Characteristic of Ultrastructure of Mice B16 Melanoma Cells under the Influence of Different Lighting Regimes
Abstract
:1. Introduction
2. Results
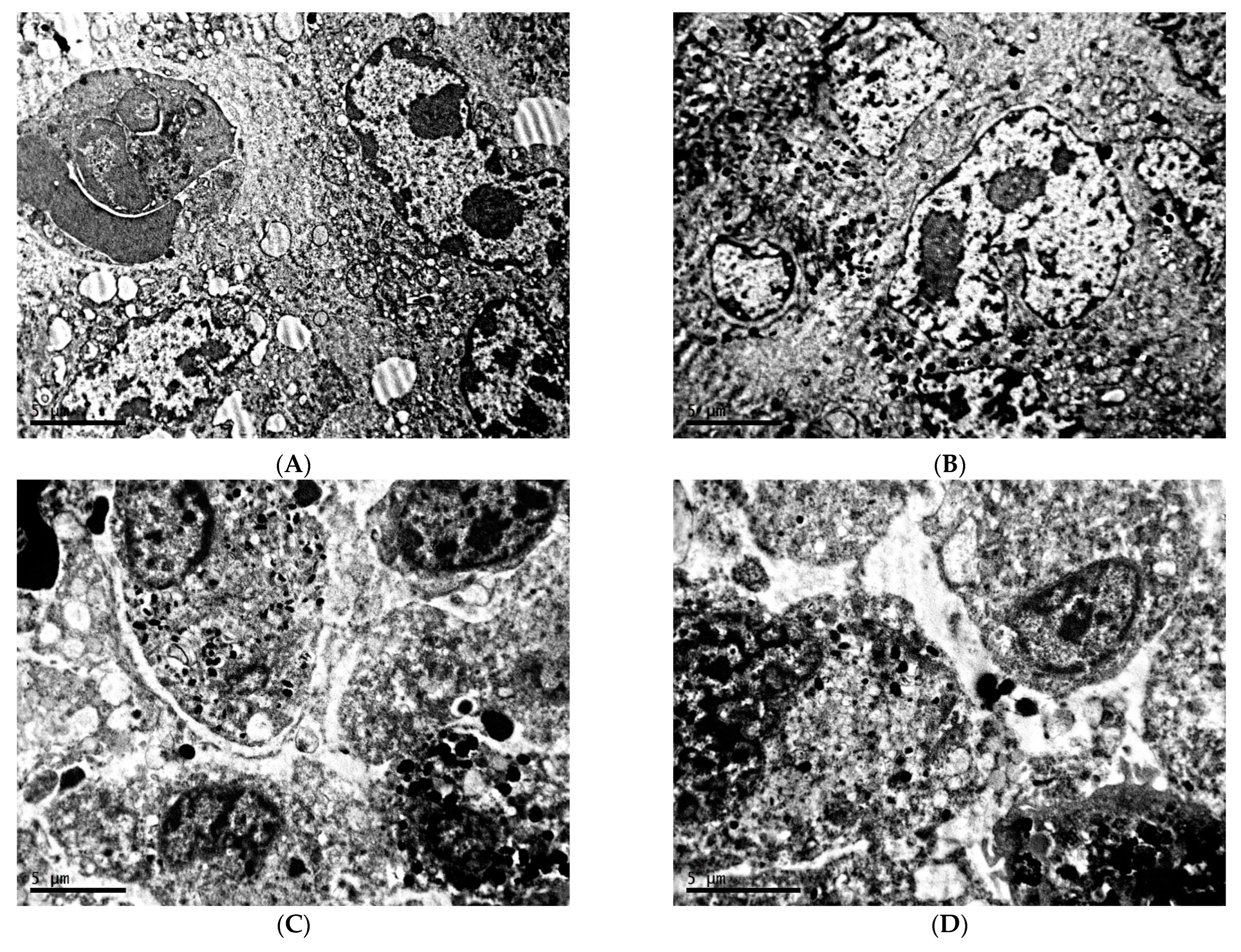
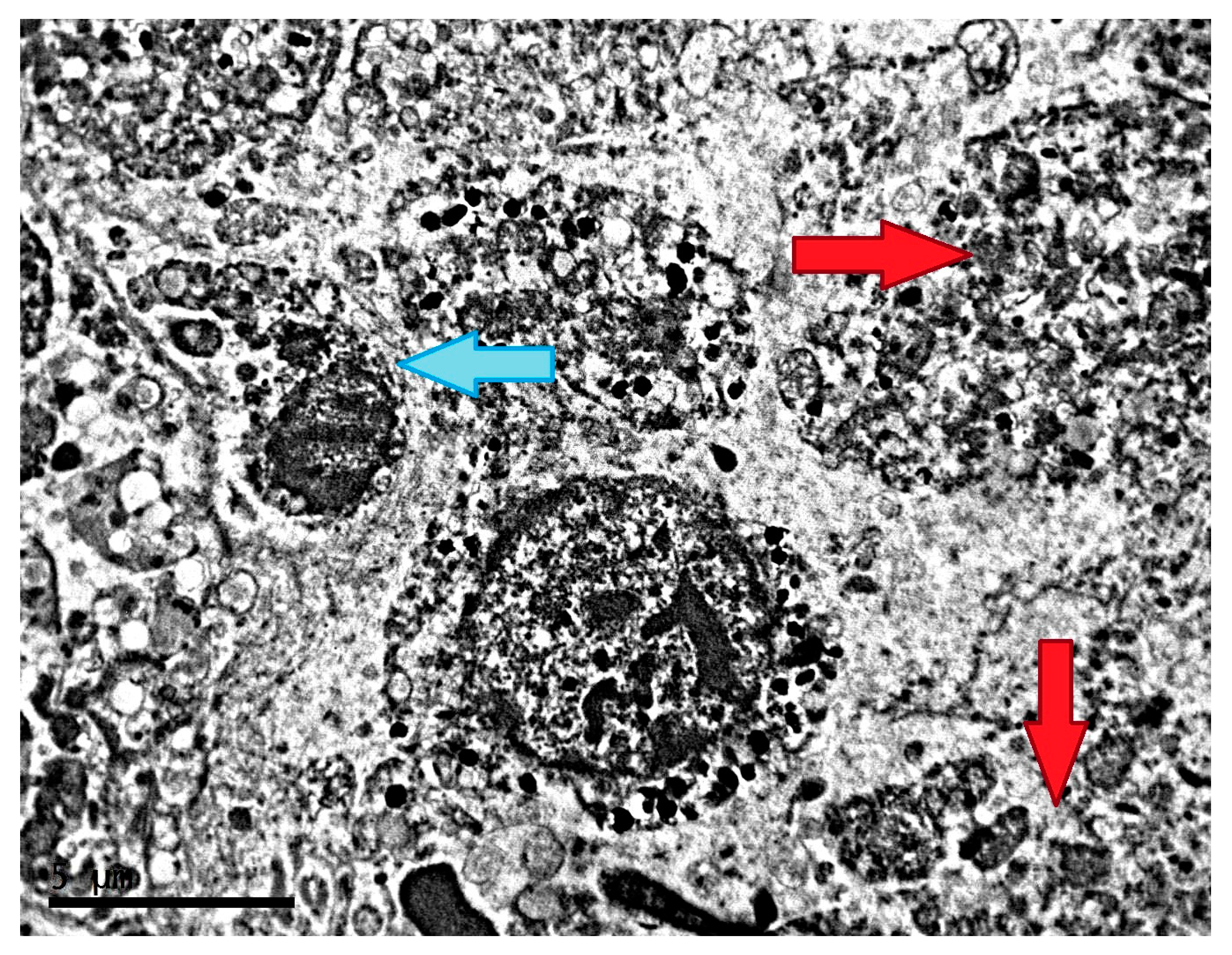
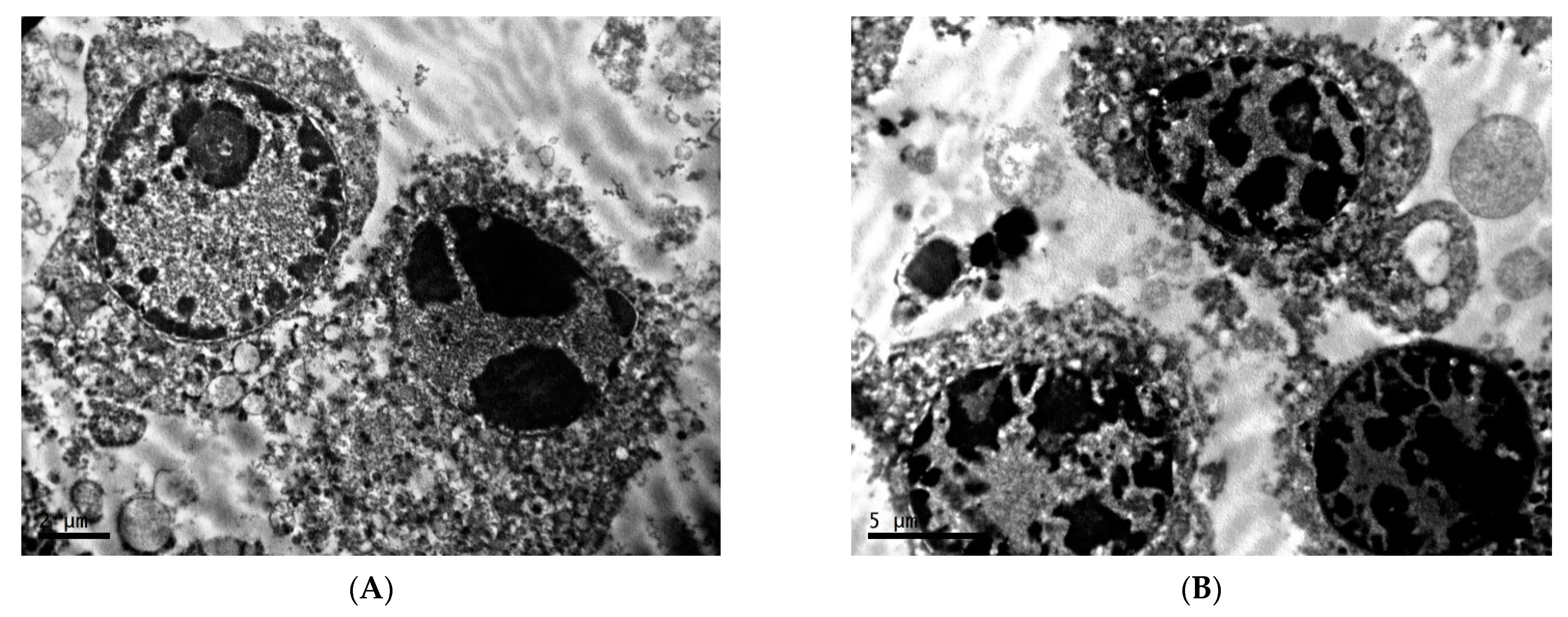
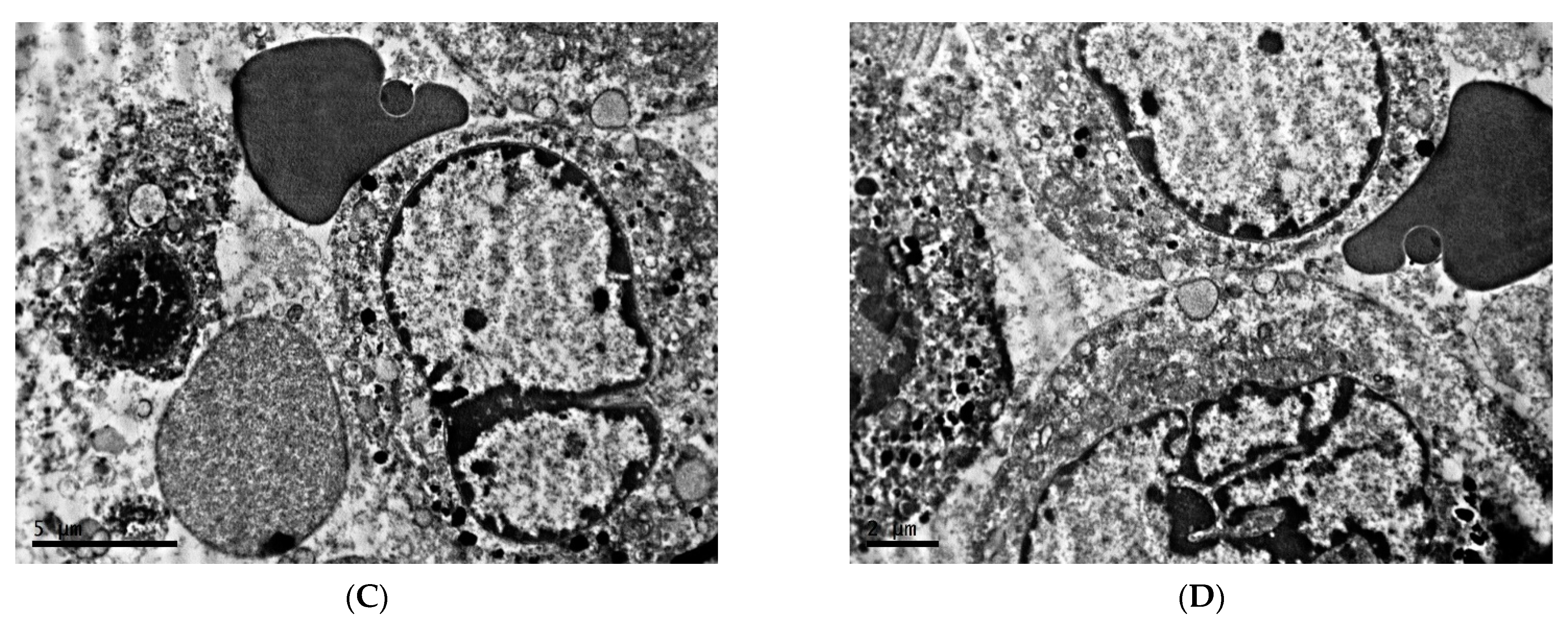
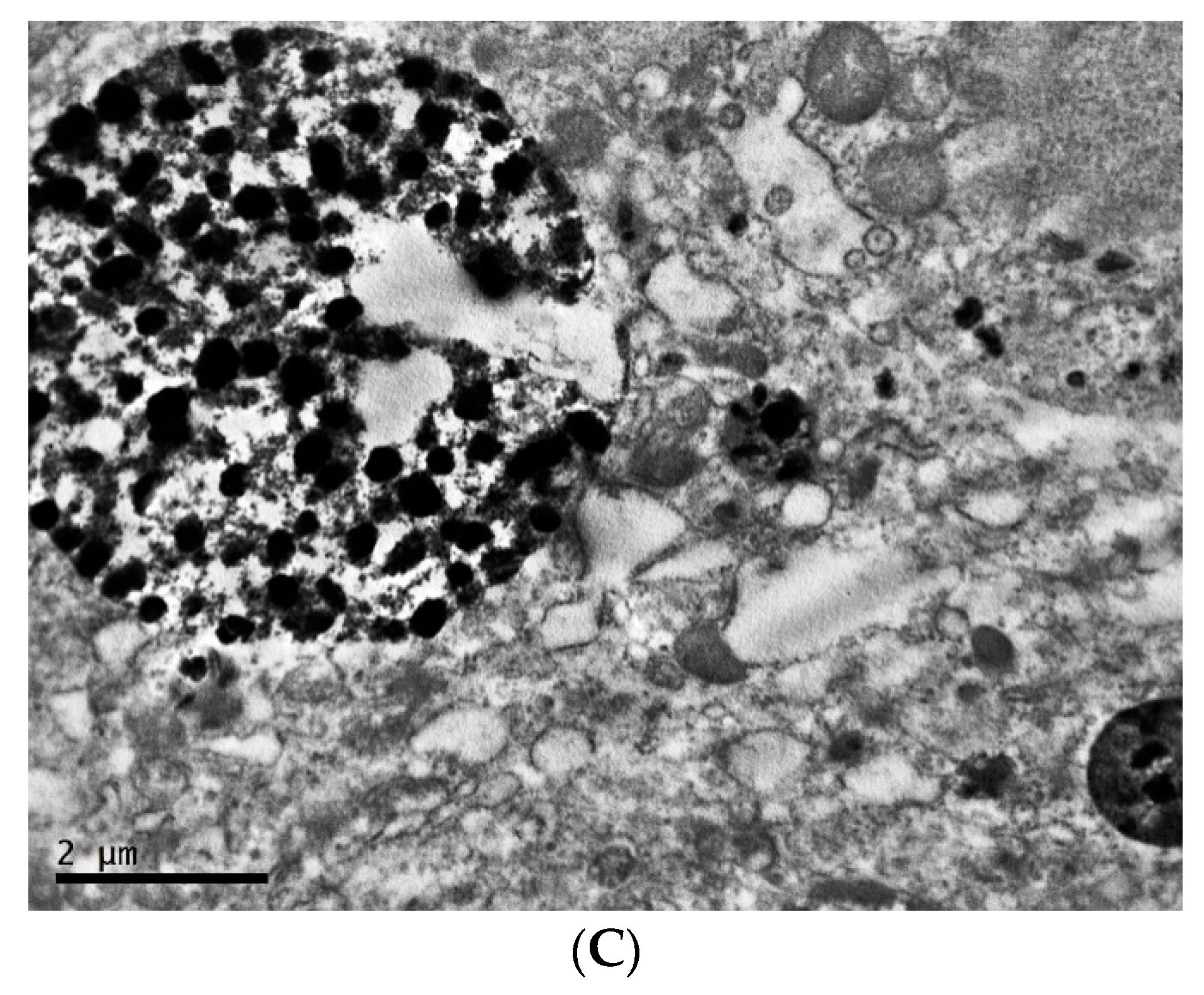
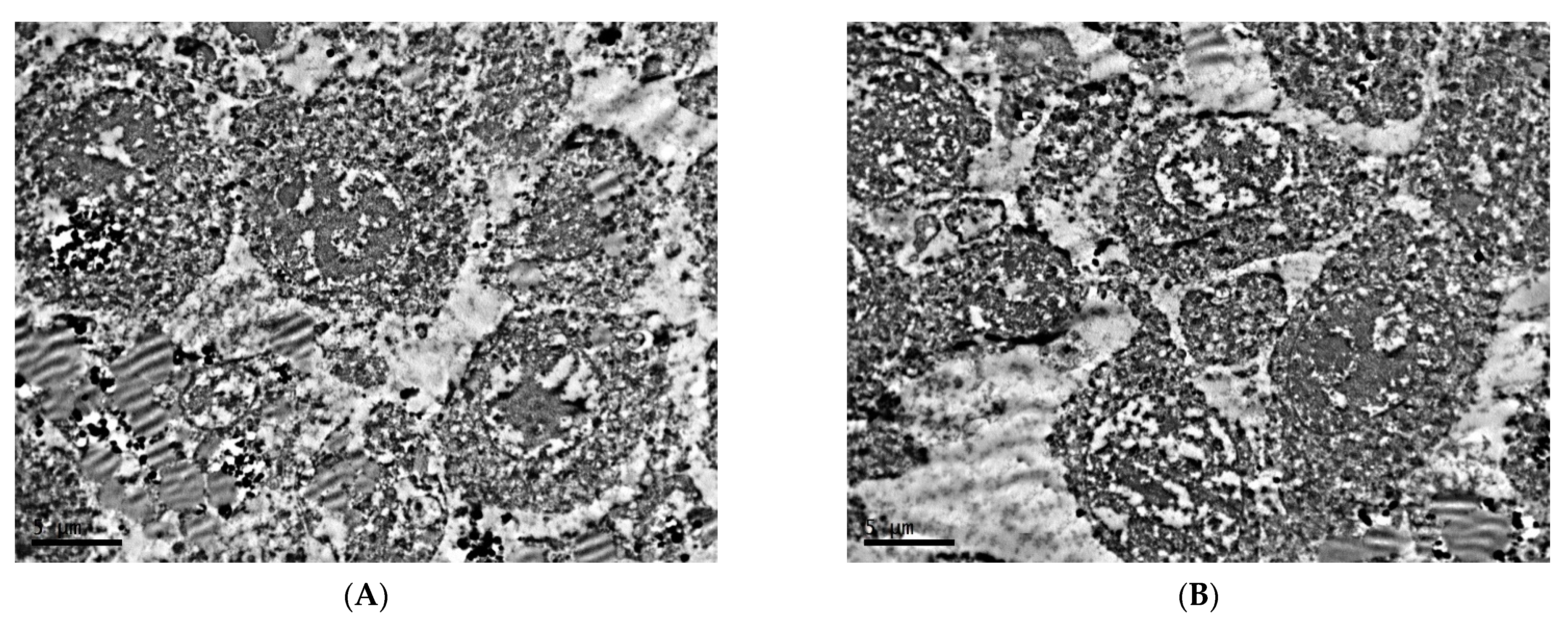
2.1. Ultrastructure of Transplantable Malignant Melanoma B16 in Conditions of Fixed Light Regime
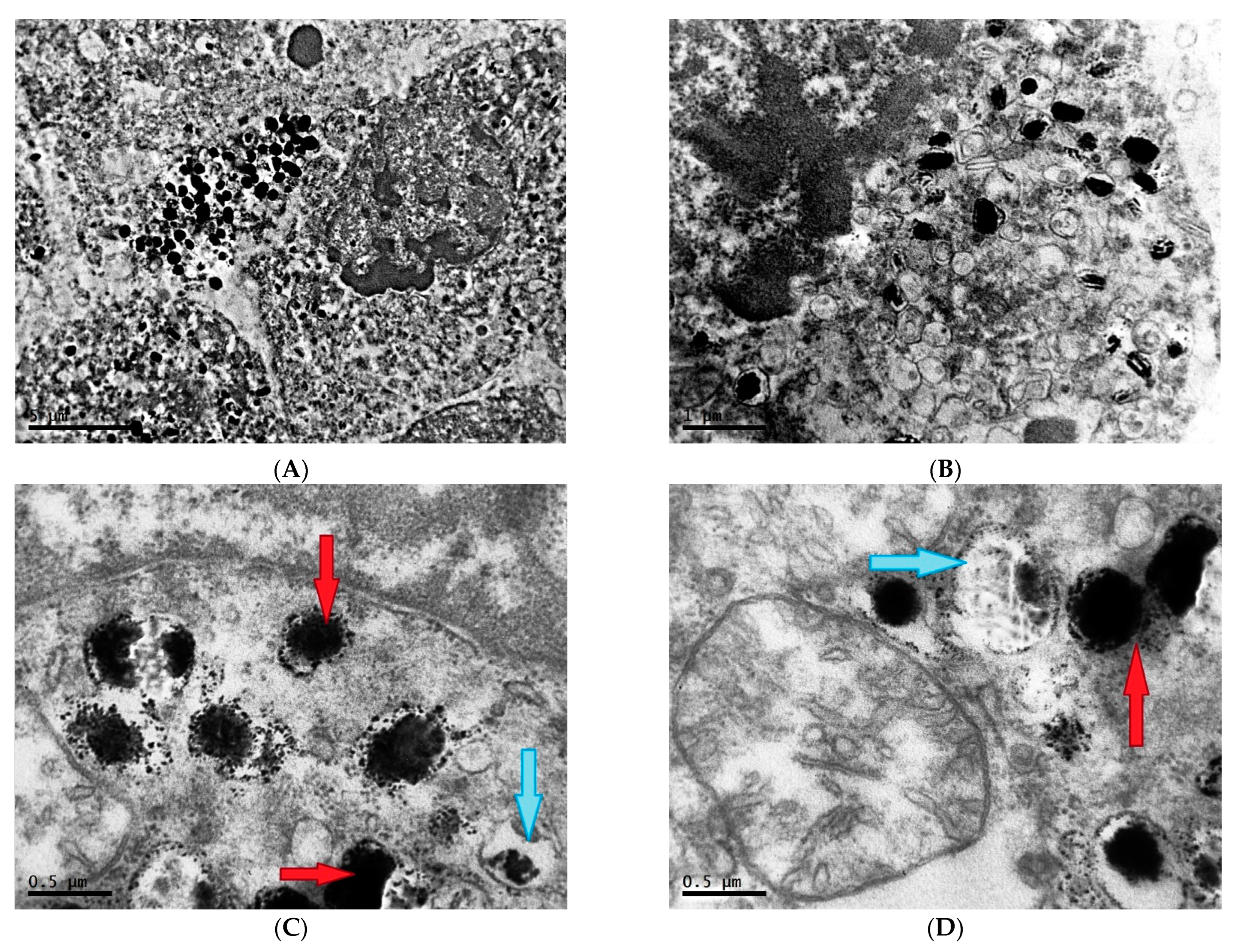
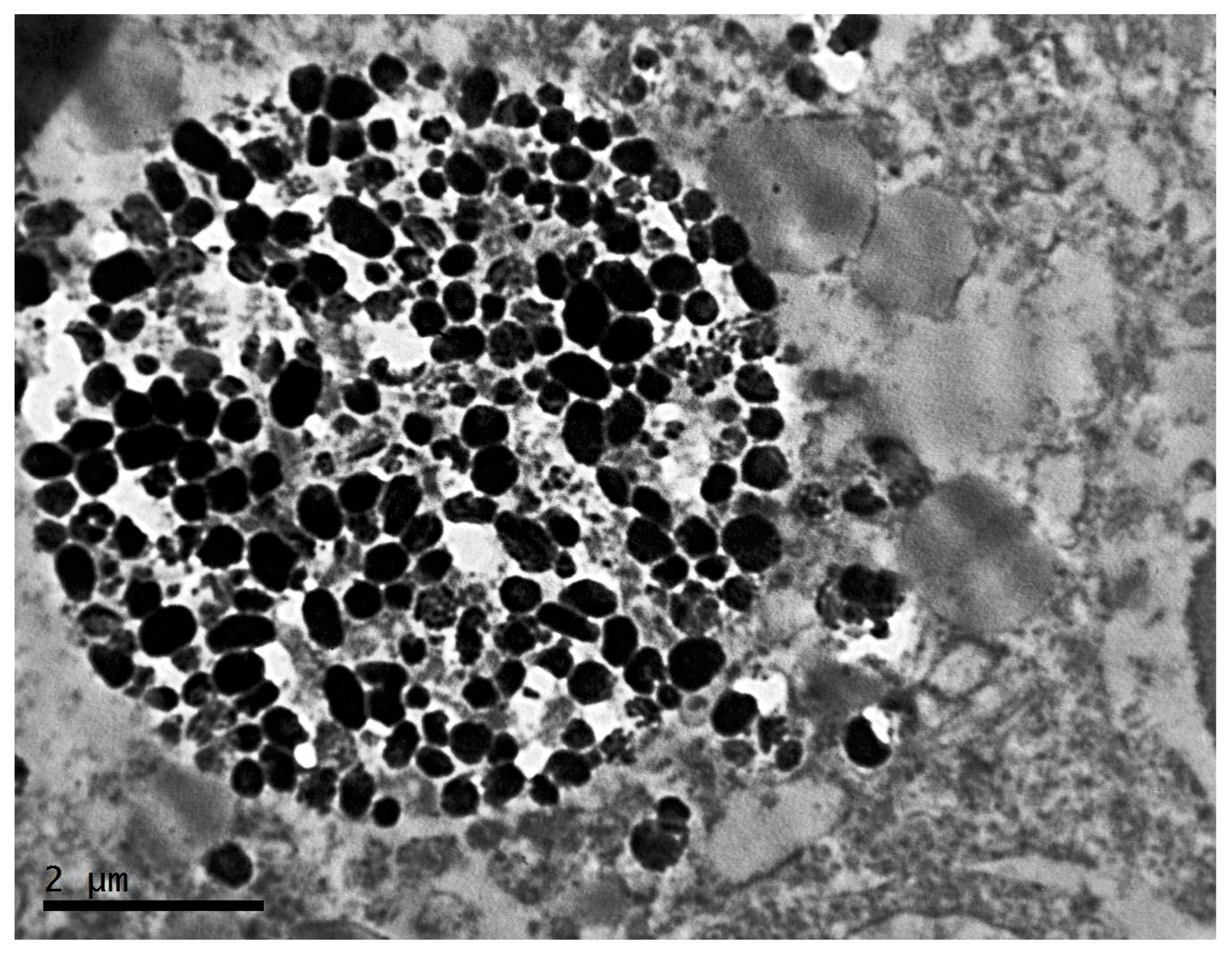
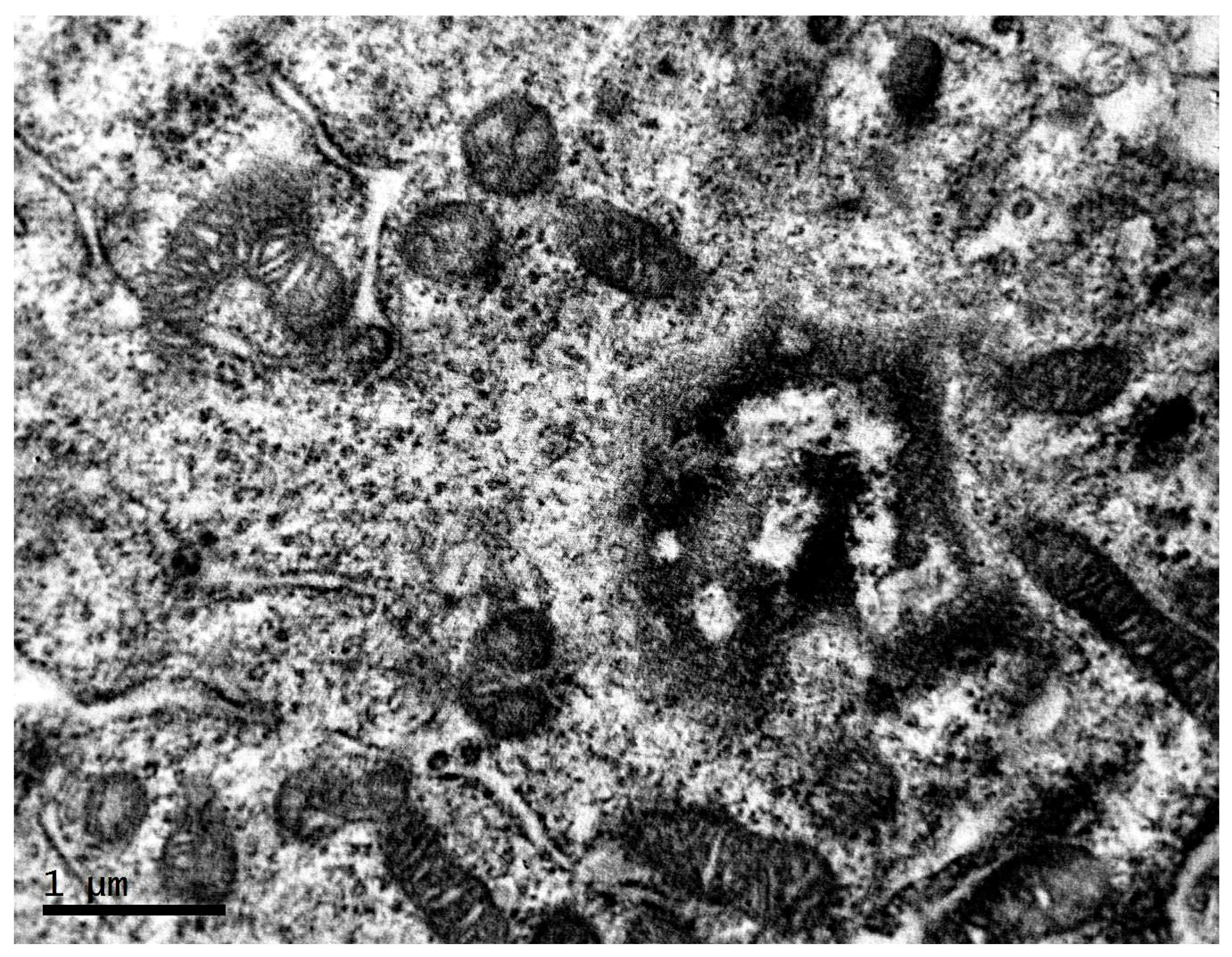
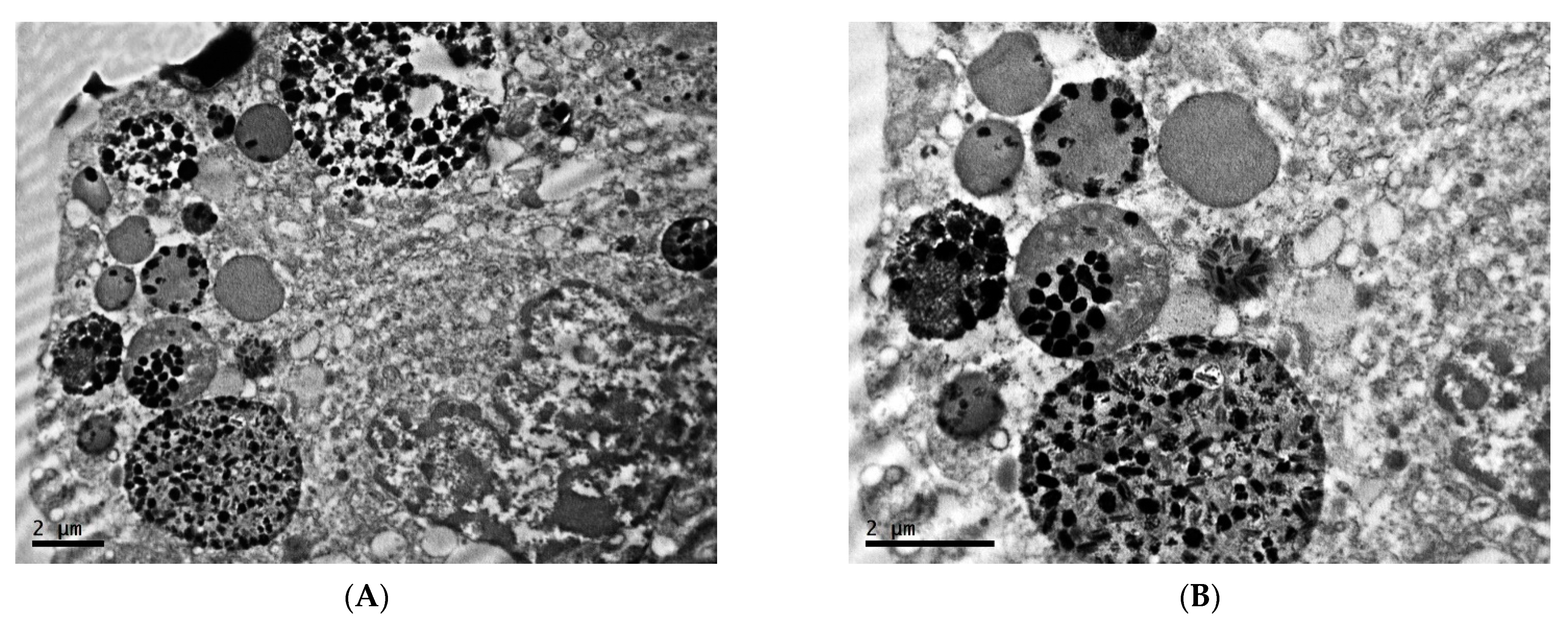
2.2. Ultrastructure of Transplantable Malignant Melanoma B16 in Conditions of Constant Lighting Regime
3. Discussion and Conclusions
4. Materials and Methods
4.1. Object of Study
4.2. Design of Study
4.3. Electron Microscopy
4.4. Statistical Evaluation
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dunlap, J.C. Molecular Bases for Circadian Clocks. Cell 1999, 96, 271–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hughes, A.T.L.; Attarian, H.P.; Hirayama, J. Editorial: The circadian circus—How our clocks keep us ticking. Front. Neurosci. 2022, 16, 973727. [Google Scholar] [CrossRef]
- Anisimov, V.N. Light pollution, reproductive function and cancer risk. Neuro Endocrinol. Lett. 2006, 27, 35–52. [Google Scholar]
- Cox, K.H.; Takahashi, J.S. Circadian clock genes and the transcriptional architecture of the clock mechanism. J. Mol. Endocrinol. 2019, 63, R93–R102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vitaterna, M.H.; Shimomura, K.; Jiang, P. Genetics of Circadian Rhythms. Neurol. Clin. 2019, 37, 487–504. [Google Scholar] [CrossRef] [PubMed]
- Farshadi, E.; van der Horst, G.; Chaves, I. Molecular Links between the Circadian Clock and the Cell Cycle. J. Mol. Biol. 2020, 432, 3515–3524. [Google Scholar] [CrossRef]
- Shostak, A. Circadian clock, cell division, and cancer: From molecules to organism. Int. J. Mol. Sci. 2017, 18, 873. [Google Scholar] [CrossRef]
