Neuroimaging in the Rare Sleep Disorder of Kleine–Levin Syndrome: A Systematic Review
Abstract
1. Introduction
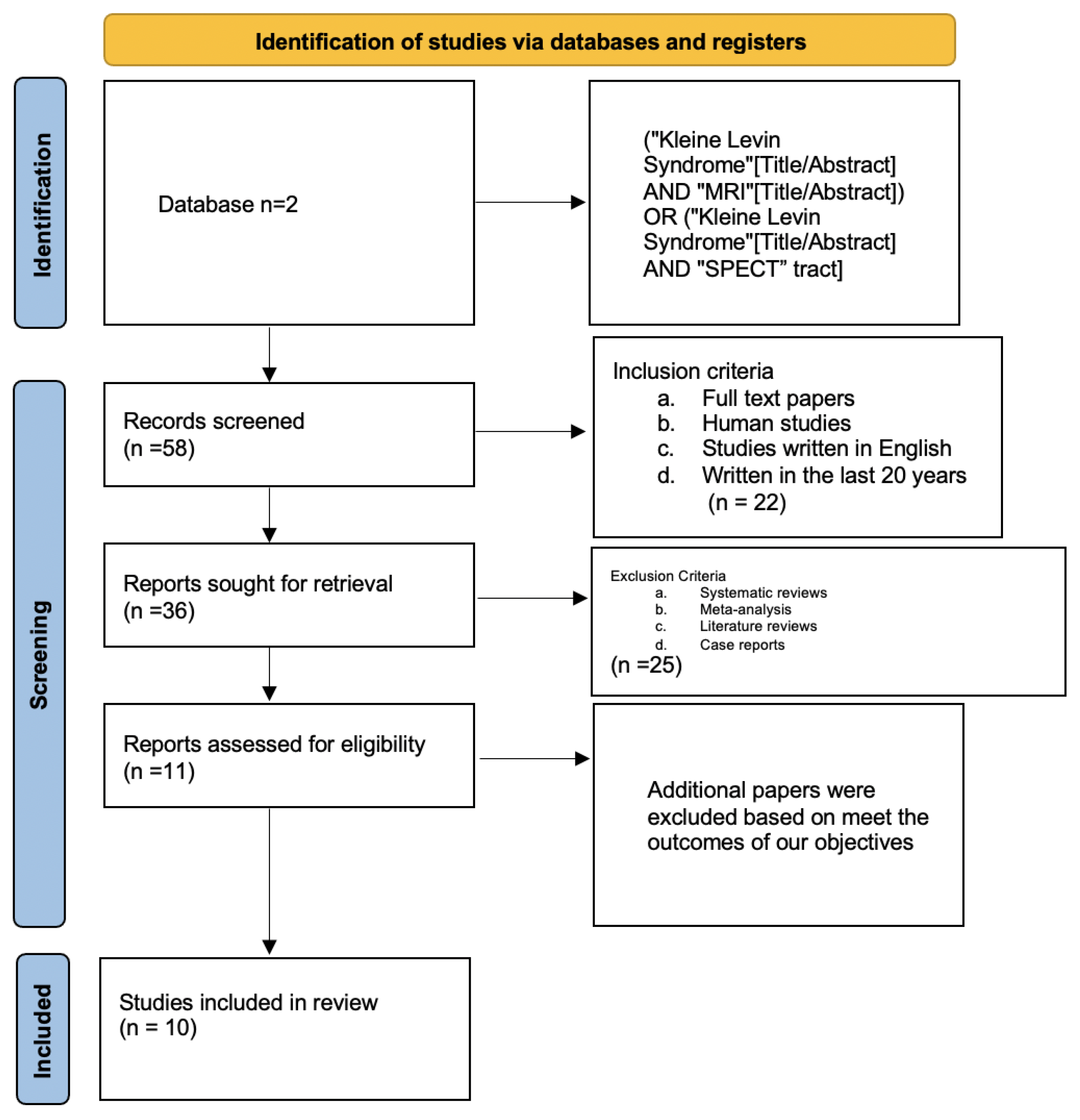
2. Methods
2.1. Protocol
2.1.1. Eligibility Criteria and Study Selection
2.1.2. Database and Search Strategy
2.1.3. Data Extraction and Analysis
2.1.4. Bias Assessment
3. Results
3.1. Figures, Tables, and Schemes
3.2. Study Characteristics
3.3. Study Outcomes
3.4. Bias Analysis
4. Discussion
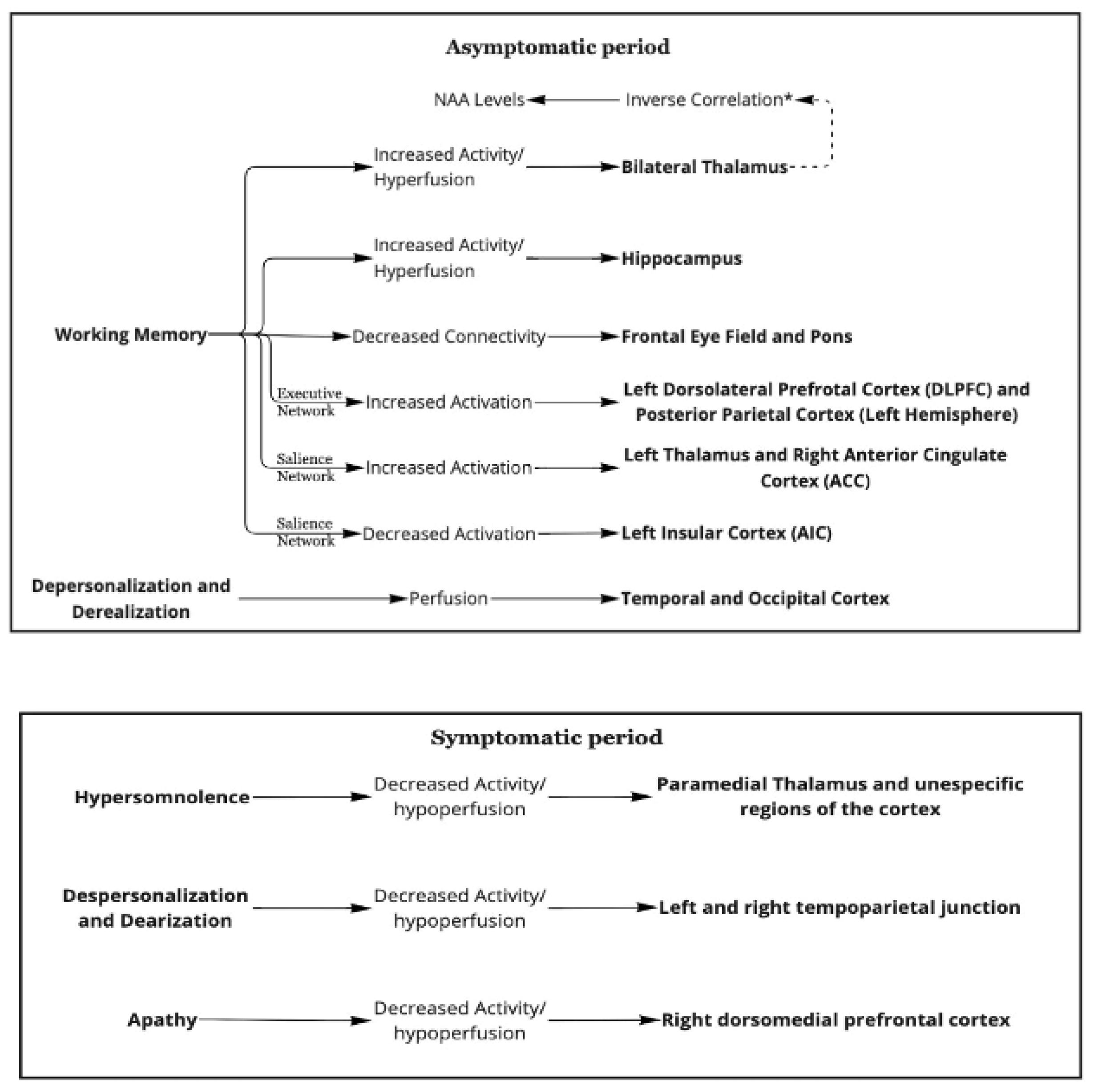
4.1. Asymptomatic Period
4.1.1. Working Memory
4.1.2. Thalamic Activation and Hippocampal Activation
4.1.3. Thalamic Activation and N.A.A. Levels
4.1.4. Frontal Eye Fields and Pons
4.1.5. The Executive and Salient Networks
4.1.6. Depersonalization/Derealization
4.1.7. Initial Markers
4.2. Symptomatic Periods
4.2.1. Hypersomnolence
4.2.2. Depersonalization and Derealization
4.2.3. Apathy
4.2.4. Hypersexuality
4.2.5. Striatum Role and Additional Findings
4.3. Disease Progression and Remission
4.4. Study Type
5. Overview
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Levin, M. Narcolepsy (Gélineau’s syndrome) and other varieties of morbid somnolence. Arch. Neurol. Psychiatry 1929, 22, 1172–1200. [Google Scholar] [CrossRef]
- Gadoth, N.; Oksenberg, A. Kleine-Levin syndrome: An update and mini-review. Brain Dev. 2017, 39, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Arnulf, I.; Lin, L.; Gadoth, N.; File, J.; Lecendreux, M.; Franco, P.; Zeitzer, J.; Lo, B.; Faraco, J.H.; Mignot, E. Kleine-Levin syndrome: A systematic study of 108 patients. Ann. Neurol. 2008, 63, 482–493. [Google Scholar] [CrossRef] [PubMed]
- BaHammam, A.S.; GadElRab, M.O.; Owais, S.M.; Alswat, K.; Hamam, K.D. Clinical characteristics and HLA typing of a family with Kleine-Levin syndrome. Sleep Med. 2008, 9, 575–578. [Google Scholar] [CrossRef]
- Afolabi-Brown, O.; Mason, T.B.A. Kleine-Levin Syndrome. Paediatr. Respir. Rev. 2018, 25, 9–13. [Google Scholar] [CrossRef]
- Arnulf, I.; Zeitzer, J.M.; File, J.; Farber, N.; Mignot, E. Kleine-Levin syndrome: A systematic review of 186 cases in the literature. Brain 2005, 128, 2763–2776. [Google Scholar] [CrossRef]
- AlShareef, S.M.; Smith, R.M.; BaHammam, A.S. Kleine-Levin syndrome: Clues to aetiology. Sleep Breath. 2018, 22, 613–623. [Google Scholar] [CrossRef]
- Ramdurg, S. Kleine-Levin syndrome: Etiology, diagnosis, and treatment. Ann. Indian Acad. Neurol. 2010, 13, 241–246. [Google Scholar] [CrossRef]
- De Oliveira, M.M.; Conti, C.; Prado, G.F. Pharmacological treatment for Kleine-Levin syndrome. Cochrane Database Syst. Rev. 2016, 2016, CD006685. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. PRISMA Group: Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Brooke, B.S.; Schwartz, T.A.; Pawlik, T.M. MOOSE Reporting Guidelines for Meta-analyses of Observational Studies. JAMA Surg. 2021, 156, 787–788. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-S.; Guilleminault, C.; Kao, P.-F.; Liu, F.-Y. SPECT findings in the Kleine-Levin syndrome. Sleep 2005, 28, 955–960. [Google Scholar] [CrossRef]
