Exploring the Cross-Sectional Association Between Hypothyroidism and Circadian Syndrome: Insights from NHANES 2007–2012
Abstract
1. Introduction
2. Results
2.1. Baseline Characteristics
2.2. Hypothyroidism Prevalence
2.3. Association of Hypothyroidism Status with CircS and MetS
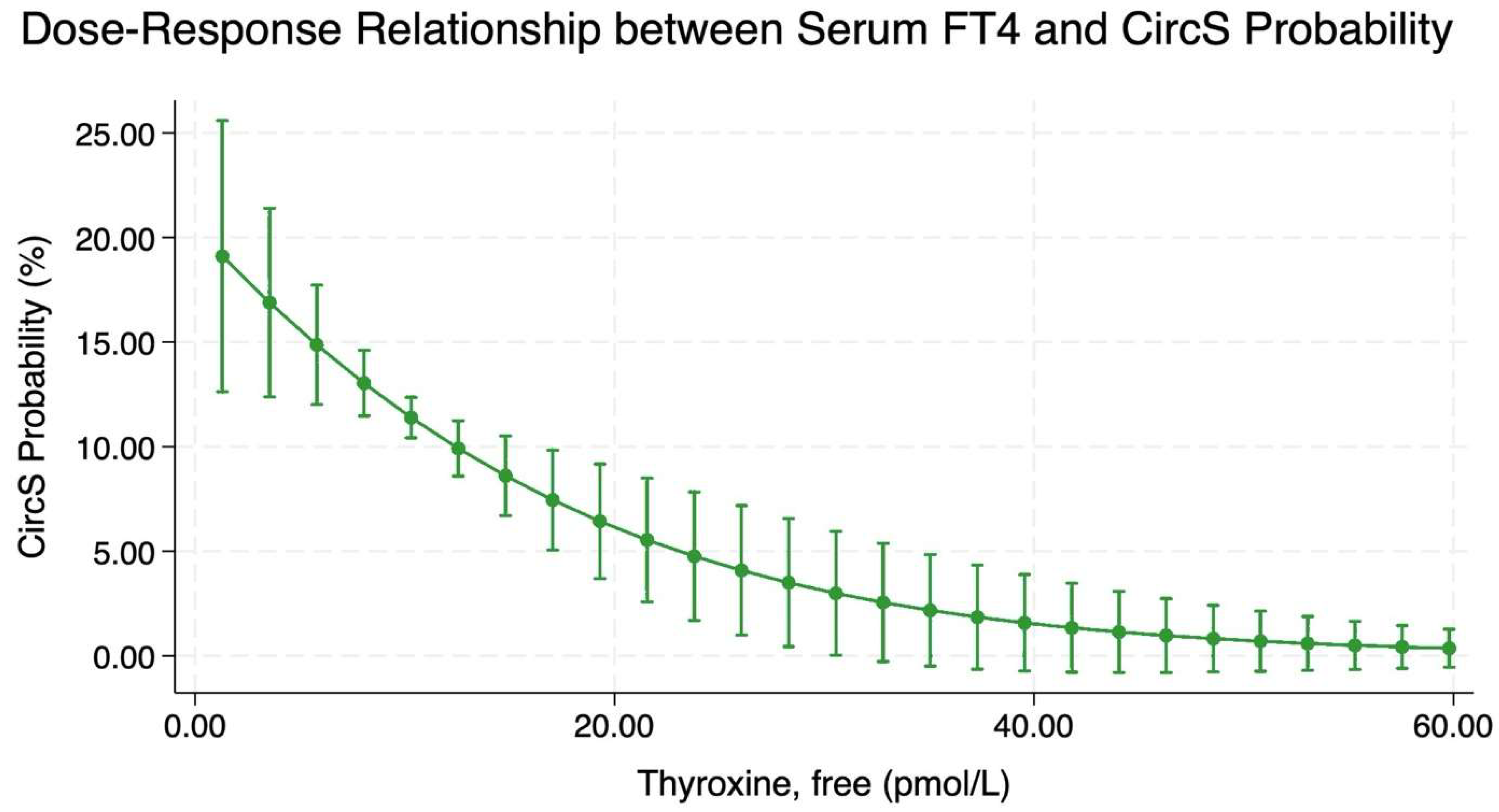
2.4. Dose–Response Relationship Between Serum Free Thyroxine and CircS Probability
3. Discussion
4. Materials and Methods
4.1. Study Design and Sample
4.2. Exposure Measure: Hypothyroidism Status
4.3. Outcome Measure: Circadian Syndrome Status
4.4. Covariates
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CircS | Circadian Syndrome |
| MetS | Metabolic Syndrome |
| FT4 | Free Thyroxine |
| TSH | Thyroid-Stimulating Hormone |
| NHANES | National Health and Nutrition Examination Survey |
| NDM | Non-Drug Managed |
| CVDs | Cardiovascular Diseases |
| T2DM | Type 2 Diabetes Mellitus |
| TG | Triglycerides |
| HDL | High-Density Lipoprotein |
| NAFLD | Non-Alcoholic Fatty Liver Disease |
| SCN | Suprachiasmatic Nucleus |
| CKD | Chronic Kidney Disease |
| AST | Aspartate Aminotransferase |
| APRI | Aspartate Aminotransferase to Platelet Ratio Index |
| PHQ-9 | Patient Health Questionnaire-9 |
| FPG | Fasting Plasma Glucose |
| US-FLI | United States Fatty Liver Index |
| ROC | Receiver Operating Characteristic |
| AUC | Area Under the Curve |
| MET | Metabolic Equivalent of Task |
| DAG | Directed Acyclic Graph |
| HEI-2020 | Healthy Eating Index-2020 |
| PIR | Poverty Income Ratio |
| STROBE | Strengthening the Reporting of Observational Studies in Epidemiology |
| CDC | Centers for Disease Control and Prevention |
References
- Arabi, A.; Nasrallah, D.; Mohsen, S.; Abugharbieh, L.; Al-Hashimi, D.; AlMass, S.; Albasti, S.; Al-Ajmi, S.A.; Khan, M.N.; Zughaier, S.M. Association between Serum Vitamin D Status and Circadian Syndrome: A Cross-Sectional Study. Nutrients 2024, 16, 2111. [Google Scholar] [CrossRef]
- Alberti, K.G.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.; Loria, C.M.; Smith, S.C., Jr. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar] [CrossRef]
- Hastings, M.H.; Maywood, E.S.; Brancaccio, M. Generation of circadian rhythms in the suprachiasmatic nucleus. Nat. Rev. Neurosci. 2018, 19, 453–469. [Google Scholar] [CrossRef]
