Prevalence and Clinical Impact of Restless Legs Syndrome in Pediatric Populations with Attention-Deficit/Hyperactivity Disorder: A Systematic Review
Abstract
1. Introduction
2. Methods
2.1. Article Selection
- Articles investigating the prevalence of RLS with or without assessment of its potential clinical impact in pediatric ADHD subjects (<18 years).
- ADHD diagnosed according to DSM-IV or DSM 5 diagnostic criteria.
- RLS diagnosed during clinical interview using standardized diagnostic criteria (International Classification of Sleep Disorders or International Restless Legs Syndrome Study Group) or using questionnaires validated for clinical research.
- Any study design (cross-sectional, longitudinal, prospective, retrospective, interventional and experimental) except for literature reviews case reports and letters to editor.
- Articles written in English or French.
- Articles available in full version.
2.2. Quality Assessment of Articles
- Grade A (established scientific evidence) for level 1 of scientific evidence (high-powered randomized comparative trial, meta-analysis of randomized comparative trials, and decision analysis based on well-conducted studies);
- Grade B (presumed scientific evidence) for level 2 of scientific evidence (low-powered randomized comparative trials, well-conducted non-randomized comparative studies, and cohort studies);
- Grade C (low level of scientific evidence) for level 3 of scientific evidence (case–control studies) or level 4 of scientific evidence (comparative studies with significant biases, retrospective studies, case series, and descriptive epidemiological studies).
2.3. Data Extraction
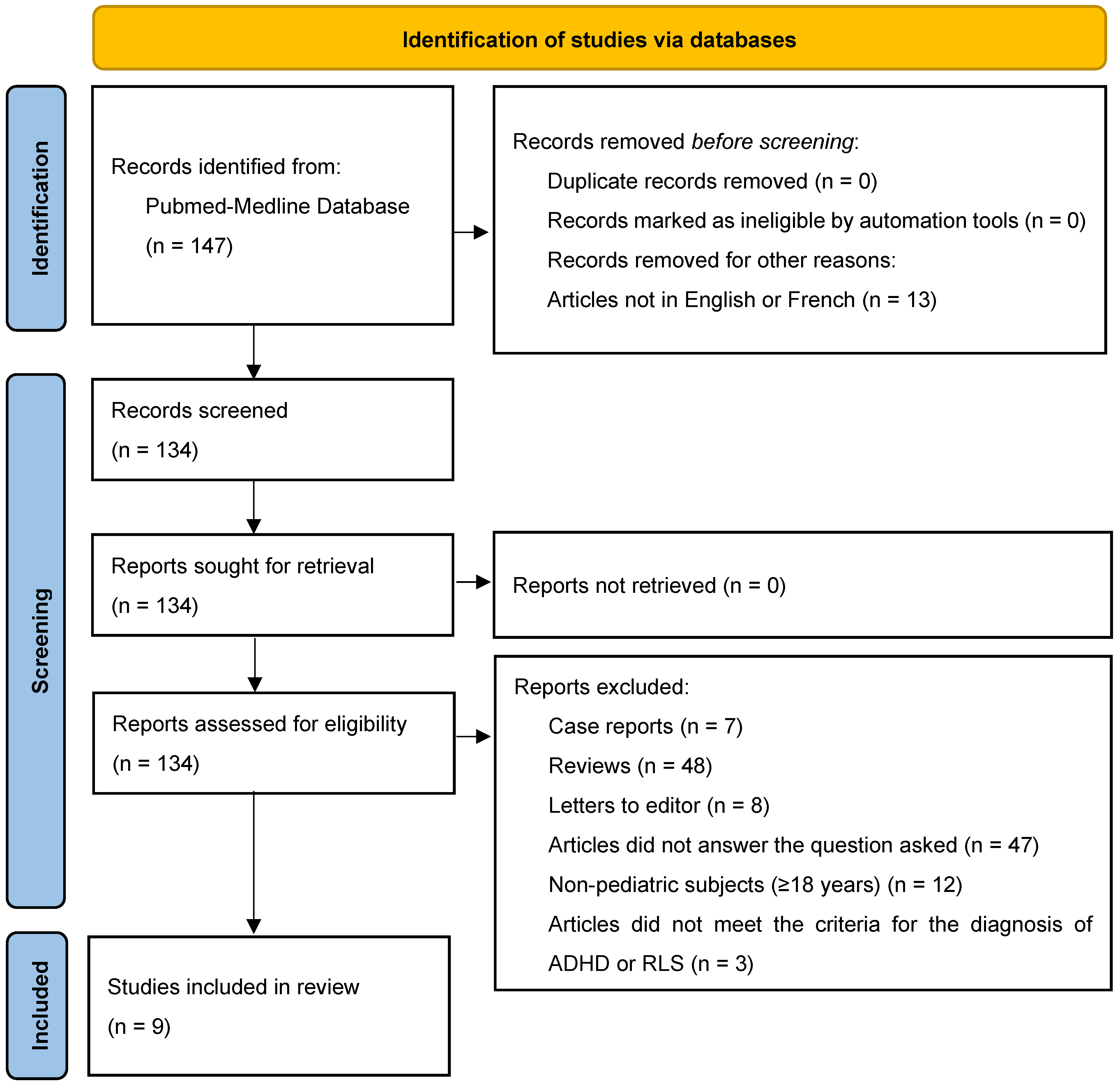
3. Results
3.1. Prevalence of RLS in Pediatric ADHD Subjects
3.2. Clinical Impact of RLS in Pediatric ADHD Subjects
3.3. Risk of Bias of Selected Studies
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; text revision; American Psychiatric Publishing: Washington, DC, USA, 2022. [Google Scholar]
- Núñez-Jaramillo, L.; Herrera-Solís, A.; Herrera-Morales, W.V. ADHD: Reviewing the Causes and Evaluating Solutions. J. Pers. Med. 2021, 11, 166. [Google Scholar] [CrossRef]
- Sharma, A.; Couture, J. A review of the pathophysiology, etiology, and treatment of attention-deficit hyperactivity disorder (ADHD). Ann. Pharmacother. 2014, 48, 209–225. [Google Scholar] [CrossRef] [PubMed]
- Posner, J.; Polanczyk, G.V.; Sonuga-Barke, E. Attention-deficit hyperactivity disorder. Lancet 2020, 395, 450–462. [Google Scholar] [CrossRef]
- Kian, N.; Samieefar, N.; Rezaei, N. Prenatal risk factors and genetic causes of ADHD in children. World J. Pediatr. 2022, 18, 308–319. [Google Scholar] [CrossRef]
- Polanczyk, G.; de Lima, M.S.; Horta, B.L.; Biederman, J.; Rohde, L.A. The worldwide prevalence of ADHD: A systematic review and metaregression analysis. Am. J. Psychiatry 2007, 164, 942–948. [Google Scholar] [CrossRef]
