Influence of Geomagnetic Disturbances at Different Times of Day on Locomotor Activity in Zebrafish (Danio Rerio)
Abstract
:1. Introduction
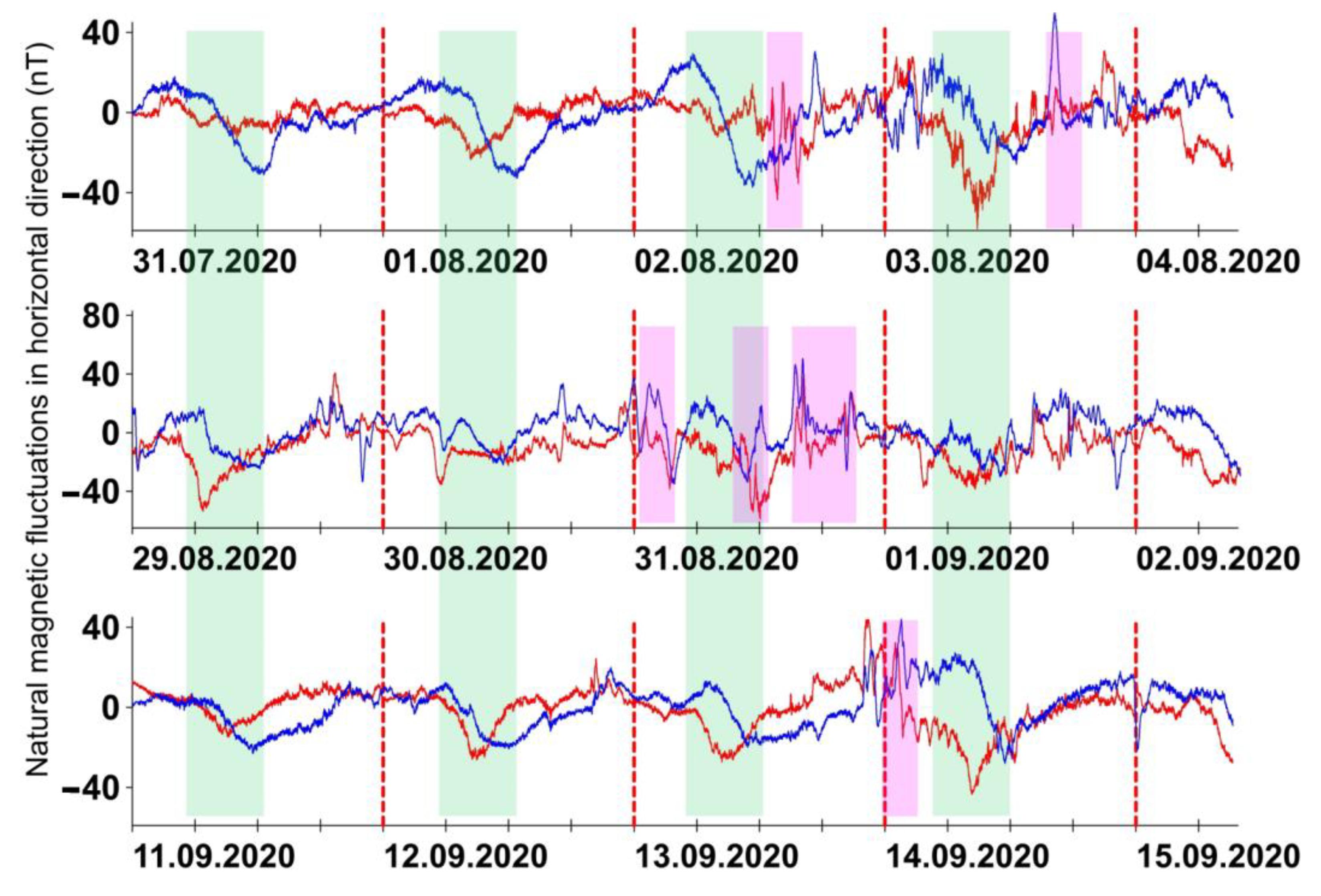
2. Results
3. Discussion
4. Materials and Methods
- -
- Average swimming speed, cm/s (distance travelled divided by observation time);
- -
- Meandering, °/cm, (sum of all turning angles divided by total distance);
- -
- Freezing time, % (time when speed is less than 1 cm/s);
- -
- Swimming time, % (time when speed is 1–10 cm/s);
- -
- Rapid movement time, % (time when speed exceeds 10 cm/s);
- -
- Wall preference index (relative time spent within a 3 cm-wide area close to the walls).
