Evaluation of the Circadian Rhythm Component Cipc (Clock-Interacting Pacemaker) in Leukemogenesis: A Literature Review and Bioinformatics Approach
Abstract
1. Introduction
2. Clock Genes and Disease
2.1. Clock Genes and Cancer
2.2. Clock Genes and Oncohematologic Neoplasms
3. CLOCK-Interacting Pacemaker (CipC)
4. Methodology
5. Results
5.1. Literature Review and Bioinformatic Analysis Approach
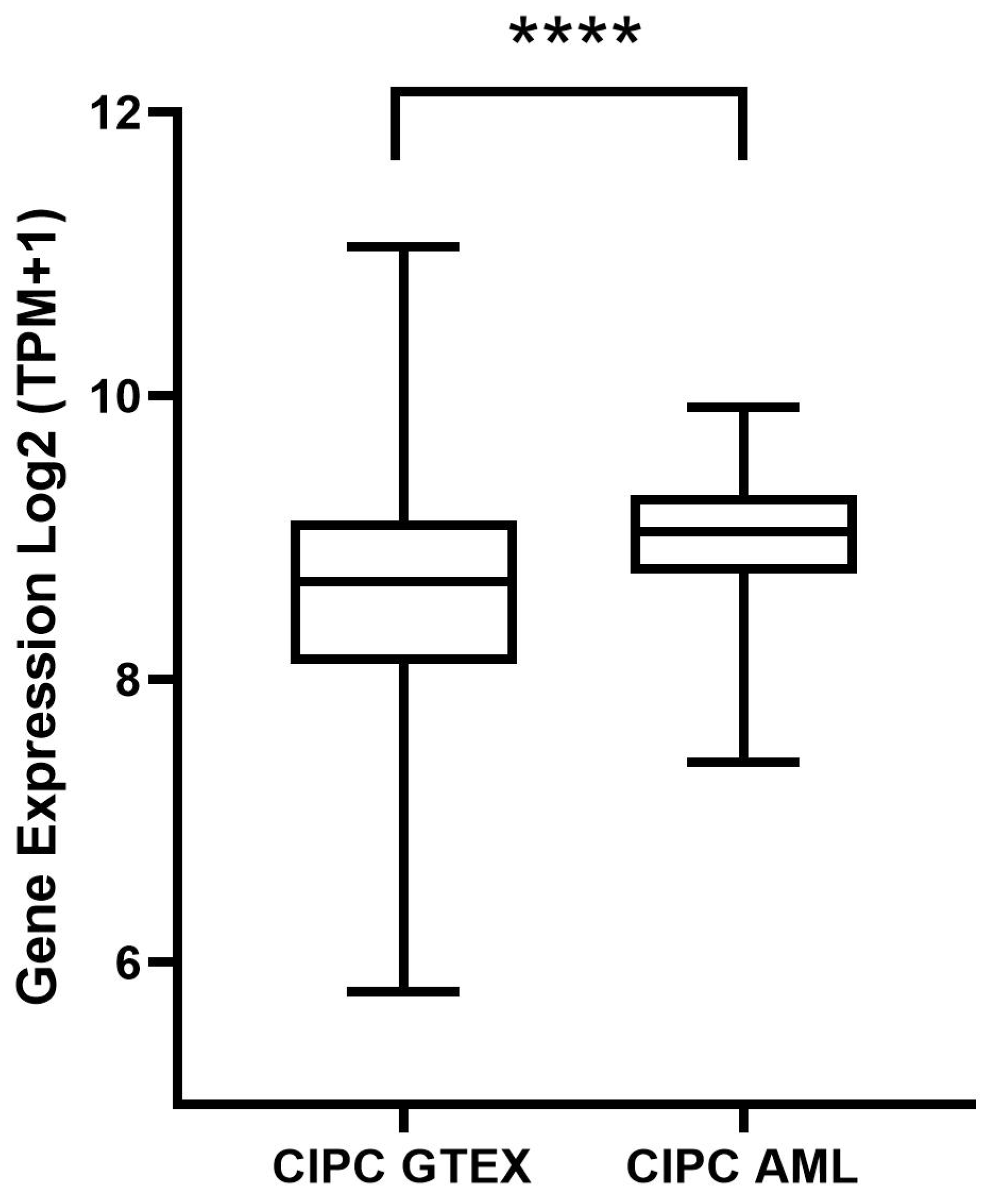
5.2. Gene Expression Analysis of CIPC and Survival Analysis
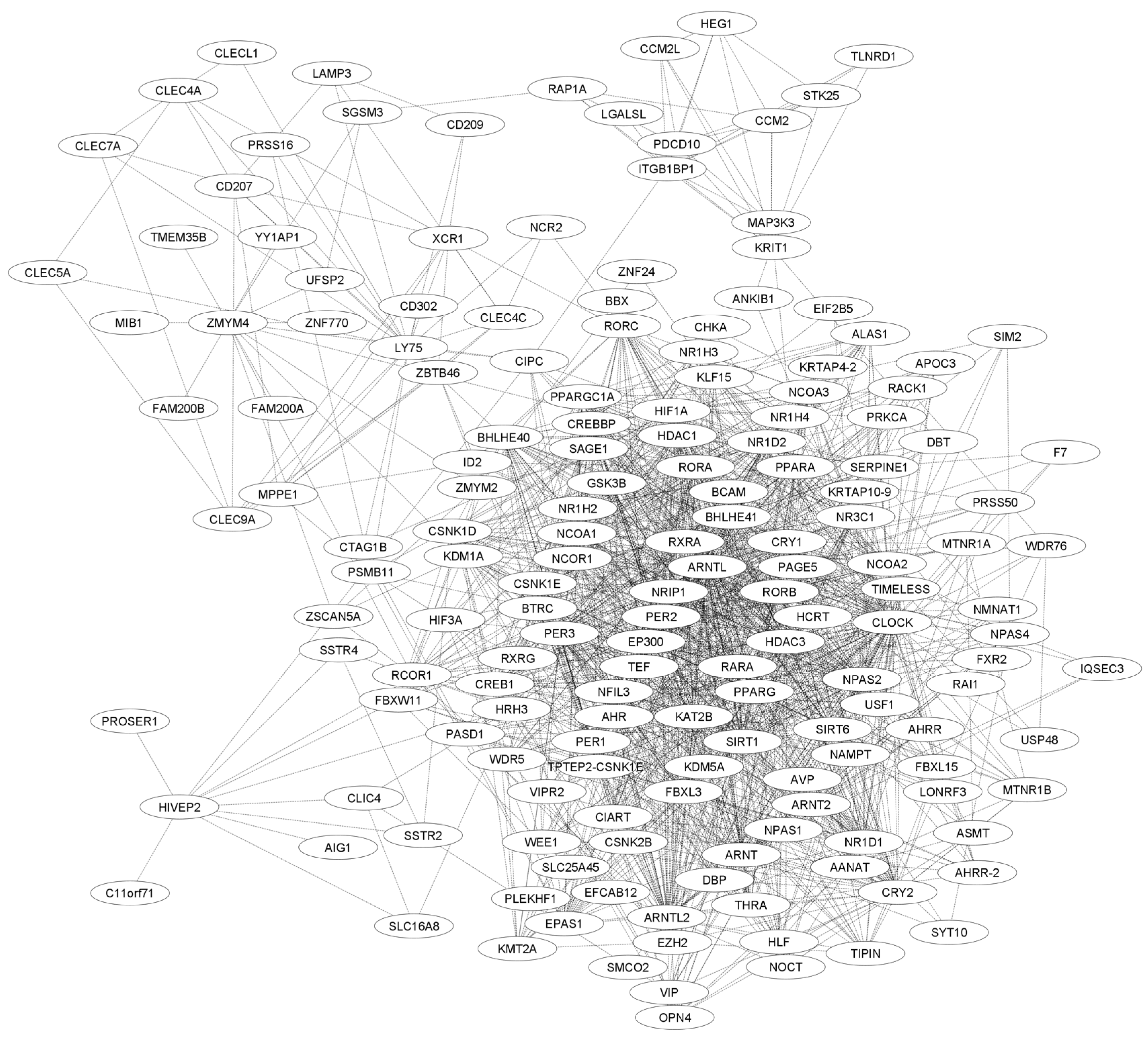
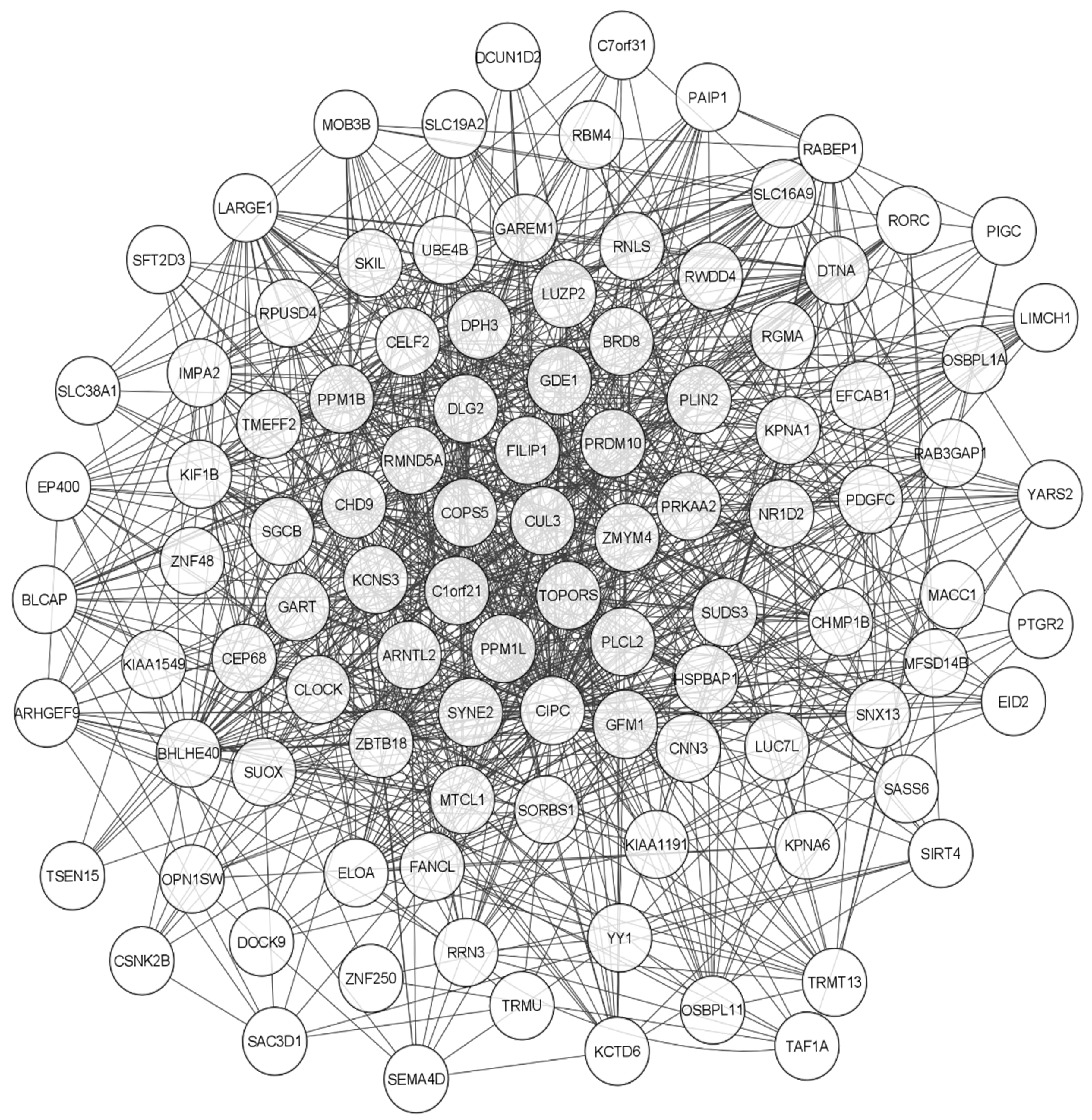
5.3. Functional Network Analysis of CIPC Using STRING and GENEMANIA
6. Discussion
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Huang, R.C. The Discoveries of Molecular Mechanisms for the Circadian Rhythm: The 2017 Nobel Prize in Physiology or Medicine. Biomed. J. 2018, 41, 5–8. [Google Scholar] [CrossRef] [PubMed]
- Jagannath, A.; Taylor, L.; Wakaf, Z.; Vasudevan, S.R.; Foster, R.G. The Genetics of Circadian Rhythms, Sleep and Health. Hum. Mol. Genet. 2017, 26, R128–R138. [Google Scholar] [CrossRef] [PubMed]
- Konopka, R.J.; Benzer, S. Clock Mutants of Drosophila Melanogaster (Eclosion/Circadian/Rhythms/X Chromosome). Proc. Natl. Acad. Sci. USA 1971, 68, 2112–2116. [Google Scholar] [CrossRef]
- Duan, J.; Greenberg, E.N.; Karri, S.S.; Andersen, B. The Circadian Clock and Diseases of the Skin. FEBS Lett. 2021, 595, 2413–2436. [Google Scholar] [CrossRef]
- Rosbash, M. Circadian Rhythms and the Transcriptional Feedback Loop (Nobel Lecture)**. Angew. Chem. Int. Ed. 2021, 60, 8650–8666. [Google Scholar] [CrossRef]
- Yang, Y.; Lindsey-Boltz, L.A.; Vaughn, C.M.; Selby, C.P.; Cao, X.; Liu, Z.; Hsu, D.S.; Sancar, A. Circadian Clock, Carcinogenesis, Chronochemotherapy Connections. J. Biol. Chem. 2021, 297, 101068. [Google Scholar] [CrossRef]
- King, D.P.; Zhao, Y.; Sangoram, A.M.; Wilsbacher, L.D.; Tanaka, M.; Antoch, M.P.; Steeves, T.D.L.; Vitaterna, M.H.; Kornhauser, J.M.; Lowrey, P.L.; et al. Positional Cloning of the Mouse Circadian Clock Gene. Cell 1997, 89, 641–653. [Google Scholar] [CrossRef] [PubMed]
- Kraft, M.; Martin, R. Chronobiology and Chronotherapy in Medicine. Disease-a-Month 1995, 41, 506–575. [Google Scholar] [CrossRef]
- Reddy, P.; Zehring, W.A.; Wheeler, D.A.; Pirrotta, V.; Hadfield, C.; Hall, J.C.; Rosbash, M. Molecular Analysis of the Period Locus in Drosophila Melanogaster and Identification of a Transcript Involved in Biological Rhythms. Cell 1984, 38, 701–710. [Google Scholar] [CrossRef]
- Sweeney, B.M. Biological Clocks-An Introduction. BioScience 1983, 33, 424–425. [Google Scholar] [CrossRef]
- Hastings, M.H.; Maywood, E.S.; Brancaccio, M. Generation of Circadian Rhythms in the Suprachiasmatic Nucleus. Nat. Rev. Neurosci. 2018, 19, 453–469. [Google Scholar] [CrossRef] [PubMed]
- Ruan, W.; Yuan, X.; Eltzschig, H.K. Circadian Rhythm as a Therapeutic Target. Nat. Rev. Drug Discov. 2021, 20, 287–307. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.L.; Li, L. Circadian Clock Regulates Inflammation and the Development of Neurodegeneration. Front. Cell. Infect. Microbiol. 2021, 11, 696554. [Google Scholar] [CrossRef]
