Who Benefits the Most from Sleep Hygiene Education? Findings from the SLeep Education for Everyone Program (SLEEP)
Abstract
1. Introduction
2. Results
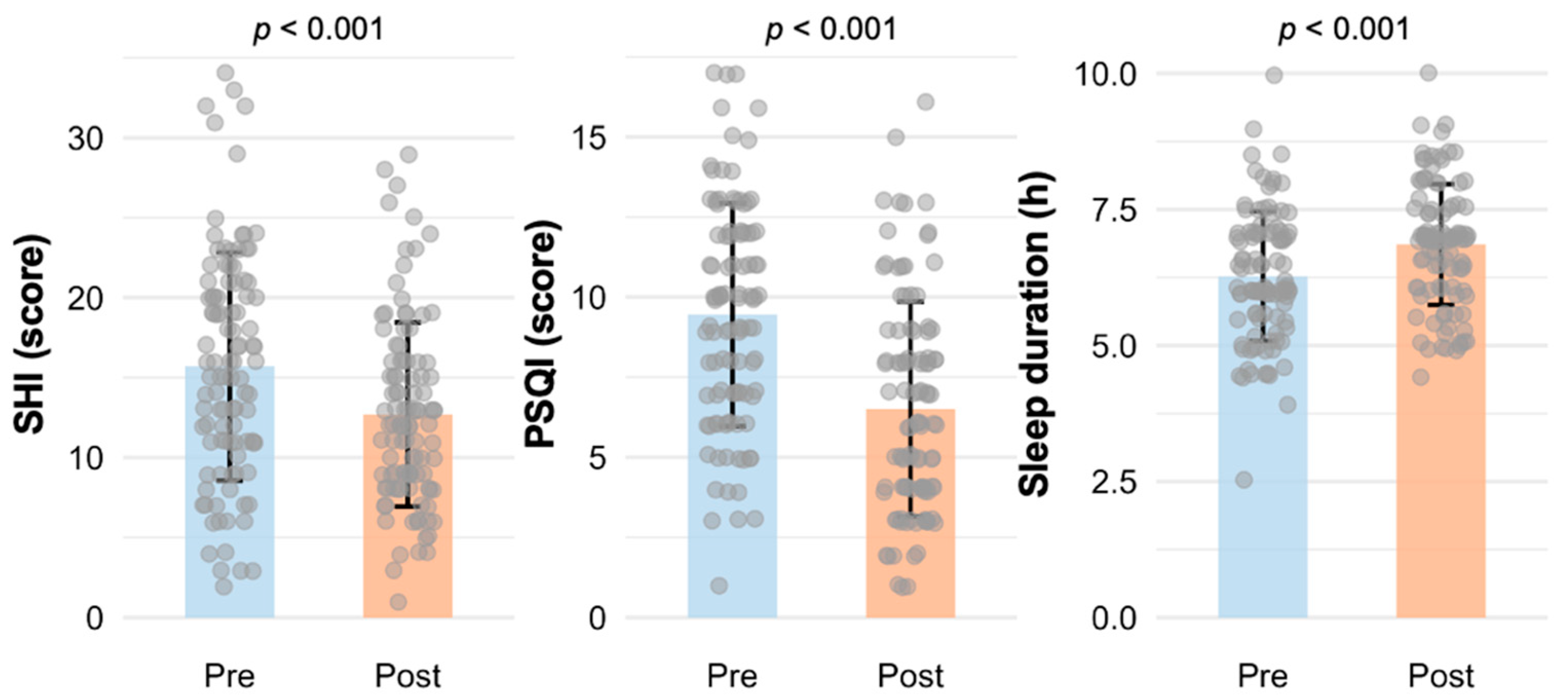
2.1. Sleep Outcomes
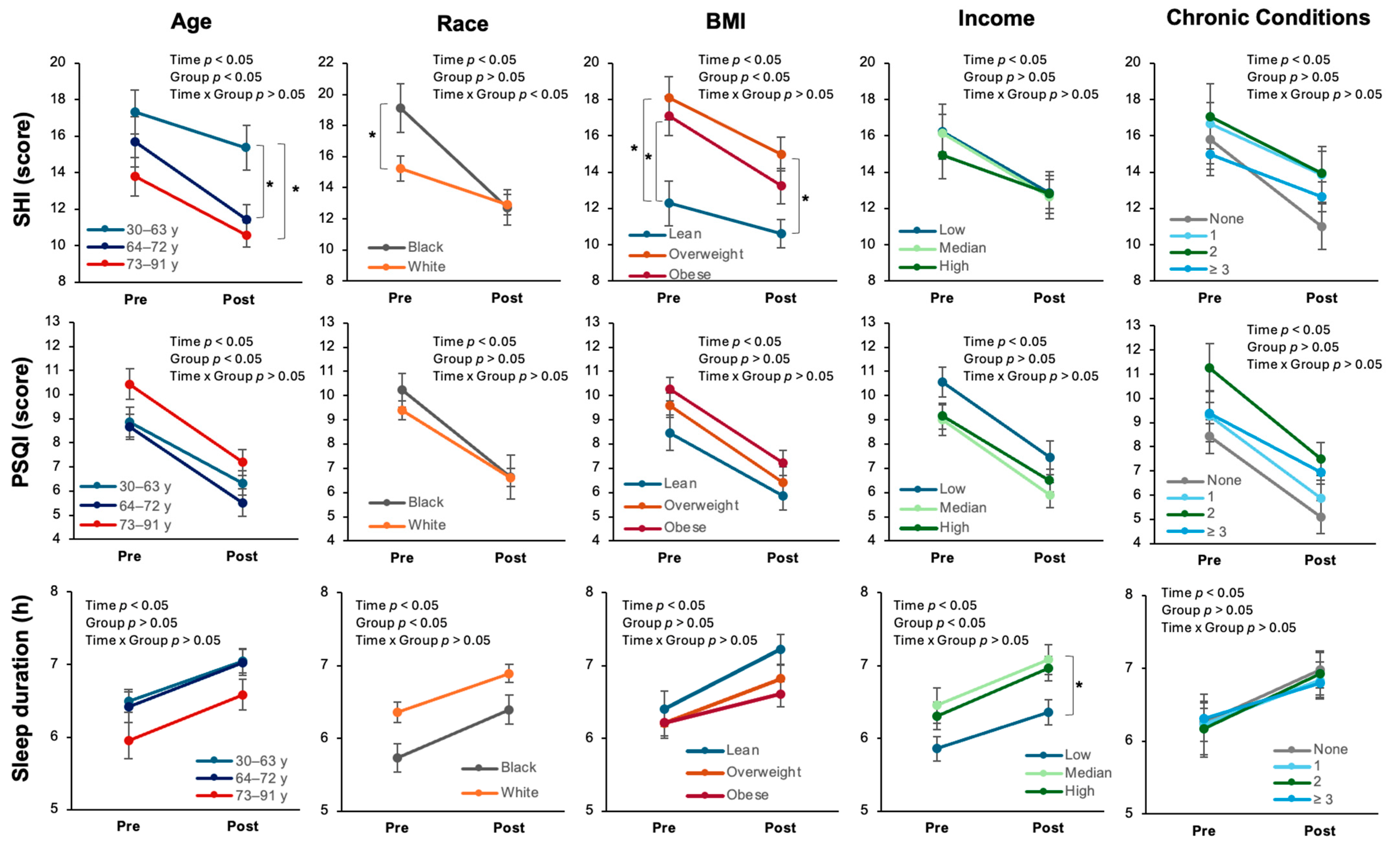
2.2. Differences in Outcomes
2.2.1. Age
2.2.2. Race
2.2.3. BMI
2.2.4. Income
2.2.5. Chronic Conditions
2.3. Sleep Improvement Predictors
3. Discussion
4. Materials and Methods
4.1. Participants
4.2. Measures
4.2.1. Sleep Hygiene Index (SHI)
4.2.2. Pittsburgh Sleep Quality Index (PSQI)
4.3. Other Measures
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- CDC. FastStats: Sleep in Adults. Sleep. 2024. Available online: https://www.cdc.gov/sleep/data-research/facts-stats/adults-sleep-facts-and-stats.html (accessed on 3 April 2025).
