MicroRNA: A Key Player for the Interplay of Circadian Rhythm Abnormalities, Sleep Disorders and Neurodegenerative Diseases
Abstract
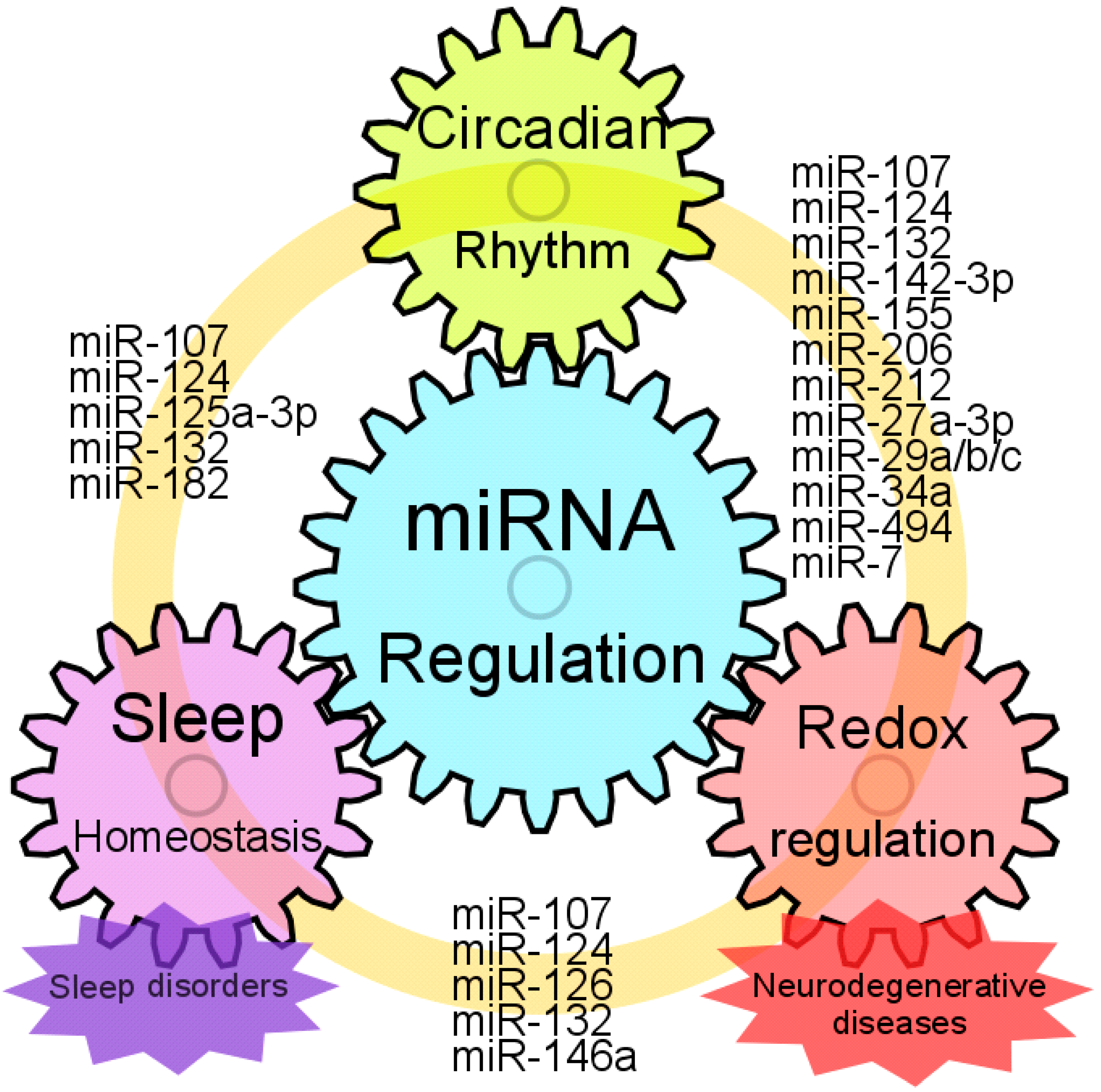
1. Introduction
2. Molecular Basis of Circadian Clock System
3. MiRNA Biosynthesis
4. Circadian Regulation of miRNA
5. Interplay of Circadian Genes and miRNAs
6. Sleep Disorder, Circadian Rhythm Disorder and miRNA Regulation
7. Circadian Rhythm and Neurodegenerative Diseases
7.1. Alzheimer’s Disease
7.2. Parkinson’s Disease
7.3. Amyotrophic Lateral Sclerosis (ALS)
7.4. Huntington’s Disease
7.5. Multiple System Atrophy
8. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Sartor, F.; Eelderink-Chen, Z.; Aronson, B.; Bosman, J.; Hibbert, L.E.; Dodd, A.N.; Kovács, Á.T.; Merrow, M. Are There Circadian Clocks in Non-Photosynthetic Bacteria? Biology 2019, 8, 41. [Google Scholar] [CrossRef] [PubMed]
- Bhadra, U.; Thakkar, N.; Das, P.; Pal Bhadra, M. Evolution of circadian rhythms: From bacteria to human. Sleep Med. 2017, 35, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Mohawk, J.A.; Green, C.B.; Takahashi, J.S. Central and Peripheral Circadian Clocks in Mammals. Annu. Rev. Neurosci. 2012, 35, 445–462. [Google Scholar] [CrossRef] [PubMed]
- Tsang, A.H.; Astiz, M.; Leinweber, B.; Oster, H. Rodent Models for the Analysis of Tissue Clock Function in Metabolic Rhythms Research. Front. Endocrinol. 2017, 8, 27. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.L.; Sehgal, A. 11-Circadian Rhythms and Disease. In Emery and Rimoin’s Principles and Practice of Medical Genetics and Genomics, 7th ed.; Pyeritz, R.E., Korf, B.R., Grody, W.W., Eds.; Elsevier, Academic Press: London, UK, 2019; pp. 299–314. [Google Scholar] [CrossRef]
- Robertson, M.P.; Joyce, G.F. The Origins of the RNA World. Cold Spring Harb. Perspect. Biol. 2012, 4. [Google Scholar] [CrossRef]
- Wright, M.W.; Bruford, E.A. Naming ‘junk’: Human non-protein coding RNA (ncRNA) gene nomenclature. Hum. Genom. 2011, 5, 90–98. [Google Scholar] [CrossRef]
- Cora’, D.; Re, A.; Caselle, M.; Bussolino, F. MicroRNA-mediated regulatory circuits: Outlook and perspectives. Phys. Biol. 2017, 14, 045001. [Google Scholar] [CrossRef]
- Cheng, H.Y.M.; Papp, J.W.; Varlamova, O.; Dziema, H.; Russell, B.; Curfman, J.P.; Nakazawa, T.; Shimizu, K.; Okamura, H.; Impey, S.; et al. microRNA modulation of circadian-clock period and entrainment. Neuron 2007, 54, 813–829. [Google Scholar] [CrossRef]
- Piletič, K.; Kunej, T. MicroRNA epigenetic signatures in human disease. Arch. Toxicol. 2016, 90, 2405–2419. [Google Scholar] [CrossRef]
- Takahashi, J.S. Transcriptional architecture of the mammalian circadian clock. Nat. Rev. Genet. 2017, 18, 164–179. [Google Scholar] [CrossRef]
