Influence of Maternal Working Hours on Children’s Sleep: A Preliminary Study on Disparities Between Day and Night Shifts
Abstract
1. Introduction
2. Results
Activity Parameters
3. Discussion
4. Materials and Methods
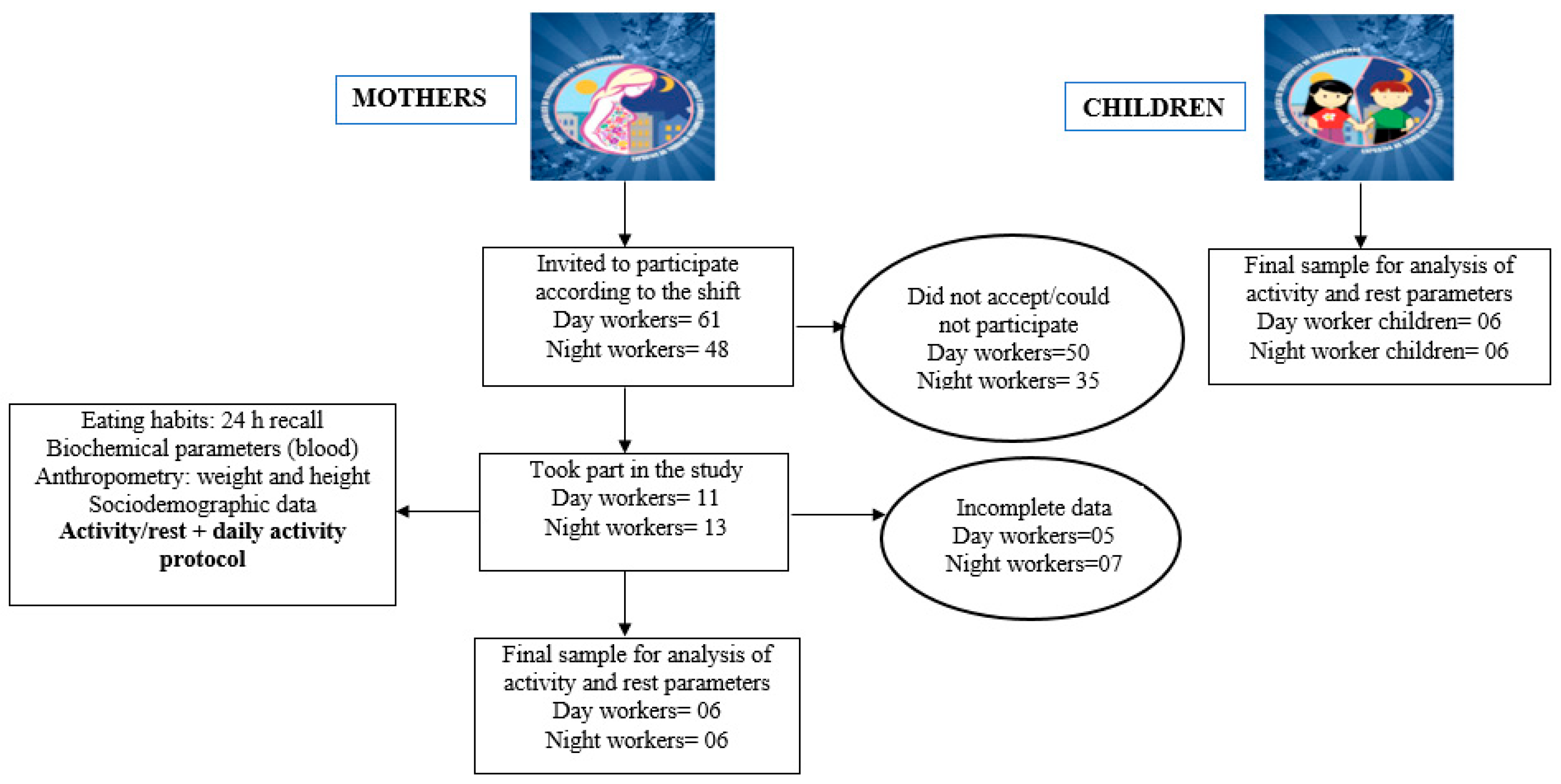
4.1. Participants
4.2. Study Protocol
4.3. Anthropometric Data
4.4. Activity and Sleep Data
4.5. Data Analysis
5. Conclusions
6. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roeths, T.; Roth, T. Sleep-wake state and memory function. Sleep 2000, 23 (Suppl. S3), S64–S68. [Google Scholar] [PubMed]
- Barone, M.T.U.; de Castro Moreno, C.R.; Prates, E.J.S.; Silveira, J. Sleep disorders are an overlooked risk factor for non-communicable diseases. Br. Med. J. 2023, 383, 2721. [Google Scholar] [CrossRef]
- Engeda, J.; Mezuk, B.; Ratliff, S.; Ning, Y. Association between duration and quality of sleep and the risk of pre-diabetes: Evidence from NHANES. Diabet. Med. 2013, 30, 676–680. [Google Scholar] [CrossRef]
- Tan, N.Y.Q.; Chan, J.; Cheng, C.-Y.; Wong, T.Y.; Sabanayagam, C. Sleep duration and diabetic kidney disease. Front. Endocrinol. 2018, 9, 808. [Google Scholar] [CrossRef]
- Antza, C.; Kostopoulos, G.; Mostafa, S.; Nirantharakumar, K.; Tahrani, A. The links between sleep duration, obesity and type 2 diabetes mellitus. J. Endocrinol. 2021, 252, 125–141. [Google Scholar] [CrossRef]
- Li, C.; Shang, S. Relationship between Sleep and Hypertension: Findings from the NHANES (2007–2014). Int. J. Environ. Res. Public Health 2021, 18, 7867. [Google Scholar] [CrossRef] [PubMed]
- Moon, C.; Hagen, E.W.; Johnson, H.M.; Brown, R.L.; Peppard, P.E. Longitudinal sleep characteristics and hypertension status: Results from the Wisconsin Sleep Cohort Study. J. Hypertens. 2021, 39, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.A.; Howarth, N.E. Sleep and cardiovascular disease. Emerg. Top. Life Sci. 2023, 7, 457–466. [Google Scholar] [CrossRef] [PubMed]
- De Jonge, L.; Zhao, X.; Mattingly, M.S.; Zuber, S.M.; Piaggi, P.; Csako, G.; Cizza, G.; NIDDK Sleep Extension Study Group. Poor sleep quality and sleep apnea are associated with higher resting energy expenditure in obese individuals with short sleep duration. J. Clin. Endocrinol. Metab. 2012, 97, 2881–2889. [Google Scholar] [CrossRef]
