Abstract
Background: Given the beneficial effects of exercise in different populations and the close relationship between healthy ageing and sleep quality, our objective was to determine if physical exercise delivered through a structured program improves sleep quality in older adults. Methods: Embase, PubMed/MEDLINE, Web of Science, and Cochrane Register of Clinical Trials (CENTRAL) were searched to 15 January 2023. Studies that applied physical exercise programs in older adults were reviewed. Two independent reviewers analysed the studies, extracted the data, and assessed the quality of evidence. Results: Of the 2599 reports returned by the initial search, 13 articles reporting on 2612 patients were included in the data synthesis. The articles used interventions based on yoga (n = 5), multicomponent exercise (n = 3), walking (n = 2), cycling (n = 1), pilates (n = 1), elastic bands (n = 1), and healthy beat acupunch (n = 1). In the intervention group, we found significant improvement in Pittsburgh sleep quality index of −2.49 points (95% CI −3.84 to −1.14) in comparison to the control group (p = 0.0003) and sleep efficiency measured with objective instruments (MD 1.18%, 95% CI 0.86 to 1.50%, p < 0.0001). Conclusion: Our results found that physical exercise programs in older adults improve sleep quality and efficiency measured with objective instruments.
1. Introduction
The number of older people is growing worldwide. Currently, about 10% of the global population is over 65 years old [1], and it is projected that between 2015 and 2050, the proportion of the world’s population over 60 years old will nearly double from 12 to 22% [2]. The challenge for this population is to achieve healthy ageing [2]. In addition, the clinical guidelines widely recommend physical activity as a central component of healthy ageing [3,4]. Regular exercise leads to ageing actively and satisfactorily because it is associated with physical, functional, psychological, and cognitive improvement [5].
Sufficient lifetime physical activity, healthy food habits, and significant sleep quality are major contributors to healthy ageing [6,7]. On the other hand, poor sleep quality and insufficient habitual physical activity behaviour are reported to deteriorate health along with the ageing process [8]. Furthermore, poor sleep quality has been related to negative health outcomes, and an increase in the speed of multimorbidity development [9] and these deteriorations have been associated with losses in cognitive and functional capacity [10,11].
Sleep disorders, such as altered sleep duration and increased sleep fragmentation, are common among older adults [12]. The most frequent sleep disorders that have been described in older adults are difficulty initiating and maintaining sleep and waking up early in the morning [13]. These sleep disorders in this population may be attributed to a lack of physical exercise, poor sleep habits, and inactive lifestyles with repetitive daily routines [14].
Poor sleep quality in older adults is expressed, among other ways, as insomnia, a sleep–wake disorder characterised by difficulty initiating or maintaining sleep or both, and older adults are more likely to be prescribed medication for insomnia treatment [15]. Medication classes commonly used to treat insomnia—including benzodiazepines and z-drugs (such as zolpidem)—can have adverse effects, including cognitive and memory impairment, rebound insomnia upon cessation, and increased risk of motor vehicle accidents, falls, dependency, and addiction [16,17]. Thus, these medications may not be appropriate for use in older adults due to unfavourable risk–benefit ratios [18], particularly for those with a history of falls or fractures [19]. Furthermore, individuals receiving insomnia treatment had an increased risk of falls and mortality and higher healthcare resource utilisation and costs than matched beneficiaries without sleep disorders [18].
Exercise is a widely accepted approach adopted to improve physical function, cardiovascular health, and mental health, and it may also be beneficial for sleep [20,21,22,23,24]. The exercise can be performed in different environments, such as rehabilitation centres, gyms, public parks, or at home [20]. The exercise program is considered home-based if the physical exercise is performed in an informal and flexible place such as the individual’s house. Each program should have clear goals, including monitoring, follow-up visits, calls from health professionals, or self-monitoring diaries [25].
Given the beneficial effects of exercise in different populations and the close relationship between healthy ageing and sleep quality, our objective was to determine if physical exercise delivered through a structured program improves sleep quality in older adults.
2. Methods
2.1. Protocol and Registration
A systematic review was reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [26]. The review was registered in the International Prospective Register of Systematic Reviews (PROSPERO) CRD42022369802.
2.2. Criteria for Considering Studies in This Review
Randomised controlled trials (RCTs) that included older adults (>65 yr) [27] performing a structured exercise program were searched. The included studies aimed to determine the effects of exercise on sleep quality. The search strategy was based on the PICO model (population: older adults; intervention: exercise program; control: no intervention or placebo; and outcome: sleep quality, sleepiness, apnoea–hypopnoea index) [28].
2.3. Search Strategies and Data Resources
The Embase, PubMed/MEDLINE, Web of Science, and Cochrane Register of Clinical Trials (CENTRAL) were reviewed on 23 October 2022 and updated on 15 January 2023. Manual searches with the following terms were conducted: ((elderly) OR (older people) OR (older adults) OR (ageing)) AND ((Exercise) OR (physical training) OR (physical activity) OR (resistance training) OR (aerobic) OR (gym) OR (gymnastic) OR (strength) OR (balance)) AND ((sleep quality) OR (sleepiness) OR (apnea-hypopnea index)) (supplementary material 1). No language or publication restrictions were imposed.
The terms selected were combined using Boolean logical operators (OR, AND, NOT). Additionally, a manual search of the references included in the selected articles was conducted. All references were analysed using Rayyan web software [29].
2.4. Reviewing Procedure and Data Extraction
The selected articles were reviewed independently by investigators with experience in meta-analysis and training in the literature review. First, the titles and abstracts of all identified studies were reviewed in pairs by four investigators (LSN-MOY-OM-RTC). Studies deemed irrelevant based on the title and abstract were excluded. Any possible disagreements were resolved by a third reviewer (MSR). Second, the first step’s full-text versions of the articles selected were reviewed and rechecked against the eligibility criteria (LSN-RTC). Again, any potential disagreements were resolved by a third reviewer (MSR). There were no disagreements between the investigators. Finally, study authors were contacted as required to determine study eligibility.