- Landgraf, D.; Wang, L.L.; Diemer, T.; Welsh, D.K. NPAS2 Compensates for Loss of CLOCK in Peripheral Circadian Oscillators. PLoS Genet. 2016, 12, e1005882. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Viveros, L.; Bouchard-Cannon, P.; Hegazi, S.; Cheng, A.H.; Pastore, S.; Cheng, H.-Y.M. Molecular modulators of the circadian clock: Lessons from flies and mice. Cell. Mol. Life Sci. 2017, 74, 1035–1059. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Target recognition and regulatory functions. Cell 2009, 136, 215–233. [Google Scholar] [CrossRef] [PubMed]
- Mehta, N.; Cheng, H.Y. Micro-managing the circadian clock: The role of microRNAs in biological timekeeping. J. Mol. Biol. 2013, 425, 3609–3624. [Google Scholar] [CrossRef] [PubMed]
- Shende, V.R.; Neuendorff, N.; Earnest, D.J. Role of miR-142-3p in the post-transcriptional regulation of the clock gene Bmal1 in the mouse SCN. PLoS ONE 2013, 8, e65300. [Google Scholar] [CrossRef] [PubMed]
- Gatfield, D.; Le Martelot, G.; Vejnar, C.E.; Gerlach, D.; Schaad, O.; Fleury-Olela, F.; Ruskeepaa, A.L.; Oresic, M.; Esau, C.C.; Zdobnov, E.M.; et al. Integration of microRNA miR-122 in hepatic circadian gene expression. Genes Dev. 2009, 23, 1313–1326. [Google Scholar] [CrossRef]
- Mendoza-Viveros, L.; Chiang, C.K.; Ong, J.L.K.; Hegazi, S.; Cheng, A.H.; Bouchard-Cannon, P.; Fana, M.; Lowden, C.; Zhang, P.; Bothorel, B.; et al. miR-132/212 Modulates Seasonal Adaptation and Dendritic Morphology of the Central Circadian Clock. Cell Rep. 2017, 19, 505–520. [Google Scholar] [CrossRef]
- Yan, Y.; Salazar, T.E.; Dominguez, J.M., II; Nguyen, D.V.; Li Calzi, S.; Bhatwadekar, A.D.; Qi, X.; Busik, J.V.; Boulton, M.E.; Grant, M.B. Dicer Expression Exhibits a Tissue-Specific Diurnal Pattern That Is Lost during Aging and in Diabetes. PLoS ONE 2013, 8, e80029. [Google Scholar] [CrossRef]
- Du, N.H.; Arpat, A.B.; De Matos, M.; Gatfield, D. MicroRNAs shape circadian hepatic gene expression on a transcriptome-wide scale. eLife 2014, 3, e02510. [Google Scholar] [CrossRef]
- Chen, R.; D’Alessandro, M.; Lee, C. miRNAs are required for generating a time delay critical for the circadian oscillator. Curr. Biol. 2013, 23, 1959–1968. [Google Scholar] [CrossRef]
- Na, Y.J.; Sung, J.H.; Lee, S.C.; Lee, Y.J.; Choi, Y.J.; Park, W.Y.; Shin, H.S.; Kim, J.H. Comprehensive analysis of microRNA-mRNA co-expression in circadian rhythm. Exp. Mol. Med. 2009, 41, 638–647. [Google Scholar] [CrossRef] [PubMed]
- Vollmers, C.; Schmitz, R.J.; Nathanson, J.; Yeo, G.; Ecker, J.R.; Panda, S. Circadian oscillations of protein-coding and regulatory RNAs in a highly dynamic mammalian liver epigenome. Cell Metab. 2012, 16, 833–845. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.H.; Kojima, S.; Shimomura, K.; Koike, N.; Buhr, E.D.; Furukawa, T.; Ko, C.H.; Gloston, G.; Ayoub, C.; Nohara, K.; et al. Period2 3′-UTR and microRNA-24 regulate circadian rhythms by repressing PERIOD2 protein accumulation. Proc. Natl. Acad. Sci. USA 2017, 114, E8855–E8864. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhu, X.; Cheng, S.; Xie, Y.; Wang, Z.; Liu, Y.; Jiang, Z.; Xiao, J.; Guo, H.; Wang, Y. MiR-29a/b/c regulate human circadian gene hPER1 expression by targeting its 3′UTR. Acta Biochim. Biophys. Sin. 2014, 46, 313–317. [Google Scholar] [CrossRef] [PubMed]