- Cao, X.; Yang, Y.; Selby, C.P.; Liu, Z.; Sancar, A. Molecular Mechanism of the Repressive Phase of the Mammalian Circadian Clock. Proc. Natl. Acad. Sci. USA 2021, 118, e2021174118. [Google Scholar] [CrossRef] [PubMed]
- Sinturel, F.; Gos, P.; Petrenko, V.; Hagedorn, C.; Kreppel, F.; Storch, K.F.; Knutti, D.; Liani, A.; Weitz, C.; Emmenegger, Y.; et al. Circadian Hepatocyte Clocks Keep Synchrony in the Absence of a Master Pacemaker in the Suprachiasmatic Nucleus or Other Extrahepatic Clocks. Genes Dev. 2021, 35, 329–334. [Google Scholar] [CrossRef]
- Ray, S.; Valekunja, U.K.; Stangherlin, A.; Howell, S.A.; Snijders, A.P.; Damodaran, G.; Reddy, A.B. Circadian Rhythms in the Absence of the Clock Gene Bmal1. Science 2020, 367, 800–806. [Google Scholar] [CrossRef]
- Takahashi, J.S. Transcriptional Architecture of the Mammalian Circadian Clock. Nat. Rev. Genet. 2016, 18, 164. [Google Scholar] [CrossRef]
- Allada, R.; Bass, J. Circadian Mechanisms in Medicine. N. Engl. J. Med. 2021, 384, 550–561. [Google Scholar] [CrossRef]
- Chen, H.; Gao, L.; Yang, D.; Xiao, Y.; Zhang, M.; Li, C.; Wang, A.; Jin, Y. Coordination between the Circadian Clock and Androgen Signaling Is Required to Sustain Rhythmic Expression of Elovl3 in Mouse Liver. J. Biol. Chem. 2019, 294, 7046–7056. [Google Scholar] [CrossRef]
- Rutter, J.; Reick, M.; Wu, L.C.; Mcknight, S.L. Regulation of Clock and NPAS2 DNA Binding by the Redox State of NAD Cofactors. Science 2001, 293, 510–514. [Google Scholar] [CrossRef]
- Poggiogalle, E.; Jamshed, H.; Peterson, C.M. Circadian Regulation of Glucose, Lipid, and Energy Metabolism in Humans. Metabolism 2018, 84, 11–27. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhang, S.; Wang, H.; Wang, Z.; Zhang, X.; Wang, Y.; Yuan, J. Association of Rotating Night Shift Work, CLOCK, MTNR1A, MTNR1B Genes Polymorphisms and Their Interactions with Type 2 Diabetes among Steelworkers: A Case–Control Study. BMC Genom. 2023, 24, 232. [Google Scholar] [CrossRef]
- Marcheva, B.; Ramsey, K.M.; Buhr, E.D.; Kobayashi, Y.; Su, H.; Ko, C.H.; Ivanova, G.; Omura, C.; Mo, S.; Vitaterna, M.H.; et al. Disruption of the Clock Components CLOCK and BMAL1 Leads to Hypoinsulinaemia and Diabetes. Nature 2010, 466, 627–631. [Google Scholar] [CrossRef]
- Cai, Y.; Liu, S.; Sothern, R.B.; Xu, S.; Chan, P. Expression of Clock Genes Per1 and Bmal1 in Total Leukocytes in Health and Parkinson’s Disease. Eur. J. Neurol. 2010, 17, 550–554. [Google Scholar] [CrossRef]
- Niu, L.; Zhang, F.; Xu, X.; Yang, Y.; Li, S.; Liu, H.; Le, W. Chronic Sleep Deprivation Altered the Expression of Circadian Clock Genes and Aggravated Alzheimer’s Disease Neuropathology. Brain Pathol. 2022, 32, e13028. [Google Scholar] [CrossRef]
- Farshadi, E.; van der Horst, G.T.J.; Chaves, I. Molecular Links between the Circadian Clock and the Cell Cycle. J. Mol. Biol. 2020, 432, 3515–3524. [Google Scholar] [CrossRef] [PubMed]
- Rijo-Ferreira, F.; Takahashi, J.S. Genomics of Circadian Rhythms in Health and Disease. Genome Med. 2019, 11, 82. [Google Scholar] [CrossRef] [PubMed]
- Gauger, M.A.; Sancar, A. Cryptochrome, Circadian Cycle, Cell Cycle Checkpoints, and Cancer. Cancer Res. 2005, 65, 6828–6834. [Google Scholar] [CrossRef]
