
- (ii)
- Airborne salinity and PM are anticipated to play the dominant role in the corrosion of aluminum in these atmospheres (Figure 5b).
- (i)
- Chlorides and PM are anticipated to be the most important causes of corrosion of Al alloys in the specific atmosphere.
- (ii)
- The high RH, the atmospheric precipitation (rain) and the temperature play a secondary role.
- (iii)
- Finally, the synergy of the above factors with the pollutants.
- (i)
- The high relative humidity, the atmospheric precipitation (rain) and the concentration of O3 in the atmosphere are the dominant factors in the long-term corrosion of Cu.
- (ii)
- Chloride deposition rate has a secondary, but significant, role in corrosion rate.
- (i)
- From the first year of exposure, Zn exhibits a corrosion rate dependent of the period initially exposed and an order of magnitude lower than steel, a fact that has been observed by other researchers [86]. Higher RH, during the initial time of exposure, plays a catalytic role in the corrosion rate of Zn [86], especially during the first four years of exposure. The synergistic effect of the relatively high moisture with SO2 [56] and relatively high O3 concentration [87] in the atmosphere increases its corrosion rate.
- (ii)
- The exponent “b” shows values far higher for the specimens exposed in the summer. The corrosion layer of the specimens exposed during the summer, under the influence of the airborne salinity, the particulate matters and the maximizing of concentration of the pollutants (mainly CO2 and O3) is less protective than its counterpart of the specimens exposed during the winter months, where high humidity and, especially, atmospheric precipitations (rainfall) provoke a washing out of the chlorides and pollutants from the metal surfaces.
- (i)
- Higher salinity deposition rate.
- (ii)
- Higher concentration of PM (African dust, particle resuspension and redeposition due to aircraft take-offs and landings, etc.).
- (iii)
- Higher concentration of pollutants, especially of CO2, mainly from the combustion fuels of aircraft.
- (iv)
- Higher O3 concentration.
- (v)
- The decisive effect of the rain on washing out the chlorides and the pollutants from the tested metals’ surface during winter.
4.2. Aspects of Gravimetric Data Analysis and Interpretation
- (i)
- The corrosivity of the atmosphere regarding carbon steel and Al classification as C2 “low”, according to ISO 9223.
- (ii)
- The low concentration of chlorides on the Al, Zn and steel surfaces.
- (iii)
- The pitting corrosion observed on Al surface, with maximum depth of 2 μm, after four years of exposure, at a distance of approximately 0.2 km from the seacoast.
- (iv)
- The average annual loss of Cu, less than 2 µm/year during the first four years of exposure.
- (i)
- The higher first-year corrosion rate of carbon steel specimens, exposed during summer, is primarily caused by the salinity and the background pollution of the area. The high conductivity of the steel surface by particulate matters acts as seeding for the water vapors to form water droplets. The seasonal presence of the African dust increases the effect.
- (ii)
- The higher first-year of aluminum, exposed during winter, can be attributed to an easier Al3+ diffusion through the formed oxide with the humidity presence.
4.3. Comparison of Three Different Approaches for Corrosive Environment Classification
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Simillion, H.; Dolgikh, O.; Terryn, H.; Deconinck, J. Atmospheric Corrosion Modeling. Corros. Rev. 2014, 32, 73–100. [Google Scholar] [CrossRef]
- United States Department of Transportation, National Transportation Library. Available online: https://rosap.ntl.bts.gov/view/dot/40697 (accessed on 20 February 2020).
- Biezma, M.V.; San Cristóbal, J.R. Methodology to Study Cost of Corrosion. Corros. Eng. Sci. Technol. 2005, 40, 344–352. [Google Scholar] [CrossRef]
- Thompson, N.G.; Yunovich, M.; Dunmire, D. Cost of Corrosion and Corrosion Maintenance Strategies. Corros. Rev. 2007, 25. [Google Scholar] [CrossRef]
- Xu, N.; Zhao, L.; Ding, C.; Zhang, C.; Li, R.; Zhong, Q. Laboratory Observation of Dew Formation at an Early Stage of Atmospheric Corrosion of Metals. Corros. Sci. 2002, 44, 163–170. [Google Scholar] [CrossRef]
- International Organization for Standardization. Corrosion of Metals and Alloys: Corrosivity of Atmospheres: Classification, Determination and Estimation (ISO/DIS Standard No. 9223); International Organization for Standardization: Geneva, Switzerland, 1992. [Google Scholar]
- International Organization for Standardization. Corrosion of Metals and Alloys: Corrosivity of Atmospheres: Guiding Values for the Corrosivity Categories (ISO/DIS Standard No. 9224); International Organization for Standardization: Geneva, Switzerland, 1992. [Google Scholar]
- International Organization for Standardization. Corrosion of Metals and Alloys: Corrosivity of Atmospheres: Measurement of Pollution (ISO/DIS Standard No. 9225); International Organization for Standardization: Geneva, Switzerland, 1992. [Google Scholar]
- International Organization for Standardization. Corrosion of Metals and Alloys: Corrosivity of Atmospheres: Methods of Determination of Corrosion rates of Standard Specimens for the Evaluation of Corrosivity (ISO/DIS Standard No. 9226); International Organization for Standardization: Geneva, Switzerland, 1992. [Google Scholar]