- Nagel, R.; Clijsters, L.; Agami, R. The miRNA-192/194 cluster regulates the Period gene family and the circadian clock. FEBS J. 2009, 276, 5447–5455. [Google Scholar] [CrossRef]
- Hasakova, K.; Reis, R.; Vician, M.; Zeman, M.; Herichova, I. Expression of miR-34a-5p is up-regulated in human colorectal cancer and correlates with survival and clock gene PER2 expression. PLoS ONE 2019, 14, e0224396. [Google Scholar] [CrossRef]
- Wang, Y.; Lv, K.; Chen, H.; Zhao, M.; Ji, G.; Zhang, Y.; Cao, H.; Kan, G.; Li, Y.; Qu, L. Functional annotation of extensively and divergently expressed miRNAs in suprachiasmatic nucleus of Clock (Δ19) mutant mice. Biosci. Rep. 2018, 38, BSR20180233. [Google Scholar] [CrossRef]
- Hong, Z.; Feng, Z.; Sai, Z.; Tao, S. PER3, a novel target of miR-103, plays a suppressive role in colorectal cancer in vitro. BMB Rep. 2014, 47, 500–505. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, B.; Yang, L.; Bai, Y.G.; Song, J.B.; Ge, Y.L.; Ma, H.Z.; Cheng, J.H.; Ma, J.; Xie, M.J. BMAL1 Disrupted Intrinsic Diurnal Oscillation in Rat Cerebrovascular Contractility of Simulated Microgravity Rats by Altering Circadian Regulation of miR-103/Ca(V)1.2 Signal Pathway. Int. J. Mol. Sci. 2019, 20, 3947. [Google Scholar] [CrossRef]
- Shende, V.R.; Goldrick, M.M.; Ramani, S.; Earnest, D.J. Expression and rhythmic modulation of circulating microRNAs targeting the clock gene Bmal1 in mice. PLoS ONE 2011, 6, e22586. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, P.; Chen, S.; Zhang, Z.; Liang, T.; Liu, C. Rhythmic expression of miR-27b-3p targets the clock gene Bmal1 at the posttranscriptional level in the mouse liver. FASEB J. 2016, 30, 2151–2160. [Google Scholar] [CrossRef] [PubMed]
- Curtis, A.M.; Fagundes, C.T.; Yang, G.; Palsson-McDermott, E.M.; Wochal, P.; McGettrick, A.F.; Foley, N.H.; Early, J.O.; Chen, L.; Zhang, H.; et al. Circadian control of innate immunity in macrophages by miR-155 targeting Bmal1. Proc. Natl. Acad. Sci. USA 2015, 112, 7231–7236. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Zhao, S.; Shen, J.; Guo, L.; Sun, Y.; Zhu, Y.; Ma, Z.; Zhang, X.; Hu, Y.; Xiao, W.; et al. The MiR-135b-BMAL1-YY1 loop disturbs pancreatic clockwork to promote tumourigenesis and chemoresistance. Cell Death Dis. 2018, 9, 149. [Google Scholar] [CrossRef] [PubMed]
- Horii, R.; Honda, M.; Shirasaki, T.; Shimakami, T.; Shimizu, R.; Yamanaka, S.; Murai, K.; Kawaguchi, K.; Arai, K.; Yamashita, T.; et al. MicroRNA-10a Impairs Liver Metabolism in Hepatitis C Virus-Related Cirrhosis Through Deregulation of the Circadian Clock Gene Brain and Muscle Aryl Hydrocarbon Receptor Nuclear Translocator-Like 1. Hepatol. Commun. 2019, 3, 1687–1703. [Google Scholar] [CrossRef] [PubMed]