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goncalves, I.F.S.; Souza, T.M.; Vieira, L.R.; Marchi, F.C.; Nascimento, A.P.; Farias, D.F. Toxicity testing of pesticides in zebrafish—A systematic review on chemicals and associated toxicological endpoints. Environ. Sci. Pollut. Res. 2020, 27, 10185–10204. [Google Scholar] [CrossRef]
- Audira, G.; Sampurna, B.P.; Juniardi, S.; Liang, S.T.; Lai, Y.H.; Han, L.; Hsiao, C.D. Establishing simple image-based methods and cost-effective instrument for toxicity assessment on circadian rhythm dysregulation in fish. Biol. Open 2019, 8, bio041871. [Google Scholar] [CrossRef] [Green Version]
- Pan, H.; Zhang, X.; Ren, B.; Yang, H.; Ren, Z.; Wang, W. Toxic Assessment of Cadmium Based on Online Swimming Behavior and the Continuous AChE Activity in the Gill of Zebrafish (Danio rerio). Water Air Soil. Pollut. 2017, 228, 355–363. [Google Scholar] [CrossRef]
- Sarasamma, S.; Audira, G.; Juniardi, S.; Sampurna, B.P.; Lai, Y.-H.; Hao, E.; Chen, J.-R.; Hsiao, C.-D. Evaluation of the Effects of Carbon 60 Nanoparticle Exposure to Adult Zebrafish: A Behavioral and Biochemical Approach to Elucidate the Mechanism of Toxicity. Int. J. Mol. Sci. 2018, 19, 3853. [Google Scholar] [CrossRef] [Green Version]
- Malhotra, N.; Chen, J.-R.; Sarasamma, S.; Audira, G.; Siregar, P.; Liang, S.-T.; Lai, Y.-H.; Lin, G.-M.; Ger, T.-R.; Hsiao, C.-D. Ecotoxicity Assessment of Fe3O4 Magnetic Nanoparticle Exposure in Adult Zebrafish at an Environmental Pertinent Concentration by Behavioral and Biochemical Testing. Nanomaterials 2019, 9, 873. [Google Scholar] [CrossRef] [Green Version]
- Zhao, R.; Hu, Y.; Li, B.; Chen, M.; Ren, Z. Potential effects of internal physio-ecological changes on the online biomonitoring of water quality: The behavior responses with circadian rhythms of zebrafish (Danio rerio) to different chemicals. Chemosphere 2019, 239, 124752–124760. [Google Scholar] [CrossRef]
- Persinger, M.A. Day time wheel running activity in laboratory rats following geomagnetic event of 5–6 July 1974. Int. J. Biometeorol. 1976, 20, 19–22. [Google Scholar] [CrossRef] [PubMed]
- Muraveiko, V.M.; Stepanyuk, I.A.; Zenzerov, V.S. The response of the crab Paralithodes Camtschaticus (Tilesius, 1815) to geomagnetic storms. Dokl. Biol. Sci. 2013, 448, 10–12. [Google Scholar] [CrossRef] [PubMed]
- Varanelli, C.C.; McCleave, J.D. Locomotor activity of atlantic salmon parr (Salmo salar L.) in various light conditions and in weak magnetic fields. Anim. Behav. 1974, 22, 178–186. [Google Scholar] [CrossRef]
- Deshcherevsky, A.V.; Sidorin, A.Y.; Kharin, E.P. Geomagnetic disturbances and animal activity in laboratory conditions. Biophysics 2009, 54, 389–395. [Google Scholar] [CrossRef]