- Morcillo, M.; Almeida, E.; Chico, B.; de la Fuente, D. Analysis of ISO Standard 9223 (Classification of Corrosivity of Atmospheres) in the Light of Information Obtained in the Ibero-American Micat Project. In Outdoor Atmospheric Corrosion; Townsend, H., Ed.; ASTM International: West Conshohocken, PA, USA, 2002; pp. 59–72. [Google Scholar] [CrossRef]
- Kreislova, K.; Knotkova, D. Corrosion Behaviour of Structural Metals in Respect to Long-Term Changes in the Atmospheric Environment. In Proceedings of the EUROCORR 2011, Stockholm, Sweden, 5–8 September 2011. [Google Scholar]
- Kreislova, K.; Knotkova, D. The Results of 45 Years of Atmospheric Corrosion Study in the Czech Republic. Materials 2017, 10, 394. [Google Scholar] [CrossRef]
- Tidblad, J.; Kucera, V.; Ferm, M.; Kreislova, K.; Brüggerhoff, S.; Doytchinov, S.; Screpanti, A.; Grøntoft, T.; Yates, T.; de la Fuente, D.; et al. Effects of Air Pollution on Materials and Cultural Heritage: ICP Materials Celebrates 25 Years of Research. Int. J. Corros. 2012, 2012. [Google Scholar] [CrossRef]
- Mendoza, A.; Corvo, F. Outdoor and Indoor Atmospheric Corrosion of Non-Ferrous Metals. Corros. Sci. 2000, 42, 1123–1147. [Google Scholar] [CrossRef]
- Tidblad, J. Atmospheric Corrosion of Metals in 2010–2039 and 2070–2099. Atmos. Environ. 2012, 55, 1–6. [Google Scholar] [CrossRef]
- Pourbaix, M. The Linear Bilogarithmic Law for Atmospheric Corrosion. In Atmospheric Corrosion; Ailor, W.H., Ed.; J. Wiley & Sons: New York, NY, USA, 1982; pp. 107–121. [Google Scholar] [CrossRef]
- McCuen, R.H.; Albrecht, P.; Cheng, J.G. A New Approach To Power-Model Regression Of Corrosion Penetration Data. In Corrosion Forms and Control for Infrastructure; ASTM STP 1137; Chaker, V., Ed.; American Society for Testing and Materials: West Conshohocken, PA, USA, 1992; pp. 46–76. [Google Scholar]
- Summitt, R.; Fink, F. PACER LIME, Part 2. Experimental Determination of Environmental Corrosion Severity; Michigan State University: East Lansing, MI, USA, 1980. [Google Scholar]
- Research and Technology Organization. Corrosion Fatigue and Environmentally Assisted Cracking in Aging Military Vehicles; Tech. Rep. AG-AVT-140; NATO: Neuilly, France, 2011. [Google Scholar]
- Kenny, E.D.; Esmanhoto, E.J. Tratamento Estatistico do Desempenho de Materiais Metalicos no Estado do Parana. In XVII Congresso Brasileiro de Corrosao; Anais; ABRACO, T-24: Rio de Janeiro, Brazil, 1993; pp. 297–308. [Google Scholar]
- Kenny, E.D.; Esmanhoto, E.J. Corrosao do aco-carbono por intemperismo natural no Estado do Parana. In IV Seminario de Materiais no Setor Elιtrico; Anais; Copel/UFPR: Curitiba, Brazil, 1994; pp. 377–382. [Google Scholar]
- Feliu, S.; Morcillo, A.; Feliu Jr., S. The Prediction of Atmospheric Corrosion from Meteorological and Pollution Parameters—I. Annual corrosion. Corros. Sci. 1993, 34, 403–414. [Google Scholar] [CrossRef]
- Feliu, S.; Morcillo, A.; Feliu Jr., S. The Prediction of Atmospheric Corrosion from Meteorological and Pollution Parameters—II. Long-term forecasts. Corros. Sci. 1993, 34, 415–422. [Google Scholar] [CrossRef]
- Morcillo, M.; Almeida, E.M.; Rosales, B.M. Functiones de Dano (Dosis/Respuesta) de la Corrosion Atmospherica en Iberoamerica, Corrosion y Proteccion de Metales en las Atmosferas de Iberoamerica; Programma CYTED: Madrid, Spain, 1998; pp. 629–660. [Google Scholar]
- Cai, J.; Cottis, R.A.; Lyon, S.B. Phenomenological Modelling of Atmospheric Corrosion Using an Artificial Neural Network. Corros. Sci. 1999, 41, 2001–2030. [Google Scholar] [CrossRef]
- Jančíková, Z.; Zimný, O.; Koštial, P. Prediction of Metal Corrosion by Neural Networks. Metalurgija 2013, 52, 379–381. [Google Scholar]
- Cai, Y.; Zhao, Y.; Ma, X.; Zhou, K.; Chen, Y. Influence of Environmental Factors on Atmospheric Corrosion in Dynamic Environment. Corros. Sci. 2018, 137, 163–175. [Google Scholar] [CrossRef]
- Mikhailov, A.A.; Tidblad, J.; Kucera, V. The Classification System of ISO 9223 Standard and the Dose-Response Functions Assessing the Corrosivity of Outdoor Atmospheres. Prot. Metals 2004, 40, 541–550. [Google Scholar] [CrossRef]
- University of the Aegean. Department of Environment. Available online: http://www1.aegean.gr/lid/internet/elliniki_ekdosi/TEL_DIMOSI/Paper_Periferiakotita.pdf (accessed on 20 February 2020).
- World.bymap.org/Coastline Lengths. Available online: http://world.bymap.org/Coastlines.html (accessed on 20 February 2020).
- ASTM G140-02. Standard Test Method for Determining Atmospheric Chloride Deposition Rate by Wet Candle Method; ASTM International: West Conshohocken, PA, USA, 2002; Available online: www.astm.org (accessed on 19 February 2020).
- International Organization for Standardization. Corrosion of Metals and Alloys: Corrosivity of atmospheres: Removal of Corrosion Products from Corrosion Test Specimens (ISO/DIS Standard No. 8407); International Organization for Standardization: Geneva, Switzerland, 1991. [Google Scholar]
- ASTM G1-90(1999)e1. Standard Practice for Preparing, Cleaning, and Evaluating Corrosion Test Specimens; ASTM International: West Conshohocken, PA, USA, 1999; Available online: www.astm.org (accessed on 19 February 2020).