- Engström, M.; Latini, F.; Landtblom, A.-M. Neuroimaging in the Kleine-Levin Syndrome. Curr. Neurol. Neurosci. Rep. 2018, 18, 58. [Google Scholar] [CrossRef]
- Vigren, P.; Tisell, A.; Engström, M.; Karlsson, T.; Dahlqvist, O.L.; Lundberg, P.; Landtblom, A.-M. Low Thalamic NAA-Concentration Corresponds to Strong Neural Activation in Working Memory in Kleine-Levin Syndrome. PLoS ONE 2013, 8, e56279. [Google Scholar] [CrossRef] [PubMed]
- Engström, M.; Landtblom, A.-M.; Karlsson, T. Brain and effort: Brain activation and effort-related working memory in healthy participants and patients with working memory deficits. Front. Hum. Neurosci. 2013, 7, 140. [Google Scholar] [CrossRef]
- Vigren, P.; Engström, M.; Landtblom, A.-M. SPECT in the Kleine-Levin Syndrome, a Possible Diagnostic and Prognostic Aid? Front. Neurol. 2014, 5, 178. [Google Scholar] [CrossRef]
- Kas, A.; Lavault, S.; Habert, M.-O.; Arnulf, I. Feeling unreal: A functional imaging study in patients with Kleine-Levin syndrome. Brain 2014, 137, 2077–2087. [Google Scholar] [CrossRef]
- Dudoignon, B.; Tainturier, L.-E.; Dodet, P.; Bera, G.; Groos, E.; Chaumereuil, C.; Maranci, J.; Kas, A.; Arnulf, I. Functional brain imaging using 18F-fluorodeoxyglucose positron emission tomography/computerized tomography in 138 patients with Kleine–Levin syndrome: An early marker? Brain Commun. 2021, 3, fcab130. [Google Scholar] [CrossRef]
- Engström, M.; Landtblom, A.-M.; Karlsson, T. New hypothesis on pontine-frontal eye field connectivity in Kleine-Levin syndrome. J. Sleep Res. 2016, 25, 716–719. [Google Scholar] [CrossRef]
- Dauvilliers, Y.; Bayard, S.; Lopez, R.; Comte, F.; Zanca, M.; Peigneux, P. Widespread hypermetabolism in symptomatic and asymptomatic episodes in Kleine-Levin syndrome. PLoS ONE 2014, 9, e93813. [Google Scholar] [CrossRef] [PubMed]
- Engström, M.; Karlsson, T.; Landtblom, A.-M. Thalamic Activation in the Kleine-Levin Syndrome. Sleep 2014, 37, 379–386. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Engstrom, M.; Vigren, P.; Karlsson, T.; Landtblom, A.-M. Working Memory in 8 Kleine-Levin Syndrome Patients: An fMRI Study. Sleep 2009, 32, 681. [Google Scholar] [CrossRef] [PubMed]
- Yandrapalli, S.; Puckett, Y. SPECT Imaging; StatPearls Publishing: Tampa, FL, USA, 2022. [Google Scholar]
- Glover, G.H. Overview of Functional Magnetic Resonance Imaging. Neurosurg. Clin. N. Am. 2011, 22, 133–139. [Google Scholar] [CrossRef]
- Kapoor, M.; Kasi, A. PET Scanning; StatPearls Publishing: Tampa, FL, USA, 2021. [Google Scholar]