- Gnanavel, S.; Sharma, P.; Kaushal, P.; Hussain, S. Attention deficit hyperactivity disorder and comorbidity: A review of literature. World J. Clin. Cases 2019, 7, 2420–2426. [Google Scholar] [CrossRef] [PubMed]
- Mechler, K.; Banaschewski, T.; Hohmann, S.; Häge, A. Evidence-based pharmacological treatment options for ADHD in children and adolescents. Pharmacol Ther. 2022, 230, 107940. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence. Attention Deficit Hyperactivity Disorder: Diagnosis and Management; National Institute for Health and Care Excellence (NICE): London, UK, 2019. [Google Scholar]
- Hvolby, A. Associations of sleep disturbance with ADHD: Implications for treatment. Atten. Deficit Hyperact. Disord. 2015, 7, 1–18. [Google Scholar] [CrossRef]
- Hiscock, H.; Sciberras, E.; Mensah, F.; Gerner, B.; Efron, D.; Khano, S.; Oberklaid, F. Impact of a behavioural sleep intervention on symptoms and sleep in children with attention deficit hyperactivity disorder, and parental mental health: Randomised controlled trial. BMJ 2015, 350, h68. [Google Scholar] [CrossRef] [PubMed]
- Picchietti, D.L.; Bruni, O.; de Weerd, A.; Durmer, J.S.; Kotagal, S.; Owens, J.A.; Simakajornboon, N.; International Restless Legs Syndrome Study Group (IRLSSG). Pediatric restless legs syndrome diagnostic criteria: An update by the International Restless Legs Syndrome Study Group. Sleep Med. 2013, 14, 1253–1259. [Google Scholar] [CrossRef]
- Yoganathan, S.; Chakrabarty, B. Epidemiology of Pediatric Restless Leg Syndrome. Sleep Med. Clin. 2025, 20, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Pullen, S.J.; Wall, C.A.; Angstman, E.R.; Munitz, G.E.; Kotagal, S. Psychiatric comorbidity in children and adolescents with restless legs syndrome: A retrospective study. J. Clin. Sleep Med. 2011, 7, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Picchietti, D.; Allen, R.P.; Walters, A.S.; Davidson, J.E.; Myers, A.; Ferini-Strambi, L. Restless legs syndrome: Prevalence and impact in children and adolescents—The Peds REST study. Pediatrics 2007, 120, 253–266. [Google Scholar] [CrossRef] [PubMed]
- DelRosso, L.M.; Mogavero, M.P.; Baroni, A.; Bruni, O.; Ferri, R. Restless Legs Syndrome in Children and Adolescents. Psychiatr. Clin. N. Am. 2024, 47, 147–161. [Google Scholar] [CrossRef]
- American Academy of Sleep Medicine. International Classification of Sleep Disorders, 3rd ed.; American Academy of Sleep Medicine: Darien, IL, USA, 2014. [Google Scholar]
- Bruni, O.; Angriman, M. Management of RLS in Children (Unique Features); Springer: New York, NY, USA, 2017. [Google Scholar]
- Silvestri, R.; DelRosso, L.M. Pediatric Restless Legs Syndrome. Sleep Med. Clin. 2021, 16, 305–314. [Google Scholar] [CrossRef]