- Burchett, J.; Knudsen-Clark, A.; Altman, B. MYC Ran Up the Clock: The Complex Interplay between MYC and the Molecular Circadian Clock in Cancer. Int. J. Mol. Sci. 2021, 22, 7761. [Google Scholar] [CrossRef]
- Kinouchi, K.; Sassone-Corsi, P. Metabolic rivalry: Circadian homeostasis and tumorigenesis. Nat. Rev. Cancer 2020, 20, 645–661. [Google Scholar] [CrossRef]
- Golombek, D.A.; Rosenstein, R.E. Physiology of Circadian Entrainment. Physiol. Rev. 2010, 90, 1063–1102. [Google Scholar] [CrossRef] [Green Version]
- Verlande, A.; Masri, S. Circadian Clocks and Cancer: Timekeeping Governs Cellular Metabolism. Trends Endocrinol. Metab. 2019, 30, 445–458. [Google Scholar] [CrossRef] [PubMed]
- Vainshelbaum, N.M.; Salmina, K.; Gerashchenko, B.I.; Lazovska, M.; Zayakin, P.; Cragg, M.S.; Pjanova, D.; Erenpreisa, J. Role of the Circadian Clock “Death-Loop” in the DNA Damage Response Underpinning Cancer Treatment Resistance. Cells 2022, 11, 880. [Google Scholar] [CrossRef] [PubMed]
- Xuan, W.; Khan, F.; James, C.D.; Heimberger, A.B.; Lesniak, M.S.; Chen, P. Circadian regulation of cancer cell and tumor microenvironment crosstalk. Trends Cell Biol. 2021, 31, 940–950. [Google Scholar] [CrossRef] [PubMed]
- Carlson, J.A.; Linette, G.P.; Aplin, A.; Ng, B.; Slominski, A. Melanocyte Receptors: Clinical Implications and Therapeutic Relevance. Dermatol. Clin. 2007, 25, 541–557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stankov, B.; Reiter, R.J. Melatonin receptors: Current status, facts, and hypotheses. Life Sci. 1990, 46, 971–982. [Google Scholar] [CrossRef]
- Slominski, A.; Pruski, D. Melatonin Inhibits Proliferation and Melanogenesis in Rodent Melanoma Cells. Exp. Cell Res. 1993, 206, 189–194. [Google Scholar] [CrossRef]
- Slominski, A.; Pisarchik, A.; Zbytek, B.; Tobin, D.; Wortsman, J. Functional activity of serotoninergic and melatoninergic systems expressed in the skin. J. Cell Physiol. 2003, 196, 144–153. [Google Scholar] [CrossRef]
- Slominski, A.; Tobin, D.J.; Shibahara, S.; Wortsman, J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol. Rev. 2004, 84, 1155–1228. [Google Scholar] [CrossRef]