- Naumann, A.; Bellebaum, C.; Daum, I. Cognitive deficits in narcolepsy. J. Sleep Res. 2006, 15, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Corbetta, M.; Akbudak, E.; Conturo, T.E.; Snyder, A.Z.; Ollinger, J.M.; Drury, H.A.; Linenweber, M.R.; Petersen, S.E.; Raichle, M.E.; van Essen, D.C.; et al. A common network of functional areas for attention and eye movements. Neuron 1998, 21, 761–773. [Google Scholar] [CrossRef]
- Engström, M.; Karlsson, T.; Landtblom, A.-M. Reduced Thalamic and Pontine Connectivity in Kleine–Levin Syndrome. Front. Neurol. 2014, 5, 42. [Google Scholar]
- Seeley, W.W. The Salience Network: A Neural System for Perceiving and Responding to Homeostatic Demands. J. Neurosci. 2019, 39, 9878–9882. [Google Scholar] [CrossRef]
- Central Executive Network—An Overview. ScienceDirect Topics. Available online: https://www.sciencedirect.com/topics/psychology/central-executive-network (accessed on 14 February 2022).
- Hawkes, M.A.; Arena, J.E.; Rollán, C.; Pujol-Lereis, V.A.; Romero, C.; Ameriso, S.F. Bilateral Paramedian Thalamic Infarction. Neurologist 2015, 20, 89–92. [Google Scholar] [CrossRef]
- Kichloo, A.; Jamal, S.M.; Zain, E.-A.; Wani, F.; Vipparala, N. Artery of Percheron Infarction: A Short Review. J. Investig. Med. High Impact Case Rep. 2019, 7, 2324709619867355. [Google Scholar] [CrossRef]
- Miglis, M.G.; Guilleminault, C. Kleine-Levin syndrome: A review. Nat. Sci. Sleep. 2014, 6, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Haba-Rubio, J.; Prior, J.O.; Guedj, E.; Tafti, M.; Heinzer, R.; Rossetti, A.O. Kleine-Levin syndrome: Functional imaging correlates of hypersomnia and behavioral symptoms. Neurology 2012, 79, 1927–1929. [Google Scholar] [CrossRef] [PubMed]
- Drouet, C.; Morel, O.; Verger, A.; Guedj, E.; Boulahdour, H. FDG Brain PET/CT Revealing Bilateral Thalamostriatal Activation During a Symptomatic Episode in a Patient with Kleine-Levin Syndrome. Clin. Nucl. Med. 2017, 42, e261–e262. [Google Scholar] [CrossRef] [PubMed]
- Hoexter, M.Q.; Shih, M.C.; Felício, A.C.; Tufik, S.; Bressan, R.A. Greater reduction of striatal dopamine transporter availability during the symptomatic than asymptomatic phase of Kleine-Levin syndrome. Sleep Med. 2010, 11, 959. [Google Scholar] [CrossRef] [PubMed]
- Landtblom, A.-M.; Dige, N.; Schwerdt, K.; Säfström, P.; Granérus, G. Short-term memory dysfunction in Kleine-Levin syndrome. Acta Neurol. Scand. 2003, 108, 363–367. [Google Scholar] [CrossRef] [PubMed]
| A ≥2 recurrent episodes of hypersomnia, each persisting for 2 days to 5 weeks B Episodes recur at least 1 every 18 months C There is normal sleep, cognition, behavior, and mood between episodes In addition, the patient should have at least one of the following:
|
| Author, Year, Country | Number of Participants, (M/F), Mean Age | Age | Study Type/Single-Center/Multicenter | Methods |
|---|---|---|---|---|