- Picchietti, M.A.; Picchietti, D.L. Advances in pediatric restless legs syndrome: Iron, genetics, diagnosis and treatment. Sleep Med. 2010, 11, 643–651. [Google Scholar] [CrossRef]
- Picchietti, D.L.; Stevens, H.E. Early manifestations of restless legs syndrome in childhood and adolescence. Sleep Med. 2008, 9, 770–781. [Google Scholar] [CrossRef]
- Mammarella, V.; Breda, M.; Esposito, D.; Orecchio, S.; Polese, D.; Bruni, O. Psychiatric Comorbidities in Pediatric Restless Leg Syndrome. Sleep Med. Clin. 2025, 20, 209–218. [Google Scholar] [CrossRef]
- Allen, R.P.; Barker, P.B.; Horská, A.; Earley, C.J. Thalamic glutamate/glutamine in restless legs syndrome: Increased and related to disturbed sleep. Neurology 2013, 80, 2028–2034. [Google Scholar] [CrossRef]
- Winkelman, J.W.; Schoerning, L.; Platt, S.; Jensen, J.E. Restless legs syndrome and central nervous system gamma-aminobutyric acid: Preliminary associations with periodic limb movements in sleep and restless leg syndrome symptom severity. Sleep Med. 2014, 15, 1225–1230. [Google Scholar] [CrossRef]
- Allen, R.P. Restless Leg Syndrome/Willis-Ekbom Disease Pathophysiology. Sleep Med. Clin. 2015, 10, 207–214. [Google Scholar] [CrossRef]
- Vlasie, A.; Trifu, S.C.; Lupuleac, C.; Kohn, B.; Cristea, M.B. Restless legs syndrome: An overview of pathophysiology, comorbidities and therapeutic approaches (Review). Exp. Ther. Med. 2022, 23, 185. [Google Scholar] [CrossRef]
- Connor, J.R.; Wang, X.S.; Patton, S.M.; Menzies, S.L.; Troncoso, J.C.; Earley, C.J.; Allen, R.P. Decreased transferrin receptor expression by neuromelanin cells in restless legs syndrome. Neurology 2004, 62, 1563–1567. [Google Scholar] [CrossRef]
- Gossard, T.R.; Trotti, L.M.; Videnovic, A.; St Louis, E.K. Restless Legs Syndrome: Contemporary Diagnosis and Treatment. Neurotherapeutics 2021, 18, 140–155. [Google Scholar] [CrossRef]
- Provini, F.; Chiaro, G. Neuroimaging in Restless Legs Syndrome. Sleep Med. Clin. 2015, 10, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Hein, M. Prise en charge des troubles du sommeil. EMC Psychiatr. 2021, 37, 1–12. [Google Scholar]
- Hein, M.; Mungo, A.; Hubain, P.; Loas, G. Excessive daytime sleepiness in adolescents: Current treatment strategies. Sleep Sci. 2020, 13, 157–171. [Google Scholar] [PubMed]
- Furudate, N.; Komada, Y.; Kobayashi, M.; Nakajima, S.; Inoue, Y. Daytime dysfunction in children with restless legs syndrome. J. Neurol. Sci. 2014, 336, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Newcorn, J.H.; Schulz, K.; Harrison, M.; DeBellis, M.D.; Udarbe, J.K.; Halperin, J.M. Alpha 2 adrenergic agonists—Neurochemistry, efficacy, and clinical guidelines for use in children. Pediatr. Clin. N. Am. 1998, 45, 1099–1122. [Google Scholar] [CrossRef]