Two authors (OM-RTC) extracted the data using a standardised protocol and reporting forms. The following information was extracted from each included study: design, population characteristics, and exercise program characteristics (type of exercise, setting, duration, intensity, frequency). If some relevant data were not in the article, the author was contacted to request the information.
2.5. Methodological Quality Assessment
The primary articles’ methodological quality was assessed using the Cochrane Collaboration tool to assess the risk of bias (the Cochrane Handbook for Systematic Reviews of Interventions) [28]. The tool included seven items: generation of a random sequence, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, completeness of outcome data, selection of reports, and other biases. For each item, the risk of bias for the study was rated according to three categories: low, high, or unclear risk of bias. Two reviewers (CFJ-MSM) independently assessed the risk of bias in the studies. A third author (RTC) was consulted for discrepancies that could not be resolved.
2.6. Data Synthesis and Analysis
A narrative synthesis was used to summarise the characteristics of studies, including population and intervention characteristics. Summaries of the association between the outcomes for each study in terms of mean differences (MDs) or standard mean differences (SMDs) using Review Manager 5 (RevMan, Copenhagen, Denmark: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014) were reported. Absolute values and obtained combined measures of the effect of each primary outcome through meta-analysis with a random-effect model due to the expected heterogeneity between studies were compared [28]. Statistical heterogeneity was measured with the I2 statistic and classified as low (I2 < 25%), moderate (I2 = 25–50%), or high (I2 > 50%) [28]. A sub-analysis was carried out by type and duration of training and setting for the main outcome. Additionally, the overall certainty of evidence was assessed independently by two reviewers (RTC, LSN) using the GRADE approach [30]. Disagreements were solved by consensus. Publication bias was assessed by visualising a funnel plot and Begg’s and Egger’s tests for the possible existence of study bias using the Jamovi software (version 2.3) [31].
3. Results
3.1. Study Selection
The initial search yielded 2599 potential studies. In total, 376 duplicate records were deleted. We screened 2223 titles and abstracts and excluded 2169 records that did not meet the inclusion criteria. Fifty-four of these were assessed as full text. Of these, 30 were excluded for including people under 65 years old, 14 for being conference abstracts, 3 for not being RCTs, 3 for not including exercise as intervention, and 1 for duplicated data. Ultimately, 13 studies met the criteria for eligibility and were included in the review [32,33,34,35,36,37,38,39,40,41,42,43,44]. The flow chart of the study selection process is shown in Figure 1.
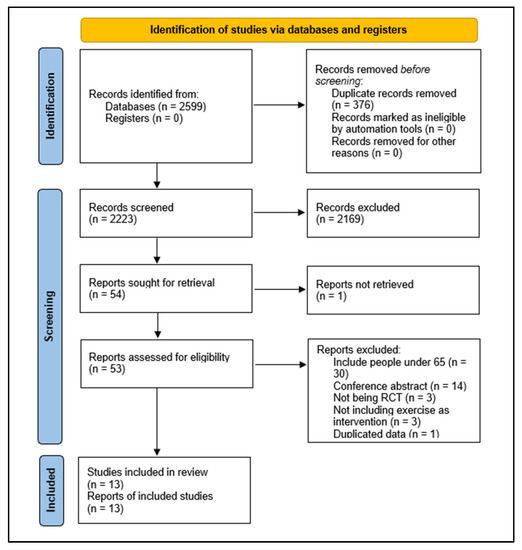
Figure 1.
Study selection process.
3.2. Characteristics of the Included Studies
Three studies were conducted in Taiwan [32,41,42], two in the USA [38,40] and Turkey [34,39], and one in Korea [43], Spain [44], the UK [37], Tunisia [36], Canada [35], and Japan [33]. All studies were published after 2010. The characteristics of included studies are in Table 1.

Table 1.
Characteristics of included studies.
3.3. Participants
In total, 2612 older adults were analysed (1304 in the intervention group and 1308 in the control group). Sample sizes varied between 21 [37] and 1635 [40] participants. The studies included 864 (33.8%) males and 1693 (66.2%) females, with mean age varying between 68 ± 2 [37] and 80.1 ± 6.4 [41] years. One study did not report sex distribution [32]. The body mass index (BMI) varied between 23.4 ± 17.7 [44] and 31.5 ± 3.6 [39] kg/m2 (Table 1). Most of the studies were conducted in older adults living in the community [35,36,37,38,40,42,43,44], and a third of the studies were conducted in older adults living in nursing homes or assisted living facilities [12,32,34,39]. Furthermore, a study was carried out on carers of people with dementia [33].
3.4. Characteristics of Training
Five articles used interventions based on yoga [32,36,38,39,43], three in multicomponent exercise [34,40,44], two in walking [33,35], one in cycling [37], one in pilates [39], one in elastic bands [41], and one in healthy beat acupunch [42]. The duration of the programs varied between 8 and 12 weeks [33,36,38,39,43,44], between 12 weeks and 12 months [32,34,37,41,42], and more than 12 months [35,40]. The details of the training programs are shown in Table 2.

Table 2.
Characteristics of physical exercise programs.
3.5. Methodological Quality Assessment
All studies had a high or unclear risk of bias in at least one domain. The majority of studies claimed to be randomised. However, only half of them explain how the randomisation was conducted [34,35,38,40,41,43]. No study reported that participants and personnel were blinded. However, this is a common feature in non-pharmacological studies, particularly in rehabilitation studies, in which it is complex to blind professionals or patients due to the nature of the intervention. One-third of studies reported that researchers and outcome assessments were blinded [35,37,38,40]. Five studies had a low risk of bias on attrition rates and selection reporting [34,35,38,41,43]. Finally, the majority of the studies had a low risk of other potential sources of bias (Figure 2). Funnel plots and Begg’s and Egger’s tests indicated a publication bias for the estimation of the effect of exercise on sleep quality (Begg = 0.08; Egger = 0.009) (Figure 3).
