Objective Measures of Immediate “Energizing” Effect of Light: Studies Review and Data Analysis
Abstract
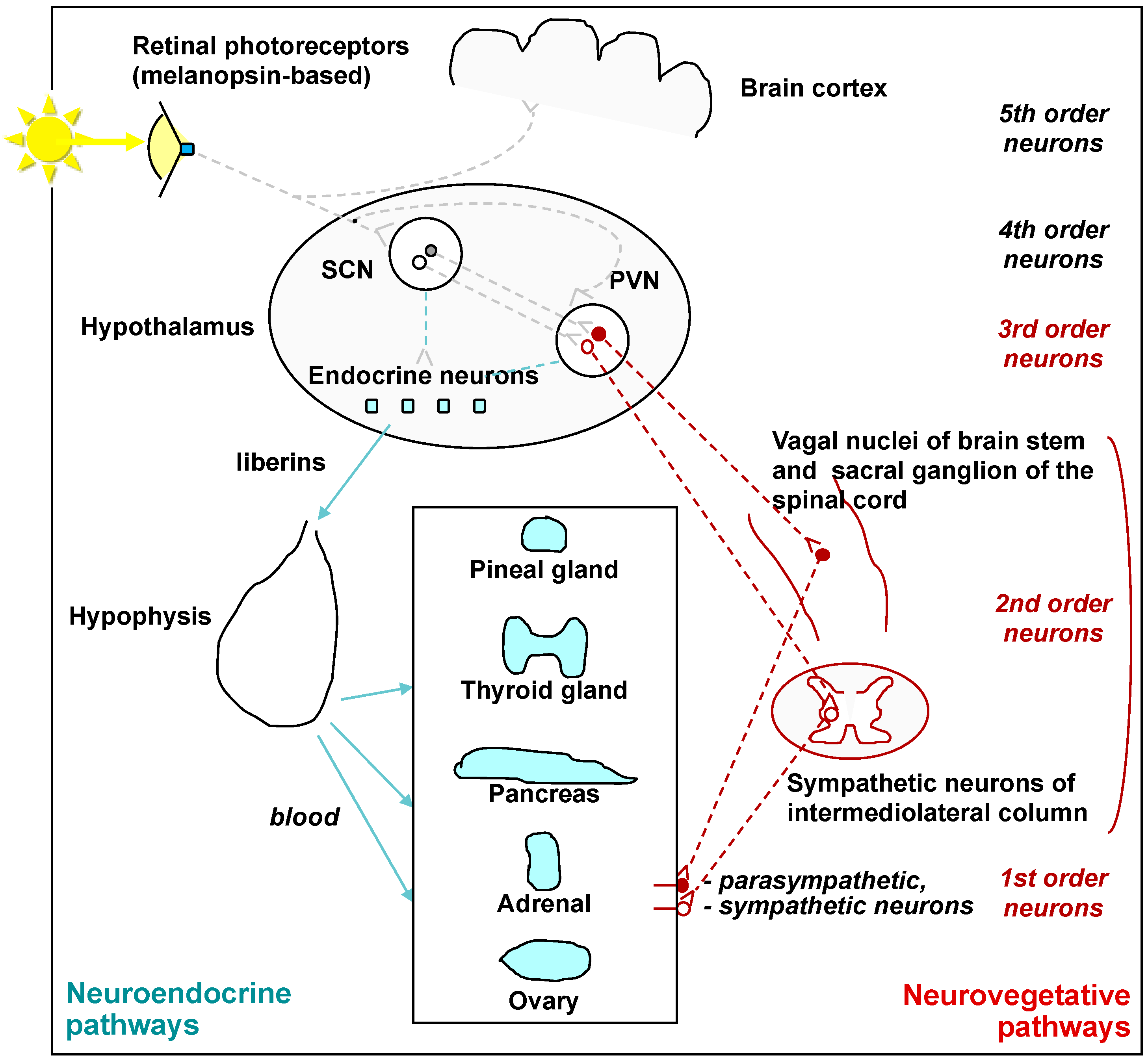
1. Introduction
- Total energy expenditure/metabolism (by oxygen consumption);
- Autonomic nervous system (ANS) activity:
- −
- Sympathetic nerve activity (by microneurography);
- −
- Galvanic skin conductance;
- −
- Skin blood flow/perfusion;
- −
- Salivary alpha-amylase;
- −
- (Nor)adrenaline concentration;
- −
- Blood pressure;
- −
- Heart rate, heart rate variability;
- −
- Pupillary light response;
- Thermogenesis (regulated in part by ANS; body temperature);
- Endocrine system activity (cortisol and thyroid hormone concentrations).
2. Methods
3. Studies Review
| N | Study | Subjects, Design | Light, Posture, Data Acquisition | Effect | |||
|---|---|---|---|---|---|---|---|
| HR | HRV | Body T | Other | ||||
| 1. | Rechlin et al., 1995 [21] | 18 (13 f, 5 m), within | 1.5 h 2500 lx vs. <200 lx 6:00–7:30; supine; ECG before/after | = HR | ↑ HF = VLF, LF | ||
| 2. | Saito et al., 1996 [15] | 5 males, within | 20 min 5000 lx vs. 500 lx (20 min pre and post 500 lx), between 10:00–12:00, BP continuously using a tonometric system | ↑ HR | ↑ sympathetic nerve activity = BP | ||
| 3. | Leproult et al., 1997 [39] | 17 males, within NCB | 3 h 5000 lx (vs. light < 300 lx 2 wk prior), between 22:00–08:00 (individually); supine and awake for 37–39 h; pill-ingested telemetric T°, blood taken every 20 min | = digestive T | = Cor = TSH | ||
| 4. | Ishimoto et al., 1998 [40] | 4 males, within | 3 h 2500 lx vs. 200 lx 10:00–13:00; sitting; esophageal, tympanic, and forehead skin T | = HR | = core T = forehead T | ||
| 5. | Scheer et al., 1999, 2004 [28,29] | 17 (6 f, 11 m): 10 males: both—alternating | 0 lx 20 min → indoor light 20 min, 5 times over 24 h, at daytime; supine 0 lx 20 min → 100 lx 10 min → 800 lx 10 min, 5 times over 39 h, including night (during middle and end of sleep); supine | ↑ HR ↑ HR at night | = SDNN = SDNN | ||
| 6. | Sakabibara et al., 2000 [41] | 12 females, within | 1 h 5000 lx vs. dim 18:00–19:00, two successive days; 5 min measurement at 19:00 only | ↑ LF = HF, LF/HF, HRV | |||
| 7. | Kim, Tokura, 2000 [42] | 5 aged females, within | 5 h 3000 lx vs. 50 lx 9:30–14:30; sitting; HR and BP using electronic sphygmomanometry | = HR | ↓ ReT at 13:50–14:30 | = BP ** | |
| 8. | Leproult et al., 2001 [43] | 8 males, within | 3 h 2000–4500 lx vs. dim < 150 lx another day -at night/morning 5:00–8:00 or -in the afternoon 13:00–16:00 supine and awake for 24 h each of the 3 days; blood (plasma) taken every 15 min | = HR = HR | ↑ Cor, = TSH = Cor, TSH | ||
| 9. | Burgess et al., 2001 [44] | 16 (8 f, 8 m), within NCB | 4.5 h 3000 lx vs. <10 lx day prior, starting at ~19:00—2 h before the estimated time of melatonin secretion onset (pre-pulse < 10 lx); supine; 2 skin thermistors on soles; BP using ‘standard’ sphygmomanometry (3 readings at each 30 min time point); measurements starting 0.5 h after the pulse onset | = HR * | = %HF | ↑ ReT in 3 h ↓ peripheral skin T | ↑ sBP in 3.5 h = dBP (n = 12) |
| 10. | Lavoie et al., 2003 [45] | 14 (8 f, 6 m), within | 4 h white 3000 lx vs. red < 15 lx 00:30–04:30; sitting; urine taken every ~2 h | ↑ ReT in 2.5 h | = Cor | ||
| 11. | Cajochen et al., 2005 [46] | 9 males, within | 2 h bright blue 460 nm vs. bright green 550 nm vs. dark, 21:00–23:00 (2 h pre-pulse 0 lx); supine; 8-site skin T using thermocouples; 20 min binned values | ↑ HR in blue light in 1.5 h | ↑ ReT in blue light in 1 h ↓ DPG at either light in 1 h | ||
| 12. | Rüger et al., 2006 [3] | Two studies 12 males: 12 males: each—within | 4 h 5000 lx vs. <10 lx (pre- and post-pulse < 10 lx) –at 0:00–4:00 or –at 12:00–16:00 sitting; saliva sampled every 30 min | ↑ HR = HR | ↑ ReT = ReT | = Cor = Cor | |
| 13. | Yokoi et al., 2006 [47] | 8 males, within | 7.4 h 2800 lx vs. 120 lx 21:10–4:30 (pre-pulse 120 lx from 19:30); supine; measurements from 21:10; ReT—n = 7, BP using photoplethysmography (n = 6) | ↑ HR | = %LF, %HF, LF/HF | ↑ ReT in 3 h | ↑ sBP in 4 h = dBP |
| 14. | Schäfer, Kratky, 2006 [48] | 12 subjects, within | 10 min 700 lx blue vs. green vs. red (15 min pre and post: 0 lx), after 21:00 on separate(?) days in winter | ↓ very LF in blue | |||
| 15. | Ishibashi et al., 2007 [49] | 7 males, within | 6.5 h 1000 lx, 3000 K vs. 5000 K vs. 6700 K 19:30–02:00 (1.5 h pre-pulse 10 lx); supine | = HR | = HF ↓ fractal-free HF at 6700 K | ||
| 16. | An et al., 2009 [50] | 12 males, within | 20 min 67 lx, blue 458 nm vs. green 550 nm, sequentially (for 35 min), in the evening (before sleep) vs. 12 h apart, baseline 19 lx → 11 min pre-pulse < 1 lx; oral temperature before/after, HR and BP using photoplethysmography | = HR | ↑ HF/(HF + LF) by blue vs. green at daytime | ↑ oral T in blue vs. green in daytime and blue in evening | = BP ** |
| 17. | Laufer et al., 2009 [51] | 12 adults 66–84 y, within | 8 min 300 lx, blue vs. red (6 min pre-pulse 0 lx) sequentially between 13:00–17:00, reverse sequence on another day; sitting | ↑ HF, ↓ LF/HF at blue | |||