- Samoilova, E.M.; Belopasov, V.V.; Ekusheva, E.V.; Zhang, C.; Troitskiy, A.V.; Baklaushev, V.P. Epigenetic Clock and Circadian Rhythms in Stem Cell Aging and Rejuvenation. J. Pers. Med. 2021, 11, 1050. [Google Scholar] [CrossRef]
- Uchida, Y.; Hirayama, J.; Nishina, H. A Common Origin: Signaling Similarities in the Regulation of the Circadian Clock and DNA Damage Responses Current Topics Review. Biol. Pharm. Bull. 2010, 33, 535–544. [Google Scholar] [CrossRef]
- Shostak, A. Circadian Clock, Cell Division, and Cancer: From Molecules to Organism. Int. J. Mol. Sci. 2017, 18, 873. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. The Hallmarks of Cancer Review Evolve Progressively from Normalcy via a Series of Pre. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Gyamfi, J.; Kim, J.; Choi, J. Cancer as a Metabolic Disorder. Int. J. Mol. Sci. 2022, 23, 1155. [Google Scholar] [CrossRef] [PubMed]
- Papagiannakopoulos, T.; Bauer, M.R.; Davidson, S.M.; Heimann, M.; Subbaraj, L.; Bhutkar, A.; Bartlebaugh, J.; Vander Heiden, M.G.; Jacks, T. Circadian Rhythm Disruption Promotes Lung Tumorigenesis. Cell Metab. 2016, 24, 324–331. [Google Scholar] [CrossRef]
- Altman, B.J.; Hsieh, A.L.; Sengupta, A.; Krishnanaiah, S.Y.; Stine, Z.E.; Walton, Z.E.; Gouw, A.M.; Venkataraman, A.; Li, B.; Goraksha-Hicks, P.; et al. MYC Disrupts the Circadian Clock and Metabolism in Cancer Cells. Cell Metab. 2015, 22, 1009–1019. [Google Scholar] [CrossRef]
- Schernhammer, E.S.; Razavi, P.; Li, T.Y.; Qureshi, A.A.; Han, J. Rotating Night Shifts and Risk of Skin Cancer in the Nurses’ Health Study. J. Natl. Cancer Inst. 2011, 103, 602–606. [Google Scholar] [CrossRef]
- Hu, M.L.; Yeh, K.T.; Lin, P.M.; Hsu, C.M.; Hsiao, H.H.; Liu, Y.C.; Lin, H.Y.H.; Lin, S.F.; Yang, M.Y. Deregulated Expression of Circadian Clock Genes in Gastric Cancer. BMC Gastroenterol. 2014, 14, 67. [Google Scholar] [CrossRef]
- Karantanos, T.; Theodoropoulos, G.; Pektasides, D.; Gazouli, M. Clock Genes: Their Role in Colorectal Cancer. World J. Gastroenterol. 2014, 20, 1986–1992. [Google Scholar] [CrossRef]
- Gréchez-Cassiau, A.; Rayet, B.; Guillaumond, F.; Teboul, M.; Delaunay, F. The Circadian Clock Component BMAL1 Is a Critical Regulator of P21 WAF1/CIP1 Expression and Hepatocyte Proliferation. J. Biol. Chem. 2008, 283, 4535–4542. [Google Scholar] [CrossRef] [PubMed]
- Qu, M.; Zhang, G.; Qu, H.; Vu, A.; Wu, R.; Tsukamoto, H.; Jia, Z.; Huang, W.; Lenz, H.J.; Rich, J.N.; et al. Circadian Regulator BMAL1::CLOCK Promotes Cell Proliferation in Hepatocellular Carcinoma by Controlling Apoptosis and Cell Cycle. Proc. Natl. Acad. Sci. USA 2023, 120, e2214829120. [Google Scholar] [CrossRef]
- Juliusson, G.; Hough, R. Leukemia. Prog. Tumor Res. 2016, 43, 87–100. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Chen, W.; Liu, K.; Wang, L.; Hu, Y.; Mao, Y.; Sun, X.; Luo, Y.; Shi, J.; Shao, K.; et al. The Global Burden of Leukemia and Its Attributable Factors in 204 Countries and Territories: Findings from the Global Burden of Disease 2019 Study and Projections to 2030. J. Oncol. 2022, 2022, 1612702. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Guang, P.; Li, F.; Liu, X.; Zhang, W.; Huang, W.; Haque, N. AML, ALL, and CML Classification and Diagnosis Based on Bone Marrow Cell Morphology Combined with Convolutional Neural Network: A STARD Compliant Diagnosis Research. Medicine 2020, 99, E23154. [Google Scholar] [CrossRef]
