The Effect of General Anaesthesia on Circadian Rhythms in Behaviour and Clock Gene Expression of Drosophila melanogaster
Abstract
1. Introduction
2. Results
2.1. Isoflurane Causes Behavioural Phase Shifts in Locomotor Activity
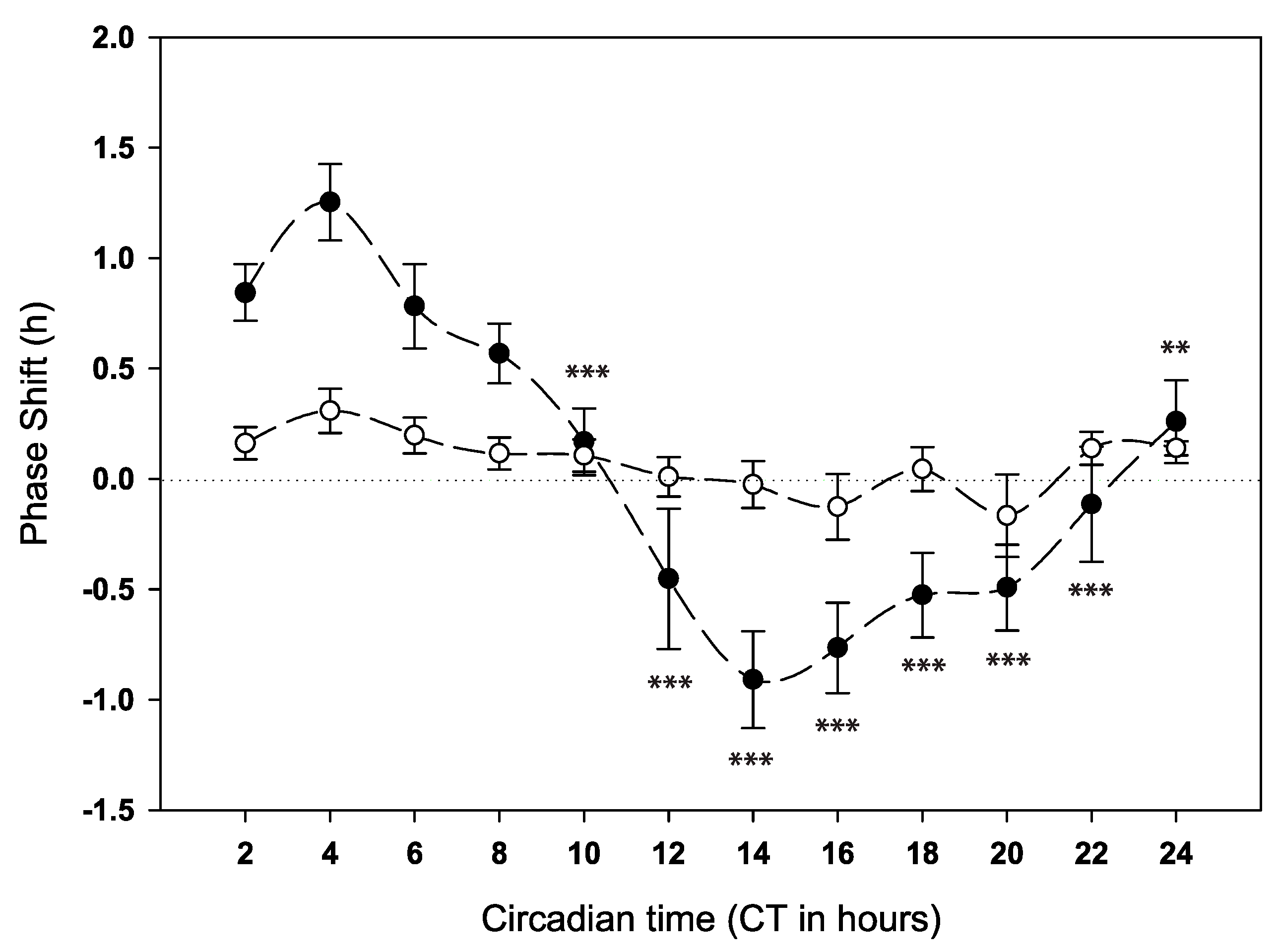
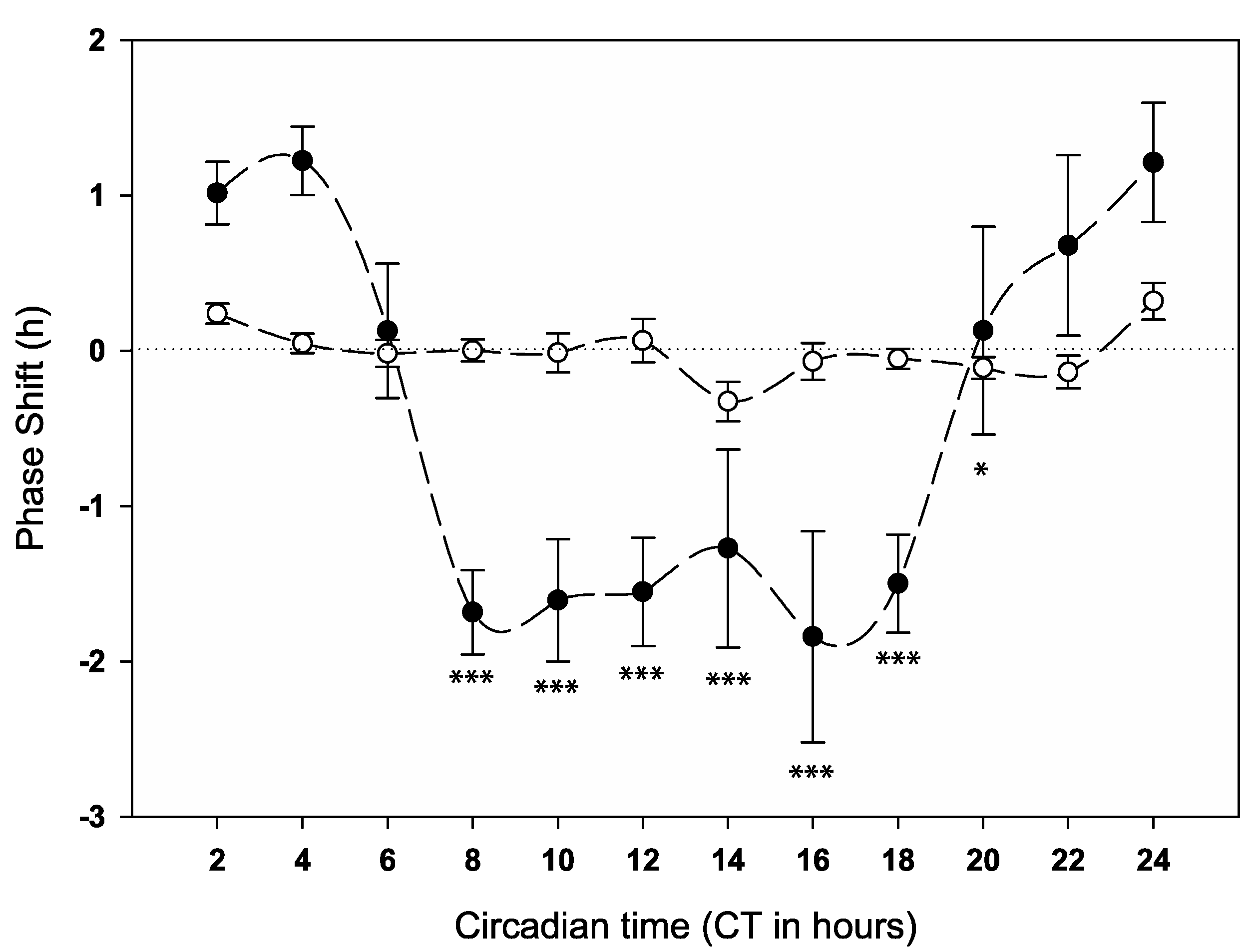
2.2. Behavioural Phase Response to Anaesthesia at Different Times of Day
2.3. General Anaesthesia Affects Molecular Clock Components in a Time–Dependent Manner
3. Discussion
4. Materials and Methods
4.1. Locomotor Activity Experiments Including Dose, Duration, and Phase Responses
4.2. Clock Gene Expression Experiments
4.3. Analysis of Bioluminescence Data
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Franks, N.P. Molecular targets underlying general anaesthesia. Br. J. Pharmacol. 2006, 147, S72–S81. [Google Scholar] [CrossRef] [PubMed]
- Veleri, S.; Brandes, C.; Helfrich-Foerster, C.; Hall, J.C.; Stanewsky, R. A Self-Sustaining, Light-Entrainable Circadian Oscillator in the Drosophila Brain. Curr. Biol. 2003, 13, 1758–1767. [Google Scholar] [CrossRef]
- Ceriani, M.F.; HogenEsch, J.B.; Yanovsky, M.; Panda, S.; Straume, M.; Kay, S.A. Genome-Wide Expression Analysis in DrosophilaReveals Genes Controlling Circadian Behavior. J. Neurosci. 2002, 22, 9305–9319. [Google Scholar] [CrossRef]
- Jaramillo, A.M.; Zheng, X.; Zhou, Y.; Amado, D.A.; Sheldon, A.; Sehgal, A.; Levitan, I.B. Pattern of distribution and cycling of SLOB, Slowpoke channel binding protein, in Drosophila. BMC Neurosci. 2004, 5, 3. [Google Scholar] [CrossRef]
- Cheeseman, J.F.; Winnebeck, E.C.; Millar, C.D.; Kirkland, L.S.; Sleigh, J.; Goodwin, M.; Pawley, M.D.M.; Bloch, G.; Lehmann, K.; Menzel, R.; et al. General anesthesia alters time perception by phase shifting the circadian clock. Proc. Natl. Acad. Sci. USA 2012, 109, 7061–7066. [Google Scholar] [CrossRef] [PubMed]