| 18. | Ishibashi et al., 2010 [52] | 10 males, within | 2.5 h 5000 lx vs. 30 lx 21:30–24:00 (0.5 h pre-pulse 650 lx); semi-supine; 7-site skin T° by thermistors, BP using electronic sphygmomanometry once at 21:15 and at 23:45, oxyspirography at 23:55 (while moving) | = HR | ↓ LF = HF, LF/(LF + HF) | = ReT, DPG ↑ proximal and distal skin T in the last 30 min | = OC = BP |
| 19. | Choi et al., 2011 [22] | 55 subjects, within | 5 min white 49.5 lx vs. blue 0.04 lx vs. red 0.4 lx (10 min pre- and post- 0 lx), each of 3 in any of 4 time slots between 09:00–11:00 and 14:00–16:00 for two successive days; sitting; 5 min ECG before and after each pulse | = HR | ↑ LF/HF in white and red vs. blue = RMSSD, HF | ||
| 20. | Litscher et al., 2013 [53] | 7 (5 f, 2 m), within | 10 min 140 lx, blue 461 nm vs. red 621 nm between 9:00–11:00 on a single day; lying; 2-site skin T using infrared camera | ↓ HR | ↓ SDNN * = LF/HF | = forehead T * ↓ nose T * | |
| 21. | Smolders et al., 2012 [54] | 32 (13 f, 19 m), within ‘mixed’ | 1 h 650 lx vs. 230 lx (0.5 h pre-pulse 120 lx) at 9:30, 11:30, 13:30, and/or 15:30 on separate days (each subject participated in at least 2 of the 4 arms) | ↑ HR | = LF, HF ↑ LF/HF at 45 min | ||
| 22. | Smolders, de Kort, 2014 [55] | 28 (16 f, 12 m), within | 30 min 1000 lx vs. 200 lx (45 min pre-pulse 92 lx) on separate days in the same timeslot between 9:00–18:00; sitting | = HR | = LF/HF | ↑ SC | |
| 23. | Huiberts et al., 2016 [56] | 39 (28 f, 11 m), within/ between | 1 h 1700 lx vs. 600 lx vs. 165 lx (0.5 h pre-pulse 120 lx) –at 09:00 (n = 18) or –at 15:45 (n = 21) sitting; ECG (n = 34), two electrodermal electrodes on hand (n = ?), BP using photoplethysmography (n = 35) | = HR ↑ HR 1700 lx | = SC, sBP = SC, sBP | ||
| 24. | Yuda et al., 2016 [57] | 10 (1 f, 9 m), within | 6 min blue 10 lx vs. green 71 lx vs. red 39 lx (3 min pre- and post- 0 lx) between 08:30–13:00 on a single day; supine | = HR | ↓ HF with blue = LF, LF/HF | ||
| 25. | Canazei et al., 2017 [58] | 31 (18 f, 13 m), within | 4 h 150 lx, 4667 K vs. 3366 K vs. 2166 K color T°, 0:00–4:00; sitting | ↑ HR * for 2.5 h at 4667 K | = SDNN, LF ↓ RMSSD *, HF * ↑ LF/HF * at 4667 K | ||
| 26. | Ivanova et al., 2017 [59] | 10 females, within | 30 min white 4300 lx vs. red 250 lx between 9:00–10:00 after coming into the lab, 25 min pre and post < 100 lx; sitting; 5 min pulsemetry + oxyspirography, blood (serum)—3 times | = HR | = OC = Cor = alpha-amylase | ||
| 27. | Te Kulve et al., 2017 [60] | 19 females, within | 4.5 h 1200 lx vs. 5 lx, 08:30–13:00 (0.5 h pre-pulse 250 lx); every 1.5 h free moving 15′ + supine 75′; pill-ingested T°, 26-site skin T°; oxyspirography, laser doppler flowmetry—continuously, while supine; BP using electronic sphygmomanometry—3 times; blood (plasma) taken at pre-pulse and every 1.5 h | = HR | ↓ digestive T° ↓ proximal skin T° = distal skin T° ↑ DPG | = OC, SBF, BP = dopamine = noradrenaline ↓ adrenaline ↓ Cor * | |
| 28. | Te Kulve et al., 2018 [61] | 16 females, within | 4.5 h 55 lx 6500 K vs. 55 lx 2700 K, 08:30–13:00 (pre-pulse 5 lx); the remaining is the same as in the cell above | = HR | = digestive T° * = skin T°, DPG | = OC, SBF, BP = Cor | |
| 29. | Te Kulve et al., 2019 [62] | 12 females, within | 1 h 750 lx vs. 5 lx 22:30–23:30 (pre- and post-pulse for 1 h); sitting; telemetric pill-ingested T°, 26-site skin T°, oxyspirography, laser doppler flowmetry | = HR | = digestive T° = DPG | = OC = SBF | |
| 30. | Smolders et al., 2018 [63] | 60 (41 f, 19 m), within/between | 1 h pulse of 20 various intensities between 20–2000 lux (0.5 h pre-pulse 100 lx) at 9:00 or 11:00 vs. 13:00 or 15:00 for two separate days; sitting; 2 electrodermal electrodes on hand | = HR | = skin conductance | ||
| 31. | Lok et al., 2019 [64] | 10 (5 f, 5 m), within | 1.5 h 2000 lx vs. 10 lx 14:30–16:00 (2.5 h pre-pulse 10 lx); sitting(?); under-tongue telemetric T pill, 10-site skin T | = HR | = under-tongue T ↑ hand skin T, DPG | ||
| 32. | Prayag et al., 2019 [27] | 28 males, within | 50 min pulse: blue-enriched 298 melanopic lx vs. red 80 mlx for the 1st min adding more centric vs. less centric white light for the next 49 min: 494 mlx vs. 276 mlx vs. 620 mlx vs. 402 mlx; 10 min pre-pulse < 5 lx = four 1 h arms between 19:00–23:00 (18:00–19:—<5 lx); sitting; ECG, 2-site skin T; analysis during min −1, 1, 2, 5, and 42 | ↑ HR for 4 lights altogether at min 2, 5, 42 | = LF, HF, LF/HF; for between-light effects see paper | ↑ DPG for 4 lights altogether at 5 and 42 min | |
| 33. | Kompier et al., 2020 [65] | 38 (19 f, 19 m), within | 45 min cool 1000 lx vs. warm 100 lx (45 min pre-pulse either cool or warm) = 4 arms on separate days during the same daytime slots; sitting; four 5 min measurements (−13, 2, 17, and 32 min) analyzed. 14-site skin T, 2 electrodermal electrodes on hand | = HR | = ‘mean’ HRV | = skin T° = DRG | = skin conductance |
| 34. | Kompier et al., 2021 [66] | 23 (13 f, 10 m), within | 45 min cool 1000 lx vs. cool 100 lx vs. warm 1000 lx vs. warm 100 lx (45 min pre-pulse warm 100 lx); the remaining is the same as in the cell above | = HR | = ‘mean’ HRV | = skin T° = DPG | = skin conductance |