- Broussard, J.L.; Klein, S. Insufficient sleep and obesity: Cause or consequence. Obesity 2022, 30, 1914–1916. [Google Scholar] [CrossRef]
- Chaput, J.P.; McHill, A.W.; Cox, R.C.; Broussard, J.L.; Dutil, C.; da Costa, B.G.G.; Sampasa-Kanyinga, H.; Wright, K.P., Jr. The role of insufficient sleep and circadian misalignment in obesity. Nat. Rev. Endocrinol. 2023, 19, 82–97. [Google Scholar] [CrossRef]
- Cappuccio, F.P.; Miller, M.A. Sleep and Cardio-Metabolic Disease. Curr. Cardiol. Rep. 2017, 19, 110. [Google Scholar] [CrossRef] [PubMed]
- Lechner, K.; von Schacky, C.; McKenzie, A.L.; Worm, N.; Nixdorff, U.; Lechner, B.; Kränkel, N.; Halle, M.; Krauss, R.M.; Scherr, J. Lifestyle factors and high-risk atherosclerosis: Pathways and mechanisms beyond traditional risk factors. Eur. J. Prev. Cardiol. 2020, 27, 394–406. [Google Scholar] [CrossRef]
- Ródenas-Munar, M.; Monserrat-Mesquida, M.; Gómez, S.F.; Wärnberg, J.; Medrano, M.; González-Gross, M.; Gusi, N.; Aznar, S.; Marín-Cascales, E.; González-Valeiro, M.A.; et al. Perceived Quality of Life Is Related to a Healthy Lifestyle and Related Outcomes in Spanish Children and Adolescents: The Physical Activity, Sedentarism, and Obesity in Spanish Study. Nutrients 2023, 15, 5125. [Google Scholar] [CrossRef] [PubMed]
- Markwald, R.R.; Melanson, E.L.; Smith, M.R.; Higgins, J.; Perreault, L.; Eckel, R.H.; Wright, K.P., Jr. Impact of insufficient sleep on total daily energy expenditure, food intake, and weight gain. Proc. Natl. Acad. Sci. USA 2013, 110, 5695–5700. [Google Scholar] [CrossRef]
- St-Onge, M.-P.; Pizinger, T.; Kovtun, K.; RoyChoudhury, A. Sleep and meal timing influence food intake and its hormonal regulation in healthy adults with overweight/obesity. Eur. J. Clin. Nutr. 2019, 72 (Suppl. S1), 76–82. [Google Scholar] [CrossRef] [PubMed]
- Papatriantafyllou, E.; Efthymiou, D.; Zoumbaneas, E.; Popescu, C.A.; Vassilopoulou, E. Sleep Deprivation: Effects on Weight Loss and Weight Loss Maintenance. Nutrients 2022, 14, 1549. [Google Scholar] [CrossRef]