- Agence Nationale D’accréditation et D’évaluation en Santé (Ed.) Analyse de la Littérature et Gradation des Recommandations: Janvier 2000; Agence Nationale D’accréditation et D’évaluation en Santé: Paris, France, 2000. [Google Scholar]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef]
- Srifuengfung, M.; Bussaratid, S.; Ratta-Apha, W.; Sanguanpanich, N.; Hosiri, T. Restless legs syndrome in children and adolescents with attention-deficit/hyperactivity disorder: Prevalence, mimic conditions, risk factors, and association with functional impairment. Sleep Med. 2020, 73, 117–124. [Google Scholar] [CrossRef]
- Konofal, E.; Cortese, S.; Marchand, M.; Mouren, M.C.; Arnulf, I.; Lecendreux, M. Impact of restless legs syndrome and iron deficiency on attention-deficit/hyperactivity disorder in children. Sleep Med. 2007, 8, 711–715. [Google Scholar] [CrossRef]
- Sierra Montoya, A.C.; Mesa Restrepo, S.C.; Cuartas Arias, J.M.; Cornejo Ochoa, W. Prevalence and Clinical Characteristics of the Restless Legs Syndrome (RLS) in Patients Diagnosed with Attention-Deficit Hyperactivity Disorder (ADHD) in Antioquia. Int. J. Psychol. Res. 2018, 11, 58–69. [Google Scholar] [CrossRef]
- Chervin, R.D.; Dillon, J.E.; Bassetti, C.; Ganoczy, D.A.; Pituch, K.J. Symptoms of sleep disorders, inattention, and hyperactivity in children. Sleep 1997, 20, 1185–1192. [Google Scholar] [CrossRef]
- Kapoor, V.; Ferri, R.; Stein, M.A.; Ruth, C.; Reed, J.; DelRosso, L.M. Restless sleep disorder in children with attention-deficit/hyperactivity disorder. J. Clin. Sleep Med. 2021, 17, 639–643. [Google Scholar] [CrossRef] [PubMed]
- Chervin, R.D.; Archbold, K.H.; Dillon, J.E.; Pituch, K.J.; Panahi, P.; Dahl, R.E.; Guilleminault, C. Associations between symptoms of inattention, hyperactivity, restless legs, and periodic leg movements. Sleep 2002, 25, 213–218. [Google Scholar] [CrossRef]
- Silvestri, R.; Gagliano, A.; Aricò, I.; Calarese, T.; Cedro, C.; Bruni, O.; Condurso, R.; Germanò, E.; Gervasi, G.; Siracusano, R.; et al. Sleep disorders in children with Attention-Deficit/Hyperactivity Disorder (ADHD) recorded overnight by video-polysomnography. Sleep Med. 2009, 10, 1132–1138. [Google Scholar] [CrossRef]
- Oner, P.; Dirik, E.B.; Taner, Y.; Caykoylu, A.; Anlar, O. Association between low serum ferritin and restless legs syndrome in patients with attention deficit hyperactivity disorder. Tohoku J. Exp. Med. 2007, 213, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Silvestri, R.; Gagliano, A.; Calarese, T.; Aricò, I.; Cedro, C.; Condurso, R.; Germanò, E.; Vita, G.; Tortorella, G. Ictal and interictal EEG abnormalities in ADHD children recorded over night by video-polysomnography. Epilepsy Res. 2007, 75, 130–137. [Google Scholar] [CrossRef]