| 35. | Schmid et al., 2021 [67] | 33 males, within | 1.5 h light pulse melanopic 287 mW 8300 K vs. 114 mW 3000 K vs. 0.4 mW 2200 K 21:45–23:15 (pre- and post-pulse < 5 lx); sitting, allowed to move; 4-site skin T (n = 30), salivary cortisol at min 0 and 90 of light pulse | = DPG | = Cor | ||
| 36. | Lok et al., 2022, 2022 [30,31] | 8 males, within, between | 13 h lights during three 18 h forced desynchrony days 1300 lx and 6 lx; sitting/moving; pill-ingested telemetric T (n = 8 subjects, crossover), 10-site skin T (n = 4 and n = 4, parallel groups), Cor in saliva sampled hourly (n = 7, crossover) | = digestive T° ↑ proximal skin T ↓ distal skin T | = Cor | ||
| N | Study | Subjects, Design | Light, Posture, Data Acquisition | Effect |
|---|---|---|---|---|
| 1. | Dijk et al., 1991 [68] | 7 males: 8 (3 f, 5 m): both—within | 3 h 2500 lx vs. 6 lx 21:00–24:00; sitting from 19:30 3 h 2500 lx vs. <1 lx (goggles) 20:30–23:30; sitting(?) pre-pulse(?) | ↑ ReT ↑ ReT |
| 2. | Badia et al., 1991 [32] | 16, alternating; 8, alternating; 19, between | bright 5000 lx vs. dim 50 lx 1.5 h bright /1.5 h dim × 3 blocks 0:00–9:00 1.5 h bright /1.5 h dim × 2 blocks 13:00–19:00 9 h bright (n = 10) vs. dim (n = 9) 21:45–7:45 sitting; tympanic T every 30 min | ↑ T (except 0:00–1:30) = tympanic T ↑ tympanic T |
| 3. | Myers, Badia, 1993 [69] | 15 males, within | 2 h 5000 vs. 1000 vs. 500 vs. 50 lx 21:00–23:00 (pre-pulse 50(?) lx); sitting; tympanic T every 15 min from 19:00 | ↑ tympanic T (in ~1 h; 5000 = 1000 = 500 > 50 lx) |
| 4. | Kim, Tokura, 1995 [70] | 7 females, within | 8 h 4000 lx vs. 10 lx 10:00–18:00 (light before 10:00 not controlled); sitting(?); measurements starting at 10:00 | = ReT |
| 5. | Morita, Tokura, 1996 [71] | 5 males, within | 5 h 1000 lx 6500 K vs. 1000 lx 3000 K vs. 50 lx dim 21:00–2:00 (2 h pre-pulse 50 lx); sitting | ↑ ReT (each bright vs. dim) |
| 6. | Morita et al., 1997 [72] | 4 males, within | 5 h blue 435 nm vs. green 545 nm vs. red 610 nm, each at 2500 lx vs. 1000 lx vs. 50 lx incandescent light 4:00–9:00 (pre-pulse sleep 22:00–04:00), 7 arms in total; posture(?) | ↑ ReT at green 2500 lx |
| 7. | Kräuchi et al., 1997 [73] | 9 males, within | 3 h 5000 lx vs. <10 lx 21:00–24:00 (7 h pre-pulse < 10 lx); supine | ↑ ReT (to the end of the pulse) |
| 8. | Aizawa, Tokura, 1997 [74] | 9 (7 f, 2 m), within | 8.5 h 4000 lx vs. 100 lx 9:30–18:00, pre-pulse light not controlled; posture(?); tympanic T every min | ↓ tympanic T 16:45–18:00 |
| 9. | Aizawa, Tokura, 1998 [75] | 5 females, within | 4 h 5500 lx vs. 150 lx 9:00–13:00 (post 150 lx until 16:00); posture(?); tympanic T 10:00–16:00 | ↓ tympanic T 11:00-onwards |
| 10. | Foret et al., 1998 [76] | 8 males, within | 4 h 700–1000 lx vs. 50 lx, 20:00–24:00, (pre-pulse light—“from windows”); sitting | = ReT * |
| 11. | Park, Tokura, 1998 [77] | 8 females, within | 13 h pulse 5000 lx vs. 200 lx 6:30–19:30 (until 06:00—sleep); sitting | = ReT |
| 12. | Zhang, Tokura, 1999 [78] | 9 females, within | 6 h 5000 lx vs. 50 lx, 6:00–12:00 30 (until 06:00—sleep); sitting | = ReT |
| 13. | Cajochen et al., 2000 [79] | 13 (at least 12 are males), between | 6.5 h ~3190 lx (n = 7) or ~23 lx (n = 6) at night (centered 3.5 h before the expected ReT minimum; pre-pulse 3 lx); supine | ↑ ReT (in 1.5 h) |
| 14. | Rüger et al., 2003 [80] | 7 males, within | 4 h 5000 lx vs. <10 lx, 0:00–4:00 (17 h pre-pulse 10 lx); sitting | ↑ ReT |
| 15. | Sato et al., 2005 [81] | 9 (4 f, 5 m), within | 2 h 2500 lx 6480 K vs. 2500 lx 3150 K vs. <50 lx in the morning (2 h after ReT minimum) after sleep; posture(?) | ↑ ReT (at 6480 K vs. both 3150 K and dim) |
| 16. | Lok et al., 2018 [35] | 50 (25 f, 25 m), between and within (alternating) | 1 h pulse of 24 or 74 or 222 or 666 or 2000 lx (n = 10 in each group) at 9:00, 11:30, 14:00, and 16:30 (1.5 h pre-pulse < 10 lx); sitting; 6-site skin T | ↑ DPG |
| N | Study | Subjects, Design | Light, Posture, Data Acquisition | Effect |
|---|---|---|---|---|
| 1. | Petterborg et al., 1991 [82] | 13 (7 f, 6 m), within | 15 min 1500 lx vs. <200 lx at 22:00 (n = 5) or 23:00 (n = 8) (pre and post < 200 lx); sitting; blood (serum) taken at different times between 20:00–24:00 or 20:00–08:00 | = Cor |
| 2. | McIntyre et al., 1992 [83] | 6 (2 f, 4 m), within | 3 h 600 lx vs. <10 lx at 0:00–3:00 (2 h pre-pulse 10 lx); sitting; blood (plasma) taken hourly 23:00–05:00 | = Cor |
| 3. | Kostoglou-Athanassiou et al., 1998 [84] | 10 males, within | 6 h 5500 lx vs. 500 lx at 20:00–02:00 (pre-pulse light not controlled); blood (serum) taken at 16:00 and every 2 h from 20:00 | ↓ Cor * |
| 4. | Scheer, Buijs, 1999 [85] | 14 males, within NCB | 1 h 800 lx vs. 0 lx the day prior –at ~07:00 (after awakening) –at ~23:00 (before sleep; pre-pulse(?) lx; n = 12) supine; saliva taken every 20 min during pulse | ↑ Cor at 20, 40 min = Cor |
| 5. | Lockley et al., 2006 [86] | 13 subjects, between | 6.5 h blue (460 nm, n = 6) or red (555 nm, n = 7) light of equal photon density starting at ~23:00 h, ~72 h pre-pulse < 2 lx; supine; pupils dilated; blood (plasma) taken every 20–30 min, Cor values expressed as a percentage of the values at corresponding clock times on the previous day | = Cor |