- Giorgi, F.; Mattei, A.; Notarnicola, I.; Petrucci, C.; Lancia, L. Can sleep quality and burnout affect the job performance of shift-work nurses? A hospital cross-sectional study. J. Adv. Nurs. 2018, 74, 698–708. [Google Scholar] [CrossRef]
- Visvalingam, N.; Sathish, T.; Soljak, M.; Chua, A.-P.; Dunleavy, G.; Divakar, U.; Nazeha, N.; Bajpai, R.; Soh, C.K.; Woon, K.K.; et al. Prevalence of and factors associated with poor sleep quality and short sleep in a working population in Singapore. Sleep Health 2020, 6, 277–287. [Google Scholar] [CrossRef]
- Kecklund, G.; Axelsson, J. Health consequences of shift work and insufficient sleep. Br. Med. J. 2016, 355, i5210. [Google Scholar] [CrossRef]
- Ganesan, S.; Magee, M.; Stone, J.E.; Mulhall, M.D.; Collins, A.; Howard, M.E.; Lockley, S.W.; Rajaratnam, S.M.W.; Sletten, T.L. The Impact of Shift Work on Sleep, Alertness and Performance in Healthcare Workers. Sci. Rep. 2019, 9, 4635. [Google Scholar] [CrossRef]
- Kanki, M.; Nath, A.P.; Xiang, R.; Yiallourou, S.; Fuller, P.J.; Cole, T.J.; Cánovas, R.; Young, M.J. Poor sleep and shift work associate with increased blood pressure and inflammation in UK Biobank participants. Nat. Commun. 2023, 14, 7096. [Google Scholar] [CrossRef] [PubMed]
- Nehme, P.A.; Amaral, F.G.; Middleton, B.; Lowden, A.; Marqueze, E.; França-Junior, I.; Antunes, J.L.F.; Cipolla-Neto, J.; Skene, D.J.; Moreno, C.R. Melatonin profiles during the third trimester of pregnancy and health status in the offspring among day and night workers: A case series. Neurobiol. Sleep Circadian Rhythm 2019, 6, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Strohmaier, S.; Devore, E.E.; Vetter, C.; Missmer, S.; Eliassen, A.H.; Rosner, B.; Rich-Edwards, J.; Field, A.E.; Schernhammer, E.S. Night Shift Work Before and During Pregnancy and Offspring Weight Outcomes Through Adolescence. Obesity 2018, 26, 1491–1500. [Google Scholar] [CrossRef]
- Zhao, Y.; Cooklin, A.; Butterworth, P.; Strazdins, L.; Leach, L.S. How does working nonstandard hours impact psychological resources important for parental functioning? Evidence from an Australian longitudinal cohort study. SSM Popul. Health 2021, 16, 100931. [Google Scholar] [CrossRef]
- Tucker, P.; Leineweber, C.; Kecklund, G. Comparing the acute effects of shiftwork on mothers and fathers. Occup. Med. 2021, 71, 414–421. [Google Scholar] [CrossRef]