| Huang et al., 2005, Taiwan [13] | 7 K.L.S. patients (7 M, 0 F) | Participants: 13.4 years Control: No cases | Cross-Sectional | SPECT studies were conducted in the asymptomatic period in all the patients (n = 7). Studies were conducted in the symptomatic period only in 5 patients. There was no control group. |
| Engstrom et al., 2009, England [23] | 8 participants (5 M, 3 W), 12 controls | Participant: 27 years, Controls: 24.1 | Cross-Sectional/Single Center. | (fMRI) applying a verbal working memory task was used in conjunction with a paper-and-pencil version of the task. Seven patients had an active disease, and one was in remission. All patients were asymptomatic at the time of the fMRI. |
| Vigren et al., 2013, Sweden [15] | 14 K.L.S. patients, 15 healthy controls | Participants: 14.7 Controls: 22.1 | Cross-sectional, Single-center | Patients diagnosed with KLS. according to ICSD were enrolled. All controls were recruited after a paper-and-pencil version of a reading-span task by Daneman and Carpenter. |
| Engström et al., 2013, England [16] | 44 participants (24 F, 20 M) 26 controls, 18 KLS patients | Participant: 24.1 years Control: 24.7 years | Cross-Sectional | Working Memory was assesed by using FMR. The participants of the study were divided in low capacity and high capacity groups according to a performance of memory task. |
| Kas et al., 2014, France [18] | 41 K.L.S. patients, 15 healthy control | Participants: 22.3 | Cross-sectional, Single-center Study | A total of 70 patients with primary K.L.S. diagnosis were enrolled after removing 35 suspected cases referred to the center. |
| Vigren et al., 2014, Sweeden [17] | 24 K.L.S. patients, no controls | Not reported | Cross-sectional, Single center Study | SPECT SCAN was used in all the patients in the symptomatic period. SPECT SCAN was also performed in the asymptomatic period in 21 patients. Patients were categorized as severe and non-severe. |
| Dudoignon et al., 2021, France [19] | 138 K.L.S. patients, no control | 21.6 | Cross-Sectional Study, Single-center | The confirmed 210 K.L.S. patients were enrolled out of 260 suspected K.L.S. patients referred to the center after a cognitive assessment, blood sampling, and an interview with K.L.S. physicians. |
| Engstrom et al., 2014, Sweden [22] | 18 K.L.S. patients, 26 healthy controls | Participants: 25.9 Controls: 24.1 | Cross-sectional study, Single center | According to the International Classification of Sleep Disorders, 18 patients diagnosed with K.L.S. were included. The healthy controls were recruited after a thorough evaluation by a clinical interview. |
| Engstrom et al., 2016, England [20] | 12 Participants (4 M, 8 F), 14 controls, | Participant: 23.8 years (SD = 9.1 years). Controls: 24.1 | Cross Sectional Study/Single Center | All participants were asymptomatic at the time of the study. The participants of the study were matched to controls. |