- DelRosso, L.M.; Mogavero, M.P.; Bruni, O.; Ferri, R. Restless Legs Syndrome and Restless Sleep Disorder in Children. Sleep Med. Clin. 2023, 18, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Ohayon, M.M.; O’Hara, R.; Vitiello, M.V. Epidemiology of restless legs syndrome: A synthesis of the literature. Sleep Med. Rev. 2012, 16, 283–295. [Google Scholar] [CrossRef]
- Wajszilber, D.; Santiseban, J.A.; Gruber, R. Sleep disorders in patients with ADHD: Impact and management challenges. Nat. Sci. Sleep 2018, 10, 453–480. [Google Scholar] [CrossRef]
- Migueis, D.P.; Lopes, M.C.; Casella, E.; Soares, P.V.; Soster, L.; Spruyt, K. Attention deficit hyperactivity disorder and restless leg syndrome across the lifespan: A systematic review and meta-analysis. Sleep Med. Rev. 2023, 69, 101770. [Google Scholar] [CrossRef]
- Cortese, S.; Konofal, E.; Lecendreux, M.; Arnulf, I.; Mouren, M.C.; Darra, F.; Dalla Bernardina, B. Restless legs syndrome and attention-deficit/hyperactivity disorder: A review of the literature. Sleep 2005, 28, 1007–1013. [Google Scholar] [CrossRef]
- Yoon, S.Y.; Jain, U.; Shapiro, C. Sleep in attention-deficit/hyperactivity disorder in children and adults: Past, present, and future. Sleep Med. Rev. 2012, 16, 371–388. [Google Scholar] [CrossRef]
- Staedt, J.; Stoppe, G.; Kögler, A.; Riemann, H.; Hajak, G.; Munz, D.L.; Emrich, D.; Rüther, E. Nocturnal myoclonus syndrome (periodic movements in sleep) related to central dopamine D2-receptor alteration. Eur. Arch. Psychiatry Clin. Neurosci. 1995, 245, 8–10. [Google Scholar] [CrossRef] [PubMed]
- Turjanski, N.; Lees, A.J.; Brooks, D.J. Striatal dopaminergic function in restless legs syndrome: 18F-dopa and 11C-raclopride PET studies. Neurology 1999, 52, 932–937. [Google Scholar] [CrossRef]
- Trenkwalder, C.; Walters, A.S.; Hening, W.A.; Chokroverty, S.; Antonini, A.; Dhawan, V.; Eidelberg, D. Positron emission tomographic studies in restless legs syndrome. Mov. Disord. 1999, 14, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Tribl, G.G.; Asenbaum, S.; Happe, S.; Bonelli, R.M.; Zeitlhofer, J.; Auff, E. Normal striatal D2 receptor binding in idiopathic restless legs syndrome with periodic leg movements in sleep. Nucl. Med. Commun. 2004, 25, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Earley, C.J.; Kuwabara, H.; Wong, D.F.; Gamaldo, C.; Salas, R.E.; Brašić, J.R.; Ravert, H.T.; Dannals, R.F.; Allen, R.P. Increased synaptic dopamine in the putamen in restless legs syndrome. Sleep 2013, 36, 51–57. [Google Scholar] [CrossRef]
- Allen, R.P.; Connor, J.R.; Hyland, K.; Earley, C.J. Abnormally increased CSF 3-Ortho-methyldopa (3-OMD) in untreated restless legs syndrome (RLS) patients indicates more severe disease and possibly abnormally increased dopamine synthesis. Sleep Med. 2009, 10, 123–128. [Google Scholar] [CrossRef]