- Arlinghaus, A.; Bohle, P.; Iskra-Golec, I.; Jansen, N.; Jay, S.; Rotenberg, L. Working Time Society consensus statements: Evidence-based effects of shift work and non-standard working hours on workers, family and community. Ind. Health 2019, 57, 184–200. [Google Scholar] [CrossRef] [PubMed]
- Kalil, A.; Dunifon, R.; Crosby, D.; Su, J.H. Work Hours, Schedules, and Insufficient Sleep Among Mothers and Their Young Children. J. Marriage Fam. 2014, 76, 891–904. [Google Scholar] [CrossRef] [PubMed]
- Ananat, E.O.; Gassman-Pines, A. Work Schedule Unpredictability: Daily Occurrence and Effects on Working Parents’ Well-Being. J. Marriage Fam. 2021, 83, 10–26. [Google Scholar] [CrossRef]
- Matsuoka, M.; Matsuishi, T.; Nagamitsu, S.; Iwasaki, M.; Iemura, A.; Obara, H.; Yamashita, Y.; Maeda, M.; Kakuma, T.; Uchimura, N. Sleep disturbance has the largest impact on children’s behavior and emotions. Front. Pediatr. 2022, 10, 1034057. [Google Scholar] [CrossRef]
- Han, W.J.; Miller, D.P.; Waldfogel, J. Parental work schedules and adolescent risky behaviors. Dev. Psychol. 2010, 46, 1245–1267. [Google Scholar] [CrossRef]
- Silva, I.; Costa, D. Consequences of Shift Work and Night Work: A Literature Review. Healthcare 2023, 11, 1410. [Google Scholar] [CrossRef]
- Perry-Jenkins, M.; Goldberg, A.E.; Pierce, C.P.; Sayer, A.G. Shift Work, Role Overload, and the Transition to Parenthood. J. Marriage Fam. 2007, 69, 123–138. [Google Scholar] [CrossRef]
- Costa, G. Shift work and health: Current problems and preventive actions. Saf. Health Work 2010, 1, 112–123. [Google Scholar] [CrossRef]
- Rosa, D.; Terzoni, S.; Dellafiore, F.; Destrebecq, A. Systematic review of shift work and nurses’ health. Occup. Med. 2019, 69, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ji, X.; Pitt, S.; Wang, G.; Rovit, E.; Lipman, T.; Jiang, F. Childhood sleep: Physical, cognitive, and behavioral consequences and implications. World J. Pediatr. 2024, 20, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Matricciani, L.; Bin, Y.S.; Lallukka, T.; Kronholm, E.; Dumuid, D.; Paquet, C.; Olds, T. Past, present, and future: Trends in sleep duration and implications for public health. Sleep Health 2017, 3, 317–323. [Google Scholar] [CrossRef]