- Nelson, K.L.; Davis, J.E.; Corbett, C.F. Sleep quality: An evolutionary concept analysis. Nurs. Forum 2022, 57, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Miyata, S.; Noda, A.; Iwamoto, K.; Kawano, N.; Okuda, M.; Ozaki, N. Poor sleep quality impairs cognitive performance in older adults. J. Sleep Res. 2013, 22, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, D.; Dregan, A. A Population-Based Investigation into the Self-Reported Reasons for Sleep Problems. PLoS ONE 2014, 9, e101368. [Google Scholar] [CrossRef]
- Becker, N.B.; Jesus, S.N.; Joao, K.A.; Viseu, J.N.; Martins, R.I. Depression and sleep quality in older adults: A meta-analysis. Psychol. Health Med. 2017, 22, 889–895. [Google Scholar] [CrossRef]
- Adjaye-Gbewonyo, D.; Ng, A.E.; Black, L.I. Sleep Difficulties in Adults: United States, 2020; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2022. [Google Scholar]
- Vásquez-Carrasco, E.; Rojas, M.; Larenas, L.; Ferrada, A.; Hernandez-Martinez, J.; Ahumada-Méndez, F.; Leiva-Bianchi, M.; Carmine, F.; Sandoval, C.; Branco, B.H.M.; et al. Effectiveness of Non-Pharmacological Interventions for Sleep Disorders in Enhancing Quality of Life, Cognitive Function, and Sleep Quality in Older Adults with Mild Cognitive Impairment: A Systematic Review and Meta-Analysis. Medicina 2025, 61, 583. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.T.; Perlis, M.L.; Park, A.; Smith, M.S.; Pennington, J.; Giles, D.E.; Buysse, D.J. Comparative Meta-Analysis of Pharmacotherapy and Behavior Therapy for Persistent Insomnia. Am. J. Psychiatry 2002, 159, 5–11. [Google Scholar] [CrossRef]
- Montgomery, P.; Dennis, J.A. Cognitive Behavioural Interventions for Sleep Problems in Adults Aged 60+; Cochrane Library: London, UK, 2003. [Google Scholar]
- Mitchell, M.D.; Gehrman, P.; Perlis, M.; Umscheid, C.A. Comparative effectiveness of cognitive behavioral therapy for insomnia: A systematic review. BMC Fam. Pract. 2012, 13, 40. [Google Scholar] [CrossRef]
- van Straten, A.; van der Zweerde, T.; Kleiboer, A.; Cuijpers, P.; Morin, C.M.; Lancee, J. Cognitive and behavioral therapies in the treatment of insomnia: A meta-analysis. Sleep Med. Rev. 2018, 38, 3–16. [Google Scholar] [CrossRef]
- Rossman, J. Cognitive-Behavioral Therapy for Insomnia: An Effective and Underutilized Treatment for Insomnia. Am. J. Lifestyle Med. 2019, 13, 544–547. [Google Scholar] [CrossRef] [PubMed]
- Hale, L.; Troxel, W.; Buysse, D.J. Sleep Health: An Opportunity for Public Health to Address Health Equity. Annu. Rev. Public Health 2020, 41, 81–99. [Google Scholar] [CrossRef]
- Altevogt, B.M.; Colten, H.R. (Eds.) Sleep Disorders and Sleep Deprivation: An Unmet Public Health Problem; National Academies Press: Washington, DC, USA, 2006. [Google Scholar]
- Tucker, R.M.; Contreras, D.A.; Carlson, B.R.; Carter, A.; Drake, C.L. Sleep Education for Elders Program (SLEEP): Promising Pilot Results of a Virtual, Health Educator-Led, Community-Delivered Sleep Behavior Change Intervention. Nat. Sci. Sleep 2021, 13, 625–633. [Google Scholar] [CrossRef]