- Bu, Y.; Yoshida, A.; Chitnis, N.; Altman, B.J.; Tameire, F.; Oran, A.; Gennaro, V.; Armeson, K.E.; McMahon, S.B.; Wertheim, G.B.; et al. A PERK-miR-211 axis suppresses circadian regulators and protein synthesis to promote cancer cell survival. Nat. Cell Biol. 2018, 20, 104–115. [Google Scholar] [CrossRef]
- Chi, S.W.; Zang, J.B.; Mele, A.; Darnell, R.B. Argonaute HITS-CLIP decodes microRNA–mRNA interaction maps. Nature 2009, 460, 479–486. [Google Scholar] [CrossRef]
- Kondratov, R.V.; Chernov, M.V.; Kondratova, A.A.; Gorbacheva, V.Y.; Gudkov, A.V.; Antoch, M.P. BMAL1-dependent circadian oscillation of nuclear CLOCK: Posttranslational events induced by dimerization of transcriptional activators of the mammalian clock system. Genes Dev. 2003, 17, 1921–1932. [Google Scholar] [CrossRef]
- Yoshitane, H.; Takao, T.; Satomi, Y.; Du, N.-H.; Okano, T.; Fukada, Y. Roles of CLOCK Phosphorylation in Suppression of E-Box-Dependent Transcription. Mol. Cell Biol. 2009, 29, 3675–3686. [Google Scholar] [CrossRef]
- Daimiel-Ruiz, L.; Klett-Mingo, M.; Konstantinidou, V.; Micó, V.; Aranda, J.F.; García, B.; Martínez-Botas, J.; Dávalos, A.; Fernández-Hernando, C.; Ordovás, J.M. Dietary lipids modulate the expression of miR-107, an miRNA that regulates the circadian system. Mol. Nutr. Food Res. 2015, 59, 552–565. [Google Scholar] [CrossRef]
- Li, A.; Lin, X.; Tan, X.; Yin, B.; Han, W.; Zhao, J.; Yuan, J.; Qiang, B.; Peng, X. Circadian gene Clock contributes to cell proliferation and migration of glioma and is directly regulated by tumor-suppressive miR-124. FEBS Lett. 2013, 587, 2455–2460. [Google Scholar] [CrossRef]
- Kiriakidou, M.; Nelson, P.T.; Kouranov, A.; Fitziev, P.; Bouyioukos, C.; Mourelatos, Z.; Hatzigeorgiou, A. A combined computational-experimental approach predicts human microRNA targets. Genes Dev. 2004, 18, 1165–1178. [Google Scholar] [CrossRef] [PubMed]
- Jacovetti, C.; Rodriguez-Trejo, A.; Guay, C.; Sobel, J.; Gattesco, S.; Petrenko, V.; Saini, C.; Dibner, C.; Regazzi, R. MicroRNAs modulate core-clock gene expression in pancreatic islets during early postnatal life in rats. Diabetologia 2017, 60, 2011–2020. [Google Scholar] [CrossRef]
- Gao, Q.; Zhou, L.; Yang, S.Y.; Cao, J.M. A novel role of microRNA 17-5p in the modulation of circadian rhythm. Sci. Rep. 2016, 6, 30070. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Sun, B.; Huang, J.; Xu, L.; Pan, J.; Fang, C.; Tao, Y.; Hu, S.; Li, R.; Han, X.; et al. The role of miR-182 in regulating pineal CLOCK expression after hypoxia-ischemia brain injury in neonatal rats. Neurosci. Lett. 2015, 591, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Yuan, P.; Yang, T.; Mu, J.; Zhao, J.; Yang, Y.; Yan, Z.; Hou, Y.; Chen, C.; Xing, J.; Zhang, H.; et al. Circadian clock gene NPAS2 promotes reprogramming of glucose metabolism in hepatocellular carcinoma cells. Cancer Lett. 2020, 469, 498–509. [Google Scholar] [CrossRef]