- Panda, S. The arrival of circadian medicine. Nat. Rev. Endocrinol. 2019, 15, 67–69. [Google Scholar] [CrossRef]
- Emrich, F.; Gomes, B.H.; Selvatici-Tolentino, L.; Lopes, R.A.; Secio-Silva, A.; Carvalho-Moreira, J.P.; Bittencourt-Silva, P.G.; Guarnieri, L.O.; Silva, A.B.P.; Drummond, L.R.; et al. Hypothyroidism alters the rhythmicity of the central clock, body temperature and metabolism: Evidence of Bmal1 transcriptional regulation by T3. J. Physiol. 2024, 602, 4865–4887. [Google Scholar] [CrossRef]
- Peliciari-Garcia, R.A.; Bargi-Souza, P.; Young, M.E.; Nunes, M.T. Repercussions of hypo and hyperthyroidism on the heart circadian clock. Chronobiol. Int. 2018, 35, 147–159. [Google Scholar] [CrossRef]
- Pingitore, A.; Gaggini, M.; Mastorci, F.; Sabatino, L.; Cordiviola, L.; Vassalle, C. Metabolic Syndrome, Thyroid Dysfunction, and Cardiovascular Risk: The Triptych of Evil. Int. J. Mol. Sci. 2024, 25, 10628. [Google Scholar] [CrossRef] [PubMed]
- Lakhani, G.; Patel, P.; Patel, T.C. A Cross-Sectional Study on the Prevalence of Subclinical Hypothyroidism in Metabolic Syndrome Patients at a Tertiary Care Hospital. Cureus 2024, 16, e67851. [Google Scholar] [CrossRef] [PubMed]
- Verma, D.P.; Chaudhary, S.C.; Singh, A.; Sawlani, K.K.; Gupta, K.K.; Usman, K.; Reddy, H.D.; Patel, M.L.; Verma, S.K.; Atam, V. Hypothyroidism in Metabolic Syndrome. Ann. Afr. Med. 2024, 23, 717–722. [Google Scholar] [CrossRef]
- Wang, B.; Huang, J.; Chen, L. Association between depression and anxiety disorders with euthyroid Hashimoto’s thyroiditis: A systematic review and meta-analysis. Compr. Psychoneuroendocrinol. 2024, 20, 100279. [Google Scholar] [CrossRef] [PubMed]
- Soares De Oliveira, L.; Ritter, M.J. Thyroid hormone and the Liver. Hepatol. Commun. 2025, 9. [Google Scholar] [CrossRef]
- Acharya, H.; Jayaraj Mangala, S.; Kalra, P. Evaluating the Spectrum of Sleep Abnormalities in Patients With Primary Hypothyroidism. Cureus 2024, 16, e69855. [Google Scholar] [CrossRef]
- Khatiwada, S.; Sah, S.K.; Kc, R.; Baral, N.; Lamsal, M. Thyroid dysfunction in metabolic syndrome patients and its relationship with components of metabolic syndrome. Clin. Diabetes Endocrinol. 2016, 2, 3. [Google Scholar] [CrossRef]