- Reiter, R.J.; Sharma, R.; Rodriguez, C.; Martin, V.; Rosales-Corral, S.; Zuccari, D.A.P.C.; Chuffa, L.G.A. Part-time cancers and role of melatonin in determining their metabolic phenotype. Life Sci. 2021, 278, 119597. [Google Scholar] [CrossRef]
- Sevilla, A.; Chéret, J.; Slominski, R.M.; Slominski, A.T.; Paus, R. Revisiting the role of melatonin in human melanocyte physiology: A skin context perspective. J. Pineal Res. 2022, 72, e12790. [Google Scholar] [CrossRef]
- Valverde, P.; Benedito, E.; Solano, F.; Oaknin, S.; Lozano, J.A.; Garcia-Borron, J.C. Melatonin Antagonizes alpha-Melanocyte-Stimulating Hormone Enhancement of Melanogenesis in Mouse Melanoma Cells by Blocking the Hormone-Induced Accumulation of the C Locus Tyrosinase. JBICJ. Biol. Inorg. Chem. 1995, 232, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Schadendorf, D.; van Akkooi, A.C.J.; Berking, C.; Griewank, K.G.; Gutzmer, R.; Hauschild, A.; Stang, A.; Roesch, A.; Ugurel, S. Melanoma. Lancet 2018, 392, 971–984. [Google Scholar] [CrossRef] [PubMed]
- Dubocovich, M.L.; Masana, M.I.; Iacob, S.; Sauri, D.M. Melatonin receptor antagonists that differentiate between the human Mel1a and Mel1b recombinant subtypes are used to assess the pharmacological profile of the rabbit retina ML1 presynaptic heteroreceptor. Naunyn-Schmiedebergs Arch. Exp. Pathol. Pharmakol. 1997, 355, 365–375. [Google Scholar] [CrossRef]
- Lissoni, P.; Barni, S.; Cattaneo, G.; Tancini, G.; Esposti, G.; Fraschini, F. Clinical Results with the Pineal Hormone Melatonin in Advanced Cancer Resistant to Standard Antitumor Therapies. Oncology 1991, 48, 448–450. [Google Scholar] [CrossRef] [PubMed]
- Souza, A.V.; Visconti, M.A.; Castrucci, A.M.L. Melatonin biological activity and binding sites in human melanoma cells. J. Pineal Res. 2003, 34, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Savvidis, C.; Koutsilieris, M. Circadian Rhythm Disruption in Cancer Biology. Mol. Med. 2012, 18, 1249–1260. [Google Scholar] [CrossRef]
- Zubidat, A.E.; Fares, B.; Fares, F.; Haim, A. Artificial light at night of different spectral compositions differentially affects tumor growth in mice: Interaction with melatonin and epigenetic pathways. Cancer Control 2018, 25, 25. [Google Scholar] [CrossRef] [Green Version]