- Yamazaki, Y.; Maute, A. Sq and EEJ—A Review on the Daily Variation of the Geomagnetic Field Caused by Ionospheric Dynamo Currents. Space Sci. Rev. 2017, 206, 299–405. [Google Scholar] [CrossRef] [Green Version]
- Brown, F.A.; Scow, K.M. Magnetic induction of a circadian cycle in hamsters. J. Interdiscipl. Cycle Res. 1978, 9, 137–145. [Google Scholar] [CrossRef]
- Krylov, V.V.; Osipova, E.A.; Pankova, N.A.; Talikina, M.G.; Chebotareva, Y.V.; Izyumov, Y.G.; Batrakova, A.A.; Nepomnyashchikh, V.A. The effect of a temporal shift in diurnal geomagnetic variation on roach Rutilus rutilus L. embryos: A comparison with effects of simulated geomagnetic storms. Biophysics 2017, 62, 675–681. [Google Scholar] [CrossRef]
- Welker, H.A.; Semm, P.; Willig, R.P.; Commentz, J.C.; Wiltschko, W.; Vollrath, L. Effects of an artificial magnetic field on serotonin N-acetyltransferase activity and melatonin content of the rat pineal gland. Exp. Brain Res. 1983, 50, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Brown, F.A. Response to pervasive geophysical factors and the biological clock problem. Cold Spring Harb. Sym. Quant. Biol. 1960, 25, 57–71. [Google Scholar] [CrossRef]
- Bliss, V.L.; Heppner, F.H. Circadian activity rhythm influenced by near zero magnetic field. Nature 1976, 261, 411–412. [Google Scholar] [CrossRef]
- Bartos, P.; Netusil, R.; Slaby, P.; Dolezel, D.; Ritz, T.; Vacha, M. Weak radiofrequency fields affect the insect circadian clock. J. R. Soc. Interface 2019, 16, 20190285. [Google Scholar] [CrossRef]
- Yoshii, T.; Ahmad, M.; Helfrich-Förster, C. Cryptochrome Mediates Light-Dependent Magnetosensitivity of Drosophila’s Circadian Clock. PLoS Biol. 2009, 7, e1000086. [Google Scholar] [CrossRef] [PubMed]
- Sallam, A.E.-D.; Hassan, S.A.; Hassaneen, E.; Ali, E.M. Environmental stress of mobile phone EM radiation on locomotor activity and melatonin circadian rhythms of rats. Biol. Rhythm. Res. 2016, 47, 597–607. [Google Scholar] [CrossRef]
- Manzella, N.; Bracci, M.; Ciarapica, V.; Staffolani, S.; Strafella, E.; Rapisarda, V.; Valentino, M.; Amati, M.; Copertaro, A.; Santarelli, L. Circadian gene expression and extremely low-frequency magnetic fields: An in vitro study. Bioelectromagnetics 2015, 36, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Agliassa, C.; Maffei, M.E. Reduction of geomagnetic field (GMF) to near null magnetic field (NNMF) affects some Arabidopsis thaliana clock genes amplitude in a light independent manner. J. Plant. Physiol. 2019, 232, 23–26. [Google Scholar] [CrossRef]
- Oliva, R.; Jansen, B.; Benscheidt, F.; Sandbichler, A.M.; Egg, M. Nuclear magnetic resonance affects the circadian clock and hypoxia-inducible factor isoforms in zebrafish. Biol. Rhythm. Res. 2019, 50, 739–757. [Google Scholar] [CrossRef] [Green Version]