- Huang, Z.; Zhao, X.; Wu, X.; Xiang, L.; Yuan, Y.; Zhou, S.; Yu, W. LncRNA UCA1 facilitated cell growth and invasion through the miR-206/CLOCK axis in glioma. Cancer Cell Int. 2019, 19, 316. [Google Scholar] [CrossRef] [PubMed]
- Linnstaedt, S.D.; Rueckeis, C.A.; Riker, K.D.; Pan, Y.; Wu, A.; Yu, S.; Wanstrath, B.; Gonzalez, M.; Harmon, E.; Green, P.; et al. MicroRNA-19b predicts widespread pain and posttraumatic stress symptom risk in a sex-dependent manner following trauma exposure. Pain 2020, 161, 47–60. [Google Scholar] [CrossRef]
- Zheng, X.; Wu, K.; Liao, S.; Pan, Y.; Sun, Y.; Chen, X.; Zhang, Y.; Xia, S.; Hu, Y.; Zhang, J. MicroRNA-transcription factor network analysis reveals miRNAs cooperatively suppress RORA in oral squamous cell carcinoma. Oncogenesis 2018, 7, 79. [Google Scholar] [CrossRef]
- Lee, K.H.; Kim, S.H.; Lee, H.R.; Kim, W.; Kim, D.Y.; Shin, J.C.; Yoo, S.H.; Kim, K.T. MicroRNA-185 oscillation controls circadian amplitude of mouse Cryptochrome 1 via translational regulation. Mol. Biol. Cell 2013, 24, 2248–2255. [Google Scholar] [CrossRef]
- Liu, L.; Pang, X.L.; Shang, W.J.; Xie, H.C.; Wang, J.X.; Feng, G.W. Over-expressed microRNA-181a reduces glomerular sclerosis and renal tubular epithelial injury in rats with chronic kidney disease via down-regulation of the TLR/NF-κB pathway by binding to CRY1. Mol. Med. 2018, 24, 49. [Google Scholar] [CrossRef]
- Tang, Z.; Xu, T.; Li, Y.; Fei, W.; Yang, G.; Hong, Y. Inhibition of CRY2 by STAT3/miRNA-7-5p Promotes Osteoblast Differentiation through Upregulation of CLOCK/BMAL1/P300 Expression. Mol. Ther. Nucleic Acids 2020, 19, 865–876. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Chen, X.; Lei, T.; Gu, Y.; Gu, J.; Huang, J.; Lu, B.; Yuan, L.; Sun, M.; Wang, Z. Integrative Analysis of NSCLC Identifies LINC01234 as an Oncogenic lncRNA that Interacts with HNRNPA2B1 and Regulates miR-106b Biogenesis. Mol. Ther. 2020, 28, 1479–1493. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Zhu, Y.; Hong, X.; Zhang, M.; Qiu, X.; Wang, Z.; Qi, Z.; Hong, X. miR-181d and c-myc-mediated inhibition of CRY2 and FBXL3 reprograms metabolism in colorectal cancer. Cell Death Dis. 2017, 8, e2958. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, M.; Jakovcevski, M.; Polacheck, T.; Drori, Y.; Luoni, A.; Röh, S.; Zaugg, J.; Ben-Dor, S.; Albrecht, C.; Chen, A. Placental miR-340 mediates vulnerability to activity based anorexia in mice. Nat. Commun. 2018, 9, 1596. [Google Scholar] [CrossRef]
- Zhao, Q.; Sun, H.; Yin, L.; Wang, L. miR-126a-5p-Dbp and miR-31a-Crot/Mrpl4 interaction pairs crucial for the development of hypertension and stroke. Mol. Med. Rep. 2019, 20, 4151–4167. [Google Scholar] [CrossRef]
