1. Introduction
The mammalian circadian system is an intricate internal timekeeping system that synchronizes physiological and behavioral functions with the 24 h environmental daily cycle. The master oscillator in mammals is the hypothalamic suprachiasmatic nuclei (SCN), which are responsible for the coordination of central and peripheral clocks. The SCN are aligned with environmental cycles such as the day/night cycle by cues known as “Zeitgebers” [
1,
2]. The most prominent Zeitgeber is light.
Photoreceptors in the retina detect light and relay the photic signal to the SCN, which transmit this signal to other central and peripheral oscillators. The molecular mechanism of the circadian oscillator is made up of a transcriptional/translational feedback loop. This process begins when the BMAL1 and CLOCK proteins (i.e., positive elements of the loop that activate the transcriptional/translational feedback loop) form a heterodimer (CLOCK:BMAL1) that drives the transcription of the negative elements of the loop, namely,
Period (
Per1,
Per2, and
Per3) and
Cryptochrome genes (
Cry1 and
Cry2), leading to the expression of their respective proteins (PER, CRY). These negative elements, in turn, form their own heterodimer complex (PER:CRY) that suppresses their own transcription by inhibiting the activity of the CLOCK:BMAL1 complex [
3,
4].
In nocturnal rodents, nocturnal light signals can reset the circadian oscillator in the SCN, which leads to phase shifts of the circadian locomotor activity rhythm [
5,
6,
7]. The responsiveness to light in nocturnal rodents is time-dependent. Wild-type (
WT) animals exhibit phase delays when photic signals are applied early in the night and phase advances when signals are applied late at night, while no significant effect is observed when light is applied at midday or midnight [
7].
The expression of the molecular clock genes
Per1 and
Per2 has been reported to be induced by light within the SCN and retina [
8,
9]. This modulation underscores their role in the light transduction pathway, light responsiveness, and subsequent re-entrainment of circadian rhythms.
Per2 is a critical component of the molecular clockwork in the SCN of mammals, playing a pivotal role in resetting the circadian clock in response to light. Studies have shown that mutant
Per2 mice, as well as total and neuronal-specific
Per2 knockout mice, are unable to delay the clock when exposed to a light pulse at early night (ZT14), unlike their wild-type (
WT) counterparts [
10,
11]. Furthermore, mutant
Per2 mice and total
Per2 knockout mice display a shorter period compared to their controls with a transition to arrhythmic circadian activity under prolonged constant darkness [
12,
13]. Notably, the shorter period length is also observed in neuronal-specific
Per2 knockout mice [
11]. Interestingly, recent findings indicate that
Per2 also participates in the CREB pathway, contributing to the activation of the
Per1 gene in response to photic signals [
14].
Circadian molecular clock genes are expressed not only in neurons of the SCN but also in various glial cells, including astrocytes [
15,
16]. Emerging evidence suggests that a functional and intact circadian molecular clockwork in astrocytes plays an essential role in regulating SCN outputs and behavioral rhythms—such as activity patterns and sleep homeostasis—in a manner that is autonomous and independent from neuronal clocks [
17,
18,
19].
Astrocytic clock genes are reported to regulate the fluctuating expression of glutamate, ATP, and adenosine, which are crucial in controlling circadian system entrainment to photic cues and the rhythmic sleep/wake cycle [
20,
21]. Disruption of the astrocytic clock has been shown to alter various animal behaviors [
22]. For instance, targeted deletion of
Per2 in astrocytes decreases anxiety-like and depression-related behaviors [
13]. Furthermore, specific deletion of
Bmal1 in astrocytes leads to disruptions in cognitive functions and circadian locomotor activity rhythms in mice, resulting in longer period lengths compared to control groups [
18,
23].
Despite these findings, the role of the astrocytic Per2 gene in regulating the re-entrainment of the circadian clock in response to photic signals remains unexplored. Therefore, the current study aims to investigate the potential role of the astrocytic Per2 gene in the photic signal transduction pathway and circadian clock resetting in mice. This investigation will utilize astrocyte-specific Per2 knockout mice (GPer2) and neuron-specific Per2 knockout mice (NPer2) to compare the function of Per2 in astrocytes in circadian periodicity and phase resetting with its function in neurons.