- Krylov, V.V.; Izvekov, E.I.; Pavlova, V.V.; Pankova, N.A.; Osipova, E.A. Magnetic fluctuations affect circadian patterns of locomotor activity in zebrafish. bioRxiv 2021. [Google Scholar] [CrossRef]
- Krylov, V.V. Biological effects related to geomagnetic activity and possible mechanisms. Bioelectromagnetics 2017, 38, 497–510. [Google Scholar] [CrossRef]
- Nikolskaya, K.; Shtemler, V.; Yeschenko, O.; Savonenko, A.; Osipov, A.; Nickolsky, S. The Sensitivity of Cognitive Processes to the Inhomogeneity of Natural Magnetic Fields. Electro. Magnetobiol. 1996, 15, 163–174. [Google Scholar] [CrossRef]
- Zhadin, M.N.; Deryugina, O.N.; Pisachenko, T.M. Influence of combined DC and AC magnetic fields on rat behavior. Bioelectromagnetics 1999, 20, 378–386. [Google Scholar] [CrossRef]
- Lundberg, L.; Sienkiewicz, Z.; Anthony, D.; Broom, K.A. Effects of 50 Hz magnetic fields on circadian rhythm control in mice. Bioelectromagnetics 2019, 40, 250–259. [Google Scholar] [CrossRef]
- Murugan, N.J.; Persinger, M.A. Comparisons of responses by planarian to micromolar to attomolar dosages of morphine or naloxone and/or weak pulsed magnetic fields: Revealing receptor subtype affinities and non-specific effects. Int. J. Radiat. Biol. 2014, 90, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Zmejkoski, D.; Petković, B.; Pavković-Lučić, S.; Prolić, Z.; Anđelković, M.; Savić, T. Different responses of Drosophila subobscura isofemale lines to extremely low frequency magnetic field (50 Hz, 0.5 mT): Fitness components and locomotor activity. Int. J. Radiat. Biol. 2017, 93, 544–552. [Google Scholar] [CrossRef]
- Hore, P.J.; Mouritsen, H. The radical-pair mechanism of magnetoreception. Annu. Rev. Biophys. 2016, 45, 299–344. [Google Scholar] [CrossRef]
- Chiou, Y.Y.; Yang, Y.; Rashid, N.; Ye, R.; Selby, C.P.; Sancar, A. Mammalian Period represses and de-represses transcription by displacing CLOCK–BMAL1 from promoters in a Cryptochrome-dependent manner. Proc. Natl. Acad. Sci. USA 2016, 113, E6072–E6079. [Google Scholar] [CrossRef] [Green Version]
- Finger, A.M.; Dibner, C.; Kramer, A. Coupled network of the circadian clocks: A driving force of rhythmic physiology. FEBS Lett. 2020, 594, 2734–2769. [Google Scholar] [CrossRef] [PubMed]
- Solov’Yov, I.A.; Chandler, D.E.; Schulten, K. Magnetic Field Effects in Arabidopsis thaliana Cryptochrome-1. Biophys. J. 2007, 92, 2711–2726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sánchez-Vázquez, F.J.; Terry, M.I.; Felizardo, V.O.; Vera, L.M. Daily Rhythms of Toxicity and Effectiveness of Anesthetics (MS222 and Eugenol) in Zebrafish (Danio Rerio). Chronobiol. Int. 2011, 28, 109–117. [Google Scholar] [CrossRef]
- Hurd, M.W.; Debruyne, J.; Straume, M.; Cahill, G.M. Circadian rhythms of locomotor activity in zebrafish. Physiol. Behav. 1998, 65, 465–472. [Google Scholar] [CrossRef]