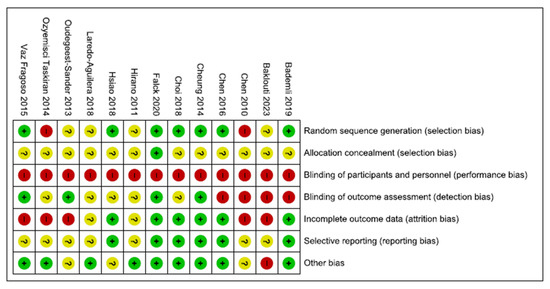
Figure 2.
Risk of bias summary. Legend: Red (−) = high risk of bias; Yellow (?) = unknown risk of bias; Green (+) = low risk of bias.
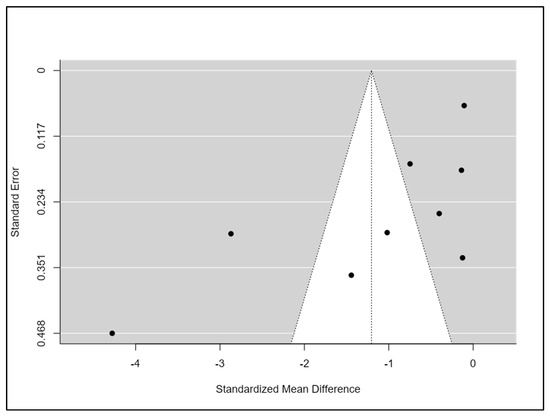
Figure 3.
Risk of bias summary.
3.6. Main Findings
Sleep Quality: A total of 13 studies reported sleep quality [32,33,34,35,36,37,38,39,40,41,42,43,44]. The instruments used were the PSQI [32,34,35,36,38,40,41,42,43], the Oviedo sleep questionnaire [44], the quality sleep score [33], the sleep dimension of Nottingham health profile (n = 1) [39], and an accelerometer/actigraphy [35,37]. A meta-analysis was pooled with nine studies [32,34,35,36,38,40,41,43,44]. These studies compared 822 patients in the intervention group (IG) versus 840 in the control group (CG). Older people in the IG had an SMD of −1.17 points (95% CI −1.79 to −0.54) in comparison to CG (p = 0.0002) (Figure 4). The heterogeneity of the comparison was high (I2 = 95%). If we analysed only the studies with PSQI [32,34,35,36,38,40,41,43], there were 802 patients compared in the IG versus 822 in the CG. Older people in the IG had an MD of −2.49 points (95% CI −3.84 to −1.14) in comparison to CG (p < 0.0001) (Figure 5). The heterogeneity of the comparison was high (I2 = 98%). The certainty of evidence, according to the GRADE methodology, was moderate. Although four studies [33,37,39,42] could not be pooled due to the way they reported the results, it is important to note that three of the four reported an improvement in sleep quality after the intervention [33,39,42].
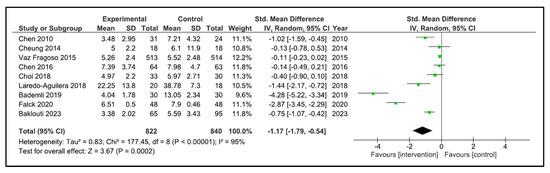
Figure 4.
Forest plot for sleep quality.
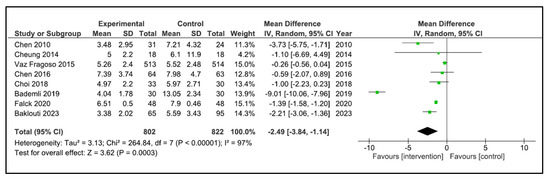
Figure 5.
Forest plot for PSQI.
When performing the analysis by time duration, the programs with duration less than 12 weeks improve the sleep quality (SMD −0.66, 95% CI −1.10 to −0.22, p = 0.003) with a high heterogeneity (I2 = 64%). However, the programs carried out between 12 weeks and 12 months (SMD −1.76, 95% CI −3.67 to 0.15, p = 0.07) and more than 12 months (SMD −1.47, 95% CI −4.18 to 1.23, p = 0.29) did not improve sleep quality. The heterogeneity was high in both cases (I2 = 97, and I2 = 95%, respectively) (Figure 6). If we analysed by time duration, but including intermediate assessment points of high-duration programs, the programs between 12 weeks and 12 months improve the sleep quality (SMD −1.94, 95% CI −3.51 to −0.38, p = 0.02) with a high heterogeneity (I2 = 97%), and the programs of more than 12 months (SMD −0.11, 95% CI −0.23 to 0.02, p = 0.09) did not improve sleep quality (Figure 7).
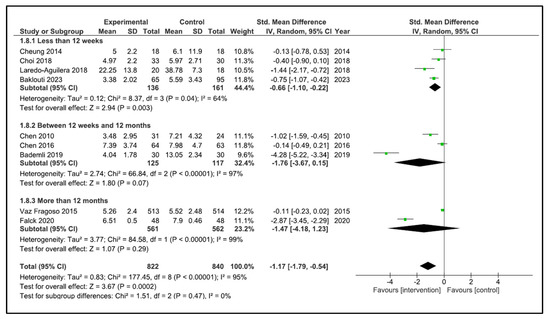
Figure 6.
Forest plot for sleep quality by duration time.
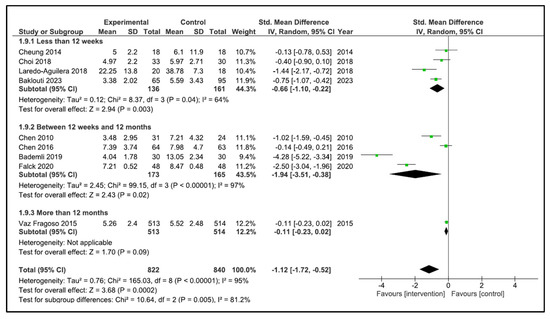
Figure 7.
Forest plot for sleep quality by duration time including intermediate assessment points.
When performing the analysis by setting, the programs carried out at home did not improve sleep quality (SMD −0.95, 95% CI −1.91 to 0.02, p = 0.06). However, the programs carried out in the facilities showed a significant improvement in sleep quality (SMD −1.39, 95% CI −2.45 to −0.33, p = 0.01) (Figure 8). No significant differences were found when performing the analysis by type of training.