- Heima, N.E.; Eekhoff, E.M.; Oosterwerff, M.M.; Lips, P.T.; van Schoor, N.M.; Simsek, S. Thyroid function and the metabolic syndrome in older persons: A population-based study. Eur. J. Endocrinol. 2013, 168, 59–65. [Google Scholar] [CrossRef]
- He, J.; Lai, Y.; Yang, J.; Yao, Y.; Li, Y.; Teng, W.; Shan, Z. The Relationship Between Thyroid Function and Metabolic Syndrome and Its Components: A Cross-Sectional Study in a Chinese Population. Front. Endocrinol. 2021, 12. [Google Scholar] [CrossRef]
- Kota, S.K.; Meher, L.K.; Krishna, S.; Modi, K. Hypothyroidism in metabolic syndrome. Indian J. Endocrinol. Metab. 2012, 16, S332–S333. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Lv, X.; Yue, F.; Wei, D.; Liu, W.; Zhang, T. Subclinical hypothyroidism and the risk of metabolic syndrome: A meta-analysis of observational studies. Endocr. Res. 2016, 41, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Zhao, Y.; Zhu, C.Y.; Wu, L.P.; Wang, Y.; Peng, Z.Y.; Deji, C.; Zhao, F.Y.; Shi, B.Y. The association between subclinical hypothyroidism and metabolic syndrome: An update meta-analysis of observational studies. Endocr. J. 2021, 68, 1043–1056. [Google Scholar] [CrossRef]
- Bode, H.; Ivens, B.; Bschor, T.; Schwarzer, G.; Henssler, J.; Baethge, C. Association of Hypothyroidism and Clinical Depression: A Systematic Review and Meta-analysis. JAMA Psychiatry 2021, 78, 1375–1383. [Google Scholar] [CrossRef]
- Jaruvongvanich, V.; Sanguankeo, A.; Upala, S. Nonalcoholic Fatty Liver Disease Is Not Associated with Thyroid Hormone Levels and Hypothyroidism: A Systematic Review and Meta-Analysis. Eur. Thyroid. J. 2017, 6, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Eshraghian, A.; Hamidian Jahromi, A. Non-alcoholic fatty liver disease and thyroid dysfunction: A systematic review. World J. Gastroenterol. 2014, 20, 8102–8109. [Google Scholar] [CrossRef]
- He, W.; An, X.; Li, L.; Shao, X.; Li, Q.; Yao, Q.; Zhang, J.-a. Relationship between Hypothyroidism and Non-Alcoholic Fatty Liver Disease: A Systematic Review and Meta-analysis. Front. Endocrinol. 2017, 8. [Google Scholar] [CrossRef]
- Zeng, X.; Li, B.; Zou, Y. The relationship between non-alcoholic fatty liver disease and hypothyroidism: A systematic review and meta-analysis. Medicine 2021, 100, e25738. [Google Scholar] [CrossRef]