- Lopez-Olmeda, J.F.; Madrid, J.A.; Sanchez-Vázquez, F.J. Light and Temperature Cycles as Zeitgebers of Zebrafish (Danio rerio) Circadian Activity Rhythms. Chronobiol. Int. 2006, 23, 537–550. [Google Scholar] [CrossRef] [PubMed]
- Bartels, J.; Heck, N.H.; Johnston, H.F. The three-hour-range index measuring geomagnetic activity. Terr. Magn. Atmos. Electr. 1939, 44, 411–454. [Google Scholar] [CrossRef]
- Perez-Escudero, A.; Vicente-Page, J.; Hinz, R.; Arganda, S.; de Polavieja, G. idTracker: Tracking individuals in a group by automatic identification of unmarked animals. Nat. Methods 2014, 11, 743–748. [Google Scholar] [CrossRef] [PubMed]
- Noldus, L.P.; Spink, A.J.; Tegelenbosch, R.A. EthoVision: A versatile video tracking system for automation of behavioral experiments. Behav. Res. Methods Instrum. Comput. 2001, 33, 398–414. [Google Scholar] [CrossRef] [Green Version]
- Shenk, J.; Lohkamp, K.J.; Wiesmann, M.; Kiliaan, A.J. Automated Analysis of Stroke Mouse Trajectory Data With Traja. Front. Neurosci. 2020, 14, 518–528. [Google Scholar] [CrossRef]
- Krylov, V.V.; Kantserova, N.P.; Lysenko, L.A.; Osipova, E.A. A simulated geomagnetic storm unsynchronizes with diurnal geomagnetic variation affecting calpain activity in roach and great pond snail. Int. J. Biometeorol. 2019, 63, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Campbell, W.H. Annual and semiannual changes of the quiet daily variations (Sq) in the geomagnetic field at North American locations. J. Geophys. Res. 1982, 87, 785–796. [Google Scholar] [CrossRef]
| Individuals | Time Interval 1 | Time Interval 2 | Time Interval 3 | ||
|---|---|---|---|---|---|
| Events after FDGV 12:00–15:00 2 August 2020 15:00–18:00 3 August 2020 n = 12 | Event before FDGV 00:00–03:00 31 August 2020 | Event Coincided with FDGV 09:00–12:00 31 August 2020 | Events after FDGV 15:00–21:00 31 August 2020 | Event before FDGV 00:00–03:00 14 September 2020 | |
| Average swimming speed (cm/s) | |||||
| Fish#1 | 2.63 ± 0.19 3.44 ± 0.12 | 0.35 ± 0.09 2.28 ± 0.30 | 2.87 ± 0.56 2.49 ± 0.13 | 2.06 ± 0.15 2.05 ± 0.14 | 0.75 ± 0.17 0.76 ± 0.09 |
| Fish#2 | 1.55 ± 0.09 1.65 ± 0.08 | 0.68 ± 0.15 1.14 ± 0.12 | 2.19 ± 0.18 2.66 ± 0.16 | 2.00 ± 0.40 1.86 ± 0.14 | 0.39 ± 0.09 0.67 ± 0.06 |
| Fish#3 | 1.78 ± 0.07 2.05 ± 0.06 | 0.64 ± 0.15 0.78 ± 0.09 | 2.79 ± 0.50 3.49 ± 0.43 | 2.21 ± 0.34 2.74 ± 0.18 | 0.35 ± 0.13 0.67 ± 0.05 |
| Fish#4 | 1.07 ± 0.14 1.50 ± 0.16 | 0.94 ± 0.30 1.61 ± 0.12 | 3.88 ± 0.40 3.41 ± 0.15 | 2.11 ± 0.25 2.84 ± 0.09 | 0.69 ± 0.26 0.89 ± 0.08 |
| The mean value | 1.76 ± 0.10 2.16 ± 0.12 | 0.65 ± 0.10 1.45 ± 0.14 | 2.93 ± 0.24 3.01 ± 0.15 | 2.09 ± 0.14 2.37 ± 0.09 | 0.54 ± 0.09 0.75 ± 0.04 |
| Freezing time (%) | |||||