- Giannotti, F.; Cortesi, F. Family and cultural influences on sleep development. Child Adolesc. Psychiatr. Clin. N. Am. 2009, 18, 849–861. [Google Scholar] [CrossRef]
- Hirshkowitz, M.; Whiton, K.; Albert, S.M.; Alessi, C.; Bruni, O.; DonCarlos, L.; Hazen, N.; Herman, J.; Adams Hillard, P.J.; Katz, E.S.; et al. National Sleep Foundation’s updated sleep duration recommendations: Final report. Sleep Health 2015, 1, 233–243. [Google Scholar] [CrossRef]
- Consensus Conference Panel; Watson, N.F.; Badr, M.S.; Belenky, G.; Bliwise, D.L.; Buxton, O.; Buysse, D.; Dinges, D.F.; Gangwisch, J.; Grandner, M.; et al. Joint Consensus Statement of the American Academy of Sleep Medicine and Sleep Research Society on the Recommended Amount of Sleep for a Healthy Adult: Methodology and Discussion. Sleep 2015, 38, 1161–1183. [Google Scholar] [CrossRef] [PubMed]
- Wells, M.E.; Vaughn, B.V. Poor sleep challenging the health of a Nation. Neurodiagn. J. 2012, 52, 233–249. [Google Scholar] [PubMed]
- Chattu, V.K.; Sakhamuri, S.M.; Kumar, R.; Spence, D.W.; BaHammam, A.S.; Pandi-Perumal, S.R. Insufficient sleep: Is it time to recognize it as a major non-communicable disease? Sleep Sci. 2018, 11, 56–64. [Google Scholar] [CrossRef]
- Kim, Y.; Wilkens, L.R.; Schembre, S.M.; Henderson, B.E.; Kolonel, L.N.; Goodman, M.T. Insufficient and excessive amounts of sleep increase the risk of premature death from cardiovascular and other diseases: The Multiethnic Cohort Study. Prev. Med. 2013, 57, 377–385. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, H.; Wang, J.; Zhang, S.; Tong, S.; Hu, J.; Che, Y.; Zhuo, L.; Wang, P.; Geng, R.; Zhou, Y.; et al. Relationship between night shift and sleep problems, risk of metabolic abnormalities of nurses: A 2 years follow-up retrospective analysis in the National Nurse Health Study (NNHS). Int. Arch. Occup. Environ. Health 2023, 96, 1361–1371. [Google Scholar] [CrossRef] [PubMed]
- Owens, J.A.; Weiss, M.R. Insufficient sleep in adolescents: Causes and consequences. Minerva Pediatr. 2017, 69, 326–336. [Google Scholar] [CrossRef]