- Vyakaranam, S.; Vanaparthy, S.; Nori, S.; Palarapu, S.; Bhongir, A.V. Study of Insulin Resistance in Subclinical Hypothyroidism. Int. J. Health Sci. Res. 2014, 4, 147–153. [Google Scholar] [PubMed]
- Grover, G.J.; Mellström, K.; Ye, L.; Malm, J.; Li, Y.L.; Bladh, L.G.; Sleph, P.G.; Smith, M.A.; George, R.; Vennström, B.; et al. Selective thyroid hormone receptor-beta activation: A strategy for reduction of weight, cholesterol, and lipoprotein (a) with reduced cardiovascular liability. Proc. Natl. Acad. Sci. USA 2003, 100, 10067–10072. [Google Scholar] [CrossRef]
- Green, M.E.; Bernet, V.; Cheung, J. Thyroid Dysfunction and Sleep Disorders. Front. Endocrinol. 2021, 12, 725829. [Google Scholar] [CrossRef] [PubMed]
- Teliti, M.; Fanfulla, F.; Croce, L.; Coperchini, F.; Rotondi, M. The interplay between subclinical hypothyroidism and poor sleep quality: A systematic review. Eur. J. Intern. Med. 2024, 126, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Gekakis, N.; Staknis, D.; Nguyen, H.B.; Davis, F.C.; Wilsbacher, L.D.; King, D.P.; Takahashi, J.S.; Weitz, C.J. Role of the CLOCK protein in the mammalian circadian mechanism. Science 1998, 280, 1564–1569. [Google Scholar] [CrossRef]
- Hogenesch, J.B.; Gu, Y.Z.; Jain, S.; Bradfield, C.A. The basic-helix-loop-helix-PAS orphan MOP3 forms transcriptionally active complexes with circadian and hypoxia factors. Proc. Natl. Acad. Sci. USA 1998, 95, 5474–5479. [Google Scholar] [CrossRef]
- Zhang, W.; Xiong, Y.; Tao, R.; Panayi, A.C.; Mi, B.; Liu, G. Emerging Insight Into the Role of Circadian Clock Gene BMAL1 in Cellular Senescence. Front. Endocrinol. 2022, 13, 915139. [Google Scholar] [CrossRef]
- Kettner, N.M.; Katchy, C.A.; Fu, L. Circadian gene variants in cancer. Ann Med 2014, 46, 208–220. [Google Scholar] [CrossRef] [PubMed]
- Amir, S.; Robinson, B. Thyroidectomy alters the daily pattern of expression of the clock protein, PER2, in the oval nucleus of the bed nucleus of the stria terminalis and central nucleus of the amygdala in rats. Neurosci. Lett. 2006, 407, 254–257. [Google Scholar] [CrossRef]