- Lawther, A.J.; Phillips, A.J.; Chung, N.-C.; Chang, A.; Ziegler, A.I.; Debs, S.; Sloan, E.K.; Walker, A.K. Disrupting circadian rhythms promotes cancer-induced inflammation in mice. Brain Behav. Immun. Heal 2022, 21, 100428. [Google Scholar] [CrossRef]
- Sancar, A.; Van Gelder, R.N. Clocks, cancer, and chronochemotherapy. Science 2021, 371, eabb0738. [Google Scholar] [CrossRef]
- Chang, N.; Tseng, M.T.; Spaulding, T.S. Induction and growth of mammary tumors after superior cervical ganglionectomy in sighted and blinded-anosmic rats. Life Sci. 1986, 38, 1821–1826. [Google Scholar] [CrossRef]
- Otálora, B.B.; Madrid, J.A.; Alvarez, N.; Vicente, V.; Rol, M.A.; Rol, A. Effects of exogenous melatonin and circadian synchronization on tumor progression in melanoma-bearing C57BL6 mice. J. Pineal Res. 2008, 44, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Slominski, R.M.; Sarna, T.; Płonka, P.M.; Raman, C.; Brożyna, A.A.; Slominski, A.T. Melanoma, Melanin, and Melanogenesis: The Yin and Yang Relationship. Front. Oncol. 2022, 12, 842496. [Google Scholar] [CrossRef] [PubMed]
- Dakup, P.P.; Porter, K.I.; Little, A.A.; Gajula, R.P.; Zhang, H.; Skornyakov, E.; Kemp, M.G.; Van Dongen, H.P.; Gaddameedhi, S. The circadian clock regulates cisplatin-induced toxicity and tumor regression in melanoma mouse and human models. Oncotarget 2018, 9, 14524–14538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alvarez-Artime, A.; Cernuda-Cernuda, R.; Naveda, F.A.; Cepas, V.; Gonzalez-Menendez, P.; Fernadez-Vega, S.; Quiros-Gonzalez, I.; Sainz, R.M.; Mayo, J.C. Melatonin-Induced Cytoskeleton Reorganization Leads to Inhibition of Melanoma Cancer Cell Proliferation. Int. J. Mol. Sci. 2020, 21, 548. [Google Scholar] [CrossRef] [Green Version]
- Favero, G.; Moretti, E.; Bonomini, F.; Reiter, R.J.; Rodella, L.F.; Rezzani, R. Promising Antineoplastic Actions of Melatonin. Front. Pharmacol. 2018, 9, 1086. [Google Scholar] [CrossRef] [Green Version]