- Boivin, D.B.; Boudreau, P.; Kosmadopoulos, A. Disturbance of the Circadian System in Shift Work and Its Health Impact. J. Biol. Rhythms 2022, 37, 3–28. [Google Scholar] [CrossRef]
- Fekedulegn, D.; Andrew, M.E.; Shi, M.; Violanti, J.M.; Knox, S.; Innes, K.E. Actigraphy-Based Assessment of Sleep Parameters. Ann. Work Expo. Health 2020, 64, 350–367. [Google Scholar] [CrossRef] [PubMed]
- Sack, R.L.; Auckley, D.; Auger, R.R.; Carskadon, M.A.; Wright, K.P., Jr.; Vitiello, M.V.; Zhdanova, I.V. American Academy of Sleep Medicine Circadian rhythm sleep disorders: Part I, basic principles, shift work and jet lag disorders. Sleep 2007, 30, 1460–1483. [Google Scholar] [CrossRef]
- Coles, L.; Thorpe, K.; Smith, S.; Hewitt, B.; Ruppanner, L.; Bayliss, O.; O’FLaherty, M.; Staton, S. Children’s sleep and fathers’ health and wellbeing: A systematic review. Sleep Med. Rev. 2022, 61, 101570. [Google Scholar] [CrossRef]
- World Health Organization. Obesity: Preventing and man-aging the global epidemic-report of a WHO consultation. World Health Organ Tech. Rep. Ser. 2000, 894, 1–253. [Google Scholar]
- World Health Organization. The WHO Child Growth Standards. 2007. Available online: https://www.who.int/tools/growth-reference-data-for-5to19-years (accessed on 30 March 2025).
- Bland, J.M.; Altman, D.G. Statistical method for assessing agreement between two methods of clinical measurement. Lancet 1986, 327, 307–310. [Google Scholar] [CrossRef]

| Variables | Day Workers and Children (n = 12) | Night Workers and Children (n = 12) | ||||||
|---|---|---|---|---|---|---|---|---|
| Mothers | Children | Mothers | Children | |||||
| n % | Mean (sd) [Range] | n % | Mean (sd) [Range] | n % | Mean (sd) [Range] | n % | Mean (sd) [Range] | |
| Age | ||||||||
| Years | 6 (100) | 47.67 (2.42) [6] | 6 (100) | 7.83 (1.47) [4] | 6 (100) | 49.6 (4.97) [8] | 6 (100) | 9.17 (0.75) [2] |
| Level of education | ||||||||
| Elementary and middle school | 0 0 | n.a. | 6 100 | n.a. | 0 0 | n.a. | 6 100 | n.a. |
| High school | 6 100 | n.a | 0 0 | n.a. | 5 83.3 | n.a. | 0 0 | n.a. |
| Undergraduate degree | 0 0 | n.a. | 0 0 | n.a. | 1 16.7 | n.a. | 0 0 | n.a. |
| Nutritional Status | ||||||||