| Dauvilliers et al., 2014, France [21] | 15 healthy control 4 K.L.S. | participants: 16.25 years Control: 28 | Cross-sectional, Single-center Study | Four K.L.S. patients underwent F-FDG-PET scanning from day 2 to day 3 after the symptomatic episode and two to three months after the last day of the symptomatic episode. Fifteen controls were included for comparison. |
| Author, Year, Country | Imaging | Hypometabolism/Less Activity/Hypoperfusion | Hypermetabolism/Greater Activity/Hyperfusion |
|---|---|---|---|
| Huang et al., 2005, Taiwan [13] | SPECT with 925 MBq (25 mCi) of technetium-99 m ethyl cysteinate dimer (Tc-99 m ECD) | All the patients had hypoperfusion of both thalami during the symptomatic period. In the symptomatic and asymptomatic period, there was hypoperfusion in the temporal lobe, frontal lobe, and basal ganglia. | |
| Engstrom et al., 2009, England [23] | fMRI—BOLD response 1.5 T body scanner—in the asymptomatic period. | Reduced frontal activity in the anterior cingulate and prefrontal cortex while performing a reading span task. | Increased thalamic activity while performing reading and span tasks. |
| Vigren et al., 2013, Sweden [15] | fMRI 1.5 T in the asymptomatic period | There was a negative correlation between activity in the thalamus and N.A.A. levels. | Decreased N.A.A. levels when there was high activity in the left thalamus. High activity in the left thalamus while performing W.M. task. Larger activation in bilateral parietal cortex compared to controls. |
| Engstrom et al., England, 2013 [16] | fMRI-BOLD- 1.5 T body scanner | Salient network: decreased activation of the left insular cortex (A.I.C.). | Salient network: Increased activation of the left thalamus, more activation of the right anterior cingulate cortex (A.C.C.) Executive network, K.L.S. patients had increased activation in the left dorsolateral prefrontal cortex (DLPFC) and increased left hemisphere activation in the region of the posterior parietal cortex (P.P.C.) tested. |
| Kas et al., 2014, France [18] | SPECT Tc-99 m ECD- in the symptomatic and asymptomatic phase | Compared to control, K.L.S. patients had hypoperfusion in the hypothalamus, the thalamus, mainly the right posterior part, the caudate nucleus, and cortical associative areas including the anterior cingulate, the orbitofrontal, and the right superior temporal cortices during the asymptomatic period, while hypoperfusion in the right dorsomedial prefrontal cortex and the right parietal-temporal junction was noted during the symptomatic period. | Depersonalization/derealization- temporal-occipital relation, r = −0.79.5, p = 0.01) in the asymtomatic period. Depersonalization/derealization- temporal-occipital relation, r = −0.45, p = 0.05) in the asymtomatic period. The perfusion during the asymptomatic period in the right parieto-temporal r = 0.53, p = 0.05 decreased with each episode. |