- International Organization for Standardization. Air Quality Determination of Mass Concentration of Sulphur Dioxide in Ambient Air; Thorin Spectrophotometric Method (ISO/DIS Standard No. 4221); International Organization for Standardization: Geneva, Switzerland, 1999. [Google Scholar]
- ASTM D4458-09. Standard Test Method for Chloride Ions in Brackish Water, Seawater, and Brines; ASTM International: West Conshohocken, PA, USA, 2009; Available online: www.astm.org (accessed on 19 February 2020).
- Klassen, R.D.; Roberge, P.R. The Effects of Wind on Local Atmospheric Corrosivity; Corrosion 2001; NACE International: Houston, TX, USA, 2001. [Google Scholar]
- Dean, S.W.; Reiser, D.B. Analysis of Data from ISO CORRAG Program; Corrosion 1998, Paper #340; NACE International: Houston, TX, USA, 1998. [Google Scholar]
- Dean, S.W.; Reiser, D.B. Comparison of the Atmospheric Corrosion Rates of Wires and Flat Panels; Corrosion 2000, Paper #455; NACE International: Houston, TX, USA, 2000. [Google Scholar]
- Dean, S.W. Classifying Atmospheric Corrosivity - a Challenge for ISO. Mater. Perform. 1992, 32, 53–58. [Google Scholar]
- Roberge, P.R.; Klassen, R.D.; Haberecht, P.W. Atmospheric Corrosivity Modeling—A Review. Mater. Des. 2002, 23. [Google Scholar] [CrossRef]
- Dean, S.W. Corrosion Testing of Metals Under natural Atmospheric Conditions. Corrosion Testing and Evaluation: Silver Anniversary Volume, ASTM STP 1000; Baboian, R., Dean, S.W., Eds.; ASTM: West Conshohocken, PA, USA, 1990; pp. 163–176. [Google Scholar]
- Knotkova, D. 2005 F.N. Speller Award Lecture: Atmospheric Corrosion—Research, Testing, and Standardization. Corrosion 2005, 61, 723–738. [Google Scholar] [CrossRef]
- Leygraf, C.; Graedel, T.E. Atmospheric Corrosion; Wiley-Interscience: New York, NY, USA, 2000. [Google Scholar]
- Hellenic Republic, Ministry of Infrastructure and Transport, Civil Aviation Authority. Available online: http://www.ypa.gr/en/our-airports/monada-ejyphrethshs-aeroskafwn-genikhs-aeroporias-m-e-g-a-p (accessed on 20 February 2020).
- Mossotti, V.G.; Eldeeb, A.R. MORPH-2, a Software Package for the Analysis of Scanning Electron Micrograph (Binary Formatted) Images for the Assessment of the Fractal Dimension of Exposed Stone Surfaces; U.S. Geological Survey: Reston, VA, USA, 2000. [Google Scholar]
- Xiao, H.; Ye, W.; Song, X.; Ma, Y.; Li, Y. Evolution of Akaganeite in Rust Layers Formed on Steel Submitted to Wet/Dry Cyclic Tests. Materials 2017, 10, 1262. [Google Scholar] [CrossRef]
- Titakis, C. Quantitative Chemical Composition of Pachi Airport Atmosphere and Effect of Pollutants in Aeronautical Materials. Master’s Thesis, National Technical University of Athens, Athens, Greece, 2013. [Google Scholar]
- Kambezidis, H.; Kalliampakos, G. Mapping Atmospheric Corrosion on Modern Materials in the Greater Athens Area. Water Air Soil Pollut. 2013, 224, 1463. [Google Scholar] [CrossRef]
- Ministry of Environment and Energy, Air Quality Department. Available online: http://www.ypeka.gr/LinkClick.aspx?fileticket=81Y3zyY9w%2BU%3D&tabid=490&language=el-GR (accessed on 20 February 2020).
- European Environmental Agency/Sulphur Dioxide (SO2): Annual Mean Concentrations in Europe. Available online: http://www.eea.europa.eu/themes/air/interactive/so2 (accessed on 20 February 2020).
- European Monitoring and Evaluation Programme. Available online: http://webdab.emep.int/cgi-bin/wedb2_controller.pl?State=ydata&reportflag=2015&countries=GR&years=2013&pollutants=total+ox.+sulphur&datatype=grid50_png (accessed on 20 February 2020).
- European Monitoring and Evaluation Programme. Available online: http://webdab.emep.int/cgi-bin/wedb2_controller.pl?State=ydata&reportflag=2015&countries=GR&years=2009&pollutants=SO2&datatype=grid50_png (accessed on 20 February 2020).
- Kalabokas, P.D.; Sideris, G.; Christolis, Μ.; Markatos, Ν. Analysis of air quality measurements in Volos, Greece (in Greek). In Proceedings of the 5th International Exposition and Conference for the Environmental Technology (HELECO 05), Athens, Greece, 3–6 February 2005. [Google Scholar]
- European Monitoring and Evaluation Programme. Available online: http://webdab.emep.int/cgi-bin/wedb2_controller.pl?State=ydata&reportflag=2015&countries=GR&years=2013&pollutants=O3&datatype=grid50_png (accessed on 20 February 2020).
- Riga-Karandinos, A.N.; Saitanis, C. Comparative Assessment of Ambient Air Quality in Two Typical Mediterranean Coastal Cities in Greece. Chemosphere 2005, 59, 1125–1136. [Google Scholar] [CrossRef]
- Im, U.; Christodoulaki, S.; Violaki, K.; Zarmpas, P.; Koçak, M.; Daskalakis, N.; Mihalopoulos, N.; Kanakidou, M. Atmospheric deposition of nitrogen and sulfur over Southern Europe with focus on the Mediterranean and the Black Sea. Atmos. Environ. 2013, 81, 660–670. [Google Scholar] [CrossRef]
- Psiloglou, B.E. Meteorological Data 2009-11, National Observatory of Athens, Institute for Environmental Research and Sustainable Development; Personal Communication: Athens, Greece, 2012. [Google Scholar]
- Hellenic National Meteorological Service. Available online: http://www.hnms.gr/hnms/english/climatology/climatology_region_diagrams_html?dr_city=Elefsina (accessed on 19 February 2020).