3. Discussion
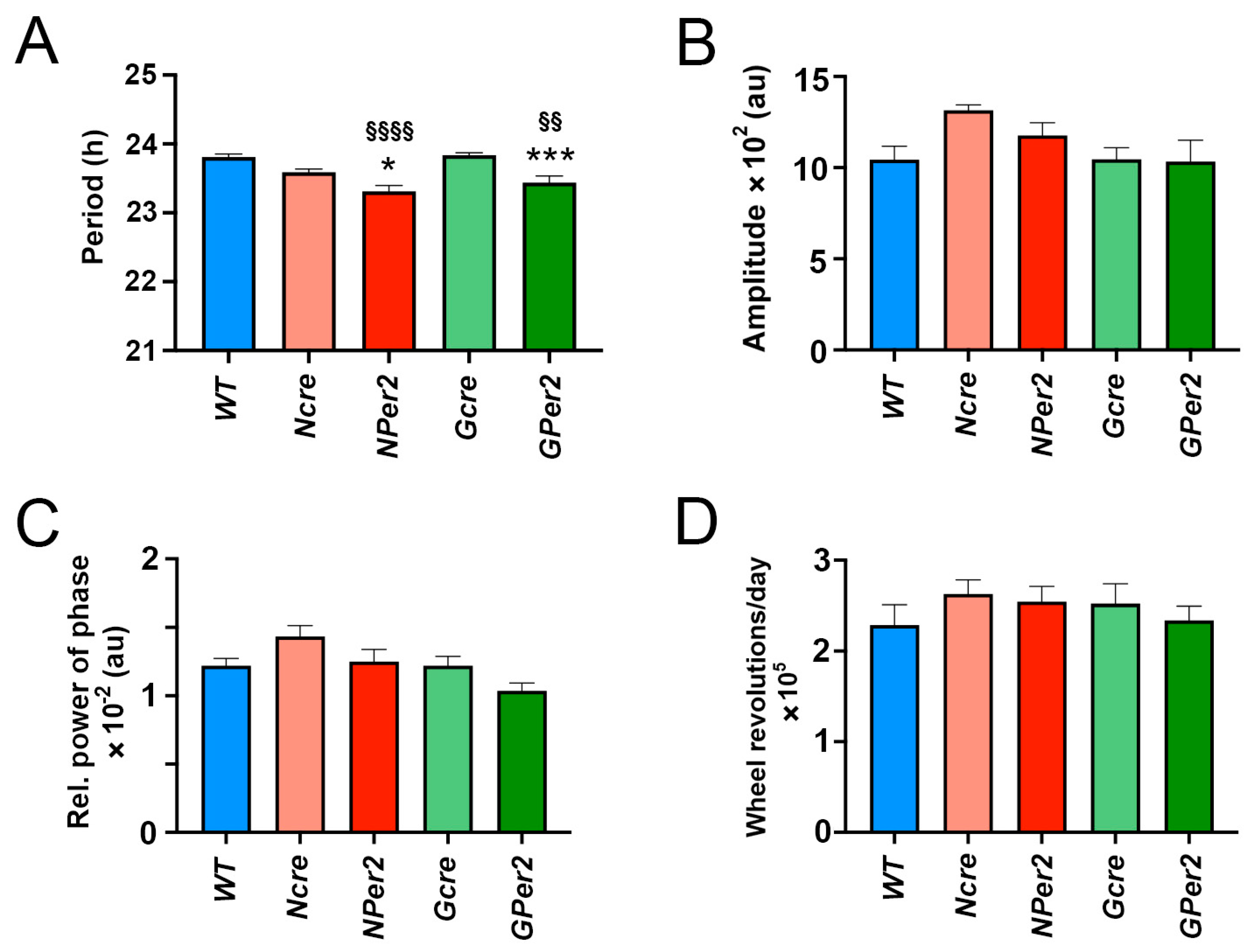
In this study, we investigated the impact of Per2 expression deficiency in astrocytes and neurons in mice on period and phase using wheel-running activity. Our findings revealed that the circadian period is shorter in mice lacking Per2 in either astrocytes or neurons. Additionally, while light-induced phase shifts in mice deficient in astrocytic Per2 were comparable to control animals, the absence of Per2 in neurons significantly affected phase shifts.