- Haggerty, D.; Contreras, D.A.; Carter, A.; Drake, C.; Tucker, R.M. SLeep Education for Everyone Program (SLEEP) Results in Sustained Improvements in Sleep Outcomes at Six Months. Behav. Sleep. Med. 2023, 21, 601–607. [Google Scholar] [CrossRef]
- Gruber, R.; Somerville, G.; Bergmame, L.; Fontil, L.; Paquin, S. School-based sleep education program improves sleep and academic performance of school-age children. Sleep Med. 2016, 21, 93–100. [Google Scholar] [CrossRef]
- Bani Issa, W.; Hijazi, H.; Radwan, H.; Saqan, R.; Al-Sharman, A.; Samsudin, A.B.R.; Fakhry, R.; Al-Yateem, N.; Rossiter, R.C.; Ibrahim, A.; et al. Evaluation of the effectiveness of sleep hygiene education and FITBIT devices on quality of sleep and psychological worry: A pilot quasi-experimental study among first-year college students. Front Public Health. 2023, 11, 1182758. [Google Scholar] [CrossRef]
- Hirshkowitz, M.; Whiton, K.; Albert, S.M.; Alessi, C.; Bruni, O.; DonCarlos, L.; Hazen, N.; Herman, J.; Katz, E.S.; Kheirandish-Gozal, L.; et al. National Sleep Foundation’s sleep time duration recommendations: Methodology and results summary. Sleep Health 2015, 1, 40–43. [Google Scholar] [CrossRef] [PubMed]
- Read, J.R.; Sharpe, L.; Modini, M.; Dear, B.F. Multimorbidity and depression: A systematic review and meta-analysis. J. Affect. Disord. 2017, 221, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Roughead, E.E.; Vitry, A.I.; Caughey, G.E.; Gilbert, A.L. Multimorbidity, Care Complexity and Prescribing for the Elderly. Aging Health 2011, 7, 695–705. [Google Scholar] [CrossRef]
- Ye, X.; Wang, X. Associations of multimorbidity with body pain, sleep duration, and depression among middle-aged and older adults in China. Health Qual. Life Outcomes 2024, 22, 23. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yang, Q.; He, P.; Jin, X.; Mao, X.; Hu, Y.; Jing, L. The relationship between multiple chronic diseases and sleep quality among the older people ≥ 60 years in China. Sleep Breath. 2025, 29, 179. [Google Scholar] [CrossRef]
- Idalino, S.C.; Canever, J.B.; Cândido, L.M.; Wagner, K.J.; de Souza Moreira, B.; Danielewicz, A.L.; De Avelar, N.C. Association between sleep problems and multimorbidity patterns in older adults. BMC Public Health 2023, 23, 978. [Google Scholar] [CrossRef]
- Masnoon, N.; Shakib, S.; Kalisch-Ellett, L.; Caughey, G.E. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017, 17, 230. [Google Scholar] [CrossRef] [PubMed]
- Maher, R.L.; Hanlon, J.; Hajjar, E.R. Clinical consequences of polypharmacy in elderly. Expert Opin. Drug Saf. 2014, 13, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Do, D. Trends in the use of medications with insomnia side effects and the implications for insomnia among US adults. J. Sleep Res. 2020, 29, e13075. [Google Scholar] [CrossRef]
- Rodrigues, M.C.S.; Oliveira, C.d. Drug-drug interactions and adverse drug reactions in polypharmacy among older adults: An integrative review. Rev. Lat. Am. Enferm. 2016, 24, e2800. [Google Scholar] [CrossRef]