- Allen, R.P.; Picchietti, D.L.; Auerbach, M.; Cho, Y.W.; Connor, J.R.; Earley, C.J.; Garcia-Borreguero, D.; Kotagal, S.; Manconi, M.; Ondo, W.; et al. Evidence-based and consensus clinical practice guidelines for the iron treatment of restless legs syndrome/Willis-Ekbom disease in adults and children: An IRLSSG task force report. Sleep Med. 2018, 41, 27–44. [Google Scholar] [CrossRef]
- Calarge, C.; Farmer, C.; DiSilvestro, R.; Arnold, L.E. Serum ferritin and amphetamine response in youth with attention-deficit/hyperactivity disorder. J. Child. Adolesc. Psychopharmacol. 2010, 20, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.H.; Hsu, J.F.; Huang, Y.S. Sleep Problems in Children with Attention Deficit/Hyperactivity Disorder: Current Status of Knowledge and Appropriate Management. Curr. Psychiatry Rep. 2016, 18, 76. [Google Scholar] [CrossRef] [PubMed]
- Cortese, S.; Brown, T.E.; Corkum, P.; Gruber, R.; O’Brien, L.M.; Stein, M.; Weiss, M.; Owens, J. Assessment and management of sleep problems in youths with attention-deficit/hyperactivity disorder. J. Am. Acad. Child. Adolesc. Psychiatry 2013, 52, 784–796. [Google Scholar] [CrossRef] [PubMed]
| Studies | Country of Study Study Design Evidence Level | Sample Size | Sample Age | Diagnostic Criteria for ADHD | Diagnostic Criteria for RLS | PSG | Other Relevant Parameters | RLS Prevalence | Main Results | Recommendation Level |
|---|---|---|---|---|---|---|---|---|---|---|
| Srifuengfung et al. (2020) [36] | Thailand Cross-sectional observational study Level 4 | 217 | 3–18 years (average age 9.5 years) | DSM-IV TR or DSM 5 | Revised IRLSG criteria (2013) | No | Impact on functioning (6 areas) | 11% |
| Grade C |
| Konofal et al. (2007) [37] | France Cross-sectional and retrospective observational study Level 4 | 22 | 5–9 years (average age 7.3 years) | DSM-IV | IRLSSG criteria (2003) | No | CPRS, family history of RLS, personal history of iron supplementation, serum ferritin | 54% |
| Grade C |
| Sierra Montoya et al. (2018) [38] | Colombia Cross-sectional observational study Level 4 | 177 | 4–18 years (average age 10.25 years) | DSM-IV | IRLSSG criteria (2003) | No | ADHD subtype, other sleep disorders | 13.5% |
| Grade C |
| Chervin et al. (1997) [39] | United States Cross-sectional observational study Level 4 | 143 (27 ADHD subjects, 43 others psychiatric disorders, 73 controls) | 2–18 years (average age 9 years) | DSM-IV | PSQ | Yes | PSQ, IHS | 15% |
| Grade C |
| Kapoor et al. (2021) [40] | United States Retrospective study Level 4 | 66 | 11.6 ± 3.6 years | DSM 5 | ICSD-3 | Yes | 19.7% |
| Grade C | |
| Chervin et al. (2002) [41] | United States Cross-sectional observational study Level 4 | 866 (98 ADHD) | 2–14 years (average age 6.8 years) | DSM-IV | PSQ | No | PSQ, inattention and hyperactivity indices (T-score), CPRS | 24% |
| Grade C |
| Silvestri et al. (2009) [42] | Italy Cross-sectional observational study Level 4 | 55 | 8.9 ± 2.7 years | DSM-IV | IRLSSG criteria (1995) | Yes | ADHD subtype, CPRS, CTRS, SNAP-IV, structured sleep interview | 25.4% |