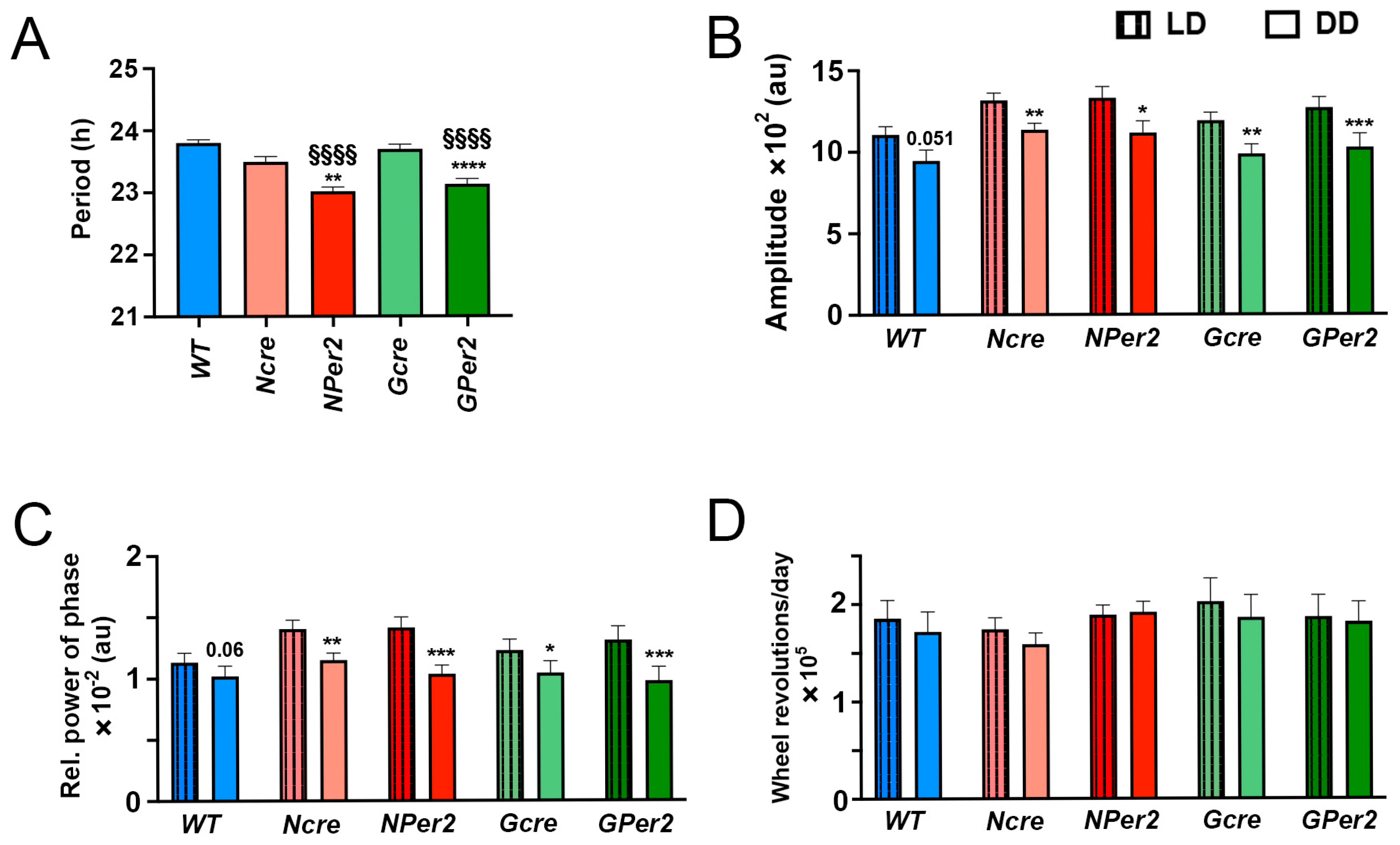
The observed shorter period in
NPer2 and
GPer2 knockout (KO) mice suggests that both astrocytes and neurons contribute to circadian clock period regulation. This aligns with earlier studies demonstrating that circadian behavior is regulated not only by neurons [
29] but also by astrocytes [
18,
19,
22]. Prior research further highlighted that astrocytes sustain circadian oscillations and influence the circadian period in the SCN, albeit with lower efficacy than neurons [
30]. Interestingly, while deletion of
Per2 in astrocytes shortens the behavioral period (
Figure 3A), ablation of
Bmal1 in astrocytes lengthens it [
18,
23]. This reinforces the importance of astrocytic clock components in behavioral period regulation.