- Casagrande, M.; Forte, G.; Favieri, F.; Corbo, I. Sleep Quality and Aging: A Systematic Review on Healthy Older People, Mild Cognitive Impairment and Alzheimer’s Disease. Int. J. Environ. Res. Public Health 2022, 19, 8457. [Google Scholar] [CrossRef]
- Mander, B.A.; Winer, J.R.; Walker, M.P. Sleep and Human Aging. Neuron 2017, 94, 19–36. [Google Scholar] [CrossRef] [PubMed]
- Miner, B.; Kryger, M.H. Sleep in the Aging Population. Sleep Med. Clin. 2017, 12, 31–38. [Google Scholar] [CrossRef]
- Fox, E.C.; Wang, K.; Aquino, M.; Grandner, M.A.; Xie, D.; Branas, C.C.; Gooneratne, N.S. Sleep debt at the community level: Impact of age, sex, race/ethnicity and health. Sleep Health 2018, 4, 317–324. [Google Scholar] [CrossRef]
- Ruggiero, A.R.; Peach, H.D.; Gaultney, J.F. Association of sleep attitudes with sleep hygiene, duration, and quality: A survey exploration of the moderating effect of age, gender, race, and perceived socioeconomic status. Health Psychol. Behav. Med. 2019, 7, 19–44. [Google Scholar] [CrossRef]
- Prentice, K.R.; Beitelshees, M.; Hill, A.; Jones, C.H. Defining health equity: A modern US perspective. iScience 2024, 27, 111326. [Google Scholar] [CrossRef]
- Jean-Louis, G.; Grandner, M.A.; Seixas, A.A. Social determinants and health disparities affecting sleep. Lancet Neurol. 2022, 21, 864–865. [Google Scholar] [CrossRef]
- Laposky, A.D.; Van Cauter, E.; Diez-Roux, A.V. Reducing health disparities: The role of sleep deficiency and sleep disorders. Sleep Med. 2016, 18, 3–6. [Google Scholar] [CrossRef]
- Sosso, E.; Armel, F. Measuring Sleep Health Disparities with Polysomnography: A Systematic Review of Preliminary Findings. Clocks Sleep 2022, 4, 80–87. [Google Scholar] [CrossRef]
- Billings, M.E.; Cohen, R.T.; Baldwin, C.M.; Johnson, D.A.; Palen, B.N.; Parthasarathy, S.; Patel, S.R.; Russell, M.; Tapia, I.E.; Williamson, A.A.; et al. Disparities in Sleep Health and Potential Intervention Models: A Focused Review. Chest 2021, 159, 1232–1240. [Google Scholar] [CrossRef] [PubMed]
- Assari, S. Health Disparities due to Diminished Return among Black Americans: Public Policy Solutions. Soc. Issues Policy Rev. 2018, 12, 112–145. [Google Scholar] [CrossRef]
- Donat, M.; Brown, C.; Williams, N.; Pandey, A.; Racine, C.; McFarlane, S.I.; Jean-Louis, G. Linking sleep duration and obesity among black and white US adults. Clin. Pract. 2013, 10, 661–667. [Google Scholar] [CrossRef] [PubMed]
- Grandner, M.A.; Chakravorty, S.; Perlis, M.L.; Oliver, L.; Gurubhagavatula, I. Habitual sleep duration associated with self-reported and objectively determined cardiometabolic risk factors. Sleep Med. 2014, 15, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Barber, L.E.; Smith-Ireland, D.; Enun, B.; Johnson, D.A. Understanding sleep health in Black American adults: A qualitative analysis of barriers, facilitators, and perspectives on sleep interventions. Sleep Health 2025, 11, 265–274. [Google Scholar] [CrossRef]
- Lazar, M.; Davenport, L. Barriers to Health Care Access for Low Income Families: A Review of Literature. J. Community Health Nurs. 2018, 35, 28–37. [Google Scholar] [CrossRef]