- Salama, M.M.; Aborehab, N.M.; El Mahdy, N.M.; Zayed, A.; Ezzat, S.M. Nanotechnology in Leukemia: Diagnosis, Efficient-Targeted Drug Delivery, and Clinical Trials. Eur. J. Med. Res. 2023, 28, 566. [Google Scholar] [CrossRef]
- Iqbal, Z. Molecular Hematology in Leukemia Biology and Treatment: Past, Present, and Future. J. Appl. Hematol. 2012, 3, 55–61. [Google Scholar]
- Li, H.X. The Role of Circadian Clock Genes in Tumors. Onco Targets Ther. 2019, 12, 3645–3660. [Google Scholar] [CrossRef]
- Rasheed, A. Niche Regulation of Hematopoiesis: The Environment Is “Micro,” but the Influence Is Large. Arterioscler. Thromb. Vasc. Biol. 2022, 42, 691–699. [Google Scholar] [CrossRef]
- Sanford, A.B.A.; da Cunha, L.S.; Machado, C.B.; de Pinho Pessoa, F.M.C.; Silva, A.N.d.S.; Ribeiro, R.M.; Moreira, F.C.; de Moraes Filho, M.O.; de Moraes, M.E.A.; de Souza, L.E.B.; et al. Circadian Rhythm Dysregulation and Leukemia Development: The Role of Clock Genes as Promising Biomarkers. Int. J. Mol. Sci. 2022, 23, 8212. [Google Scholar] [CrossRef]
- Puram, R.V.; Kowalczyk, M.S.; De Boer, C.G.; Schneider, R.K.; Miller, P.G.; McConkey, M.; Tothova, Z.; Tejero, H.; Heckl, D.; Järås, M.; et al. Core Circadian Clock Genes Regulate Leukemia Stem Cells in AML. Cell 2016, 165, 303–316. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, R.; Nishino, T.; Yokoyama, A.; Nakashima, A.; Kikkawa, U.; Konishi, H. Versatile Function of the Circadian Protein CIPC as a Regulator of Erk Activation. Biochem. Biophys. Res. Commun. 2016, 469, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Qu, Z.P.; Wang, X.H.; Liu, D.C.; Gao, X.; Xu, Y. Inactivation of Cipc Alters the Expression of Per1 but Not Circadian Rhythms in Mice. Sci. China Life Sci. 2015, 58, 368–372. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.N.; Malinin, N.; Yang, F.C.; Staknis, D.; Gekakis, N.; Maier, B.; Reischl, S.; Kramer, A.; Weitz, C.J. CIPC Is a Mammalian Circadian Clock Protein without Invertebrate Homologues. Nat. Cell Biol. 2007, 9, 268–275. [Google Scholar] [CrossRef]
- Griffin, E.A., Jr.; Staknis, D.; Weitz, C.J. Weitz Light-Independent Role of CRY1 and CRY2 in the Mammalian Circadian Clock. Science 1999, 286, 768–771. [Google Scholar] [CrossRef]
- Li, M.D.; Xin, H.; Yuan, Y.; Yang, X.; Li, H.; Tian, D.; Zhang, H.; Zhang, Z.; Han, T.L.; Chen, Q.; et al. Circadian Clock-Controlled Checkpoints in the Pathogenesis of Complex Disease. Front. Genet. 2021, 12, 721231. [Google Scholar] [CrossRef]
- Hou, Z.; Su, L.; Pei, J.; Grishin, N.V.; Zhang, H. Crystal Structure of the CLOCK Transactivation Domain Exon19 in Complex with a Repressor. Structure 2017, 25, 1187–1194.e3. [Google Scholar] [CrossRef]
- Rivas, G.B.S.; Zhou, J.; Merlin, C.; Hardin, P.E. CLOCKWORK ORANGE Promotes CLOCK-CYCLE Activation via the Putative Drosophila Ortholog of CLOCK INTERACTING PROTEIN CIRCADIAN. Curr. Biol. 2021, 31, 4207–4218.e4. [Google Scholar] [CrossRef]