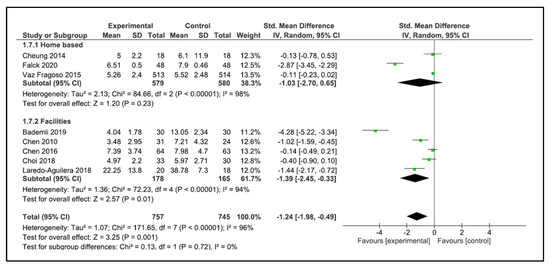
Figure 8.
Forest plot for sleep quality by setting.
Sleep efficiency: Five studies examined sleep efficiency [32,35,37,38,42]. The instruments used were accelerometry/actigraphy [35,37] and a specific domain of PSQI [32,38,42]. These studies compared 108 participants in the IG versus 100 participants in the CG. The heterogeneity was moderate (I2 = 38%). Older people in the IG had no change after intervention (SMD 0.60, 95% CI −0.12 to 1.31) in comparison to CG (p = 0.10). The heterogeneity of the comparison was high (I2 = 82%) (Figure 9). If we analysed only the studies with an objective measure of sleep efficiency [35,37], there were 59 older people compared in the IG versus 58 in the CG. Older people in the IG had a higher sleep efficiency post-intervention (MD 1.18%, 95% CI 0.86 to 1.50%) in comparison to CG (p < 0.0001) (Figure 10). The heterogeneity of the comparison was low (I2 = 0%). The certainty of evidence, according to the GRADE methodology, was low.
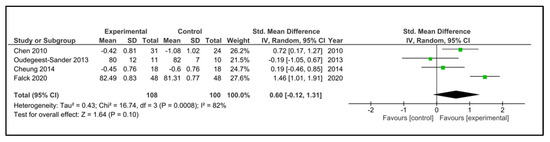
Figure 9.
Forest plot for sleep efficiency. Subjective and objective measures.
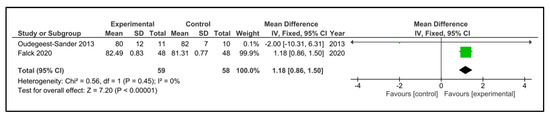
Figure 10.
Forest plot for sleep efficiency. Objective measures.
Sleepiness: Only one study examined the Epworth sleepiness scale (ESS) post-intervention [40]. No significant benefits of exercise programs were shown for prevalent cases of poor sleep quality or sleep–wake behaviours that were evaluated using the ESS.
4. Discussion
This systematic review provides new information about the effects of exercise on sleep quality measured exclusively in the older adult population, showing that exercise improves sleep quality, particularly in facilities programs, and improves sleep efficiency measured with objective instruments. However, these results must be analysed with caution because there is a high heterogeneity in interventions and baseline characteristics of the population.
4.1. Effects on Sleep Quality
Our meta-analysis reports that exercise improves sleep quality. One possible explanation could be that physical activity regulates sleep patterns [34]. With increasing age, there are substantial changes in sleep quality and quantity, including slow-wave sleep, spindle density, and sleep continuity [45]. Furthermore, it seems that for older adults with sufficient mobility and no pre-existing disease, a structured physical activity program may help reduce insomnia [46].
Poor sleep quality is closely related to mental health problems in older adults. Although this research did not explore anxiety or depression as an outcome, exercise in older adults has already been reported to have a positive effect in reducing depression and anxiety [32]. Another aspect to consider is whether the improvement in physical capacity can reduce the use of medications. Although some reports show no statistically significant association between the change in physical capacity and the use of benzodiazepines and z-hypnotics, a decrease in psychotropic drugs and antidepressants has been observed when physical capacity improves in community-dwelling older adults [47]. The high use of psychotropics, like benzodiazepines, may have detrimental effects on older adults (i.e., risk for fractures, falls, and premature mortality, among others) [48,49,50], and it is reported that only about half of the older population who take psychotropics respond to these medications [51].
The mechanisms that explain the improvement in sleep quality due to exercise in older adults are still not completely clear. One possible explanation is that exercise training provides a benefit on sleep quality by decreasing the sleep latency and the use of sleep medication in older adults [52]. Another possible mechanism is that during sleep there is a fluid accumulation in the neck, which causes an increase in the pressure on the upper airway, which can cause the onset of obstructive sleep apnoea. After exercise, there is a significant reduction in the amount of fluid in the neck [53,54]. Other proposed mechanisms include body temperature changes by effects on adenosine levels, cytokine concentration changes, increased energy consumption/metabolic rate, changes in mood/anxiety symptoms, growth hormone secretion, improved fitness level, and body composition change, among others [54,55,56,57,58].
4.2. Sleep Quality Assessment
Sleep quality is a concept that includes quantitative aspects of sleep and more subjective aspects, such as “depth” or “restfulness” of sleep [59]. The most used instrument was PSQI, an index created in the psychiatric field [59]. In our meta-analysis, we observed a change of −2.49 units in the PSQI. Limited studies evaluate the minimal clinically important difference (MCID) for this instrument, suggesting a change between 1.54 and −3 [60,61]; however, these data have not been determined for older adults. Due to the lack of universal acceptance of an MCID for PSQI, any statistically significant effect between groups at the end of treatment was considered important in this study.
Sleep is a complex phenomenon, and the literature suggests that objective and subjective indices measure different components of sleep in older adults [62]. Specifically, subjective sleep quality, according to the PSQI, appears to be poorly correlated with sleep measured by actigraphy or polysomnography, and the lack of relationship between objective and subjective sleep measures appears to be unrelated to age, gender, education, or cognitive status [35]. Therefore, both types of evaluation should complement each other. Based on the evidence, an objective and subjective assessment of sleep quality is suggested, particularly because among the selected studies, only one [35] concluded that a multimodal lifestyle could improve subjective, but not objective, sleep in older adults with mild cognitive impairment and poor sleep.