- Schull, J.; McEachron, D.L.; Adler, N.T.; Fiedler, L.; Horvitz, J.; Noyes, A.; Olson, M.; Shack, J. Effects of thyroidectomy, parathyroidectomy and lithium on circadian wheelrunning in rats. Physiol. Behav. 1988, 42, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Ikegami, K.; Refetoff, S.; Van Cauter, E.; Yoshimura, T. Interconnection between circadian clocks and thyroid function. Nat. Rev. Endocrinol. 2019, 15, 590–600. [Google Scholar] [CrossRef]
- Vandenbroucke, J.P.; von Elm, E.; Altman, D.G.; Gøtzsche, P.C.; Mulrow, C.D.; Pocock, S.J.; Poole, C.; Schlesselman, J.J.; Egger, M. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and elaboration. Epidemiology 2007, 18, 805–835. [Google Scholar] [CrossRef]
- Johnson, C.L.; Paulose-Ram, R.; Ogden, C.L.; Carroll, M.D.; Kruszon-Moran, D.; Dohrmann, S.M.; Curtin, L.R. National health and nutrition examination survey: Analytic guidelines, 1999–2010. Vital Health Stat. 2 2013, 1–24. [Google Scholar]
- Ross, D.S. Diagnosis of and Screening for Hypothyroidism in Nonpregnant Adults; UpToDate: Waltham, MA, USA, 2024. [Google Scholar]
- Kroenke, K.; Spitzer, R.L.; Williams, J.B. The PHQ-9: Validity of a brief depression severity measure. J. Gen. Intern. Med. 2001, 16, 606–613. [Google Scholar] [CrossRef]
- Akbar, Z.; Shi, Z. Dietary Patterns and Circadian Syndrome among Adults Attending NHANES 2005–2016. Nutrients 2023, 15, 3396. [Google Scholar] [CrossRef]
- Alsheikh, R.; Aldulaimi, H.; Hinawi, R.; Al-Sadi, F.; Al-Baker, A.; Alkuwari, A.; Sameer, M.; Al-Abdulla, G.; Shi, Z.; Rathnaiah Babu, G. Association of serum magnesium and calcium with metabolic syndrome: A cross-sectional study from the Qatar-biobank. Nutr. Metab. 2025, 22, 8. [Google Scholar] [CrossRef]
- Drinking Levels Defined. Available online: https://www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/moderate-binge-drinking (accessed on 22 March 2024).
- Minhas, A.M.K.; Jain, V.; Li, M.; Ariss, R.W.; Fudim, M.; Michos, E.D.; Virani, S.S.; Sperling, L.; Mehta, A. Family income and cardiovascular disease risk in American adults. Sci. Rep. 2023, 13, 279. [Google Scholar] [CrossRef]
- Developing the Healthy Eating Index. Available online: https://epi.grants.cancer.gov/hei/developing.html#:~:text=For%20the%20first%20time%2C%20the,2%20and%20older%3B%20and%20the (accessed on 22 March 2024).