| Vigren et al., 2014, Sweden [17] | SPECT with 650 MBq 99 m-Tc-HMPAO | A total of 48% have abnormal perfusion. Severe patients: 5/13 had temporal and/or frontal hypoperfusion. Non-severe patients: 7/12 had temporal and/or frontal hypoperfusion. Patients with active disease: 7/16 had temporal and/or frontal hypoperfusion. Patients with remission: 5/9 had temporal and/or frontal hypoperfusion. | |
| Dudoignon et al., 2021, France [19] | FDG-PET FDG-PET/CT using Gemini Dual PET/CT 30 min post- injection of 2 MBq/kg FDG -in the asymptomatic period | A total of 70% of 138 had hypometabolism in the left temporo-occipital junction A total of 63% hypometabolism bilaterally posterior associative cortex A total of 50% have the entire homolateral, bilateral posterior associative cortex, and hippocampus. | Prefrontal, dorsolateral cortex was noted in 34.8% of patients, more often on the right than the left side. |
| Engstrom 2014 [22] | fMRI 1.5 T- in the asymptomatic period in the asymptomatic state | K.L.S. patients illustrated reduced activation in the medial frontal and anterior cingulate cortices during (p < 0.001). | Increased thalamic activation in 61.4% of patients. |
| Engstrom et al., 2016, England [20] | fMRI/SPECT in asymptomatic patients | Patients with Kleine–Levin syndrome showed less activity in between the pons and the frontal eye fields as compared to controls at the asymptomatic period (p = 0.041). | |
| Dauvillers et al., 2014, France [21] | PET with F-fluorodeoxy glucose (F-FDG) | K.L.S. patients exhibited hypometabolism in occipital and temporal gyri and in the inferior parietal areas compared to control during the symptomatic phase. | As compared to healthy individuals, the 4 K.L.S. patients demonstrated hypermetabolism in paracentral, precentral, postcentral areas, medial frontal gyrus, thalamus, and putamen during symptomatic periods. In the asymptomatic phase, the 4 K.L.S. patients revealed having more hypermetabolism in frontal and temporal cortices, posterior cingulate, and precuneus as compared to controls. |
| Author, Year | Confounding | Selection of Participants | Classification | Deviations | Missing Data | Measurements | Selection of Reported Results |
|---|---|---|---|---|---|---|---|
| Dudoignon [19] | Medium risk | Moderate risk | Low risk | Low risk | Moderate risk | Low risk | Low risk |
| Kas et al., 2014 [18] | Low risk | High risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Dauvillers et al., 2013 [21] | Low risk | Moderate risk | Low risk | Low risk | Low risk | Moderate risk | Low risk |
| Engstrom et al., 2013 [16] | Low risk | Moderate risk | Low risk | Low risk | Low risk | Moderate risk | Low risk |