One of the outcomes analysed was sleep efficiency, which was objectively reported through accelerometry and actigraphy [35,37] and subjectively through one of the PSQI dimensions [32,38,42]. We found no differences between the control group and the intervention group. However, when analysing only the objective instruments, there was a significant difference in favour of the intervention. Some authors recommend using subjective data as an adjunct to actigraphic data in estimating total sleep time and sleep efficiency in sleep-disordered patients, especially those with disorders of excessive somnolence [62]. Therefore, articles that only report subjective measures should be analysed with caution.
4.3. Exercise Programs
Meta-analyses showed very high heterogeneity. This is probably because the populations are very different and range from healthy subjects to older adults with pathologies. However, one aspect that was even more heterogeneous was the intervention. Although most of the programs had a similar frequency (three to five times per week), the duration was highly variable, with programs that lasted between 8 and 12 weeks [36,43,44], up to programs that far exceeded 12 months [35,38,40]. On the other hand, work intensities were not reported in most of the studies. Although it is possible to work at a relatively known intensity in aerobic or strength exercise, it is not so easy to determine the intensity with activities such as pilates, healthy beat acupunch, or yoga, which was used in almost half of the studies [32,36,38,39,42,43].
The most common interventions included were yoga [32,36,38,39,43] and multicomponent exercise [34,40,44]; however, one of the interventions that caught our attention is acupunch [42]. Acupunch is a non-invasive practice where a natural parabola is produced by swinging of the relaxed wrist, elbow, and shoulder joints to direct cuffing and tapping of the fist/palm onto the targeted acupoint along the meridians to transport “qi” [63]. The acupunch itself cannot be considered an exercise or an incentive for physical activity. However, the authors describe the healthy beat acupunch as a modification of the original acupunch by adding the physical fitness guidelines for older adults and essential elements of a comprehensive exercise program for older adults [64].
When analysing by setting, we found that home-based programs (defined as at least 50% of their completion at home) were not effective in improving sleep quality [35,38,40], whereas programs carried out in a facility, such as a nursing home or community centre, were effective if they managed to improve the quality of sleep significantly [32,34,41,43,44]. One of the critical points that could explain the improvement in this type of setting is the degree of supervision. One point in which the programs in facilities agree is the close supervision and guidance in the exercise program [34,44]. Another interesting finding is that when analysing the duration of the programs, those that lasted less than 12 weeks were more effective than those that lasted more than 12 weeks. On the other hand, by adding the analysis by intermediate evaluation points, we were able to find that programs lasting between 12 weeks and 12 months were also effective. However, we believe that it is risky to carry out an analysis since programs older than 12 weeks are few and we think that with the increase in the sample size more conclusive results can be obtained.
An important point to consider is the type of physical training. Although most of the studies comply with the principles of a structured exercise program, with frequency, intensity, time, and duration, as usually happens, physical exercise programs are confused with those that encourage physical activity that consists of increasing moderate to vigorous physical activity (MVPA) [65]. These programs usually increase physical activity through walking. Although both programs show benefits in chronic diseases [65], the principles of physical training cannot be fully applied, so they cannot be analysed as a dose response.
4.4. Clinical Implications
This study shows that exercise has effects on sleep quality. Clinicians and decision makers can review these data and carry out exercise programs focused on older adults who report sleep problems with or without pathologies and who report the use of medications for the management of sleep disorders or to improve mental health. This may be particularly relevant at community care levels, such as nursing homes or primary care. On the other hand, it is recommended to carry out supervised programs that comply with the principles of training to achieve the best possible effect on the population.
4.5. Limitations
Our study has some limitations. Most of the studies included populations with different diseases or comorbidities, which may limit the extrapolation of the results and recommendations to the entire spectrum of older people, even though our results were statistically significant. The heterogeneous nature of populations implies that many subjects have different pathophysiological behaviours and conditions, a broad spectrum of severity, and the implication or impact that suffering from associated comorbidities may influence the magnitude of reported results. On the other hand, we tried to sub-analyse by health status; however, it was very complex since the definition of healthy is not uniform among the different authors and, therefore, it loses reliability. Additionally, the studies did not report adherence, which is a fundamental factor in determining the effectiveness of an intervention. Future studies must describe the adherence to exercise, since this factor must be analysed together with the results when evaluating the effectiveness of an intervention.
5. Conclusions
Physical exercise programs in older adults improve sleep quality, particularly in facilities, and sleep efficiency measured with objective instruments.
Author Contributions
L.S.-N.: Conceptualisation, Formal analysis, Methodology, Reviewing procedure and data extraction, Writing—original draft, Writing—review and editing. O.M.: Formal analysis, Reviewing procedure and data extraction, Writing—original draft, Writing—review and editing. R.T.-C.: Conceptualisation, Formal analysis, Reviewing procedure and data extraction, Writing—original draft, Writing—review and editing. M.O.-Y.: Formal analysis, Reviewing procedure and data extraction, Writing—original draft, Writing—review and editing. C.F.-J.: Formal analysis, Writing—original draft, Writing—review and editing. M.S.-M.: Formal analysis, Writing—original draft, Writing—review and editing. A.C.: Formal analysis, Writing—original draft, Writing—review and editing. E.C.-B.: Formal analysis, Writing—original draft, Writing—review and editing. M.S.-R.: Conceptualisation, Formal analysis, Writing—original draft, Writing—review and editing. L.M.P.: Conceptualisation, Formal analysis, Writing—original draft, Writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- The World Bank. United Nations Population Division. World Population Prospects: 2022 Revision. Available online: https://data.worldbank.org/indicator/SP.POP.65UP.TO.ZS (accessed on 15 January 2023).
- World Health Organization. Ageing and Health. 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/ageing-and-health (accessed on 2 May 2022).