| 6. | Jung et al., 2010 [87] | 20 (5 f, 15 m) 8 subjects: 5 subjects: 7 subjects: within NCB, between | 6.7 h 10,000 lx (vs. 3 lx the day prior) –from ~02:00 onwards; –from ~08:00 onwards; –dim 3 lx both days; ~48 h pre-pulse 3 lx; sitting; blood (plasma) taken every 30 min | ↓ Cor in ~2.5 h ↓ Cor in ~0.5 h (= Cor) |
| 7. | Figueiro, Rea, 2010 [33] | 12 (8 m, 4 f), within, alternating | 1 h blue 40 lx vs. red 40 lx vs. <3 lx, each—on separate days every 4 h for 7 times over 27 h, <3 lx the rest of the day; 2 h sitting (before and during pulse); saliva sampled at the beginning and at the end of each pulse | = Cor = alpha-amylase |
| 8. | Sahin et al., 2014 [34] | 13 (7 f, 6 m), within, alternating | 2 h white 361 lx vs. red 213 lx vs. dim < 5 lx, each—on separate days at 07:00, 11:00, and 15:00, <5 lx the rest of the day; saliva sampled at pulse hours 0, 1, and 2 | = Cor = alpha-amylase |
| 9. | Danilenko, Sergeeva, 2015 [88] | 16 females, within | 45 min cool white 1300 lx vs. red 1100 lx in the morning after coming to lab in dark glasses; pre-pulse sitting for 5 min; blood (serum) taken at pulse min 0, 22, and 44 | = Cor |
| 10. | Petrowsky et al., 2019 [89] | Two studies 30 males: 23 males: within | 1 h 05:00–06:00 (right after awakening) –bright 414 lx vs. dim < 2 lx –blue 201 lx vs. green 806 lx vs. red 235 lx equal photon density saliva every 15 min 7 times | ↑ Cor at 45–90 min ↑ Cor by blue and green at 60–75 min |
3.1. Oxygen Consumption
3.2. Sympathetic Nerve Activity
3.3. Skin Conductance
3.4. Skin Blood Flow
3.5. Noradrenaline
3.6. Blood Pressure
3.7. Heart Rate
3.8. Heart Rate Variability
3.9. Body Temperature
3.10. Alpha-Amylase
3.11. Cortisol
3.12. Thyroid-Stimulating Hormone
4. Data Synthesis and Analysis
4.1. Data Synthesis and Description
4.2. Statistical Analysis
4.3. Measures Rating
5. Discussion
6. Conclusions
Supplementary Materials
Funding
Acknowledgments
Conflicts of Interest
References
- Lewy, A.J.; Sack, R.L. Light therapy and psychiatry. Proc. Soc. Exp. Biol. Med. 1986, 183, 11–18. [Google Scholar] [CrossRef]
- Danilenko, K.V.; Lebedinskaia, M.Y.; Gadetskaia, E.V.; Markov, A.A.; Ivanova, Y.A.; Aftanas, L.I. A 6-day combined wake and light therapy trial for unipolar depression. J. Affect. Disord. 2019, 259, 355–361. [Google Scholar] [CrossRef]
- Rüger, M.; Gordijn, M.C.M.; Beersma, D.G.M.; de Vries, B. Time-of-day-dependent effects of bright light exposure on human psychophysiology: Comparison of daytime and nighttime exposure. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 290, 1413–1420. [Google Scholar] [CrossRef]
- Lok, R.; Smolders, K.C.H.J.; Beersma, D.G.M.; de Kort, Y.A.W. Light, alertness, and alerting effects of white light: A literature overview. J. Biol. Rhythms 2018, 33, 589–601. [Google Scholar] [CrossRef]
- Pinchasov, B.B.; Shurgaja, A.M.; Grischin, O.V.; Putilov, A.A. Mood and energy regulation in seasonal and non-seasonal depression before and after midday treatment with physical exercise or bright light. Psychiatry Res. 2000, 94, 29–42. [Google Scholar] [CrossRef]
- Putilov, A.A.; Danilenko, K.V. Sympatho-adrenal and energy-regulating systems in winter depression. Photodermatol. Photoimmunol. Photomed. 1998, 29, 367–386. [Google Scholar]
- LeGates, T.A.; Fernandez, D.C.; Hattar, S. Light as a central modulator of circadian rhythms, sleep and affect. Nat. Rev. Neurosci. 2014, 15, 443–454. [Google Scholar] [CrossRef]
- Daneault, V.; Dumont, M.; Massé, É.; Vandewalle, G.; Carrier, J. Light-sensitive brain pathways and aging. J. Physiol. Anthropol. 2016, 35, 9. [Google Scholar] [CrossRef]
- Fleury, G.; Masís-Vargas, A.; Kalsbeek, A. Metabolic implications of exposure to light at night: Lessons from animal and human studies. Obesity 2020, 28, S18–S28. [Google Scholar] [CrossRef]
- Gerendai, I.; Kocsis, K.; Halász, B. Supraspinal connections of the ovary: Structural and functional aspects. Microsc. Res. Tech. 2002, 59, 474–483. [Google Scholar] [CrossRef]
- Gerendai, I.; Tóth, I.E.; Boldogkői, Z.; Halász, B. Recent findings on the organization of central nervous system structures involved in the innervation of endocrine glands and other organs; observations obtained by the transneuronal viral double-labeling technique. Endocrine 2009, 36, 179–188. [Google Scholar] [CrossRef]
- Kalsbeek, A.; Palm, I.F.; la Fleur, S.E.; Scheer, F.A.J.L.; Perreau-Lenz, S.; Ruiter, M.; Kreier, F.; Cailotto, C.; Buijs, R.M. SCN outputs and the hypothalamic balance of life. J. Biol. Rhythms 2006, 21, 458–469. [Google Scholar] [CrossRef]
- Oldham, M.A.; Ciraulo, D.A. Bright light therapy for depression: A review of its effects on chronobiology and the autonomic nervous system. Chronobiol. Int. 2014, 31, 305–319. [Google Scholar] [CrossRef]
- Danilenko, K.V. The role of Light Exposure in the Regulation of Daily, Monthly and Annual Cycles in Humans. Ph.D. Thesis, Institute of Physiology SB RAMS, Novosibirsk, Russia, 2009. [Google Scholar]
- Saito, Y.; Shimizu, T.; Takahashi, Y.; Mishima, K.; Takahashi, K.; Ogawa, Y.; Kogawa, S.; Hishikawa, Y. Effect of bright light exposure on muscle sympathetic nerve activity in human. Neurosci. Lett. 1996, 219, 135–137. [Google Scholar] [CrossRef]