- Hellenic Meteorological Service. Available online: http://www.emy.gr/hnms/english/index_html (accessed on 19 February 2020).
- Titakis, C.; Vassiliou, P.; Ziomas, I. Atmospheric Corrosion of Carbon Steel, Aluminum, Copper and Zinc in a Coastal Military Airport in Greece, Nafsivios Chora, 2018 ed.; Part A: Mechanical and Marine Engineering; Hellenic Naval Academy: Pireas, Greece, 2018; pp. A35–A57. [Google Scholar]
- ASTM G16-95. Standard Guide for Applying Statistics to Analysis of Corrosion Data; ASTM International: West Conshohocken, PA, USA, 1999; Available online: www.astm.org (accessed on 19 February 2020).
- ASTM G101-01. Standard Guide for Estimating the Atmospheric Corrosion Resistance of Low Alloy Steels; ASTM International: West Conshohocken, PA, USA, 2001; Available online: www.astm.org (accessed on 19 February 2020).
- Spence, J.W.; Haynie, F.H.; Lipfert, F.W.; Cramer, S.D.; McDonald, L.G. Atmospheric Corrosion Model for Galvanized Steel Structures. Corrosion 1992, 48, 1009–1019. [Google Scholar] [CrossRef]
- Kobus, J. Long-Term Atmospheric Corrosion Monitoring. Mater. Corros. 2000, 51, 104–108. [Google Scholar] [CrossRef]
- Abdul-Wahab, S.A. Statistical Prediction of Atmospheric Corrosion From Atmospheric-Pollution Parameters. Pract. Period. Hazard. Toxic Radioact. Waste Manag. 2003, 7, 190–200. [Google Scholar] [CrossRef]
- Bhattachariee, S.; Roy, N.; Dey, A.K.; Banerjee, M.K. Statistical appraisal of the atmospheric corrosion of mild steel. Corros. Sci. 1993, 34, 573–581. [Google Scholar] [CrossRef]
- Santana Rodríguez, J.J.; Santana Hernández, F.J.; González González, J.E. The effect of environmental and meteorological variables on atmospheric corrosion of carbon steel, copper, zinc and aluminium in a limited geographic zone with different types of environment. Corros. Sci. 2003, 45, 799–815. [Google Scholar] [CrossRef]
- Dean, S.W.; Reiser, D.B. Analysis of Long-Term Atmospheric Corrosion Results from ISO CORRAG Program. In ASTM STP 1421, Outdoor Atmospheric Corrosion; Townsend, H.E., Ed.; ASTM International: West Conshohocken, PA, USA, 2002; pp. 3–18. [Google Scholar]
- Townsend, H.E. Effects of Alloying Elements on the Corrosion of Steel in Industrial Atmospheres. Corrosion 2001, 57, 497–501. [Google Scholar] [CrossRef]