- Xu, S.; Witmer, P.D.; Lumayag, S.; Kovacs, B.; Valle, D. MicroRNA (miRNA) Transcriptome of Mouse Retina and Identification of a Sensory Organ-specific miRNA Cluster. J. Biol. Chem. 2007, 282, 25053–25066. [Google Scholar] [CrossRef]
- Surendran, S.; Jideonwo, V.N.; Merchun, C.; Ahn, M.; Murray, J.; Ryan, J.; Dunn, K.W.; Kota, J.; Morral, N. Gene targets of mouse miR-709: Regulation of distinct pools. Sci. Rep. 2016, 6, 18958. [Google Scholar] [CrossRef]
- Mauvoisin, D.; Wang, J.; Jouffe, C.; Martin, E.; Atger, F.; Waridel, P.; Quadroni, M.; Gachon, F.; Naef, F. Circadian clock-dependent and -independent rhythmic proteomes implement distinct diurnal functions in mouse liver. Proc. Natl. Acad. Sci. USA 2014, 111, 167–172. [Google Scholar] [CrossRef]
- Menet, J.S.; Rodriguez, J.; Abruzzi, K.C.; Rosbash, M. Nascent-Seq reveals novel features of mouse circadian transcriptional regulation. eLife 2012, 1, e00011. [Google Scholar] [CrossRef]
- Robles, M.S.; Cox, J.; Mann, M. In-vivo quantitative proteomics reveals a key contribution of post-transcriptional mechanisms to the circadian regulation of liver metabolism. PLoS Genet. 2014, 10, e1004047. [Google Scholar] [CrossRef]
- Davis, C.J.; Bohnet, S.G.; Meyerson, J.M.; Krueger, J.M. Sleep loss changes microRNA levels in the brain: A possible mechanism for state-dependent translational regulation. Neurosci. Lett. 2007, 422, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Karabulut, S.; Korkmaz Bayramov, K.; Bayramov, R.; Ozdemir, F.; Topaloglu, T.; Ergen, E.; Yazgan, K.; Taskiran, A.S.; Golgeli, A. Effects of post-learning REM sleep deprivation on hippocampal plasticity-related genes and microRNA in mice. Behav. Brain Res. 2019, 361, 7–13. [Google Scholar] [CrossRef]
- Hijmans, J.G.; Levy, M.A.; Garcia, V.; Lincenberg, G.M.; Diehl, K.J.; Greiner, J.J.; Stauffer, B.L.; DeSouza, C.A. Insufficient sleep is associated with a pro-atherogenic circulating microRNA signature. Exp. Physiol. 2019, 104, 975–982. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.J.; Clinton, J.M.; Krueger, J.M. MicroRNA 138, let-7b, and 125a inhibitors differentially alter sleep and EEG delta-wave activity in rats. J. Appl. Physiol. 2012, 113, 1756–1762. [Google Scholar] [CrossRef]
- Wang, Q.; Bozack, S.N.; Yan, Y.; Boulton, M.E.; Grant, M.B.; Busik, J.V. Regulation of retinal inflammation by rhythmic expression of MiR-146a in diabetic retina. Investig. Ophthalmol. Vis. Sci. 2014, 55, 3986–3994. [Google Scholar] [CrossRef]
- Zhu, L.; Zee, P.C. Circadian rhythm sleep disorders. Neurol. Clin. 2012, 30, 1167–1191. [Google Scholar] [CrossRef] [PubMed]
- Iwase, T.; Kajimura, N.; Uchiyama, M.; Ebisawa, T.; Yoshimura, K.; Kamei, Y.; Shibui, K.; Kim, K.; Kudo, Y.; Katoh, M.; et al. Mutation screening of the human Clock gene in circadian rhythm sleep disorders. Psychiatry Res. 2002, 109, 121–128. [Google Scholar] [CrossRef]