- de Assis, L.V.M.; Moraes, M.N.; Castrucci, A.M.D.L. The molecular clock in the skin, its functionality, and how it is disrupted in cutaneous melanoma: A new pharmacological target? Cell. Mol. Life Sci. 2019, 76, 3801–3826. [Google Scholar] [CrossRef] [PubMed]
- Cheville, N.F. Ultrastructural Pathology: The Comparative Cellular Basis of Disease; John Wiley & Sons: Hoboken, NJ, USA, 2009; p. 997. [Google Scholar]
- Pearson, R.D. Evolutionary theory, regeneration and cancer. Med. Hypotheses 1986, 19, 7–14. [Google Scholar] [CrossRef]
- Baba, A.I.; Câtoi, C. Comparative Oncology; The Publishing House of the Romanian Academy: Bucharest, Romania, 2007. [Google Scholar]
- Rana, V.; Sharma, S.; Kamala, R.; Nair, D.; Ragavendra, T.R.; Mhatre, S.; Sabharwal, R.; Choudhury, B.K. Round cell tumors: Classification and immunohistochemistry. Indian J. Med. Paediatr. Oncol. 2017, 38, 349–353. [Google Scholar] [CrossRef]
- Thakuri, P.S.; Liu, C.; Luker, G.D.; Tavana, H. Biomaterials-Based Approaches to Tumor Spheroid and Organoid Modeling. Adv. Heal. Mater. 2017, 7, e1700980. [Google Scholar] [CrossRef]
- Piérard, G.E. Cell Proliferation in Cutaneous Malignant Melanoma: Relationship with Neoplastic Progression. ISRN Dermatol. 2012, 2012, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Vazquez, F.; Lim, J.-H.; Chim, H.; Bhalla, K.; Girnun, G.; Pierce, K.; Clish, C.B.; Granter, S.R.; Widlund, H.R.; Spiegelman, B.M.; et al. PGC1α expression defines a subset of human melanoma tumors with increased mitochondrial capacity and resistance to oxidative stress. Cancer Cell 2013, 23, 287–301. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, P.; Fovez, Q.; Germain, N.; Khamari, R.; Kluza, J. Mitochondrial spare respiratory capacity: Mechanisms, regulation, and significance in non-transformed and cancer cells. FASEB J. 2020, 34, 13106–13124. [Google Scholar] [CrossRef]
- Pfleger, J.; He, M.; Abdellatif, M. Mitochondrial complex II is a source of the reserve respiratory capacity that is regulated by metabolic sensors and promotes cell survival. Cell Death Dis. 2015, 6, e1835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, C.K.; George, J.; Chhabra, G.; Nihal, M.; Chang, H.; Ahmad, N. Genetic Manipulation of Sirtuin 3 Causes Alterations of Key Metabolic Regulators in Melanoma. Front. Oncol. 2021, 11, 676077. [Google Scholar] [CrossRef] [PubMed]