- Liang, J.; Huang, S.; Jiang, N.; Kakaer, A.; Chen, Y.; Liu, M.; Pu, Y.; Huang, S.; Pu, X.; Zhao, Y.; et al. Association Between Joint Physical Activity and Dietary Quality and Lower Risk of Depression Symptoms in US Adults: Cross-sectional NHANES Study. JMIR Public Health Surveill. 2023, 9, e45776. [Google Scholar] [CrossRef] [PubMed]
- (WHO), W.H.O. Global Physical Activity Questionnaire (GPAQ). Available online: https://www.who.int/publications/m/item/global-physical-activity-questionnaire (accessed on 26 December 2024).
- Lin, Z.H.; Xin, Y.N.; Dong, Q.J.; Wang, Q.; Jiang, X.J.; Zhan, S.H.; Sun, Y.; Xuan, S.Y. Performance of the aspartate aminotransferase-to-platelet ratio index for the staging of hepatitis C-related fibrosis: An updated meta-analysis. Hepatology 2011, 53, 726–736. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine Committee to Review Dietary Reference Intakes for Vitamin D and Calcium. The National Academies Collection: Reports funded by National Institutes of Health. In Dietary Reference Intakes for Calcium and Vitamin D; Ross, A.C., Taylor, C.L., Yaktine, A.L., Del Valle, H.B., Eds.; National Academies Press (US) Copyright © 2011; National Academy of Sciences: Washington, DC, USA, 2011. [Google Scholar]
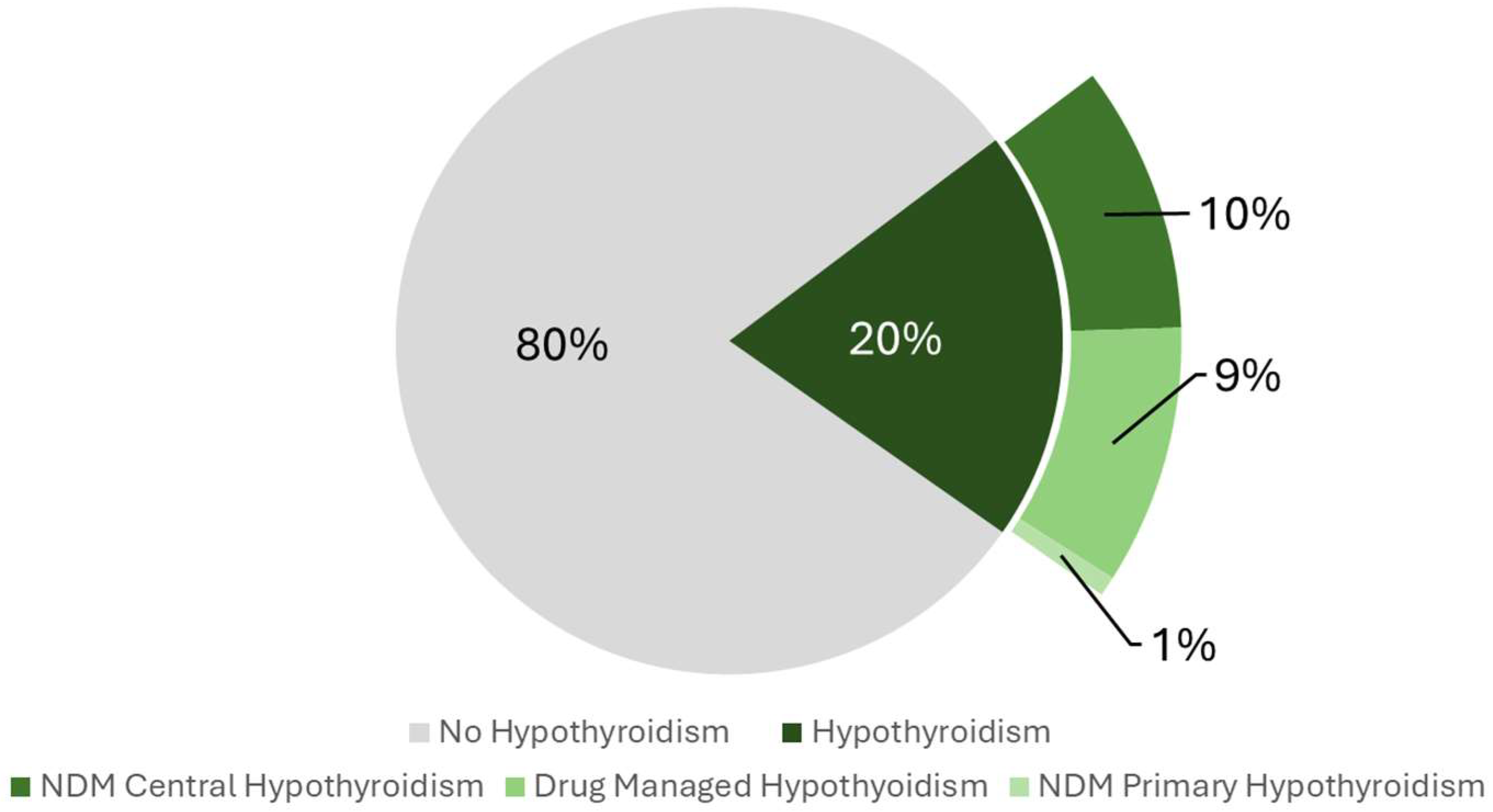
| Characteristics | Categories | No Circadian Syndrome (n = 3576) | Circadian Syndrome (n = 474) | p-Value |
|---|---|---|---|---|
| Age in years, Median (IQR) | 44.00 (31.00, 60.00) | 57.00 (46.00, 67.00) | <0.001 1 | |
| Sex | ||||
| Male | 1795 (50.2%) | 208 (43.9%) | 0.01 2 | |
| Female | 1781 (49.8%) | 266 (56.1%) | ||
| Race | ||||
| Hispanic | 922 (25.8%) | 133 (28.1%) | <0.001 2 | |
| White | 1683 (47.1%) | 256 (54.0%) | ||
| Black | 695 (19.4%) | 73 (15.4%) | ||
| Others/multi-racial | 276 (7.7%) | 12 (2.5%) | ||
| Poverty Income Ratio (PIR) | ||||
| PIR < 1 | 686 (19.2%) | 126 (26.6%) | <0.001 2 | |
| PIR 1–1.9 | 922 (25.8%) | 160 (33.8%) | ||
| PIR 2–2.9 | 582 (16.3%) | 68 (14.3%) | ||
| PIR 3–3.9 | 406 (11.4%) | 38 (8.0%) | ||
| PIR 4–4.9 | 312 (8.7%) | 27 (5.7%) | ||
| PIR ≥ 5 | 668 (18.7%) | 55 (11.6%) | ||
| Education Level | ||||
| Below high school | 844 (23.6%) | 164 (34.6%) | <0.001 2 | |
| Highschool | 1806 (50.5%) | 249 (52.5%) | ||
| Graduate | 926 (25.9%) | 61 (12.9%) | ||
| Serum Vitamin D Level | ||||
| Adequacy | 2464 (68.9%) | 321 (67.7%) | 0.45 2 | |
| Inadequacy | 812 (22.7%) | 105 (22.2%) | ||