- Ebisawa, T.; Uchiyama, M.; Kajimura, N.; Mishima, K.; Kamei, Y.; Katoh, M.; Watanabe, T.; Sekimoto, M.; Shibui, K.; Kim, K.; et al. Association of structural polymorphisms in the human period3 gene with delayed sleep phase syndrome. EMBO Rep. 2001, 2, 342–346. [Google Scholar] [CrossRef] [PubMed]
- Ebisawa, T. Circadian Rhythms in the CNS and Peripheral Clock Disorders: Human Sleep Disorders and Clock Genes. J. Pharmacol. Sci. 2007, 103, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Toh, K.L.; Jones, C.R.; He, Y.; Eide, E.J.; Hinz, W.A.; Virshup, D.M.; Ptácek, L.J.; Fu, Y.H. An hPer2 phosphorylation site mutation in familial advanced sleep phase syndrome. Science 2001, 291, 1040–1043. [Google Scholar] [CrossRef]
- Xu, Y.; Toh, K.L.; Jones, C.R.; Shin, J.Y.; Fu, Y.H.; Ptáček, L.J. Modeling of a Human Circadian Mutation Yields Insights into Clock Regulation by PER2. Cell 2007, 128, 59–70. [Google Scholar] [CrossRef]
- Hirano, A.; Shi, G.; Jones, C.R.; Lipzen, A.; Pennacchio, L.A.; Xu, Y.; Hallows, W.C.; McMahon, T.; Yamazaki, M.; Ptáček, L.J.; et al. A Cryptochrome 2 mutation yields advanced sleep phase in humans. eLife 2016, 5, e16695. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.; Steinberg, J.; Patel, P. Insomnia in the Elderly: A Review. J. Clin. Sleep Med. 2018, 14, 1017–1024. [Google Scholar] [CrossRef] [PubMed]
- Serretti, A.; Benedetti, F.; Mandelli, L.; Lorenzi, C.; Pirovano, A.; Colombo, C.; Smeraldi, E. Genetic dissection of psychopathological symptoms: Insomnia in mood disorders and CLOCK gene polymorphism. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2003, 121b, 35–38. [Google Scholar] [CrossRef] [PubMed]
- Serretti, A.; Cusin, C.; Benedetti, F.; Mandelli, L.; Pirovano, A.; Zanardi, R.; Colombo, C.; Smeraldi, E. Insomnia improvement during antidepressant treatment and CLOCK gene polymorphism. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2005, 137b, 36–39. [Google Scholar] [CrossRef]
- Pirovano, A.; Lorenzi, C.; Serretti, A.; Ploia, C.; Landoni, S.; Catalano, M.; Smeraldi, E. Two new rare variants in the circadian “clock” gene may influence sleep pattern. Genet. Med. 2005, 7, 455–457. [Google Scholar] [CrossRef]
- Gao, C.; Shi, Q.; Wei, J.; Zhou, W.; Xiao, K.; Wang, J.; Shi, Q.; Dong, X.P. The associations of two SNPs in miRNA-146a and one SNP in ZBTB38-RASA2 with the disease susceptibility and the clinical features of the Chinese patients of sCJD and FFI. Prion 2018, 12, 34–41. [Google Scholar] [CrossRef]
- Saus, E.; Soria, V.; Escaramís, G.; Vivarelli, F.; Crespo, J.M.; Kagerbauer, B.; Menchón, J.M.; Urretavizcaya, M.; Gratacòs, M.; Estivill, X. Genetic variants and abnormal processing of pre-miR-182, a circadian clock modulator, in major depression patients with late insomnia. Hum. Mol. Genet. 2010, 19, 4017–4025. [Google Scholar] [CrossRef]