- Nahhas, A.; Abdel-Malek, Z.A.; Kohli, I.; Braunberger, T.L.; Lim, H.W.; Hamzavi, I.H. The potential role of antioxidants in mitigating skin hyperpigmentation resulting from ultraviolet and visible light-induced oxidative stress. Photodermatol. Photoimmunol. Photomed. 2018, 35, 420–428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jansen, R.; Wang, S.Q.; Burnett, M.; Osterwalder, U.; Lim, H.W. Photoprotection. J. Am. Acad. Dermatol. 2013, 69, 853.e1–853.e12. [Google Scholar] [CrossRef]
- Colin-Gonzalez, A.; Aguilera, G.; Serratos, I.; Escribano, B.; Santamaria, A.; Tunez, I. On the Relationship Between the Light/Dark Cycle, Melatonin and Oxidative Stress. Curr. Pharm. Des. 2015, 21, 3477–3488. [Google Scholar] [CrossRef]
- Rusanova, I.; Martínez-Ruiz, L.; Florido, J.; Rodríguez-Santana, C.; Guerra-Librero, A.; Acuña-Castroviejo, D.; Escames, G. Protective Effects of Melatonin on the Skin: Future Perspectives. Int. J. Mol. Sci. 2019, 20, 4948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Potez, M.; Trappetti, V.; Bouchet, A.; Fernandez-Palomo, C.; Güç, E.; Kilarski, W.W.; Hlushchuk, R.; Laissue, J.; Djonov, V. Characterization of a B16-F10 melanoma model locally implanted into the ear pinnae of C57BL/6 mice. PLoS ONE 2018, 13, e0206693. [Google Scholar] [CrossRef]
- Lambert, M.W.; Maddukuri, S.; Karanfilian, K.M.; Elias, M.L.; Lambert, W.C. The physiology of melanin deposition in health and disease. Clin. Dermatol. 2019, 37, 402–417. [Google Scholar] [CrossRef]
- Galván, I.; Solano, F. The evolution of eu- and pheomelanic traits may respond to an economy of pigments related to environmental oxidative stress. Pigment Cell Melanoma Res. 2009, 22, 339–342. [Google Scholar] [CrossRef] [PubMed]
- Kalegari, P.; Leme, D.M.; Disner, G.R.; Cestari, M.M.; Bellan, D.L.; Meira, W.V.; Mazepa, E.; Martinez, G.R. High Melanin Content in Melanoma Cells Contributes to Enhanced DNA Damage after Rose Bengal Photosensitization. Photochem. Photobiol. 2022, 98, 1355–1364. [Google Scholar] [CrossRef]