- Philip, A.; Schweitzer, P.E. Fundamentals of Metallic Corrosion: Atmospheric and Media Corrosion of Metals; CRC Press: London, UK, 2006. [Google Scholar]
- Okada, H. Atmospheric Corrosion of Steels. J. Soc. Mater. Sci. 1968, 17, 705–709. [Google Scholar] [CrossRef]
- Okada, H.; Hosoi, Y.; Yuawa, K.-i.; Naio, H. Structure of the Rust Formed on Low Alloy Steels in Atmospheric Corrosion. Tetsu-to-Hagane 1969, 55, 355–365. [Google Scholar] [CrossRef]
- Misawa, T.; Hashimoto, K.; Shimodaira, S. On the Mechanism of Atmospheric Rusting of Iron and Protective Rust Layer on Low Alloy Steels. Corros. Eng. 1974, 23, 17–27. [Google Scholar] [CrossRef][Green Version]
- Misawa, T.; Asami, K.; Hashimoto, K.; Shimodaira, S. The Mechanism of Atmospheric Rusting and the Protective Amorphous Rust on Low Alloy Steel. Corros. Sci. 1974, 14, 279–290. [Google Scholar] [CrossRef]
- Misawa, T. Research Status and Unsolved Problems in Rusting of Iron and Steels. Corros. Eng. 1983, 32, 657–667. [Google Scholar] [CrossRef]
- Manivannan, M.; Rajendran, S. Investigation of Inhibitive Action of urea-Zn2+ System in the Corrosion Control of Carbon Steel in Sea Water. Int. J. Eng. Sci. Technol. 2011, 3, 8048–8060. [Google Scholar]
- Nasrazadani, S.; Raman, A. The Application of Infrared Spectroscopy to the Study of Rust Systems—II. Study of Cation Deficiency in Magnetite (Fe3O4) Produced During Its Transformation To Maghemite (γ-Fe2O3) and Hematite (α-Fe2O3). Corros. Sci. 1993, 34, 1355–1365. [Google Scholar] [CrossRef]
- Raman, A.; Kuban, B.; Razvan, A. The Application Of Infrared Spectroscopy To The Study Of Atmospheric Rust Systems—I. Standard Spectra and Illustrative Applications to Identify Rust Phases In Natural Atmospheric Corrosion Products. Corros. Sci. 1991, 32, 1295–1306. [Google Scholar] [CrossRef]
- Yamashita, M.; Miyuki, H.; Matsuda, Y.; Nagano, H.; Misawa, T. The Long Term Growth of the Protective Rust Layer Formed on Weathering Steel By Atmospheric Corrosion During a Quarter Of a Century. Corros. Sci. 1994, 36, 283–299. [Google Scholar] [CrossRef]