- Ko, M.L.; Jian, K.; Shi, L.; Ko, G.Y.-P. Phosphatidylinositol 3 kinase-Akt signaling serves as a circadian output in the retina. J. Neurochem. 2009, 108, 1607–1620. [Google Scholar] [CrossRef]
- Young, M.W.; Kay, S.A. Time zones: A comparative genetics of circadian clocks. Nat. Rev. Genet. 2001, 2, 702–715. [Google Scholar] [CrossRef]
- Lowrey, P.L.; Takahashi, J.S. Genetics of Circadian Rhythms in Mammalian Model Organisms. BT—The Genetics of Circadian Rhythms. In Advances in Genetics; Elsevier: San Diego, CA, USA, 2011; Volume 74, pp. 175–230. [Google Scholar] [CrossRef]
- Meyer-Bernstein, E.L.; Sehgal, A. Book Review: Molecular Regulation of Circadian Rhythms in Drosophila and Mammals. Neuroscientist 2001, 7, 496–505. [Google Scholar] [CrossRef]
- Stanewsky, R. Clock mechanisms in Drosophila. Cell Tissue Res. 2002, 309, 11–26. [Google Scholar] [CrossRef]
- Hardin, P.E. Molecular Genetic Analysis of Circadian Timekeeping in Drosophila BT—The Genetics of Circadian Rhythms. In Advances in Genetics; Elsevier: San Diego, CA, USA, 2011; Volume 74, pp. 141–173. [Google Scholar] [CrossRef]
- Aschoff, J. Exogenous and Endogenous Components in Circadian Rhythms. Cold Spring Harb. Symp. Quant. Biol. 1960, 25, 11–28. [Google Scholar] [CrossRef] [PubMed]
- Pittendrigh, C.S. On temperature independence in the clock system controlling emergence time in drosophila. Proc. Natl. Acad. Sci. USA 1954, 40, 1018–1029. [Google Scholar] [CrossRef] [PubMed]
- Nickalls, R.W.D.; Mapleson, W.W. Age-related iso-MAC charts for isoflurane, sevoflurane and desflurane in man. Br. J. Anaesth. 2003, 91, 170–174. [Google Scholar] [CrossRef] [PubMed]
- Nunn, M.J.F. Isoflurane as a routine anaesthetic in general surgical practice. Br. J. Anaesth. 1985, 57, 461–475. [Google Scholar] [CrossRef] [PubMed]