- Kim, Y.J. Hyperin and Quercetin Modulate Oxidative Stress-Induced Melanogenesis. Biol. Pharm. Bull. 2012, 35, 2023–2027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakagawa, H.; Hori, Y.; Sato, S.; Fizpatrick, T.B.; Martuza, R.L. The Nature and Origin of the Melanin Macroglobule. J. Investig. Dermatol. 1984, 83, 134–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pettinato, G.; Manivel, J.C.; D’Amore, E.S.; Jaszcz, W.; Gorlin, R.J. Melanotic neuroectodermal tumor of infancy. A reexamination of a histogenetic problem based on immunohistochemical, flow cytometric, and ultrastructural study of 10 cases. Am. J. Surg. Pathol. 1991, 15, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Bleehen, S.S. Ultrastructural studies on tumours and cell cultures of the Harding—Passey mouse melanoma*. Br. J. Dermatol. 1974, 90, 637–648. [Google Scholar] [CrossRef]
- Lazova, R.; Pawelek, J.M. Why do melanomas get so dark? Exp. Dermatol. 2009, 18, 934–938. [Google Scholar] [CrossRef]
- Horikoshi, T.; Jimbow, K.; Sugiyama, S. Comparison of macromelanosomes and autophagic giant melanosome complexes in nevocellular nevi, lentigo simplex and malignant melanoma. J. Cutan. Pathol. 1982, 9, 329–339. [Google Scholar] [CrossRef]
- Lazova, R.; Klump, V.; Pawelek, J. Autophagy in cutaneous malignant melanoma. J. Cutan. Pathol. 2010, 37, 256–268. [Google Scholar] [CrossRef]
- Pawelek, J.M. Cancer cell fusion with migratory bone marrow-derived cells as an explanation for metastasis: New therapeutic paradigms. Futur. Oncol. 2008, 4, 449–452. [Google Scholar] [CrossRef]
- Checinska, A.; Soengas, M.S. The gluttonous side of malignant melanoma: Basic and clinical implications of macroautophagy. Pigment. Cell Melanoma Res. 2011, 24, 1116–1132. [Google Scholar] [CrossRef] [PubMed]
- Decree of the Chief State Sanitary Doctor of the Russian Federation dated January 28, 2021 No. 2 “On approval of sanitary rules and norms SanPiN 1.2.3685-21” Hygienic standards and requirements for ensuring the safety and (or) harmlessness of environmental factors for humans “. (Registered 01/29/2021 No. 62296).