- Bosch, J.A. The use of saliva markers in psychobiology: Mechanisms and methods. Monogr. Oral. Sci. 2014, 24, 99–108. [Google Scholar]
- Strahler, J.; Skoluda, N.; Kappert, M.B.; Nater, U.M. Simultaneous measurement of salivary cortisol and alpha-amylase: Application and recommendations. Neurosci. Biobehav. Rev. 2017, 83, 657–677. [Google Scholar] [CrossRef]
- Bosch, J.A.; Veerman, E.C.; de Geus, E.J.; Proctor, G.B. α-Amylase as a reliable and convenient measure of sympathetic activity: Don’t start salivating just yet! Psychoneuroendocrinology 2011, 36, 449–453. [Google Scholar] [CrossRef]
- Danilenko, K.V.; Kobelev, E.; Semenova, E.A.; Aftanas, L.I. Summer-winter difference in 24-h melatonin rhythms in subjects on a 5-workdays schedule in Siberia without daylight saving time transitions. Physiol. Behav. 2019, 212, 112686. [Google Scholar] [CrossRef]
- Stoica, E.; Enulescu, O. Catecholamine response to light in migraine. Cephalalgia 1988, 8, 31–36. [Google Scholar] [CrossRef]
- Rechlin, T.; Weis, M.; Schneider, K.; Zimmermann, U.; Kaschka, W.P. Does bright-light therapy influence autonomic heart-rate parameters? J. Affect. Disord. 1995, 34, 131–137. [Google Scholar] [CrossRef]
- Choi, C.J.; Kim, K.S.; Kim, C.M.; Kim, S.H.; Choi, W.S. Reactivity of heart rate variability after exposure to colored lights in healthy adults with symptoms of anxiety and depression. Int. J. Psychophysiol. 2011, 79, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Münch, M.; Wirz-Justice, A.; Brown, S.A.; Kantermann, T.; Martiny, K.; Stefani, O.; Vetter, C.; Wright, K.P., Jr.; Wulff, K.; Skene, D.J. The role of daylight for humans: Gaps in current knowledge. Clocks Sleep 2020, 2, 61–85. [Google Scholar] [CrossRef] [PubMed]
- Danilenko, K.V. Metabolic effects of light. Neuropsychobiology 2017, 74, 234. [Google Scholar]
- Vetter, C.; Pattison, P.M.; Houser, K.; Herf, M.; Phillips, A.J.; Wright, K.P.; Skene, D.J.; Brainard, G.C.; Boivin, D.B.; Glickman, G. A review of human physiological responses to light: Implications for the development of integrative lighting solutions. Leukos 2022, 18, 387–414. [Google Scholar] [CrossRef]
- Siraji, M.A.; Kalavally, V.; Schaefer, A.; Haque, S. Effects of daytime electric light exposure on human alertness and higher cognitive functions: A systematic review. Front. Psychol. 2022, 12, 765750. [Google Scholar] [CrossRef]
- Prayag, A.S.; Jost, S.; Avouac, P.; Dumortier, D.; Gronfier, C. Dynamics of non-visual responses in humans: As fast as lightning? Front. Neurosci. 2019, 13, 126. [Google Scholar] [CrossRef]
- Scheer, F.A.J.L.; van Doornen, L.J.P.; Buijs, R.M. Light and diurnal cycle affect human heart rate: Possible role for the circadian pacemaker. J. Biol. Rhythms 1999, 14, 202–212. [Google Scholar] [CrossRef]
- Scheer, F.A.; van Doornen, L.J.; Buijs, R.M. Light and diurnal cycle affect autonomic cardiac balance in human; possible role for the biological clock. Auton. Neurosci. 2004, 110, 44–48. [Google Scholar] [CrossRef]
- Lok, R.; Woelders, T.; van Koningsveld, M.J.; Oberman, K.; Fuhler, S.G.; Beersma, D.G.M.; Hut, R.A. Bright light increases alertness and not cortisol in healthy men: A forced desynchrony study under dim and bright light (I). J. Biol. Rhythms 2022, 37, 403–416. [Google Scholar] [CrossRef]
- Lok, R.; Woelders, T.; van Koningsveld, M.J.; Oberman, K.; Fuhler, S.G.; Beersma, D.G.M.; Hut, R.A. Bright light decreases peripheral skin temperature in healthy men: A forced desynchrony study under dim and bright light (II). J. Biol. Rhythms 2022, 37, 417–428. [Google Scholar] [CrossRef]
- Badia, P.; Myers, B.; Boecker, M.; Culpepper, J.; Harsh, J.R. Bright light effects on body temperature, alertness, EEG and behavior. Physiol. Behav. 1991, 50, 583–588. [Google Scholar] [CrossRef]
- Figueiro, M.G.; Rea, M.S. The effects of red and blue lights on circadian variations in cortisol, alpha amylase, and melatonin. Int. J. Endocrinol. 2010, 2010, 829351. [Google Scholar] [CrossRef] [PubMed]
- Sahin, L.; Wood, B.M.; Plitnick, B.; Figueiro, M.G. Daytime light exposure: Effects on biomarkers, measures of alertness, and performance. Behav. Brain. Res. 2014, 274, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Lok, R.; Woelders, T.; Gordijn, M.C.M.; Hut, R.A.; Beersma, D.G.M. White light during daytime does not improve alertness in well-rested individuals. J. Biol. Rhythms 2018, 33, 637–648. [Google Scholar] [CrossRef]
- Tsunoda, M.; Endo, T.; Hashimoto, S.; Honma, S.; Honma, K.-I. Effects of light and sleep stages on heart rate variability in humans. Psychiatry Clin. Neurosci. 2001, 55, 286. [Google Scholar] [CrossRef] [PubMed]
- Badia, P.; Culpepper, J.; Myers, B.; Boecker, M.; Harsh, J. Psychophysiological and behavioral effects of bright and dim light. Sleep Res. 1990, 19, 387. [Google Scholar]
- Beck-Friis, J.; Borg, G.; Wetterberg, L. Rebound increase of nocturnal serum melatonin levels following evening suppression by bright light exposure in healthy men: Relation to cortisol levels and morning exposure. Anna. N. Y. Acad. Sci. 1985, 453, 371–375. [Google Scholar] [CrossRef]