- Yoshitane, H.; Takao, T.; Satomi, Y.; Du, N.-H.; Okano, T.; Fukada, Y. Roles of CLOCK Phosphorylation in Suppression of E-Box-Dependent Transcription. Mol. Cell. Biol. 2009, 29, 3675–3686. [Google Scholar] [CrossRef]
- Yoshitane, H.; Fukada, Y. CIPC-Dependent Phosphorylation of CLOCK and NPAS2 in Circadian Clockwork. Sleep Biol. Rhythm. 2009, 7, 226–234. [Google Scholar] [CrossRef]
- Zheng, J.; Lou, J.; Li, Y.; Qian, P.; He, W.; Hao, Y.; Xue, T.; Li, Y.; Song, Y.H. Satellite Cell-Specific Deletion of Cipc Alleviates Myopathy in Mdx Mice. Cell Rep. 2022, 39, 110939. [Google Scholar] [CrossRef] [PubMed]
- Goldman, M.J.; Craft, B.; Hastie, M.; Repečka, K.; McDade, F.; Kamath, A.; Banerjee, A.; Luo, Y.; Rogers, D.; Brooks, A.N.; et al. Visualizing and Interpreting Cancer Genomics Data via the Xena Platform. Nat. Biotechnol. 2020, 38, 675–678. [Google Scholar] [CrossRef] [PubMed]
- Harrison, P.W.; Amode, M.R.; Austine-Orimoloye, O.; Azov, A.G.; Barba, M.; Barnes, I.; Becker, A.; Bennett, R.; Berry, A.; Bhai, J.; et al. Ensembl 2024. Nucleic Acids Res. 2024, 52, D891–D899. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Franceschini, A.; Wyder, S.; Forslund, K.; Heller, D.; Huerta-Cepas, J.; Simonovic, M.; Roth, A.; Santos, A.; Tsafou, K.P.; et al. STRING V10: Protein-Protein Interaction Networks, Integrated over the Tree of Life. Nucleic Acids Res. 2015, 43, D447–D452. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A Software Environment for Integrated Models of Biomolecular Interaction Networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Mostafavi, S.; Ray, D.; Warde-Farley, D.; Grouios, C.; Morris, Q. GeneMANIA: A Real-Time Multiple Association Network Integration Algorithm for Predicting Gene Function. Genome Biol. 2008, 9, S4. [Google Scholar] [CrossRef]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Systematic and Integrative Analysis of Large Gene Lists Using DAVID Bioinformatics Resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef]
- Sherman, B.T.; Hao, M.; Qiu, J.; Jiao, X.; Baseler, M.W.; Lane, H.C.; Imamichi, T.; Chang, W. DAVID: A Web Server for Functional Enrichment Analysis and Functional Annotation of Gene Lists (2021 Update). Nucleic Acids Res. 2022, 50, W216–W221. [Google Scholar] [CrossRef]
- Song, B.; Chen, Y.; Liu, Y.; Wan, C.; Zhang, L.; Zhang, W. NPAS2 Regulates Proliferation of Acute Myeloid Leukemia Cells via CDC25A-Mediated Cell Cycle Progression and Apoptosis. J. Cell. Biochem. 2019, 120, 8731–8741. [Google Scholar] [CrossRef]
- Zhu, Y.; Stevens, R.G.; Leaderer, D.; Hoffman, A.; Holford, T.; Zhang, Y.; Brown, H.N.; Zheng, T. Non-Synonymous Polymorphisms in the Circadian Gene NPAS2 and Breast Cancer Risk. Breast Cancer Res. Treat. 2007, 107, 421–425. [Google Scholar] [CrossRef]
- Zhu, Y.; Leaderer, D.; Guss, C.; Brown, H.N.; Zhang, Y.; Boyle, P.; Stevens, R.G.; Hoffman, A.; Qin, Q.; Han, X.; et al. Ala394Thr Polymorphism in the Clock Gene NPAS2: A Circadian Modifier for the Risk of Non-Hodgkin’s Lymphoma NIH Public Access. Int. J. Cancer 2007, 120, 432–435. [Google Scholar] [CrossRef]