| Eutrophy | 2 33.3 | n.a. | 5 83.3 | n.a. | 2 33.3 | n.a. | 4 66.7 | n.a. |
| Overweight | 2 33.3 | n.a. | 1 16.7 | n.a. | 1 16.7 | n.a. | 2 33.3 | n.a. |
| Obesity | 2 33.3 | n.a. | 0 0 | n.a. | 3 50.0 | n.a. | 0 0 | n.a. |
| Net family income | ||||||||
| ≤BRL 4000.00 | 6 100 | n.a. | n.a. | n.a. | 5 83.3 | n.a. | n.a. | n.a. |
| ≥BRL 4001.00 | 0 0 | n.a. | n.a. | n.a. | 1 16.7 | n.a. | n.a. | n.a. |
| Professional category | ||||||||
| Nurse | 0 0 | n.a. | n.a. | n.a. | 1 16.7 | n.a. | n.a. | n.a. |
| Nursing assistant/technician | 6 100 | n.a. | n.a. | n.a. | 5 83.3 | n.a. | n.a. | n.a. |
| Current marital status | ||||||||
| Single | 2 33.3 | n.a. | n.a. | n.a. | 0 0 | n.a. | n.a. | n.a. |
| Married/lives with partner | 4 66.7 | n.a. | n.a. | n.a. | 4 66.7 | n.a. | n.a. | n.a. |
| Separated/divorced/widowed | 0 0 | n.a. | n.a. | n.a. | 2 33.3 | n.a. | n.a. | n.a. |
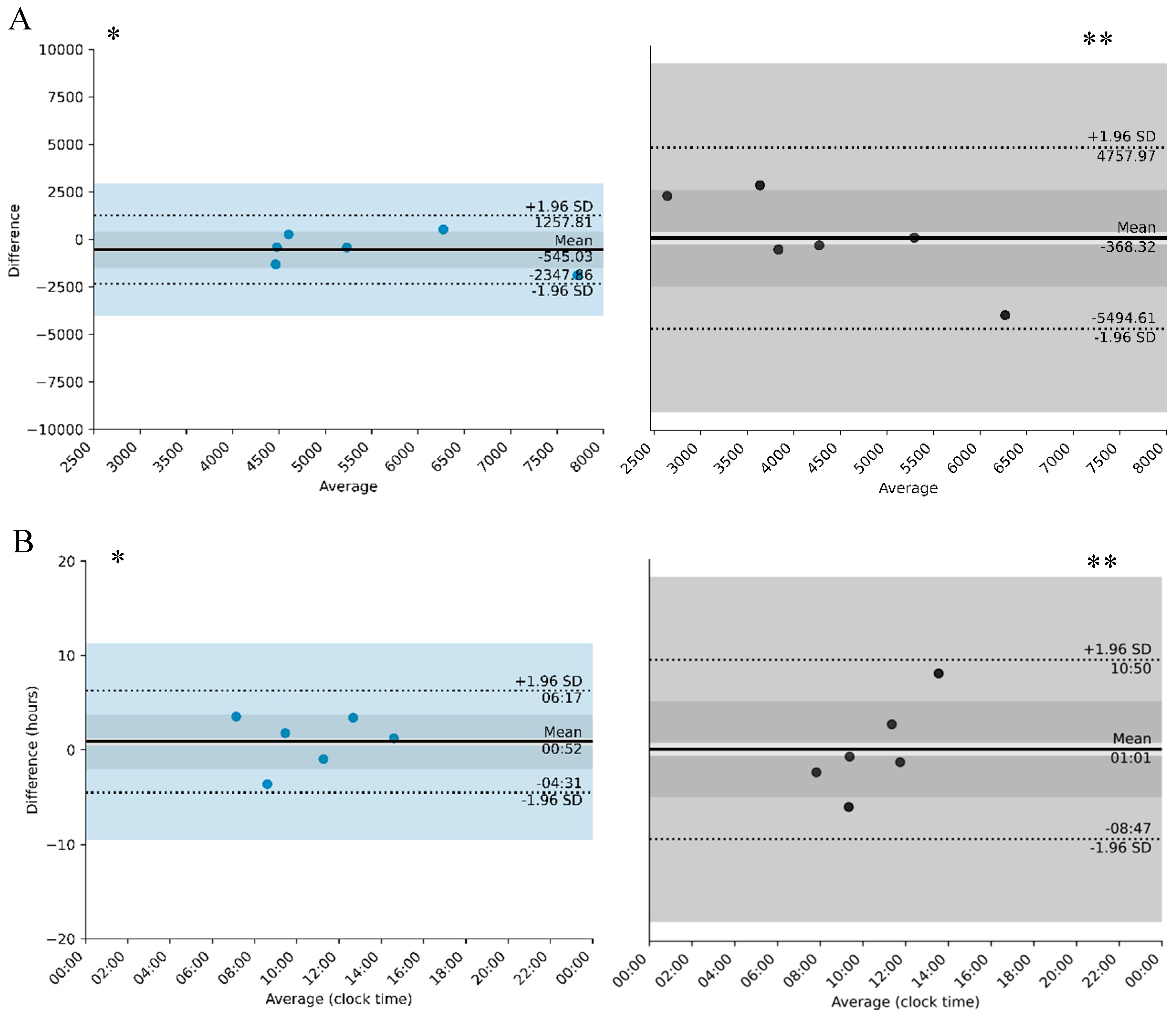
| Total time of sleep (h) | n.a. | 6 h 20 (1 h 02) [5 h 43–8 h 04] | n.a. | 7 h 13 (01 h 25) [5 h 9–9 h 06] | n.a. | 7 h 06 (01 h 54) [5 h 8–10 h 12] | n.a. | 7 h 35 (01 h 42) [5 h 19–10 h 20] |
| Diurnal motor activity, M10 (counts/min) | n.a. | 4920.7 (1133.5) [3807.2–6773.5] | n.a. | 5734.4 (1539.9) [4474.2–8671.0] | n.a. | 4138.1 (727.6) [3645.5–5124.7] | n.a. | 4506.4 (2489.5) [1630.4–8629.4] |
| Nocturnal motor activity, L5 (counts/min) | n.a. | 332.21 (105.24) [252.2–493.6] | n.a. | 390.78 (271.89) [150.9–741.3] | n.a. | 1250.74 (290.15) [911.3–1767.1] | n.a. | 562.36 (320.90) [204.1–1152.1] |
| Actigraphy | Parameter | Day Workers (Mothers and Children) | Night Workers (Mothers and Children) | ||||
| Agreement Range | Average Difference in Group | Average | Agreement Range | Average Difference | Average | ||
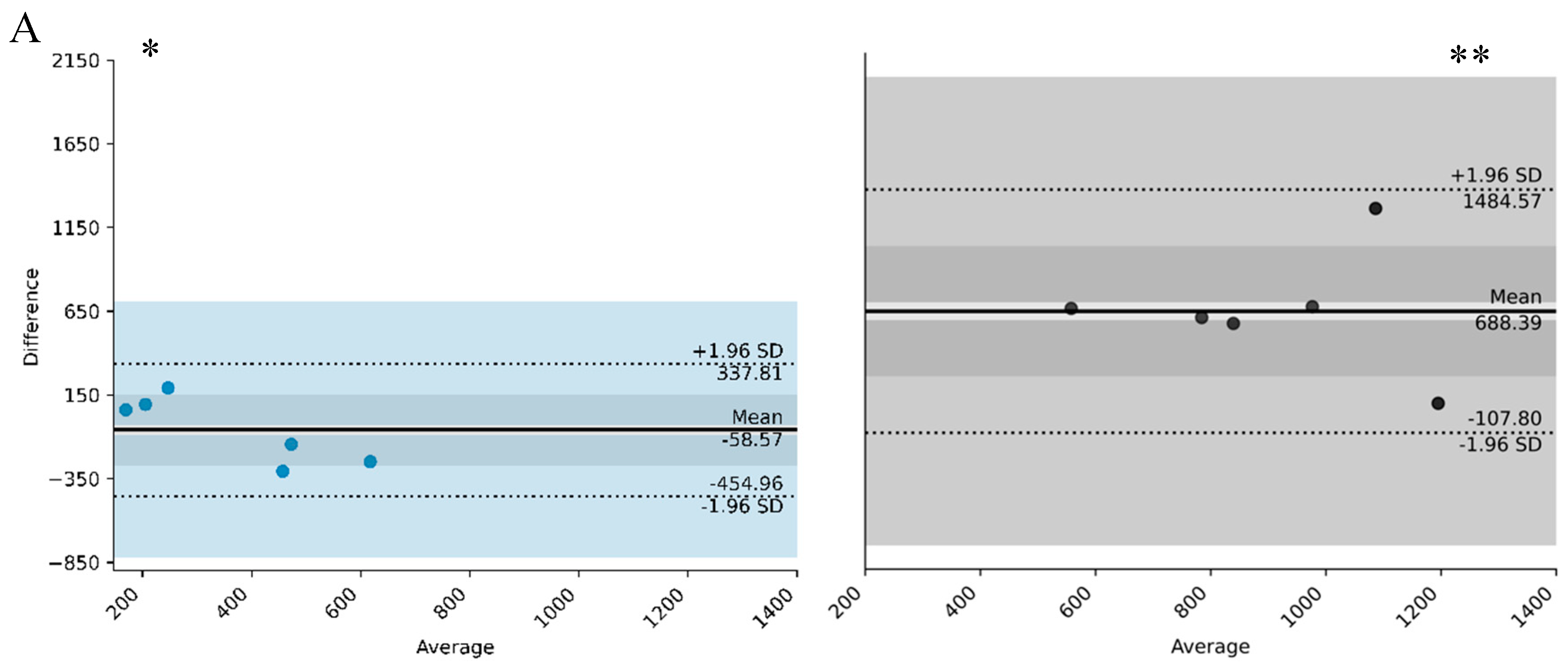
| L5 (counts/min) | −454.96 and 337.81 | −58.57 | 500 | −107.8 and 1485.57 | 688.39 | 1200 | |
| L5 onset (h) | 1 h 42 and 3 h 24 | 51 min | 11:00–12:00 * | −4 h 26 and 8 h 18 | 1 h 47 * | 11:00 and 7:00 | |
| M10 (counts/min) | −2347.86 and 1257.81 | 545.03 | 4500 and 7500 ** | −5494.61 and −4757.97 | −368.32 | 2500 and 6500 ** | |
| M 10 onset (h) | −4 h 22 and 6 h 17 | 53 min | 7:00 and 2:30 * | −8 h 47 and 10 h 51 | 1 h 2 | 7:00 and 2:00 * | |