- Mitsakou, C.; Kallos, G.; Papantoniou, N.; Spyrou, C.; Solomos, S.; Astitha, M.; Housiadas, C. Saharan Dust Levels in Greece and Received Inhalation Doses. Atmos. Chem. Phys. 2008, 8, 7181–7192. [Google Scholar] [CrossRef]
- International Organization for Standardization. Anodized Aluminium and Aluminium Alloys - Rating System for the Evaluation of Pitting Corrosion—Chart Method (ISO/DIS Standard No. 8993); International Organization for Standardization: Geneva, Switzerland, 1992. [Google Scholar]
- Odnevall, X.; Leygraf, C. The formation of Zn4Cl2(OH)4SO4•5H2O in an Urban and an Industrial Atmosphere. Corros. Sci. 1994, 36, 1551–1559. [Google Scholar] [CrossRef]
- Morcillo, M.; Chico, B.; de la Fuente, D.; Simancas, J. Looking Back On Contributions In The Field Of Atmospheric Corrosion Offered by the MICAT Ibero-American Testing Network. Int. J. Corros. 2012, 1–24. [Google Scholar] [CrossRef]
- Ericsson, R. The Influence of Sodium Chloride on the Atmospheric Corrosion of Steel. Mater. Corros. 1978, 29, 400–403. [Google Scholar] [CrossRef]
- Oesch, S. The effect of SO2, NO2, NO and O3 on the Corrosion of Unalloyed Carbon Steel and Weathering Steel—The Reults of Laboratory Exposures. Corros. Sci. 1996, 38, 1357–1368. [Google Scholar] [CrossRef]
- Chung, S.C.; Lin, A.S.; Chang, J.R.; Shih, H.C. EXAFS Study of Atmospheric Corrosion Products on Zinc at the Initial Stage. Corros. Sci. 2000, 42, 1599–1610. [Google Scholar] [CrossRef]
- Svensson, J.E.; Johansson, L.G. A Laboratory Study of the Effect of Ozone, Nitrogen Dioxide, and Sulfur Dioxide on the Atmospheric Corrosion of Zinc. J. Electrochem. Soc. 1993, 140, 2210–2216. [Google Scholar] [CrossRef]
- Slunder, C.; Boyd, W.K. Zinc: Its Corrosion Resistance, 2nd ed.; International Lead Zinc Research Organization, Inc.: New York, NY, USA, 1986. [Google Scholar]
- United States Army. Aviation Unit and Aviation Intermediate Maintenance Manual Ch-47D Helicopter-TM 55-1520-240-23-2, Change No. 25. Headquarters; Department of the US Army: Washington, DC, USA, 2000; pp. 2–973.



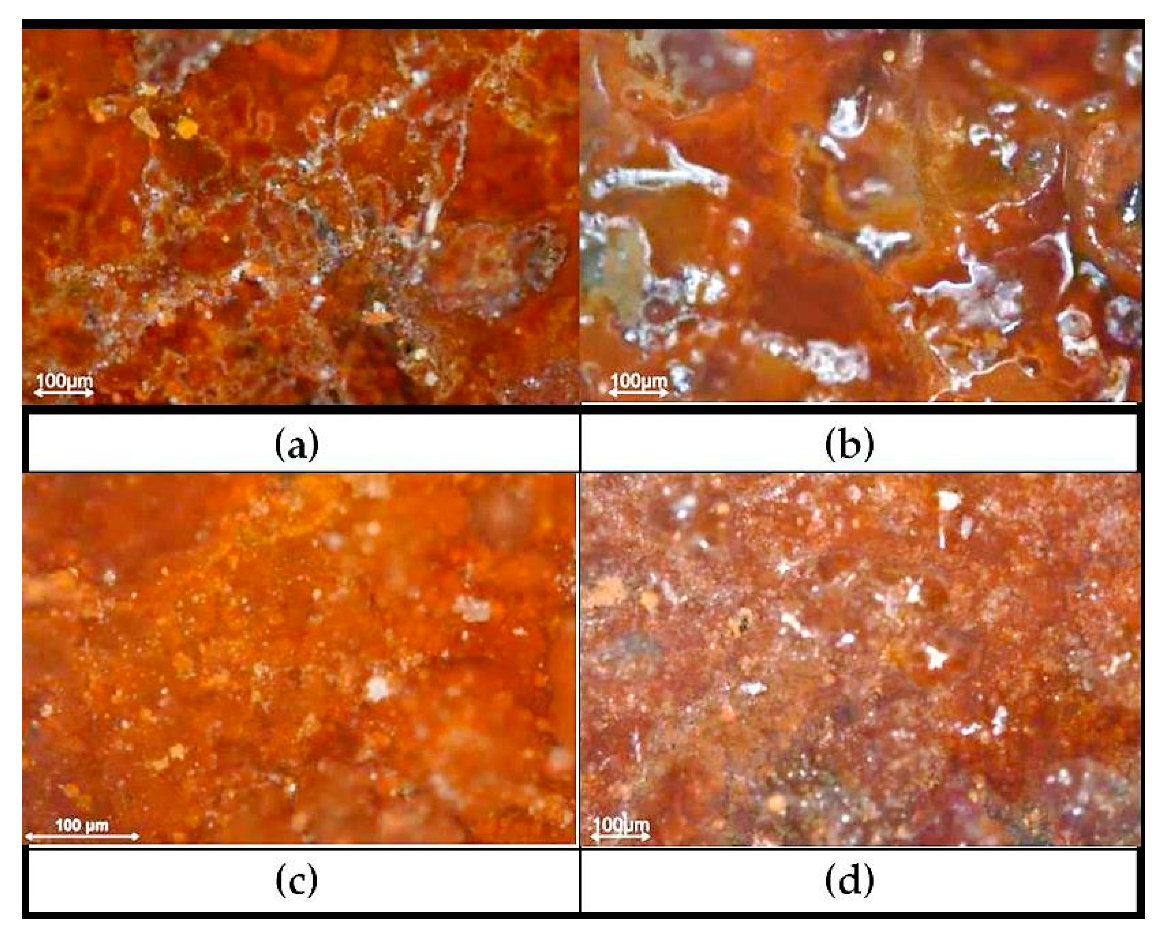
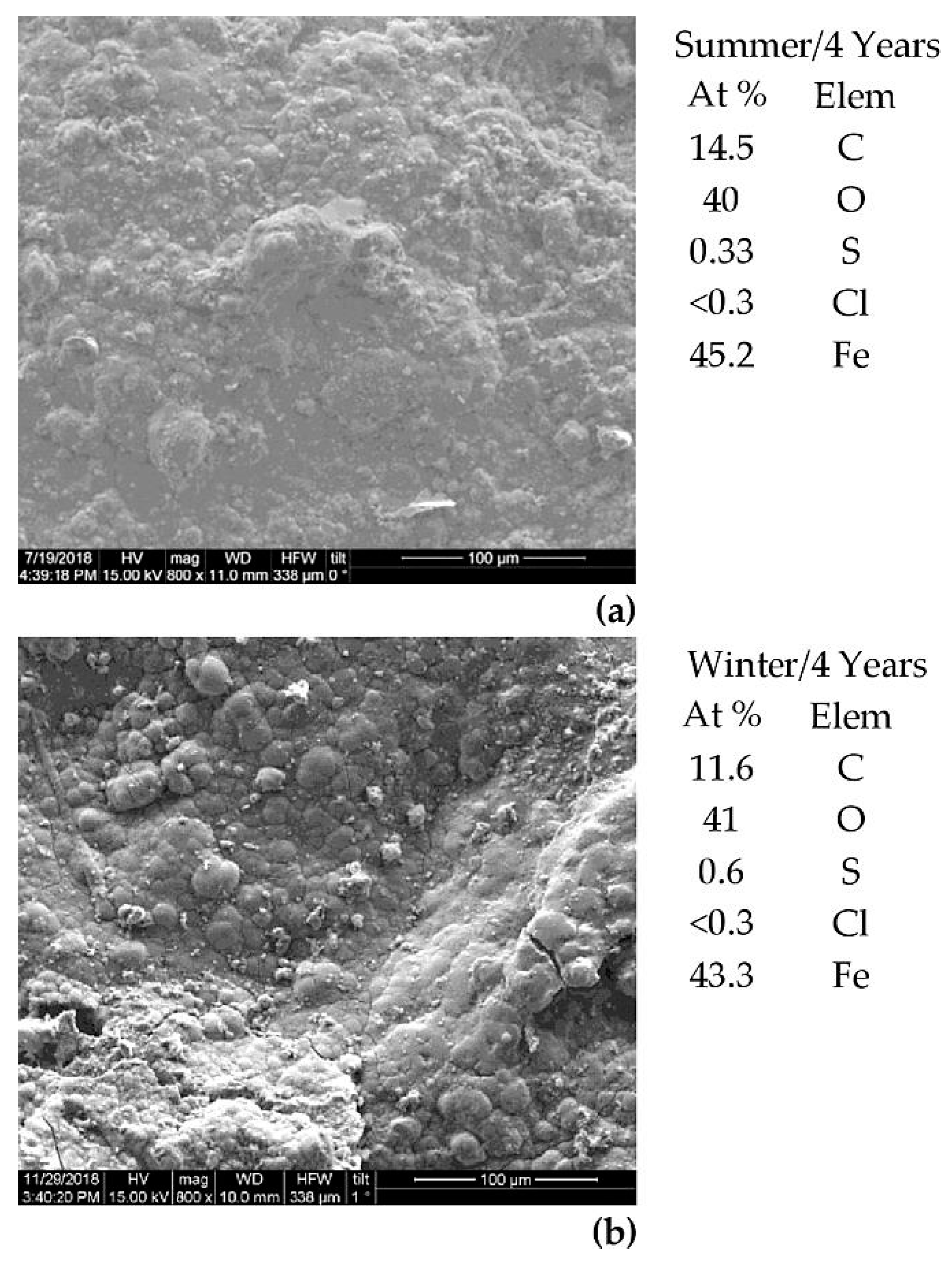


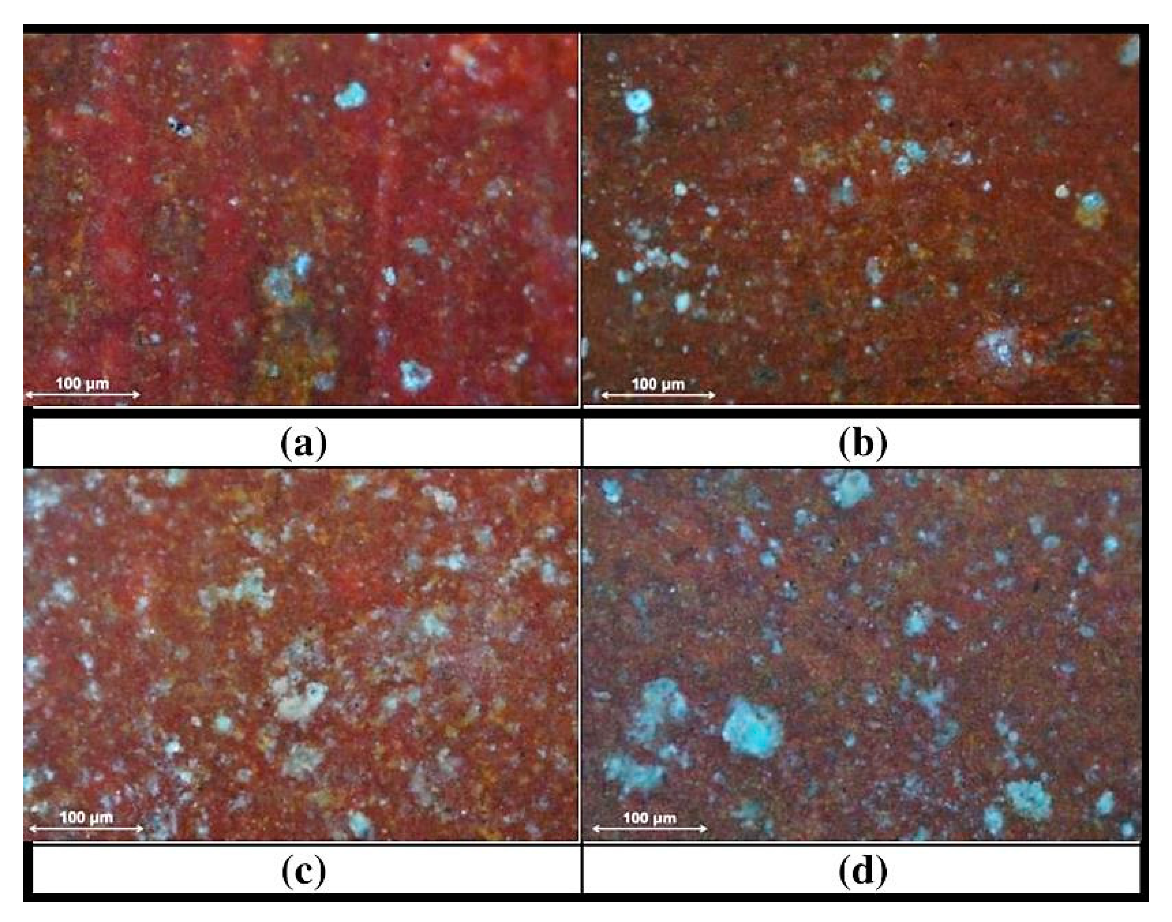
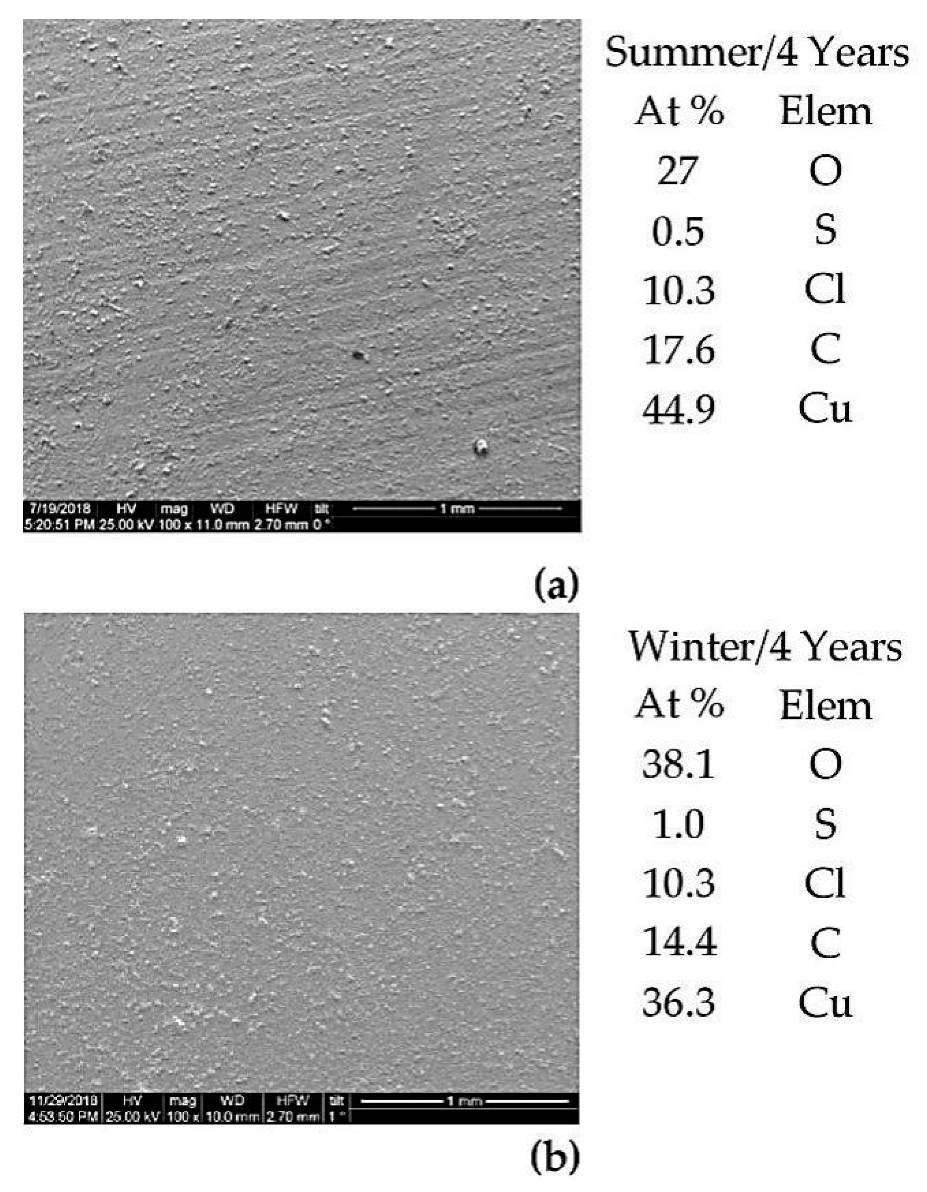