- Leproult, R.; van Reeth, O.; Byrne, M.M.; Sturis, J.; van Cauter, E. Sleepiness, performance, and neuroendocrine function during sleep deprivation: Effects of exposure to bright light or exercise. J. Biol. Rhythms 1997, 12, 245–258. [Google Scholar] [CrossRef]
- Ishimoto, A.; Kim, H.E.; Rutkowska, D.; Tanaka, S.; Tokura, H. Physiological significance of 3-h bright and dim light exposure prior to taking a bath for core and forehead skin temperatures and heart rate during 1-h bathing of 38.5 °C. J. Therm. Biol. 1998, 23, 353–357. [Google Scholar] [CrossRef]
- Sakakibara, S.; Honma, H.; Koshaka, M.; Fukuda, N.; Kawai, I.; Kobayashi, R.; Koyama, T. Autonomic nervous function after evening bright light therapy: Spectral analysis of heart rate variability. Psychiatry Clin. Neurosci. 2000, 54, 363–364. [Google Scholar] [CrossRef]
- Kim, H.E.; Tokura, H. Influence of light intensities on dressing behavior in elderly people. J. Physiol. Anthropol. Appl. Hum. Sci. 2000, 19, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Leproult, R.; Colecchia, E.F.; L’Hermite-Balériaux, M.; van Cauter, E. Transition from dim to bright light in the morning induces an immediate elevation of cortisol levels. J. Clin. Endocrinol. Metab. 2001, 86, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Burgess, H.J.; Sletten, T.; Savic, N.; Gilbert, S.S.; Dawson, D. Effects of bright light and melatonin on sleep propensity, temperature, and cardiac activity at night. J. Appl. Physiol. 2001, 91, 1214–1222. [Google Scholar] [CrossRef] [PubMed]
- Lavoie, S.; Paquet, J.; Selmaoui, B.; Rufiange, M.; Dumont, M. Vigilance levels during and after bright light exposure in the first half of the night. Chronobiol. Int. 2003, 20, 1019–1038. [Google Scholar] [CrossRef] [PubMed]
- Cajochen, C.; Münch, M.; Kobialka, S.; Kräuchi, K.; Steiner, R.; Oelhafen, P.; Orgül, S.; Wirz-Justice, A. High sensitivity of human melatonin, alertness, thermoregulation, and heart rate to short wavelength light. J. Clin. Endocrinol. Metab. 2005, 90, 1311–1316. [Google Scholar] [CrossRef] [PubMed]
- Yokoi, M.; Aoki, K.; Shimomura, Y.; Iwanaga, K.; Katsuura, T. Exposure to bright light modifies HRV responses to mental tasks during nocturnal sleep deprivation. J. Physiol. Anthropol. 2006, 25, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, A.; Kratky, K.W. The effect of colored illumination on heart rate variability. Forsch Komplementmed. 2006, 13, 167–173. [Google Scholar] [CrossRef]
- Ishibashi, K.; Kitamura, S.; Kozaki, T.; Yasukouchi, A. Inhibition of heart rate variability during sleep in humans by 6700 K presleep light exposure. J. Physiol. Anthropol. 2007, 26, 39–43. [Google Scholar] [CrossRef] [PubMed][Green Version]
- An, M.; Huang, J.; Shimomura, Y.; Katsuura, T. Time-of-day-dependent effects of monochromatic light exposure on human cognitive function. J. Physiol. Anthropol. 2009, 28, 217–223. [Google Scholar] [CrossRef]
- Laufer, L.; Lang, E.; Izso, L.; Nemeth, E. Psychophysiological effects of coloured lighting in older adults. Light. Res. Technol. 2009, 41, 371–378. [Google Scholar] [CrossRef]
- Ishibashi, K.; Arikura, S.; Kozaki, T.; Higuchi, S.; Yasukouchi, A. Thermoregulatory effect in humans of suppressed endogenous melatonin by pre-sleep bright-light exposure in a cold environment. Chronobiol. Int. 2010, 27, 782–806. [Google Scholar] [CrossRef] [PubMed]
- Litscher, D.; Wang, L.; Gaischek, I.; Litscher, G. The influence of new colored light stimulation methods on heart rate variability, temperature, and wellbeing: Results of a pilot study in humans. Evid. Based Complement. Alternat. Med. 2013, 2013, 674183. [Google Scholar] [CrossRef]
- Smolders, K.C.; de Kort, Y.A.; Cluitmans, P.J. A higher illuminance induces alertness even during office hours: Findings on subjective measures, task performance and heart rate measures. Physiol. Behav. 2012, 107, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Smolders, K.C.; de Kort, Y.A. Bright light and mental fatigue: Effects on alertness, vitality, performance and physiological arousal. J. Environ. Psychol. 2014, 39, 77–91. [Google Scholar] [CrossRef]
- Huiberts, L.M.; Smolders, K.C.; de Kort, Y.A. Non-image forming effects of illuminance level: Exploring parallel effects on physiological arousal and task performance. Physiol. Behav. 2016, 164, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Yuda, E.; Ogasawara, H.; Yoshida, Y.; Hayano, J. Suppression of vagal cardiac modulation by blue light in healthy subjects. J. Physiol. Anthropol. 2016, 35, 24. [Google Scholar] [CrossRef] [PubMed]
- Canazei, M.; Pohl, W.; Bliem, H.R.; Weiss, E.M. Acute effects of different light spectra on simulated night-shift work without circadian alignment. Chronobiol. Int. 2017, 34, 303–317. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, I.A.; Danilenko, K.V.; Aftanas, L.I. Investigation of an immediate effect of bright light on oxygen consumption, heart rate, cortisol, and α-amylase in seasonal affective disorder subjects and healthy controls. Neuropsychobiology 2017, 74, 219–225. [Google Scholar] [CrossRef]