- Bull, F.C.; Al-Ansari, S.S.; Biddle, S.; Borodulin, K.; Buman, M.P.; Cardon, G.; Carty, C.; Chaput, J.-P.; Chastin, S.; Chou, R.; et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br. J. Sports Med. 2020, 54, 1451–1462. [Google Scholar] [CrossRef]
- Piercy, K.L.; Troiano, R.P.; Ballard, R.M.; Carlson, S.A.; Fulton, J.E.; Galuska, D.A.; George, S.M.; Olson, R.D. The Physical Activity Guidelines for Americans. JAMA 2018, 320, 2020–2028. [Google Scholar] [CrossRef]
- Mora, J.C.; Valencia, W.M. Exercise and Older Adults. Clin. Geriatr. Med. 2018, 34, 145–162. [Google Scholar] [CrossRef]
- Daskalopoulou, C.; Stubbs, B.; Kralj, C.; Koukounari, A.; Prince, M.; Prina, A.M. Physical activity and healthy ageing: A systematic review and meta-analysis of longitudinal cohort studies. Ageing Res. Rev. 2017, 38, 6–17. [Google Scholar] [CrossRef]
- Gopinath, B.; Kifley, A.; Flood, V.M.; Mitchell, P. Physical Activity as a Determinant of Successful Aging over Ten Years. Sci. Rep. 2018, 8, 10522. [Google Scholar] [CrossRef]
- Speakman, J.R.; Westerterp, K.R. Associations between energy demands, physical activity, and body composition in adult humans between 18 and 96 y of age. Am. J. Clin. Nutr. 2010, 92, 826–834. [Google Scholar] [CrossRef]
- Sindi, S.; Pérez, L.M.; Vetrano, D.L.; Triolo, F.; Kåreholt, I.; Sjöberg, L.; Darin-Mattsson, A.; Kivipelto, M.; Inzitari, M.; Calderón-Larrañaga, A. Sleep disturbances and the speed of multimorbidity development in old age: Results from a longitudinal population-based study. BMC Med. 2020, 18, 382. [Google Scholar] [CrossRef]
- Landi, F.; Calvani, R.; Picca, A.; Tosato, M.; Martone, A.M.; Angelo, E.D.; Serafini, E.; Bernabei, R.; Marzetti, E. Impact of habitual physical activity and type of exercise on physical performance across ages in community-living people. PLoS ONE 2018, 13, e0191820. [Google Scholar] [CrossRef] [PubMed]
- Chaput, J.-P.; Dutil, C.; Featherstone, R.; Ross, R.; Giangregorio, L.; Saunders, T.J.; Janssen, I.; Poitras, V.J.; Kho, M.E.; Ross-White, A.; et al. Sleep timing, sleep consistency, and health in adults: A systematic review. Appl. Physiol. Nutr. Metab. Physiol. Appl. Nutr. Metab. 2020, 45, S232–S247. [Google Scholar] [CrossRef]
- Chen, K.-M.; Huang, H.-T.; Cheng, Y.-Y.; Li, C.-H.; Chang, Y.-H. Sleep quality and depression of nursing home older adults in wheelchairs after exercises. Nurs. Outlook 2015, 63, 357–365. [Google Scholar] [CrossRef]
- Ohayon, M.M. Epidemiology of insomnia: What we know and what we still need to learn. Sleep Med. Rev. 2002, 6, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Foley, D.; Ancoli-Israel, S.; Britz, P.; Walsh, J. Sleep disturbances and chronic disease in older adults: Results of the 2003 National Sleep Foundation Sleep in America Survey. J. Psychosom. Res. 2004, 56, 497–502. [Google Scholar] [CrossRef]
- Bertisch, S.M.; Herzig, S.J.; Winkelman, J.W.; Buettner, C. National use of prescription medications for insomnia: NHANES 1999–2010. Sleep 2014, 37, 343–349. [Google Scholar] [CrossRef]
- Atkin, T.; Comai, S.; Gobbi, G. Drugs for Insomnia beyond Benzodiazepines: Pharmacology, Clinical Applications, and Discovery. Pharmacol. Rev. 2018, 70, 197–245. [Google Scholar] [CrossRef]
- Mokhar, A.; Tillenburg, N.; Dirmaier, J.; Kuhn, S.; Härter, M.; Verthein, U. Potentially inappropriate use of benzodiazepines and z-drugs in the older population-analysis of associations between long-term use and patient-related factors. PeerJ 2018, 6, e4614. [Google Scholar] [CrossRef]
- Amari, D.T.; Juday, T.; Frech, F.H.; Wang, W.; Wu, Z.; Atkins, N.; Wickwire, E.M. Falls, healthcare resources and costs in older adults with insomnia treated with zolpidem, trazodone, or benzodiazepines. BMC Geriatr. 2022, 22, 484. [Google Scholar] [CrossRef]
- By the 2019 American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2019 Updated AGS Beers Criteria® for Potentially Inappropriate Medication Use in Older Adults. J. Am. Geriatr. Soc. 2019, 67, 674–694. [Google Scholar] [CrossRef]
- Solis-Navarro, L.; Gismero, A.; Fernández-Jané, C.; Torres-Castro, R.; Solá-Madurell, M.; Bergé, C.; Pérez, L.M.; Ars, J.; Martín-Borràs, C.; Vilaró, J.; et al. Effectiveness of home-based exercise delivered by digital health in older adults: A systematic review and meta-analysis. Age Ageing 2022, 51, afac243. [Google Scholar] [CrossRef]
- Izquierdo, M.; Merchant, R.A.; Morley, J.E.; Anker, S.D.; Aprahamian, I.; Arai, H.; Aubertin-Leheudre, M.; Bernabei, R.; Cadore, E.L.; Cesari, M.; et al. International Exercise Recommendations in Older Adults (ICFSR): Expert Consensus Guidelines. J. Nutr. Health Aging 2021, 25, 824–853. [Google Scholar] [CrossRef]
- Fiuza-Luces, C.; Santos-Lozano, A.; Joyner, M.; Carrera-Bastos, P.; Picazo, O.; Zugaza, J.L.; Izquierdo, M.; Ruilope, L.M.; Lucia, A. Exercise benefits in cardiovascular disease: Beyond attenuation of traditional risk factors. Nat. Rev. Cardiol. 2018, 15, 731–743. [Google Scholar] [CrossRef]
- Torres-Castro, R.; Vilaró, J.; Martí, J.-D.; Garmendia, O.; Gimeno-Santos, E.; Romano-Andrioni, B.; Embid, C.; Montserrat, J.M. Effects of a Combined Community Exercise Program in Obstructive Sleep Apnea Syndrome: A Randomized Clinical Trial. J. Clin. Med. 2019, 8, 361. [Google Scholar] [CrossRef]
- Castelli, L.; Galasso, L.; Mulè, A.; Bruno, E.; Shokohyar, S.; Esposito, F.; Montaruli, A.; Roveda, E. Physical activity, chronotype and sleep in a sample of Italian elderly population. Sport Sci. Health 2019, 16, 55–64. [Google Scholar] [CrossRef]
- Ashworth, N.L.; Chad, K.E.; Harrison, E.; Reeder, B.A.; Marshall, S.C. Home versus center based physical activity programs in older adults. Cochrane Database Syst. Rev. 2005, 2005, CD004017. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann. Intern. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef]
- Orimo, H. Reviewing the definition of elderly. Geriatr. Gerontol. Int. 2006, 6, 149–158. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions Version 6.0; Cochrane: London, UK, 2019. [Google Scholar]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
- Guyatt, G.H.; Oxman, A.D.; Vist, G.E.; Kunz, R.; Falck-Ytter, Y.; Alonso-Coello, P.; Schünemann, H.J. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008, 336, 924–926. [Google Scholar] [CrossRef]
- Project T Jamovi. Jamovi. 2022. Available online: https://www.jamovi.org (accessed on 22 January 2023).