| Fe | C | Mn | S | P | Si | Ni | Cr | Cu | Al | Sn | Mo | Co | As | Nb | N | O | Other |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 99.44 | 0.07 | 0.32 | 0.03 | 0.007 | 0.007 | 0.02 | 0.02 | 0.04 | 0.01 | 0.004 | 0.003 | 0.003 | 0.0017 | 0.001 | 0.004 | 0.016 | 0.002 |
| Metal | Chemical Bath | Time | Temperature |
|---|---|---|---|
| Aluminum | 50 mL H3PO4, 30 g CrO3, distilled water to make up 1 L. | 10 min | 80 °C to boiling |
| Steel | 250 mL HCl with inhibitor, distilled water to make up 1 L. | 10 min | 20–25 °C |
| Zinc | 150 mL NH4OH, distilled water to make up 1 L. | 5 min | 20–25 °C |
| Copper | 500 mL HCl, distilled water to make up 1 L. | 3 min | 20–25 °C |
| Wettest Month (with Highest Rainfall) | Driest Months (with Lowest Rainfall) | Mean Annual Rainfall (Period 1958–1997) | Monthly Prevailing Wind (Period 1975–1991) | Calm | Mean Annual Wind Speed (Period 2009–2011) |
|---|---|---|---|---|---|
| December | July and August | 37.29 cm | NORTHWEST (ΝW) | 31% | 11.22 km/h (3.17 m/s) |
| Metal | Exposure Start | Average Mass Loss (g/m2) | Calculated Kinetic Equations Constants | |||||
|---|---|---|---|---|---|---|---|---|
| 1 Year | 2 Years | 4 Years | 30 Years | a | b | R2 | ||
| Carbon Steel | Summer | 149.1 | 216.4 | 248 | 709.4 | 759.9 × 10−6 | 0.49 | 0.9 |
| Winter | 126.5 | 281 | 267 | 772.7 | 907.5 × 10−6 | 0.48 | 0.86 | |
| Al | Summer | 0.31 | 0.44 | 0.61 | 1.39 | 2.80 × 10−6 | 0.42 | 0.96 |
| Winter | 0.57 | 0.65 | 0.64 | 0.91 | 22.2 × 10−6 | 0.16 | 0.92 | |
| Cu | Summer | 18.9 | 25.8 | 45.0 | 153.3 | 4.7 × 10−5 | 0.62 | 0.99 |
| Winter | 16.7 | 26.4 | 44.6 | 161.1 | 3.9 × 10−5 | 0.65 | 0.99 | |
| Zn | Summer | 4.4 | 7.1 | 13.5 | 74 | 0.29 × 10−5 | 0.85 | 0.99 |
| Winter | 8.4 | 12.9 | 15.4 | 34.6 | 8.9 × 10−5 | 0.39 | 0.98 | |
| Metal | Exposure Start | Corrosion Rate (μm/year) | ||
|---|---|---|---|---|
| Cu | Summer | 1 | 1.43 | 1.23 |
| Winter | 1.86 | 1.47 | 1.24 | |
| Exposure Start/Time of Exposure in Years | Summer | Winter |
|---|---|---|
| Before the Exposure | 65.2 nm | |
| 2 Years | 131 nm | 136 nm |
| 4 Years | 189.1 nm | 214 nm |
| Exposure Start | 2 Years of Exposure | 4 Years of Exposure | ||
|---|---|---|---|---|
| Maximum Pitting Depth in μm | Rating | Maximum Pitting Depth in μm | Rating | |