- Steelman, L.S.; Abrams, S.L.; Whelan, J.; Bertrand, F.E.; Ludwig, D.E.; Bäsecke, J.; Libra, M.; Stivala, F.; Milella, M.; Tafuri, A.; et al. Contributions of the Raf/MEK/ERK, PI3K/PTEN/Akt/MTOR and Jak/STAT Pathways to Leukemia. Leukemia 2008, 22, 686–707. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Chen, X.; Zhang, Y.; Ren, F.; Ma, Y. MEK/ERK and PI3K/AKT Pathway Inhibitors Affect the Transformation of Myelodysplastic Syndrome into Acute Myeloid Leukemia via H3K27me3 Methylases and Demethylases. Int. J. Oncol. 2023, 63, 140. [Google Scholar] [CrossRef] [PubMed]
- Janssen, M.; Schmidt, C.; Bruch, P.-M.; Blank, M.F.; Rohde, C.; Waclawiczek, A.; Heid, D.; Renders, S.; Göllner, S.; Vierbaum, L.; et al. Venetoclax Synergizes with Gilteritinib in FLT3 Wild-Type High-Risk Acute Myeloid Leukemia by Suppressing MCL-1. Blood 2022, 140, 2594–2610. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Lu, X.X.; Wang, J.R.; Yang, T.Y.; Li, X.M.; He, X.S.; Li, Y.; Ye, W.L.; Wu, Y.; Gan, W.J.; et al. TRAF6 Inhibits Colorectal Cancer Metastasis through Regulating Selective Autophagic CTNNB1/β-Catenin Degradation and Is Targeted for GSK3B/GSK3β-Mediated Phosphorylation and Degradation. Autophagy 2019, 15, 1506–1522. [Google Scholar] [CrossRef]
- Rinke, J.; Chase, A.; Cross, N.C.P.; Hochhaus, A.; Ernst, T. EZH2 in Myeloid Malignancies. Cells 2020, 9, 1639. [Google Scholar] [CrossRef]
- Safaei, S.; Baradaran, B.; Hagh, M.F.; Alivand, M.R.; Talebi, M.; Gharibi, T.; Solali, S. Double Sword Role of EZH2 in Leukemia. Biomed. Pharmacother. 2018, 98, 626–635. [Google Scholar] [CrossRef]
- Zhong, Y.; Ye, Q.; Chen, C.; Wang, M.; Wang, H. Ezh2 Promotes Clock Function and Hematopoiesis Independent of Histone Methyltransferase Activity in Zebrafish. Nucleic Acids Res. 2018, 46, 3382–3399. [Google Scholar] [CrossRef]
- Abdulmawjood, B.; Costa, B.; Roma-rodrigues, C.; Baptista, P.V.; Fernandes, A.R. Genetic Biomarkers in Chronic Myeloid Leukemia: What Have We Learned so Far? Int. J. Mol. Sci. 2021, 22, 12516. [Google Scholar] [CrossRef]
- Chi, C.; Liang, X.; Cui, T.; Gao, X.; Liu, R.; Yin, C. SKIL/SnoN Attenuates TGF-Β1/SMAD Signaling-Dependent Collagen Synthesis in Hepatic Fibrosis. Biomol. Biomed. 2023, 23, 1014–1025. [Google Scholar] [CrossRef]
- Ye, X.; Chen, G.; Jin, J.; Zhang, B.; Wang, Y.; Cai, Z.; Ye, F. The Development of Inhibitors Targeting the Mixed Lineage Leukemia 1 (MLL1)-WD Repeat Domain 5 Protein (WDR5) Protein- Protein Interaction. Curr. Med. Chem. 2020, 27, 5530–5542. [Google Scholar] [CrossRef] [PubMed]
- Ge, Z.; Song, E.J.; Kawasawa, Y.I.; Li, J.; Dovat, S.; Song, C. WDR5 High Expression and Its Effect on Tumorigenesis in Leukemia. Oncotarget 2016, 7, 37740–37754. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Hu, Q.; Fan, K.; Yang, C.; Gao, Y. CSNK2B Contributes to Colorectal Cancer Cell Proliferation by Activating the MTOR Signaling. J. Cell Commun. Signal. 2021, 15, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Kong, Q.; Li, Y.; Liang, Q.; Xie, J.; Li, X.; Fang, J. SIRT6-PARP1 Is Involved in HMGB1 PolyADP-Ribosylation and Acetylation and Promotes Chemotherapy-Induced Autophagy in Leukemia. Cancer Biol. Ther. 2020, 21, 320–331. [Google Scholar] [CrossRef]