- Chen, K.-M.; Chen, M.-H.; Lin, M.-H.; Fan, J.-T.; Lin, H.-S.; Li, C.-H. Effects of yoga on sleep quality and depression in elders in assisted living facilities. J. Nurs. Res. 2010, 18, 53–61. [Google Scholar] [CrossRef]
- Hirano, A.; Suzuki, Y.; Kuzuya, M.; Onishi, J.; Ban, N.; Umegaki, H. Influence of regular exercise on subjective sense of burden and physical symptoms in community-dwelling caregivers of dementia patients: A randomized controlled trial. Arch. Gerontol. Geriatr. 2011, 53, e158–e163. [Google Scholar] [CrossRef]
- Bademli, K.; Lok, N.; Canbaz, M.; Lok, S. Effects of Physical Activity Program on cognitive function and sleep quality in elderly with mild cognitive impairment: A randomized controlled trial. Perspect. Psychiatr. Care 2018, 55, 401–408. [Google Scholar] [CrossRef]
- Falck, R.S.; Davis, J.C.; Best, J.R.; Chan, P.C.; Li, L.C.; Wyrough, A.B.; Bennett, K.J.; Backhouse, D.; Liu-Ambrose, T. Effect of a Multimodal Lifestyle Intervention on Sleep and Cognitive Function in Older Adults with Probable Mild Cognitive Impairment and Poor Sleep: A Randomized Clinical Trial. J. Alzheimer’s Dis. 2020, 76, 179–193. [Google Scholar] [CrossRef] [PubMed]
- Baklouti, S.; Fekih-Romdhane, F.; Guelmami, N.; Bonsaksen, T.; Baklouti, H.; Aloui, A.; Masmoudi, L.; Souissi, N.; Jarraya, M. The effect of web-based Hatha yoga on psychological distress and sleep quality in older adults: A randomized controlled trial. Complement. Ther. Clin. Pract. 2023, 50, 101715. [Google Scholar] [CrossRef] [PubMed]
- Oudegeest-Sander, M.H.; Eijsvogels, T.H.M.; Verheggen, R.J.H.M.; Poelkens, F.; Hopman, M.T.E.; Jones, H.; Thijssen, D.H.J. Impact of physical fitness and daily energy expenditure on sleep efficiency in young and older humans. Gerontology 2013, 59, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Cheung, C.; Wyman, J.F.; Resnick, B.; Savik, K. Yoga for managing knee osteoarthritis in older women: A pilot randomized controlled trial. BMC Complement. Altern. Med. 2014, 14, 160. [Google Scholar] [CrossRef]
- Taşkiran, Ö.Ö.; Cicioğlu, İ.; Golmoghani-Zadeh, N.; Atilgan, A.D.; Bağci, E.; Günay, M.; Atalay, F. Do Pilates and yoga affect quality of life and physical performance of elderly living in a nursing home a preliminary study. Turk. J. Geriatr./Türk Geriatr. Derg. 2014, 17, 262–271. [Google Scholar]
- Vaz Fragoso, C.A.; Miller, M.E.; King, A.C.; Kritchevsky, S.B.; Liu, C.K.; Myers, V.H.; Nadkarni, N.K.; Pahor, M.; Spring, B.J.; Gill, T.M.; et al. Effect of Structured Physical Activity on Sleep-Wake Behaviors in Sedentary Elderly Adults with Mobility Limitations. J. Am. Geriatr. Soc. 2015, 63, 1381–1390. [Google Scholar] [CrossRef]
- Chen, K.-M.; Li, C.-H.; Huang, H.-T.; Cheng, Y.-Y. Feasible modalities and long-term effects of elastic band exercises in nursing home older adults in wheelchairs: A cluster randomized controlled trial. Int. J. Nurs. Stud. 2016, 55, 4–14. [Google Scholar] [CrossRef]
- Hsiao, C.-Y.; Chen, K.-M.; Tsai, H.-Y.; Huang, H.-T.; Cheng, Y.-Y.; Tsai, A.Y. Self-Perceived Health and Sleep Quality of Community Older Adults after Acupunch Exercises. Am. J. Geriatr. Psychiatry 2018, 26, 511–520. [Google Scholar] [CrossRef]
- Choi, M.-J.; Sohng, K.-Y. The Effects of Floor-seated Exercise Program on Physical Fitness, Depression, and Sleep in Older Adults: A Cluster Randomized Controlled Trial. Int. J. Gerontol. 2018, 12, 116–121. [Google Scholar] [CrossRef]
- Laredo-Aguilera, J.A.; Carmona-Torres, J.M.; García-Pinillos, F.; Latorre-Román, P.Á. Effects of a 10-week functional training programme on pain, mood state, depression, and sleep in healthy older adults. Psychogeriatrics 2018, 18, 292–298. [Google Scholar] [CrossRef]
- Scullin, M.K.; Bliwise, D.L. Sleep, cognition, and normal aging: Integrating a half century of multidisciplinary research. Perspect. Psychol. Sci. J. Assoc. Psychol. Sci. 2015, 10, 97–137. [Google Scholar] [CrossRef] [PubMed]
- Inoue, S.; Yorifuji, T.; Sugiyama, M.; Ohta, T.; Ishikawa-Takata, K.; Doi, H. Does habitual physical activity prevent insomnia? A cross-sectional and longitudinal study of elderly Japanese. J. Aging Phys. Act. 2013, 21, 119–139. [Google Scholar] [CrossRef] [PubMed]