- Treschalina, E.M.; Zhukova, O.S.; Gerasimova, G.K.; Andronova, N.G.; Garin, A.M. Methodical recommendations for the preclinical study of the antitumor activity of drugs. In Guidelines for Conducting Preclinical Studies of Drugs. Part One; Grif i K: Moscow, Russia, 2012; pp. 642–657. (In Russian) [Google Scholar]
- Burghoff, S.; Gong, X.; Viethen, C.; Jacoby, C.; Flögel, U.; Bongardt, S.; Schorr, A.; Hippe, A.; Homey, B.; Schrader, J. Growth and metastasis of B16-F10 melanoma cells is not critically dependent on host CD73 expression in mice. BMC Cancer 2014, 14, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Underwood, W.; Anthony, R.; Cartner, S.; Corey, D.; Grandin, T.; Greenacre, C.; Gwaltney-Brant, S.; McCrackin, M.A.; Meyer, R.; Miller, D. AVMA Guidelines for the Euthanasia of Animals; American Veterinary Medical Association: Schaumburg, IL, USA, 2013. [Google Scholar]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Areshidze, D.A.; Kozlova, M.A.; Chernikov, V.P.; Borisov, A.V.; Mischenko, D.V. Characteristic of Ultrastructure of Mice B16 Melanoma Cells under the Influence of Different Lighting Regimes. Clocks & Sleep 2022, 4, 745-760. https://doi.org/10.3390/clockssleep4040056
Areshidze DA, Kozlova MA, Chernikov VP, Borisov AV, Mischenko DV. Characteristic of Ultrastructure of Mice B16 Melanoma Cells under the Influence of Different Lighting Regimes. Clocks & Sleep. 2022; 4(4):745-760. https://doi.org/10.3390/clockssleep4040056
Chicago/Turabian StyleAreshidze, D. A., M. A. Kozlova, V. P. Chernikov, A. V. Borisov, and D. V. Mischenko. 2022. "Characteristic of Ultrastructure of Mice B16 Melanoma Cells under the Influence of Different Lighting Regimes" Clocks & Sleep 4, no. 4: 745-760. https://doi.org/10.3390/clockssleep4040056
APA StyleAreshidze, D. A., Kozlova, M. A., Chernikov, V. P., Borisov, A. V., & Mischenko, D. V. (2022). Characteristic of Ultrastructure of Mice B16 Melanoma Cells under the Influence of Different Lighting Regimes. Clocks & Sleep, 4(4), 745-760. https://doi.org/10.3390/clockssleep4040056