- Nagata, J.M.; Palar, K.; Gooding, H.C.; Garber, A.K.; Bibbins-Domingo, K.; Weiser, S.D. Food Insecurity and Chronic Disease in US Young Adults: Findings from the National Longitudinal Study of Adolescent to Adult Health. J. Gen. Intern. Med. 2019, 34, 2756–2762. [Google Scholar] [CrossRef]
- Tubbs, A.S.; Ghani, S.B.; Valencia, D.; Jean-Louis, G.; Killgore, W.D.; Fernandez, F.X.; Grandner, M.A. Racial/ethnic minorities have greater declines in sleep duration with higher risk of cardiometabolic disease: An analysis of the U.S. National Health Interview Survey. Sleep Epidemiol. 2022, 2, 100022. [Google Scholar] [CrossRef]
- Patel, S.R.; Blackwell, T.; Redline, S.; Ancoli-Israel, S.; Cauley, J.A.; Hillier, T.A.; Lewis, C.E.; Orwoll, E.S.; Stefanick, M.L.; Taylor, B.C.; et al. The association between sleep duration and obesity in older adults. Int. J. Obes. 2008, 32, 1825–1834. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.R.; Hu, F.B. Short sleep duration and weight gain: A systematic review. Obesity 2008, 16, 643–653. [Google Scholar] [CrossRef]
- Contreras, D.A.; Williams, E.; Tucker, R.M. Equivalent Improvements in Sleep Duration and Sleep Quality Regardless of Program Delivery Modality: The SLeep Education for Everyone Program (SLEEP). Clocks Sleep 2023, 5, 226–233. [Google Scholar] [CrossRef]
- Mastin, D.F.; Bryson, J.; Corwyn, R. Assessment of sleep hygiene using the Sleep Hygiene Index. J. Behav. Med. 2006, 29, 223–227. [Google Scholar] [CrossRef]
- Buysse, D.J.; Reynolds, C.F.; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef] [PubMed]
| n (%) | Mean (SD) | |
|---|---|---|
| Age | 66.7 y (12.3) | |
| 99 (95.2%) 5 (4.8%) | |
| Sex | ||
| 93 (89.4%) 10 (9.6%) 1 (1.0%) | -- |
| Race | ||
| 80 (76.9%) 19 (18.3%) 3 (2.9%) 2 (1.9%) | -- |
| BMI | 29.9 kg/m2 (8.1) | |
| 0 (0%) 31 (29.8%) 25 (24.0%) 45 (43.3%) 3 (2.9%) | |
| Number of Chronic Diseases | ||
| 21 (20.2%) 15 (14.4%) 16 (15.4%) 52 (50.0%) | -- |
| Median Income in Dollars 1 | ||
| 25 (24.0%) 52 (50.0%) 25 (24.0%) 2 (1.9%) | -- |
| Outcome | Group Variable | Effect | Estimate (β) | SE | 95% CI | p-Value | |
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| SHI | |||||||
| Age | Time | −2.38 | 1.91 | −6.17 | 1.41 | <0.001 ** | |
| Group | −1.14 | 1.50 | −4.11 | 1.82 | 0.028 * | ||
| Time × Group | −0.42 | 0.89 | −2.18 | 1.34 | 0.429 | ||
| Race | Time | −14.46 | 4.93 | −24.26 | −4.65 | 0.003 * | |
| Group | −8.08 | 3.03 | −14.07 | −2.09 | 0.173 | ||
| Time × Group | 4.05 | 1.74 | 0.58 | 7.51 | 0.020 * | ||
| BMI | Time | 0.68 | 2.81 | −4.90 | 6.27 | 0.809 | |
| Group | 3.20 | 1.45 | 0.33 | 6.07 | 0.032 * | ||
| Time × Group | −1.19 | 0.86 | −2.88 | 0.51 | 0.165 | ||
| Income | Time | −4.25 | 2.14 | −8.50 | 0.00 | 0.047 * | |
| Group | −1.05 | 1.7 | −4.42 | 2.32 | 0.837 | ||
| Time × Group | 0.59 | 1.00 | −1.40 | 2.58 | 0.555 | ||
| Chronic Conditions | Time | −4.75 | 1.34 | −7.41 | −2.09 | <0.001 ** | |
| Group | −1.36 | 1.00 | −3.33 | 0.61 | 0.888 | ||
| Time × Group | 0.86 | 0.58 | −0.30 | 2.02 | 0.140 | ||
| PSQI | |||||||
| Age | Time | −2.23 | 0.90 | −4.02 | −0.45 | <0.001 ** | |
| Group | 1.42 | 0.73 | −0.02 | 2.86 | 0.045 * | ||