- Carlsen, T.; Stensvold, D.; Wisløff, U.; Ernstsen, L.; Halvorsen, T. The association of change in peak oxygen uptake with use of psychotropics in community-dwelling older adults—The Generation 100 study. BMC Geriatr. 2022, 22, 575. [Google Scholar] [CrossRef]
- Jennum, P.; Baandrup, L.; Ibsen, R.; Kjellberg, J. Increased all-cause mortality with use of psychotropic medication in dementia patients and controls: A population-based register study. Eur. Neuropsychopharmacol. 2015, 25, 1906–1913. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Satoh, H.; Miki, A.; Urushihara, H.; Sawada, Y. Medications associated with falls in older people: Systematic review of publications from a recent 5-year period. Eur. J. Clin. Pharmacol. 2015, 71, 1429–1440. [Google Scholar] [CrossRef]
- Bakken, M.S.; Engeland, A.; Engesæter, L.B.; Ranhoff, A.H.; Hunskaar, S.; Ruths, S. Risk of hip fracture among older people using anxiolytic and hypnotic drugs: A nationwide prospective cohort study. Eur. J. Clin. Pharmacol. 2014, 70, 873–880. [Google Scholar] [CrossRef]
- Gutsmiedl, K.; Krause, M.; Bighelli, I.; Schneider-Thoma, J.; Leucht, S. How well do elderly patients with major depressive disorder respond to antidepressants: A systematic review and single-group meta-analysis. BMC Psychiatry 2020, 20, 102. [Google Scholar] [CrossRef]
- Yang, P.-Y.; Ho, K.-H.; Chen, H.-C.; Chien, M.-Y. Exercise training improves sleep quality in middle-aged and older adults with sleep problems: A systematic review. J. Physiother. 2012, 58, 157–163. [Google Scholar] [CrossRef]
- Mendelson, M.; Lyons, O.D.; Yadollahi, A.; Inami, T.; Oh, P.; Bradley, T.D. Effects of exercise training on sleep apnoea in patients with coronary artery disease: A randomised trial. Eur. Respir. J. 2016, 48, 142–150. [Google Scholar] [CrossRef]
- Torres-Castro, R.; Vasconcello-Castillo, L.; Puppo, H.; Cabrera-Aguilera, I.; Otto-Yáñez, M.; Rosales-Fuentes, J.; Vilaró, J. Effects of Exercise in Patients with Obstructive Sleep Apnoea. Clocks Sleep 2021, 3, 227–235. [Google Scholar] [CrossRef]
- Sejbuk, M.; Mirończuk-Chodakowska, I.; Witkowska, A.M. Sleep Quality: A Narrative Review on Nutrition, Stimulants, and Physical Activity as Important Factors. Nutrients 2022, 14, 1912. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; van Egmond, L.T.; Cedernaes, J.; Benedict, C. The role of exercise-induced peripheral factors in sleep regulation. Mol. Metab. 2020, 42, 101096. [Google Scholar] [CrossRef] [PubMed]
- Kredlow, M.A.; Capozzoli, M.C.; Hearon, B.A.; Calkins, A.W.; Otto, M.W. The effects of physical activity on sleep: A meta-analytic review. J. Behav. Med. 2015, 38, 427–449. [Google Scholar] [CrossRef] [PubMed]
- Ritsche, K.; Nindl, B.C.; Wideman, L. Exercise-Induced growth hormone during acute sleep deprivation. Physiol. Rep. 2014, 2, e12166. [Google Scholar] [CrossRef]
- Buysse, D.J.; Reynolds, C.F., III; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Hughes, C.M.; McCullough, C.A.; Bradbury, I.; Boyde, C.; Hume, D.; Yuan, J.; Quinn, F.; McDonough, S. Acupuncture and reflexology for insomnia: A feasibility study. Acupunct. Med. 2009, 27, 163–168. [Google Scholar] [CrossRef]
- Lu, T.; Li, Y.; Pan, J.; Wu, D. Study on minimal important difference of the Pittsburgh sleep quality index based on clinical trial of traditional Chinese medicine. J. Guangzhou Univ. Tradit. Chin. Med. 2013, 30, 574–578. [Google Scholar]
- Landry, G.J.; Best, J.R.; Liu-Ambrose, T. Measuring sleep quality in older adults: A comparison using subjective and objective methods. Front. Aging Neurosci. 2015, 7, 166. [Google Scholar] [CrossRef]
- Jones, C.; Chen, K.-M.; Weeks, B.; Qi, M.; Moyle, W. Healthy Beat Acupunch exercise program: Validation and feasibility study for older adults with reduced physical capacity or probable sarcopenia. Explore 2021, 17, 498–504. [Google Scholar] [CrossRef]
- Tsai, H.-Y.; Chen, K.-M.; Huang, H.-T. Acupunch Exercise Program Development and Feasibility Evaluation for Older Adults. Rehabil. Nurs. J. 2020, 45, 195–203. [Google Scholar] [CrossRef]
- Anderson, E.; Durstine, J.L. Physical activity, exercise, and chronic diseases: A brief review. Sport Med. Health Sci. 2019, 1, 3–10. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).