| Ensgtrom et al., 2016 [20] | Low risk | Low risk | Moderate risk | Low risk | Low risk | Moderate risk | Low risk |
| Engstrom et al., 2014 [22] | Low risk | Moderate risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Engstrom et al., 2009 [23] | Low risk | High risk | Low risk | Low risk | Moderate risk | Low risk | Moderate risk |
| Vigren et al., 2013 [15] | Low risk | Moderate risk | Low risk | Low risk | Low risk | Moderate risk | Low risk |
| Vigren et al., 2014 [17] | Low risk | High risk | Low risk | Low risk | Moderate risk | Low risk | Low risk |
| Imaging Study | How It Works |
|---|---|
| Single-photon emission C.T. (SPECT) [24] | It creates a 3D image by the representation of a radioactive tracer (e.g., technetium-99 m) inserted in the body, allowing the identification of functionality and perfusion of different tissues, in this case, the brain. |
| Functional M.R.I. (fMRI) [25] | It measures hemodynamic response induced by neuronal activity and measured though a blood oxygen level dependent (BOLD) signal which depends on oxy/deoxy haemoglobin concentration. |
| Fluorodeoxyglucose positron emission tomography (FDG-PET) [26] | F.D.G. is a glucose analog metabolized by tissues with a high glucose demand (e.g., cancers, heart, and brain) and is measured using a tracer. In that way, this study allows us to identify brain activity by measuring the uptake of F.D.G. and indirectly measuring the blood flow through the brain. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortiz, J.F.; Argudo, J.M.; Yépez, M.; Moncayo, J.A.; Tamton, H.; Aguirre, A.S.; Patel, G.; Sen, M.; Mistry, A.; Yuen, R.; et al. Neuroimaging in the Rare Sleep Disorder of Kleine–Levin Syndrome: A Systematic Review. Clocks & Sleep 2022, 4, 287-299. https://doi.org/10.3390/clockssleep4020025
Ortiz JF, Argudo JM, Yépez M, Moncayo JA, Tamton H, Aguirre AS, Patel G, Sen M, Mistry A, Yuen R, et al. Neuroimaging in the Rare Sleep Disorder of Kleine–Levin Syndrome: A Systematic Review. Clocks & Sleep. 2022; 4(2):287-299. https://doi.org/10.3390/clockssleep4020025
Chicago/Turabian StyleOrtiz, Juan Fernando, Jennifer M. Argudo, Mario Yépez, Juan Andrés Moncayo, Hyder Tamton, Alex S. Aguirre, Ghanshyam Patel, Meghdeep Sen, Ayushi Mistry, Ray Yuen, and et al. 2022. "Neuroimaging in the Rare Sleep Disorder of Kleine–Levin Syndrome: A Systematic Review" Clocks & Sleep 4, no. 2: 287-299. https://doi.org/10.3390/clockssleep4020025
APA StyleOrtiz, J. F., Argudo, J. M., Yépez, M., Moncayo, J. A., Tamton, H., Aguirre, A. S., Patel, G., Sen, M., Mistry, A., Yuen, R., Eissa-Garces, A., Ojeda, D., & Ruxmohan, S. (2022). Neuroimaging in the Rare Sleep Disorder of Kleine–Levin Syndrome: A Systematic Review. Clocks & Sleep, 4(2), 287-299. https://doi.org/10.3390/clockssleep4020025