- Huang, X.; Spencer, G.J.; Lynch, J.T.; Ciceri, F.; Somerville, T.D.D.; Somervaille, T.C.P. Enhancers of Polycomb EPC1 and EPC2 Sustain the Oncogenic Potential of MLL Leukemia Stem Cells. Leukemia 2013, 28, 1081–1091. [Google Scholar] [CrossRef]

| STUDY MODEL | FUNCTION | REFERENCE |
|---|---|---|
| DROSOPHILA | CIPC represents an alternative and specific mechanism of transcriptional repression within the molecular clock, distinct from the CRY– and PER–mediated pathways. Its unique structural interaction with the CLOCK protein at exon 19 plays a crucial role in the complexity and precision of circadian regulation in mammals and some invertebrates. | [57] |
| DROSOPHILA | CIPC functions as a negative regulator of the CLOCK–CYCLE (CLK-CYC) complex, and its expression is suppressed by CLOCKWORK ORANGE (CWO) to facilitate effective circadian transcriptional activation. This modulation of CIPC expression by CWO represents an additional and crucial mechanism of transcriptional control within the circadian clock. | [58] |
| MICE | CIPC binds to CLOCK at an important site, inhibiting the transcriptional activity of the CLOCK–BMAL1 heterodimer in mammalian cells. | [54] |
| MICE AND NIH 3T3 CELLS (mice fibroblast cell line) | CIPC stimulates CLOCK phosphorylation and increases CLOCK and BMAL1 levels. Stabilization of BMAL1 is not observed in the absence of coexpressed CLOCK. Coexpression of CIPC with CLOCK without BMAL1 expression had a marginal effect on phosphorylation levels. In CLOCKΔ19, a CLOCK mutant without the CIPC–binding region, CIPC influenced the stability of BMAL1 in the CLOCKΔ19–BMAL1 complex without efficiently binding to CLOCKΔ19. | [59] |
| MICE AND NIH 3T3 CELLS | CIPC stimulates the phosphorylation of CLOCK in the CLOCK–BMAL1 complex as well as NPAS2 in the NPAS2–BMAL1 complex, probably through the same mechanisms. | [60] |
| MICE -/- (knockout) and WILD-TYPE MICE | CIPC does not function in determining the period in locomotor rhythms. It was observed that only the PER1 peak in CIPC-/- mice was reduced to half the level compared to wild-type mice. | [53] |
| HEK293 CELLS (human kidney cell line) | Identification of amino acid residues Lys186 and Lys187 as essential for CIPC nuclear signaling. Identification of CIPC–binding proteins such as the enzyme carbamoyl-phosphate synthetase 2, aspartate transcarbamoylase, and dihydroorotase (CAD). Erk activation caused by phorbol 12-myristate 13-acetate (PMA) was inhibited with CIPC expression. CIPC subcellular localization was dramatically altered in cells synchronized at the G1/S boundary using a double thymidine blockade, suggesting translocation to the cytosol. | [52] |
| MDX MICE | CIPC is upregulated during myoblast differentiation. CIPC deficiency leads to activation of the ERK1/2 and JNK1/2 signaling pathways, which activates the transcription factor SP1 and triggers the transcription of Paired Box 7 (PAX7) and Myogenic Differentiation 1 (MYOD) | [61] |
| ID | GENE | PATHWAYS |
|---|---|---|
| ENSG00000183495 | E1A binding protein p400 (EP400) | Cellular responses to stress, cellular senescence, DNA damage/telomere stress-induced senescence |