| Time × Group | −0.42 | 0.42 | −1.24 | 0.41 | 0.616 | ||
| Race | Time | −5.37 | 2.40 | −10.14 | −0.61 | 0.025 * | |
| Group | −1.45 | 1.53 | −4.47 | 1.58 | 0.715 | ||
| Time × Group | 0.83 | 0.85 | −0.85 | 2.52 | 0.325 | ||
| BMI | Time | −2.36 | 1.34 | −5.02 | 0.30 | 0.077 | |
| Group | 0.94 | 0.72 | −0.48 | 2.36 | 0.091 | ||
| Time × Group | −0.21 | 0.41 | −1.02 | 0.60 | 0.600 | ||
| Income | Time | −3.65 | 1.02 | −5.68 | −1.63 | <0.001 ** | |
| Group | −0.76 | 0.84 | −2.42 | 0.90 | 0.498 | ||
| Time × Group | 0.31 | 0.48 | −0.64 | 1.25 | 0.521 | ||
| Chronic Conditions | Time | −3.54 | 0.64 | −4.82 | −2.27 | <0.001 ** | |
| Group | 0.01 | 0.49 | −0.98 | 0.97 | 0.132 | ||
| Time × Group | 0.26 | 0.28 | −0.30 | 0.82 | 0.357 | ||
| Sleep Duration | |||||||
| Age | Time | 0.49 | 0.29 | −0.09 | 1.07 | <0.001 ** | |
| Group | −0.37 | 0.24 | −0.85 | 0.11 | 0.109 | ||
| Time × Group | 0.06 | 0.13 | −0.21 | 0.33 | 0.870 | ||
| Race | Time | 0.94 | 0.74 | −0.54 | 2.41 | 0.206 | |
| Group | 0.70 | 0.48 | −0.26 | 1.65 | 0.072 | ||
| Time × Group | −0.13 | 0.26 | −0.65 | 0.39 | 0.615 | ||
| BMI | Time | 0.62 | 0.11 | 0.41 | 0.84 | <0.001 ** | |
| Group | 0.19 | 0.23 | −0.27 | 0.65 | 0.174 | ||
| Time × Group | −0.24 | 0.13 | −0.49 | 0.01 | 0.052 | ||
| Income | Time | 0.42 | 0.32 | −0.22 | 1.07 | 0.188 | |
| Group | 0.06 | 0.27 | −0.48 | 0.59 | 0.236 | ||
| Time × Group | 0.08 | 0.15 | −0.22 | 0.38 | 0.582 | ||
| Chronic Conditions | Time | 0.73 | 0.20 | 0.32 | 1.13 | <0.001 ** | |
| Group | 0.12 | 0.16 | −0.20 | 0.44 | 0.855 | ||
| Time × Group | 0.07 | 0.09 | −0.24 | 0.11 | 0.441 | ||
| Model | B | SE | Beta | p |
|---|---|---|---|---|
| SHI—post | ||||
| −0.177 | 0.041 | −0.407 | <0.001 ** |
| 3.309 | 1.106 | 0.268 | 0.004 * | |
| 0.186 | 0.075 | 0.235 | 0.015 * | |
| PSQI—post | ||||
| 0.552 | 0.251 | 0.209 | 0.031 * |
| 0.427 | 0.085 | 0.474 | <0.001 ** | |
| Sleep Duration—post | ||||
| 0.556 | 0.080 | 0.607 | <0.001 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tisdale, A.; Kim, N.; Contreras, D.A.; Williams, E.; Tucker, R.M. Who Benefits the Most from Sleep Hygiene Education? Findings from the SLeep Education for Everyone Program (SLEEP). Clocks & Sleep 2025, 7, 40. https://doi.org/10.3390/clockssleep7030040
Tisdale A, Kim N, Contreras DA, Williams E, Tucker RM. Who Benefits the Most from Sleep Hygiene Education? Findings from the SLeep Education for Everyone Program (SLEEP). Clocks & Sleep. 2025; 7(3):40. https://doi.org/10.3390/clockssleep7030040
Chicago/Turabian StyleTisdale, Alyssa, Nahyun Kim, Dawn A. Contreras, Elizabeth Williams, and Robin M. Tucker. 2025. "Who Benefits the Most from Sleep Hygiene Education? Findings from the SLeep Education for Everyone Program (SLEEP)" Clocks & Sleep 7, no. 3: 40. https://doi.org/10.3390/clockssleep7030040
APA StyleTisdale, A., Kim, N., Contreras, D. A., Williams, E., & Tucker, R. M. (2025). Who Benefits the Most from Sleep Hygiene Education? Findings from the SLeep Education for Everyone Program (SLEEP). Clocks & Sleep, 7(3), 40. https://doi.org/10.3390/clockssleep7030040