- Kulve, M.T.; Schlangen, L.J.M.; Schellen, L.; Frijns, A.J.H.; van Lichtenbelt, W.D.M. The impact of morning light intensity and environmental temperature on body temperatures and alertness. Physiol. Behav. 2017, 175, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Kulve, M.T.; Schlangen, L.; Schellen, L.; Souman, J.L.; van Lichtenbelt, W.M. Correlated colour temperature of morning light influences alertness and body temperature. Physiol. Behav. 2018, 185, 1–13. [Google Scholar] [CrossRef]
- Kulve, M.T.; Schlangen, L.J.M.; van Lichtenbelt, W.D.M. Early evening light mitigates sleep compromising physiological and alerting responses to subsequent late evening light. Sci. Rep. 2019, 9, 16064. [Google Scholar] [CrossRef] [PubMed]
- Smolders, K.C.H.J.; Peeters, S.T.; Vogels, I.M.L.C.; de Kort, Y.A.W. Investigation of dose-response relationships for effects of white light exposure on correlates of alertness and executive control during regular daytime working hours. J. Biol. Rhythms 2018, 33, 649–661. [Google Scholar] [CrossRef] [PubMed]
- Lok, R.; van Koningsveld, M.J.; Gordijn, M.C.M.; Beersma, D.G.M.; Hut, R.A. Daytime melatonin and light independently affect human alertness and body temperature. J. Pineal Res. 2019, 67, e12583. [Google Scholar] [CrossRef] [PubMed]
- Kompier, M.E.; Smolders, K.C.H.J.; van Lichtenbelt, W.D.M.; de Kort, Y.A.W. Effects of light transitions on measures of alertness, arousal and comfort. Physiol. Behav. 2020, 223, 112999. [Google Scholar] [CrossRef]
- Kompier, M.E.; Smolders, K.C.H.J.; de Kort, Y.A.W. Abrupt light transitions in illuminance and correlated colour temperature result in different temporal dynamics and interindividual variability for sensation, comfort and alertness. PLoS ONE 2021, 16, e0243259. [Google Scholar] [CrossRef] [PubMed]
- Schmid, S.R.; Höhn, C.; Bothe, K.; Plamberger, C.P.; Angerer, M.; Pletzer, B.; Hoedlmoser, K. How smart is it to go to bed with the phone? The impact of short-wavelength light and affective states on sleep and circadian rhythms. Clocks Sleep 2021, 3, 558–580. [Google Scholar] [CrossRef] [PubMed]
- Dijk, D.J.; Cajochen, C.; Borbély, A.A. Effect of a single 3-hour exposure to bright light on core body temperature and sleep in humans. Neurosci. Lett. 1991, 121, 59–62. [Google Scholar] [CrossRef]
- Myers, B.L.; Badia, P. Immediate effects of different light intensities on body temperature and alertness. Physiol. Behav. 1993, 54, 199–202. [Google Scholar] [CrossRef]
- Kim, H.E.; Tokura, H. Influence of different light intensities during the daytime on evening dressing behavior in the cold. Physiol. Behav. 1995, 58, 779–783. [Google Scholar]
- Morita, T.; Tokura, H. Effects of lights of different color temperature on the nocturnal changes in core temperature and melatonin in humans. Appl. Hum. Sci. 1996, 15, 243–246. [Google Scholar] [CrossRef]
- Morita, T.; Tokura, H.; Wakamura, T.; Park, S.J.; Teramoto, Y. Effects of the morning irradiation of light with different wavelengths on the behavior of core temperature and melatonin in humans. Appl. Hum. Sci. 1997, 16, 103–105. [Google Scholar] [CrossRef] [PubMed]
- Kräuchi, K.; Cajochen, C.; Danilenko, K.V.; Wirz-Justice, A. The hypothermic effect of late evening melatonin does not block the phase delay induced by concurrent bright light in human subjects. Neurosci. Lett. 1997, 232, 57–61. [Google Scholar] [CrossRef]
- Aizawa, S.; Tokura, H. Exposure to bright light for several hours during the daytime lowers tympanic temperature. Int. J. Biometeorol. 1997, 41, 90–93. [Google Scholar] [CrossRef] [PubMed]
- Aizawa, S.; Tokura, H. The influence of bright light exposure for several hours during daytime on tympanic temperature level at sweating onset. J. Therm. Biol. 1998, 23, 99–106. [Google Scholar]
- Foret, J.; Daurat, A.; Tirilly, G. Effect of bright light at night on core temperature, subjective alertness and performance as a function of exposure time. Scand. J. Work Environ. Health 1998, 24, 115–120. [Google Scholar]
- Park, S.J.; Tokura, H. Effects of different light intensities during the daytime on circadian rhythm of core temperature in humans. Appl. Hum. Sci. 1999, 17, 253–257. [Google Scholar] [CrossRef]
- Zhang, P.; Tokura, H. Thermoregulatory responses in humans during exercise after exposure to two different light intensities. Eur. J. Appl. Physiol. 1999, 79, 285–289. [Google Scholar] [CrossRef]
- Cajochen, C.; Zeitzer, J.M.; Czeisler, C.A.; Dijk, D.J. Dose-response relationship for light intensity and ocular and electroencephalographic correlates of human alertness. Behav. Brain Res. 2000, 115, 75–83. [Google Scholar] [CrossRef]
- Rüger, M.; Gordijn, M.C.; Beersma, D.G.; de Vries, B.; Daan, S. Acute and phase-shifting effects of ocular and extraocular light in human circadian physiology. J. Biol. Rhythms 2003, 18, 409–419. [Google Scholar] [CrossRef]
- Sato, M.; Sakaguchi, T.; Morita, T. The effects of exposure in the morning to light of different color temperatures on the behavior of core temperature and melatonin secretion in humans. Biol. Rhythm Res. 2005, 36, 287–292. [Google Scholar] [CrossRef]
- Petterborg, L.J.; Kjellman, B.F.; Thalén, B.E.; Wetterberg, L. Effect of a 15 minute light pulse on nocturnal serum melatonin levels in human volunteers. J. Pineal Res. 1991, 10, 9–13. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, I.M.; Norman, T.R.; Burrows, G.D.; Armstrong, S.M. Melatonin, cortisol and prolactin response to acute nocturnal light exposure in healthy volunteers. Psychoneuroendocrinology 1992, 17, 243–248. [Google Scholar] [CrossRef]