| ENSG00000136603 | SKI like proto-oncogene (SKIL) | TGF–beta signaling pathway, transcriptional activity of SMAD2/SMAD3 heterotrimer |
| ENSG00000196363 | WD repeat domain 5 (WDR5) | Epigenetic regulation of gene expression, chromatin-modifying enzymes, pleural mesothelioma |
| ENSG00000204435 | Casein kinase 2 beta (CSNK2B) | NF-kappa B signaling pathway, PD-L1 expression and PD-1 checkpoint pathway in cancer, breast cancer pathway, lncRNA in canonical Wnt signaling and colorectal cancer, ncRNAs involved in Wnt signaling in hepatocellular carcinoma, pleural mesothelioma |
| ENSG00000106462 | Enhancer of zeste 2 polycomb repressive complex 2 subunit (EZH2) | Polycomb repressive complex, microRNAs in cancer, cellular senescence, lncRNA in canonical Wnt signaling and colorectal cancer, ncRNAs involved in Wnt signaling in hepatocellular carcinoma, pleural mesothelioma |
| ENSG00000082701 | Glycogen synthase kinase 3 beta (GSK3B) | Pathways in cancer, colorectal cancer, endometrial cancer, prostate cancer, breast cancer, hepatocellular carcinoma, gastric cancer, PI3K/AKT signaling in cancer, lncRNA in canonical Wnt signaling and colorectal cancer, ncRNAs involved in Wnt signaling in hepatocellular carcinoma |
| ENSG00000171720 | Histone deacetylase 3 (HDAC3) | Viral carcinogenesis, signaling by NOTCH1 in cancer, HDACs deacetylate histones, chromatin-modifying enzymes |
| ENSG00000077463 | Sirtuin 6 (SIRT6) | Central carbon metabolism in cancer |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Cunha, L.S.; Nogueira, B.M.D.; de Pinho Pessoa, F.M.C.; Machado, C.B.; de Sousa Oliveira, D.; de Moraes Filho, M.O.; de Moraes, M.E.A.; Khayat, A.S.; Moreira-Nunes, C.A. Evaluation of the Circadian Rhythm Component Cipc (Clock-Interacting Pacemaker) in Leukemogenesis: A Literature Review and Bioinformatics Approach. Clocks & Sleep 2025, 7, 33. https://doi.org/10.3390/clockssleep7030033
da Cunha LS, Nogueira BMD, de Pinho Pessoa FMC, Machado CB, de Sousa Oliveira D, de Moraes Filho MO, de Moraes MEA, Khayat AS, Moreira-Nunes CA. Evaluation of the Circadian Rhythm Component Cipc (Clock-Interacting Pacemaker) in Leukemogenesis: A Literature Review and Bioinformatics Approach. Clocks & Sleep. 2025; 7(3):33. https://doi.org/10.3390/clockssleep7030033
Chicago/Turabian Styleda Cunha, Leidivan Sousa, Beatriz Maria Dias Nogueira, Flávia Melo Cunha de Pinho Pessoa, Caio Bezerra Machado, Deivide de Sousa Oliveira, Manoel Odorico de Moraes Filho, Maria Elisabete Amaral de Moraes, André Salim Khayat, and Caroline Aquino Moreira-Nunes. 2025. "Evaluation of the Circadian Rhythm Component Cipc (Clock-Interacting Pacemaker) in Leukemogenesis: A Literature Review and Bioinformatics Approach" Clocks & Sleep 7, no. 3: 33. https://doi.org/10.3390/clockssleep7030033
APA Styleda Cunha, L. S., Nogueira, B. M. D., de Pinho Pessoa, F. M. C., Machado, C. B., de Sousa Oliveira, D., de Moraes Filho, M. O., de Moraes, M. E. A., Khayat, A. S., & Moreira-Nunes, C. A. (2025). Evaluation of the Circadian Rhythm Component Cipc (Clock-Interacting Pacemaker) in Leukemogenesis: A Literature Review and Bioinformatics Approach. Clocks & Sleep, 7(3), 33. https://doi.org/10.3390/clockssleep7030033