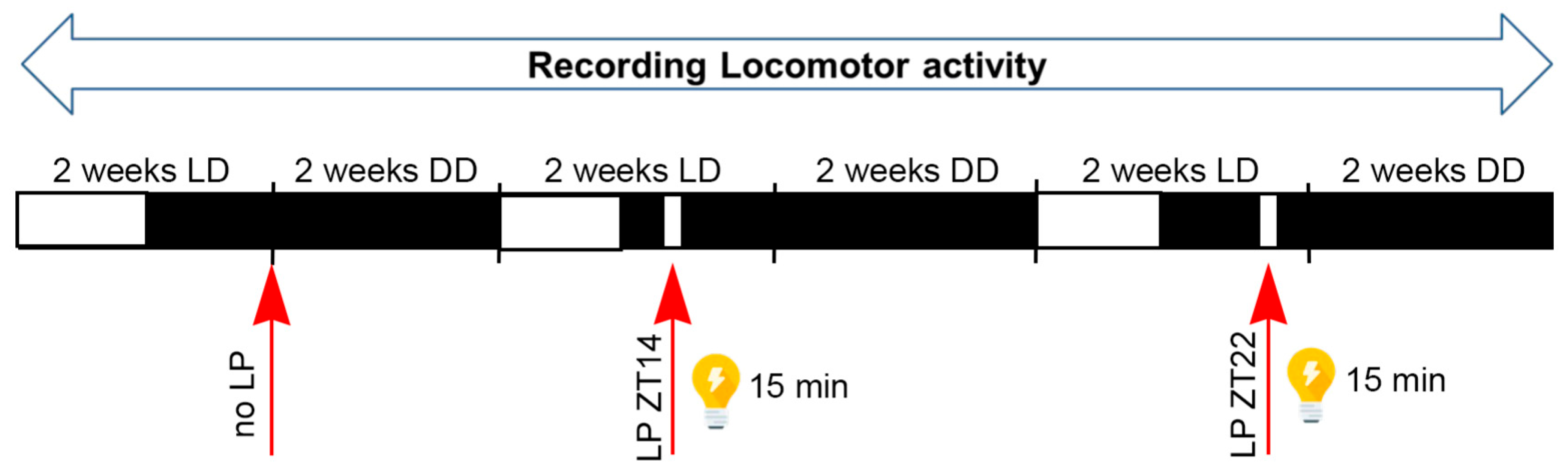
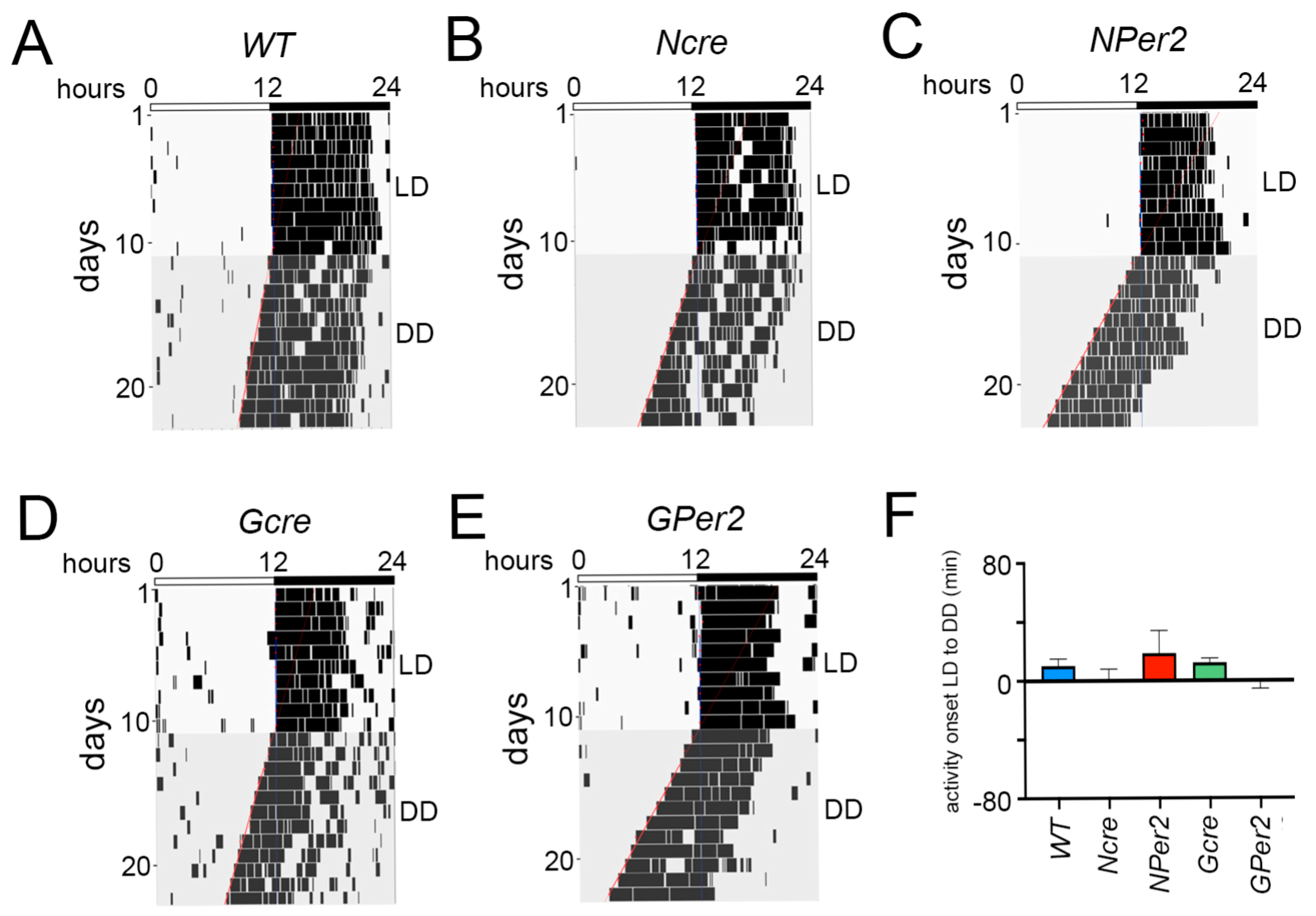
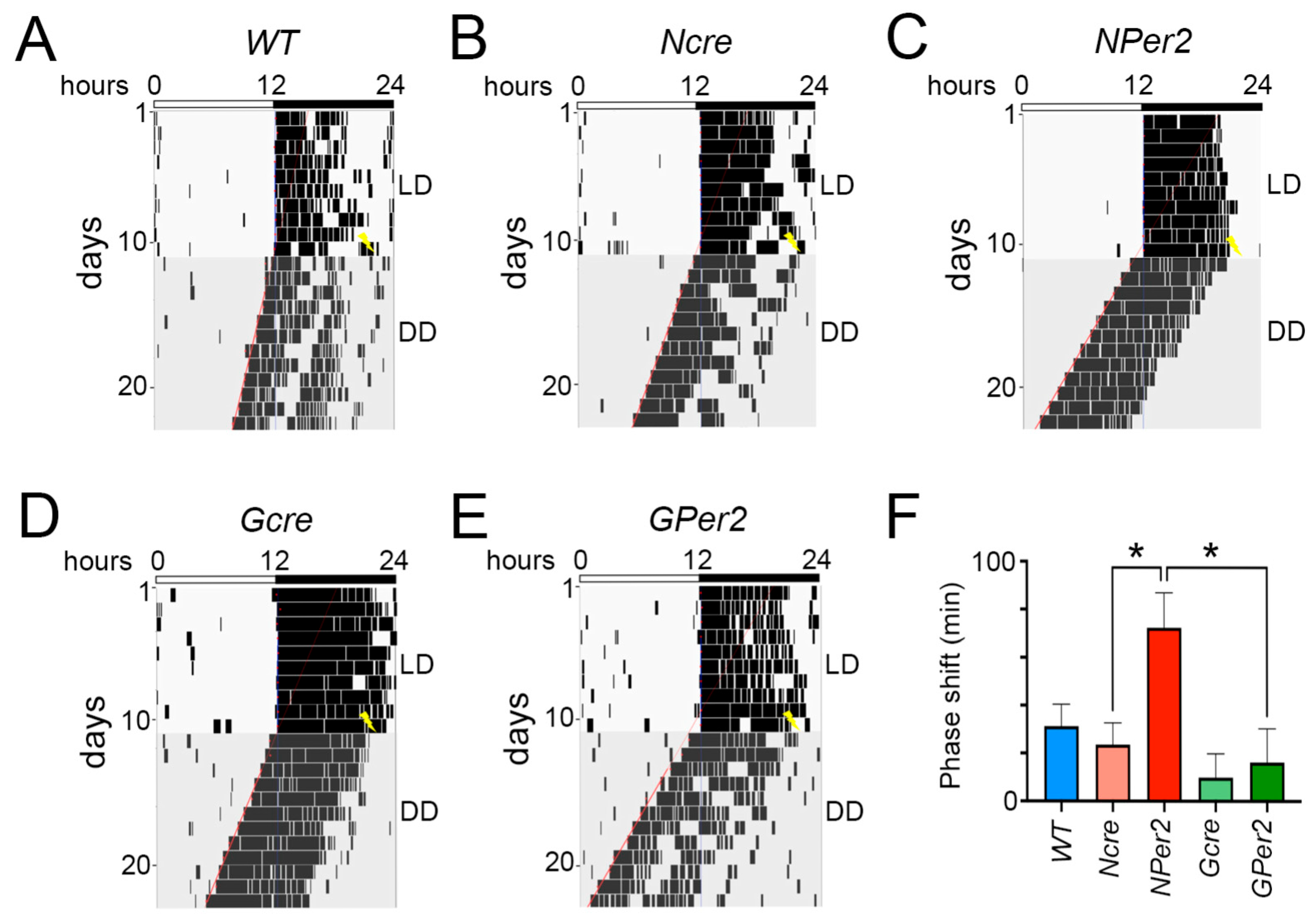
To examine phase shifts, we employed an Aschoff type II protocol and first assessed whether transitions from light/dark (LD) to constant darkness (DD) without a light pulse were consistent across genotypes. No significant differences were observed in activity onset post LD to DD transition among all groups of animals (
Figure 2). This suggests that the shorter period observed in
NPer2 and
GPer2 mice (
Figure 3A) did not significantly affect the phase shifts observed (Figs. 4 and 6). Moreover, total activity levels across genotypes showed no significant differences that could potentially impact activity onset following a light pulse (
Figure 3D). However, all genotypes exhibited reduced amplitude and stability of circadian locomotor activity rhythm in DD (
Figure 3B,C).
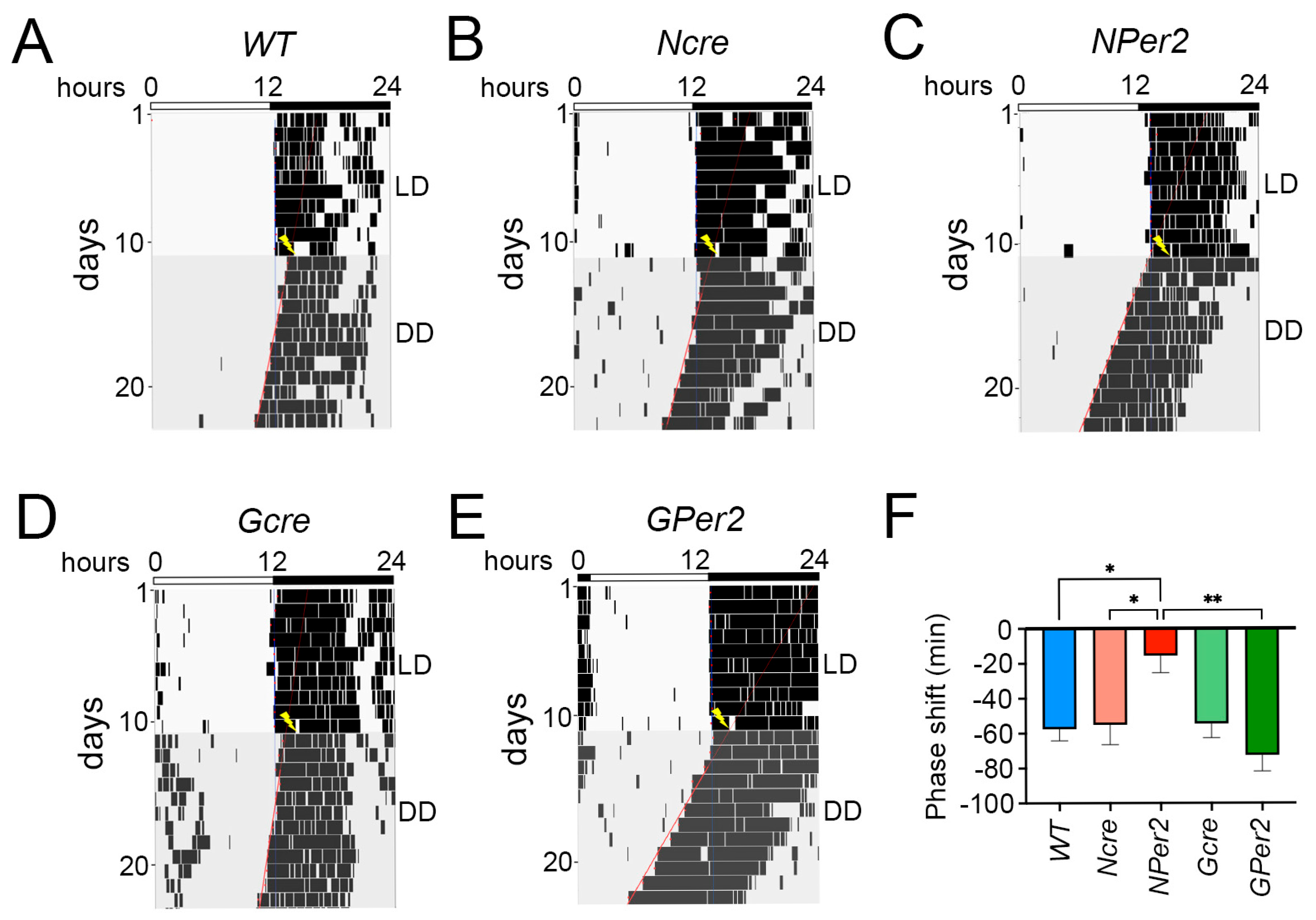
When light was applied at ZT14, all genotypes except