| Fish#1 | 20.09 ± 3.20 12.03 ± 1.06 | 93.63 ± 2.79 41.75 ± 3.70 | 17.36 ± 12.21 10.92 ± 3.08 | 28.13 ± 6.39 18.57 ± 3.60 | 72.90 ± 7.79 73.51 ± 3.70 |
| Fish#2 | 28.01 ± 5.07 29.53 ± 2.94 | 81.31 ± 6.15 68.21 ± 3.63 | 31.55 ± 8.83 12.84 ± 1.53 | 40.98 ± 8.44 37.03 ± 3.78 | 90.78 ± 3.01 80.20 ± 2.45 |
| Fish#3 | 20.61 ± 2.79 13.32 ± 0.96 | 84.01 ± 4.86 78.24 ± 2.67 | 11.68 ± 5.83 9.80 ± 6.08 | 22.92 ± 7.72 8.00 ± 1.51 | 90.55 ± 4.90 79.24 ± 1.91 |
| Fish#4 | 59.63 ± 6.03 45.55 ± 4.12 | 68.49 ± 12.36 54.28 ± 3.89 | 6.47 ± 3.98 6.89 ± 1.39 | 23.71 ± 5.95 8.79 ± 1.43 | 78.33 ± 12.15 71.74 ± 3.70 |
| The mean value | 32.08 ± 3.21 25.11 ± 2.36 | 81.86 ± 3.95 60.62 ± 3.31 | 16.77 ± 4.34 10.11 ± 1.72 | 28.94 ± 3.64 18.10 ± 2.18 | 83.14 ± 3.97 76.17 ± 1.61 |
| Swimming time (%) | |||||
| Fish#1 | 79.35 ± 3.11 85.95 ± 0.97 | 6.25 ± 2.70 56.61 ± 3.39 | 81.68 ± 12.02 88.44 ± 3.00 | 70.03 ± 7.06 81.09 ± 3.57 | 27.06 ± 7.77 26.45 ± 3.69 |
| Fish#2 | 71.96 ± 5.07 70.35 ± 2.93 | 18.41 ± 6.11 31.47 ± 3.65 | 68.26 ± 8.92 85.89 ± 1.46 | 58.53 ± 8.30 62.82 ± 3.76 | 9.17 ± 2.99 19.70 ± 2.41 |
| Fish#3 | 79.23 ± 2.77 86.45 ± 0.95 | 15.80 ± 4.82 21.62 ± 2.60 | 87.93 ± 5.72 87.57 ± 5.67 | 76.24 ± 7.56 91.45 ± 1.45 | 9.44 ± 4.90 20.67 ± 1.92 |
| Fish#4 | 40.06 ± 5.96 54.21 ± 4.14 | 31.34 ± 12.27 45.49 ± 3.90 | 91.56 ± 3.80 91.39 ± 1.31 | 75.97 ± 5.86 90.65 ± 1.40 | 21.53 ± 12.15 28.13 ± 3.72 |
| The mean value | 67.65 ± 3.19 74.24 ± 2.31 | 17.95 ± 3.92 38.80 ± 3.20 | 82.36 ± 4.26 88.32 ± 1.62 | 70.19 ± 3.66 81.50 ± 2.15 | 16.80 ± 3.96 23.74 ± 1.61 |
| Rapid movement time (%) | |||||
| Fish#1 | 0.56 ± 0.13 2.03 ± 0.30 | 0.12 ± 0.09 1.64 ± 0.52 | 0.96 ± 0.24 0.67 ± 0.15 | 1.85 ± 0.85 0.33 ± 0.05 | 0.05 ± 0.02 0.04 ± 0.02 |
| Fish#2 | 0.02 ± 0.02 0.12 ± 0.04 | 0.29 ± 0.12 0.32 ± 0.06 | 0.19 ± 0.14 1.27 ± 0.31 | 0.49 ± 0.18 0.15 ± 0.05 | 0.05 ± 0.05 0.10 ± 0.04 |
| Fish#3 | 0.16 ± 0.06 0.22 ± 0.06 | 0.19 ± 0.12 0.14 ± 0.10 | 0.39 ± 0.21 2.63 ± 1.27 | 0.83 ± 0.46 0.54 ± 0.24 | 0.01 ± 0.01 0.08 ± 0.02 |
| Fish#4 | 0.31 ± 0.16 0.24 ± 0.11 | 0.17 ± 0.11 0.23 ± 0.04 | 1.97 ± 0.75 1.72 ± 0.47 | 0.32 ± 0.18 0.56 ± 0.14 | 0.15 ± 0.07 0.13 ± 0.03 |
| The mean value | 0.26 ± 0.06 0.65 ± 0.14 | 0.19 ± 0.05 0.58 ± 0.18 | 0.88 ± 0.24 1.57 ± 0.36 | 0.87 ± 0.26 0.40 ± 0.07 | 0.06 ± 0.02 0.09 ± 0.01 |
| Meandering (°/cm) | |||||
| Fish#1 | 58.29 ± 3.51 45.46 ± 1.66 | 101.93 ± 15.01 63.16 ± 5.39 | 31.00 ± 6.04 32.29 ± 1.30 | 26.09 ± 2.16 37.04 ± 2.03 | 76.76 ± 14.60 119.31 ± 23.26 |
| Fish#2 | 31.65 ± 2.23 35.13 ± 2.40 | 57.87 ± 17.30 76.49 ± 12.58 | 23.83 ± 2.12 28.85 ± 2.75 | 43.72 ± 11.87 34.02 ± 2.29 | 68.34 ± 6.90 56.54 ± 6.03 |
| Fish#3 | 23.99 ± 2.88 28.77 ± 1.12 | 61.21 ± 15.98 54.42 ± 5.55 | 25.31 ± 1.64 43.15 ± 10.36 | 42.65 ± 10.51 30.77 ± 1.43 | 80.05 ± 32.96 59.14 ± 17.93 |