| Summer | 2 | C4 | 2 | D5 |
| Winter | 1.0 | B5 | 1.0 | D2 |
| Corrosion Category | Carbon Steel (g/m2year) | Aluminum (g/m2year) |
|---|---|---|
| C1 | ≤10 | Negligible |
| C2 | 11–200 | ≤0.6 |
| C3 | 201–400 | 0.6–2 |
| C4 | 401–650 | 2–5 |
| C5 | 651–1500 | 5–10 |
| Metal (g/m2): | Carbon Steel | Aluminum | ||||
|---|---|---|---|---|---|---|
| /Period of Initial Exposure | Mean Value | Standard Deviation | Max. Value | Mean Value | Standard Deviation | Max. Value |
| Summer | 148.1 | 25.7 | 195.2 | 0.308 | 0.016 | 0.331 |
| Winter | 123.5 | 15.6 | 140.7 | 0.562 | 0.007 | 0.571 |
| ISO Classification of the LGMG Atmosphere | C2 «LOW» | C2 «LOW» | ||||
| Critical Parameters/Airport | Distance to Sea/CDA Limit | Total Annual Rainfall/CDA Limit | Ozone (O3) Concentration/CDA Limit |
|---|---|---|---|
| Pachi | 0.2km < 4km | 37.29 < 125 cm/year | 55 > 36 μg/m3 |
| Site of Exposure | Classification of the Corrosivity of LGMG Atmosphere According to: | Metal Specimens | |
|---|---|---|---|
| Carbon Steel | Aluminum | ||
| LGMG | ISO | C2 «LOW» | C2 «LOW» |
| CDA | AA «very severe» | ||
| «Europe and Asia Corrosion Map» | «SEVERE» | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Titakis, C.; Vassiliou, P. Evaluation of 4-Year Atmospheric Corrosion of Carbon Steel, Aluminum, Copper and Zinc in a Coastal Military Airport in Greece. Corros. Mater. Degrad. 2020, 1, 159-186. https://doi.org/10.3390/cmd1010008
Titakis C, Vassiliou P. Evaluation of 4-Year Atmospheric Corrosion of Carbon Steel, Aluminum, Copper and Zinc in a Coastal Military Airport in Greece. Corrosion and Materials Degradation. 2020; 1(1):159-186. https://doi.org/10.3390/cmd1010008
Chicago/Turabian StyleTitakis, Charalampos, and Panayota Vassiliou. 2020. "Evaluation of 4-Year Atmospheric Corrosion of Carbon Steel, Aluminum, Copper and Zinc in a Coastal Military Airport in Greece" Corrosion and Materials Degradation 1, no. 1: 159-186. https://doi.org/10.3390/cmd1010008
APA StyleTitakis, C., & Vassiliou, P. (2020). Evaluation of 4-Year Atmospheric Corrosion of Carbon Steel, Aluminum, Copper and Zinc in a Coastal Military Airport in Greece. Corrosion and Materials Degradation, 1(1), 159-186. https://doi.org/10.3390/cmd1010008