| IS | −0.30 and 0.28 | −0.01 | 0.5 and 0.65 ** | −0.20 and 0.27 | 0.03 | 0.20 and 0.40 ** | |
| IV | −0.19 and 0.2 | 0.04 | 0.6 and 0.9 * | −0.36 and 0.67 | 0.15 | 0.5 and 1.1 | |
| Relative amplitude (RA) | −0.13 and 0.16 | 0.01 | 0.7 and 0.9 ** | −0.41 and −0.04 | −0.22 | 0.5 and 0.7 ** | |
| Bedtime (h) | −2 h 14 and 2 h 17 | 8 min | 11:00–12:00 * | −11 h 40 and 15 h 37 | 2 h 17 | 11:00 and 8:00 * | |
| Wake time (h) | −4 h 37 and 3 h 18 | −40 min | 7:00 and 10:00 * | −9 h 45 and 11 h 53 | 1 h 4 | 7:00 and 3:00 * | |
| Total time of sleep (h) | −2 h 51 and 1 h 3 | −54 min | 5:00 and 8:00 * | −5 h 20 and 4 h 22 | 29 min | 5:00 and 9:00 * | |
| Sleep efficiency (%) | −11.24 and 10.10 | −0.57 | 75 and 90 ** | −29.34 and 21.65 | −3.85 | 75 and 90 ** | |
| WASO (min) | −88.04 and 76.37 | −5.83 | 40 and 110 ** | −213.93 and 283.67 | 34.67 | 70 and 190 | |
| WEP | −19.93 and 21.52 | 0.80 | 13 and 26 ** | −29.82 and 18.60 | −5.61 | 13 and 31 ** | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nehme, P.A.; Santos, J.; Benedito-Silva, A.A.; Cipolla-Neto, J.; Moreno, C.R.C. Influence of Maternal Working Hours on Children’s Sleep: A Preliminary Study on Disparities Between Day and Night Shifts. Clocks & Sleep 2025, 7, 60. https://doi.org/10.3390/clockssleep7040060
Nehme PA, Santos J, Benedito-Silva AA, Cipolla-Neto J, Moreno CRC. Influence of Maternal Working Hours on Children’s Sleep: A Preliminary Study on Disparities Between Day and Night Shifts. Clocks & Sleep. 2025; 7(4):60. https://doi.org/10.3390/clockssleep7040060
Chicago/Turabian StyleNehme, Patrícia Andrade, Jefferson Santos, Ana Amélia Benedito-Silva, José Cipolla-Neto, and Claudia R. C. Moreno. 2025. "Influence of Maternal Working Hours on Children’s Sleep: A Preliminary Study on Disparities Between Day and Night Shifts" Clocks & Sleep 7, no. 4: 60. https://doi.org/10.3390/clockssleep7040060
APA StyleNehme, P. A., Santos, J., Benedito-Silva, A. A., Cipolla-Neto, J., & Moreno, C. R. C. (2025). Influence of Maternal Working Hours on Children’s Sleep: A Preliminary Study on Disparities Between Day and Night Shifts. Clocks & Sleep, 7(4), 60. https://doi.org/10.3390/clockssleep7040060