| Fish#4 | 47.67 ± 10.43 51.15 ± 8.07 | 53.57 ± 8.86 51.59 ± 5.50 | 18.91 ± 1.10 20.71 ± 0.64 | 25.72 ± 2.39 29.13 ± 2.68 | 73.19 ± 10.43 69.23 ± 8.82 |
| The mean value | 40.40 ± 3.42 40.13 ± 2.45 | 68.64 ± 7.94 61.42 ± 4.27 | 24.76 ± 1.80 31.25 ± 3.03 | 34.55 ± 4.11 32.74 ± 1.13 | 74.59 ± 8.94 76.05 ± 9.01 |
| Wall preference index | |||||
| Fish#1 | 0.79 ± 0.07 0.81 ± 0.02 | 0.08 ± 0.06 0.57 ± 0.05 | 0.62 ± 0.09 0.49 ± 0.03 | 0.44 ± 0.07 0.53 ± 0.04 | 0.57 ± 0.16 0.61 ± 0.08 |
| Fish#2 | 0.41 ± 0.05 0.52 ± 0.03 | 0.18 ± 0.10 0.29 ± 0.04 | 0.44 ± 0.04 0.51 ± 0.03 | 0.41 ± 0.09 0.34 ± 0.04 | 0.09 ± 0.07 0.26 ± 0.07 |
| Fish#3 | 0.48 ± 0.04 0.59 ± 0.04 | 0.34 ± 0.10 0.30 ± 0.04 | 0.40 ± 0.13 0.59 ± 0.09 | 0.39 ± 0.11 0.51 ± 0.06 | 0.49 ± 0.15 0.57 ± 0.09 |
| Fish#4 | 0.45 ± 0.09 0.49 ± 0.04 | 0.29 ± 0.15 0.45 ± 0.04 | 0.64 ± 0.08 0.57 ± 0.04 | 0.45 ± 0.08 0.63 ± 0.03 | 0.62 ± 0.15 0.36 ± 0.06 |
| The mean value | 0.53 ± 0.04 0.60 ± 0.02 | 0.22 ± 0.05 0.40 ± 0.03 | 0.52 ± 0.05 0.54 ± 0.03 | 0.42 ± 0.04 0.50 ± 0.03 | 0.44 ± 0.08 0.45 ± 0.05 |
| Parameter | Events after FDGV n = 8 | Event before FDGV n = 8 | Event Coincided with FDGV n = 4 |
|---|---|---|---|
| Avg. swimming speed (cm/s) | 1.93 ± 0.17 2.27 ± 0.24 * | 0.60 ± 0.08 1.10 ± 0.20 † | 2.93 ± 0.35 3.01 ± 0.26 |
| Freezing time (%) | 30.51 ± 4.78 21.60 ± 4.99 ** | 82.50 ± 3.18 68.40 ± 4.82 * | 16.77 ± 5.41 10.11 ± 1.24 |
| Swimming time (%) | 68.92 ± 4.76 77.87 ± 4.89 ** | 17.37 ± 3.17 31.27 ± 4.66 * | 82.36 ± 5.12 88.32 ± 1.15 |
| Rapid movement time (%) | 0.57 ± 0.20 0.53 ± 0.22 | 0.13 ± 0.03 0.34 ± 0.19 | 0.88 ± 0.40 1.57 ± 0.42 |
| Meandering (°/cm) | 37.47 ± 4.41 36.43 ± 2.84 | 71.62 ± 5.43 68.74 ± 7.78 | 24.76 ± 2.49 31.25 ± 4.65 |
| Wall preference index | 0.48 ± 0.05 0.55 ± 0.05 * | 0.33 ± 0.07 0.43 ± 0.05 | 0.52 ± 0.06 0.54 ± 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krylov, V.V. Influence of Geomagnetic Disturbances at Different Times of Day on Locomotor Activity in Zebrafish (Danio Rerio). Clocks & Sleep 2021, 3, 624-632. https://doi.org/10.3390/clockssleep3040045
Krylov VV. Influence of Geomagnetic Disturbances at Different Times of Day on Locomotor Activity in Zebrafish (Danio Rerio). Clocks & Sleep. 2021; 3(4):624-632. https://doi.org/10.3390/clockssleep3040045
Chicago/Turabian StyleKrylov, Viacheslav V. 2021. "Influence of Geomagnetic Disturbances at Different Times of Day on Locomotor Activity in Zebrafish (Danio Rerio)" Clocks & Sleep 3, no. 4: 624-632. https://doi.org/10.3390/clockssleep3040045
APA StyleKrylov, V. V. (2021). Influence of Geomagnetic Disturbances at Different Times of Day on Locomotor Activity in Zebrafish (Danio Rerio). Clocks & Sleep, 3(4), 624-632. https://doi.org/10.3390/clockssleep3040045