- Vanin, S.; Bhutani, S.; Montelli, S.; Menegazzi, P.; Green, E.W.; Pegoraro, M.; Sandrelli, F.; Costa, R.; Kyriacou, C.P. Unexpected features of Drosophila circadian behavioural rhythms under natural conditions. Nature 2012, 484, 371–375. [Google Scholar] [CrossRef] [PubMed]
- Rubin, E.B.; Shemesh, Y.; Cohen, M.; Elgavish, S.; Robertson, H.M.; Bloch, G. Molecular and phylogenetic analyses reveal mammalian-like clockwork in the honey bee (Apis mellifera) and shed new light on the molecular evolution of the circadian clock. Genome Res. 2006, 16, 1352–1365. [Google Scholar] [CrossRef] [PubMed]
- Yoshii, T.; Wülbeck, C.; Sehadova, H.; Veleri, S.; Bichler, D.; Stanewsky, R.; Helfrich-Förster, C.; Wulbeck, C.; Sehadova, H.; Veleri, S.; et al. The Neuropeptide Pigment-Dispersing Factor Adjusts Period and Phase of Drosophila’s Clock. J. Neurosci. 2009, 29, 2597–2610. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, N.; Stanewsky, R.; Popay, T.; Warman, G.; Cheeseman, J. The Effect of General Anaesthesia on Circadian Rhythms in Behaviour and Clock Gene Expression of Drosophila melanogaster. Clocks & Sleep 2020, 2, 434-441. https://doi.org/10.3390/clockssleep2040032
Li N, Stanewsky R, Popay T, Warman G, Cheeseman J. The Effect of General Anaesthesia on Circadian Rhythms in Behaviour and Clock Gene Expression of Drosophila melanogaster. Clocks & Sleep. 2020; 2(4):434-441. https://doi.org/10.3390/clockssleep2040032
Chicago/Turabian StyleLi, Nina, Ralf Stanewsky, Tessa Popay, Guy Warman, and James Cheeseman. 2020. "The Effect of General Anaesthesia on Circadian Rhythms in Behaviour and Clock Gene Expression of Drosophila melanogaster" Clocks & Sleep 2, no. 4: 434-441. https://doi.org/10.3390/clockssleep2040032
APA StyleLi, N., Stanewsky, R., Popay, T., Warman, G., & Cheeseman, J. (2020). The Effect of General Anaesthesia on Circadian Rhythms in Behaviour and Clock Gene Expression of Drosophila melanogaster. Clocks & Sleep, 2(4), 434-441. https://doi.org/10.3390/clockssleep2040032