| Deficiency | 300 (8.4%) | 48 (10.1%) | ||
| Diet Quality | ||||
| Low Dietary Quality | 2011 (56.2%) | 282 (59.5%) | 0.18 2 | |
| High Dietary Quality | 1565 (43.8%) | 192 (40.5%) | ||
| Physical Activity Status, Median (IQR) | 22.00 (3.33, 80.00) | 8.00 (0.00, 44.00) | <0.001 1 | |
| Chronic Kidney Disease Status | ||||
| No CKD | 3513 (98.2%) | 444 (93.7%) | <0.001 2 | |
| CKD | 63 (1.8%) | 30 (6.3%) | ||
| Liver Cirrhosis Status | ||||
| No Cirrhosis | 3523 (98.5%) | 461 (97.3%) | 0.042 2 | |
| Cirrhosis | 53 (1.5%) | 13 (2.7%) | ||
| General Hypothyroidism status | ||||
| No Hypothyroidism | 2916 (81.5%) | 324 (68.4%) | <0.001 2 | |
| General Hypothyroidism | 660 (18.5%) | 150 (31.6%) | ||
| Exposure | Categories | CircS OR | p-Value | 95% CI |
|---|---|---|---|---|
| Hypothyroidism Status | ||||
| No Hypothyroidism | 1 | |||
| Hypothyroidism | 1.58 | <0.001 | 1.26–1.98 | |
| Exposure | Categories | MetS OR | p-Value | 95% CI |
|---|---|---|---|---|
| Hypothyroidism Status | ||||
| No Hypothyroidism | 1 | |||
| Hypothyroidism | 1.19 | 0.042 | 1.01–1.42 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arabi, A.; Rajha, H.E.; Alkeilani, O.; Hamdan, A.; Nasrallah, D.; Babu, G.R. Exploring the Cross-Sectional Association Between Hypothyroidism and Circadian Syndrome: Insights from NHANES 2007–2012. Clocks & Sleep 2025, 7, 52. https://doi.org/10.3390/clockssleep7040052
Arabi A, Rajha HE, Alkeilani O, Hamdan A, Nasrallah D, Babu GR. Exploring the Cross-Sectional Association Between Hypothyroidism and Circadian Syndrome: Insights from NHANES 2007–2012. Clocks & Sleep. 2025; 7(4):52. https://doi.org/10.3390/clockssleep7040052
Chicago/Turabian StyleArabi, Ahmed, Humam Emad Rajha, Osama Alkeilani, Ahmad Hamdan, Dima Nasrallah, and Giridhara R. Babu. 2025. "Exploring the Cross-Sectional Association Between Hypothyroidism and Circadian Syndrome: Insights from NHANES 2007–2012" Clocks & Sleep 7, no. 4: 52. https://doi.org/10.3390/clockssleep7040052
APA StyleArabi, A., Rajha, H. E., Alkeilani, O., Hamdan, A., Nasrallah, D., & Babu, G. R. (2025). Exploring the Cross-Sectional Association Between Hypothyroidism and Circadian Syndrome: Insights from NHANES 2007–2012. Clocks & Sleep, 7(4), 52. https://doi.org/10.3390/clockssleep7040052