NPer2 mice displayed phase delays, with significantly reduced phase delays observed in
NPer2 animals (
Figure 4). This phenotype mirrors that of mice with global
Per2 deficiency or
Per2 mutation leading to unstable protein [
10,
31]. Notably, mice lacking
Per2 in astrocytes (
GPer2 mice) exhibited normal phase delays, suggesting that astrocytic
Per2 does not influence phase delays, unlike neuronal
Per2 (
Figure 4). This finding corroborates previous studies indicating that astrocytes do not regulate circadian phase in the SCN [
30]. After a ZT14 light pulse,
NPer2 and
GPer2 mice showed shorter periods compared to controls (
Figure 5), consistent with observations in the absence of light pulses (
Figure 3). Similarly, other parameters, such as amplitude, relative power of phase, and total activity, showed no significant differences across genotypes (
Figure 5), indicating these factors did not account for phenotypic variations in light-induced phase delays between
NPer2 and
GPer2 mice.
At ZT22, light induced behavioral phase advances in all genotypes (
Figure 6). Interestingly,
NPer2 mice displayed significantly larger phase advances compared to all other genotypes, including
GPer2 mice. This phenotype parallels previous findings in mice with global
Per2 mutations, which showed greater phase advances relative to wild-type animals [
10]. The enhanced phase advance in
NPer2 mice is unlikely to result from their shorter circadian period (
Figure 7A), as
GPer2 mice also exhibit a shorter period but do not show increased phase advances (
Figure 6F). This suggests that neuronal
Per2, but not astrocytic
Per2, influences phase advances—a conclusion consistent with observations of light-induced phase delays at ZT14 (
Figure 4). These results further support the notion that astrocytes contribute to circadian period regulation but not phase modulation within the SCN [
30].
When considering the results from the light pulses at ZT14 and ZT22 together, it is striking that mice lacking neuronal Per2—despite their impaired ability to delay the circadian clock—exhibit a disproportionately strong phase-advancing response to light during the late night. This suggests that neuronal Per2 plays a dual role—facilitating phase delays while concurrently restricting excessive phase advances.
One limitation of this study is the exclusive use of the Aschoff type II protocol, which does not exclude for potential influences of the preceding light/dark cycle prior to light pulse application and subsequent release into constant darkness. Future studies employing an Aschoff type I protocol in constant darkness could address this limitation.
In summary, our experiments shed light on the distinct roles of neuronal and astrocytic Per2 in regulating rapid behavioral phase shifts caused by brief light pulses. We conclude that while astrocytic Per2 contributes to circadian period regulation, neuronal Per2 is involved in both period and phase modulation.