- Kostoglou-Athanassiou, I.; Treacher, D.F.; Wheeler, M.J.; Forsling, M.L. Bright light exposure and pituitary hormone secretion. Clin. Endocrinol. 1998, 48, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Scheer, F.A.; Buijs, R.M. Light affects morning salivary cortisol in humans. J. Clin. Endocrinol. Metab. 1999, 84, 3395–3398. [Google Scholar] [CrossRef] [PubMed]
- Lockley, S.W.; Evans, E.E.; Scheer, F.A.; Brainard, G.C.; Czeisler, C.A.; Aeschbach, D. Short-wavelength sensitivity for the direct effects of light on alertness, vigilance, and the waking electroencephalogram in humans. Sleep 2006, 29, 161–168. [Google Scholar]
- Jung, C.M.; Khalsa, S.B.; Scheer, F.A.; Cajochen, C.; Lockley, S.W.; Czeisler, C.A.; Wright, K.P., Jr. Acute effects of bright light exposure on cortisol levels. J. Biol. Rhythms 2010, 25, 208–216. [Google Scholar] [CrossRef]
- Danilenko, K.V.; Sergeeva, O.Y. Immediate effect of blue-enhanced light on reproductive hormones in women. Neuro Endocrinol. Lett. 2015, 36, 84–90. [Google Scholar]
- Petrowski, K.; Schmalbach, B.; Niedling, M.; Stalder, T. The effects of post-awakening light exposure on the cortisol awakening response in healthy male individuals. Psychoneuroendocrinology 2019, 108, 28–34. [Google Scholar] [CrossRef]
- Spitschan, M.; Stefani, O.; Blattner, P.; Gronfier, C.; Lockley, S.W.; Lucas, R.J. How to report light exposure in human chronobiology and sleep research experiments. Clocks Sleep 2019, 1, 280–289. [Google Scholar] [CrossRef]
- Task Force of the European Society of Cardiology and the North American Society of Pacing Electrophysiology. Heart rate variability: Standards of measurement, physiological interpretation and clinical use. Circulation 1996, 93, 1043–1065. [Google Scholar] [CrossRef]
- Kräuchi, K.; Cajochen, C.; Werth, E.; Wirz-Justice, A. Functional link between distal vasodilation and sleep-onset latency? Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000, 278, R741–R748. [Google Scholar] [CrossRef] [PubMed]
- Kräuchi, K.; Cajochen, C.; Wirz-Justice, A. A relationship between heat loss and sleepiness: Effects of postural change and melatonin administration. J. Appl. Physiol. 1997, 83, 134–139. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, J.E.; Brennan, S.E. Synthesizing and presenting findings using other methods. In Cochrane Handbook for Systematic Reviews of Interventions Version 6.3; (updated February 2022); Higgins, J.P.T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A., Eds.; Cochrane: London, UK, 2022; Chapter 12. [Google Scholar]

| Measure | N of Reports | Effect | Factor 1 | Mean Effect | Overall Rating 3 | ||||
|---|---|---|---|---|---|---|---|---|---|
| ↑/ = /↓ N | ↑ % | Design % | Night % | Light % | Mean | Estimated Mean (95% CI) 2 | |||
| 1. Oxygen consumption (OC) | 5 | 0/5/0 | 0 | 0 | 0 | 60 | 0.00 | – | negative |
| 2. Sympathetic nerve activity | 1 | 1/0/0 | 100 | 0 | 0 | 0 | 1.00 | – | likely s. |
| 3. Skin conductance (SC) | 6 | 1/5/0 | 17 | 0 | 0 | 0 | 0.17 | 0.18 (−0.26 ÷ 0.62) | negative |
| 4. Skin blood flow (SBF) | 3 | 0/3/0 | 0 | 0 | 0 | 33 | 0.00 | – | unlikely s. |
| 5. Noradrenaline (NA) | 1 | 0/1/0 | 0 | 0 | 0 | 100 | 0.00 | – | unlikely s. |
| 6. Blood pressure (BP) | 10 | 2/8/0 | 20 | 10 * | 10 * | 40 | 0.20 | – | negative |
| 7. Heart rate (HR) | 33 | 10/22/1 * | 30 | 3 | 18 | 30 | 0.27 | 0.42 (0.12÷0.72) | 2 |
| 8. Heart rate variability (HRV) | 20 | 7/8/5 | 35 | 15 * | 20 (*) | 15 | 0.10 | 0.16 (−0.52 ÷ 0.85) | negative |
| 9. Body temperature (T) | |||||||||
| 34 | 17/13/4 ** | 50 | 12 | 11 * | 65 (*) | 0.38 | 0.43 (0.13 ÷ 0.73) | – |
| 20 | 13/6/1 ** | 65 | 10 | 35 *** | 70 * | 0.60 | 0.52 (0.25 ÷ 0.80) | 1 |
| 14 | 4/7/3 | 29 | 14 | 29 | 57 | 0.07 | 0.13 (−0.42 ÷ 0.67) | negative |
| 15 | 4/9/2 | 27 | 13 | 7 | 60 (*) | 0.13 | 0.35 (−0.29 ÷ 0.99) | negative |
| 10. Salivary alpha-amylase | 3 | 0/3/0 | 0 | 0 | 67 | 0 | 0.00 | – | unlikely s. |
| 11. Cortisol | 24 | 4/16/4 | 17 | 21 | 50 | 42 | 0.00 | −0.01 (−0.25 ÷ 0.24) | negative |
| 12. Thyroid-stimulating hormone (TSH) | 3 | 0/3/0 | 0 | 33 | 67 | 0 | 0.00 | – | unlikely s. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Danilenko, K.V. Objective Measures of Immediate “Energizing” Effect of Light: Studies Review and Data Analysis. Clocks & Sleep 2022, 4, 475-496. https://doi.org/10.3390/clockssleep4040038
Danilenko KV. Objective Measures of Immediate “Energizing” Effect of Light: Studies Review and Data Analysis. Clocks & Sleep. 2022; 4(4):475-496. https://doi.org/10.3390/clockssleep4040038
Chicago/Turabian StyleDanilenko, Konstantin V. 2022. "Objective Measures of Immediate “Energizing” Effect of Light: Studies Review and Data Analysis" Clocks & Sleep 4, no. 4: 475-496. https://doi.org/10.3390/clockssleep4040038
APA StyleDanilenko, K. V. (2022). Objective Measures of Immediate “Energizing” Effect of Light: Studies Review and Data Analysis. Clocks & Sleep, 4(4), 475-496. https://doi.org/10.3390/clockssleep4040038