| Grade C |
| Oner et al. (2007) [43] | Türkiye Cross-sectional observational study Level 4 | 87 | 6–16 years (average age 9.3 years) | K-SADS-PL (DSM-IV) | IRLSSG criteria (2003) | No | Ferritin levels, behavioral tests (K-SAD-PL, CBCL, TRF, CPRS and CTRS) | 33.3% |
| Grade C |
| Silvestri et al. (2007) [44] | Italy Cross-sectional observational study Level 4 | 42 | 8.9 ± 2.8 years | DSM-IV | IRLSSG criteria (2003) | Yes | ADHD subtype, ADHD-RS, CTRS, CPRS, SNAP-IV, structured sleep interview | 26.0% |
| Grade C |
| Studies | D1 | D2 | D3 | D4 | D5 | D6 | D7 | Global Risk |
|---|---|---|---|---|---|---|---|---|
| Srifuengfung et al. (2020) [36] | Moderate | Low | Low | Not applicable | Low | Moderate | Low | Moderate |
| Konofal et al. (2007) [37] | Severe | Moderate | Moderate | Not applicable | Low | Moderate | Moderate | Severe |
| Sierra Montoya et al. (2018) [38] | Severe | Moderate | Moderate | Not applicable | Low | Moderate | Moderate | Severe |
| Chervin et al. (1997) [39] | Severe | Moderate | Moderate | Not applicable | Low | Moderate | Moderate | Severe |
| Kapoor et al. (2021) [40] | Moderate | Moderate | Low | Not applicable | Low | Moderate | Low | Moderate |
| Chervin et al. (2002) [41] | Moderate | Low | Moderate | Not applicable | Moderate | Moderate | Low | Moderate |
| Silvestri et al. (2009) [42] | Moderate | Moderate | Low | Not applicable | Low | Moderate | Low | Moderate |
| Oner et al. (2007) [43] | Moderate | Moderate | Moderate | Not applicable | Low | Moderate | Low | Moderate |
| Silvestri et al. (2007) [44] | Moderate | Moderate | Low | Not applicable | Low | Moderate | Low | Moderate |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghayad, T.; Mungo, A.; Hein, M. Prevalence and Clinical Impact of Restless Legs Syndrome in Pediatric Populations with Attention-Deficit/Hyperactivity Disorder: A Systematic Review. Clocks & Sleep 2025, 7, 50. https://doi.org/10.3390/clockssleep7030050
Ghayad T, Mungo A, Hein M. Prevalence and Clinical Impact of Restless Legs Syndrome in Pediatric Populations with Attention-Deficit/Hyperactivity Disorder: A Systematic Review. Clocks & Sleep. 2025; 7(3):50. https://doi.org/10.3390/clockssleep7030050
Chicago/Turabian StyleGhayad, Toni, Anaïs Mungo, and Matthieu Hein. 2025. "Prevalence and Clinical Impact of Restless Legs Syndrome in Pediatric Populations with Attention-Deficit/Hyperactivity Disorder: A Systematic Review" Clocks & Sleep 7, no. 3: 50. https://doi.org/10.3390/clockssleep7030050
APA StyleGhayad, T., Mungo, A., & Hein, M. (2025). Prevalence and Clinical Impact of Restless Legs Syndrome in Pediatric Populations with Attention-Deficit/Hyperactivity Disorder: A Systematic Review. Clocks & Sleep, 7(3), 50. https://doi.org/10.3390/clockssleep7030050