- Berkowski, J.A.; Shelgikar, A.V. Disorders of Excessive Daytime Sleepiness Including Narcolepsy and Idiopathic Hypersomnia. Sleep Med. Clin. 2016, 11, 365–378. [Google Scholar] [CrossRef]
- Coelho, F.M.; Pradella-Hallinan, M.; Predazzoli Neto, M.; Bittencourt, L.R.; Tufik, S. Prevalence of the HLA-DQB1*0602 allele in narcolepsy and idiopathic hypersomnia patients seen at a sleep disorders outpatient unit in São Paulo. Braz. J. Psychiatry 2009, 31, 10–14. [Google Scholar] [CrossRef]
- Miyagawa, T.; Toyoda, H.; Kanbayashi, T.; Imanishi, A.; Sagawa, Y.; Kotorii, N.; Kotorii, T.; Hashizume, Y.; Ogi, K.; Hiejima, H.; et al. An association analysis of HLA-DQB1 with narcolepsy without cataplexy and idiopathic hypersomnia with/without long sleep time in a Japanese population. Hum. Genome Var. 2015, 2, 15031. [Google Scholar] [CrossRef] [PubMed]
- Moreira, F.; Pedrazzoli, M.; dos Santos Coelho, F.M.; Pradella-Hallinan, M.; Lopes da Conceição, M.C.; Pereira Peregrino, A.J.; de Oliveira, E.C.; Tufik, S. Clock gene polymorphisms and narcolepsy in positive and negative HLA-DQB1*0602 patients. Mol. Brain Res. 2005, 140, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Lippert, J.; Halfter, H.; Heidbreder, A.; Röhr, D.; Gess, B.; Boentert, M.; Osada, N.; Young, P. Altered dynamics in the circadian oscillation of clock genes in dermal fibroblasts of patients suffering from idiopathic hypersomnia. PLoS ONE 2014, 9, e85255. [Google Scholar] [CrossRef] [PubMed]
- Landzberg, D.; Trotti, L.M. Is Idiopathic Hypersomnia a Circadian Rhythm Disorder? Curr. Sleep Med. Rep. 2019, 5, 201–206. [Google Scholar] [CrossRef]
- Mosakhani, N.; Sarhadi, V.; Panula, P.; Partinen, M.; Knuutila, S. Narcolepsy patients’ blood-based miRNA expression profiling: miRNA expression differences with Pandemrix vaccination. Acta Neurol. Scand. 2017, 136, 462–469. [Google Scholar] [CrossRef]
- Holm, A.; Bang-Berthelsen, C.H.; Knudsen, S.; Kornum, B.R.; Modvig, S.; Jennum, P.; Gammeltoft, S. miRNA profiles in plasma from patients with sleep disorders reveal dysregulation of miRNAs in narcolepsy and other central hypersomnias. Sleep 2014, 37, 1525–1533. [Google Scholar] [CrossRef]
- He, K.; Kapur, V.K. Sleep-Disordered Breathing and Excessive Daytime Sleepiness. Sleep Med. Clin. 2017, 12, 369–382. [Google Scholar] [CrossRef]
- Burioka, N.; Koyanagi, S.; Endo, M.; Takata, M.; Fukuoka, Y.; Miyata, M.; Takeda, K.; Chikumi, H.; Ohdo, S.; Shimizu, E. Clock gene dysfunction in patients with obstructive sleep apnoea syndrome. Eur. Respir. J. 2008, 32, 105–112. [Google Scholar] [CrossRef]
- Yang, M.Y.; Lin, P.W.; Lin, H.C.; Lin, P.M.; Chen, I.Y.; Friedman, M.; Hung, C.F.; Salapatas, A.M.; Lin, M.C.; Lin, S.F. Alternations of Circadian Clock Genes Expression and Oscillation in Obstructive Sleep Apnea. J. Clin. Med. 2019, 8, 1634. [Google Scholar] [CrossRef]
- Li, K.; Wei, P.; Qin, Y.; Wei, Y. MicroRNA expression profiling and bioinformatics analysis of dysregulated microRNAs in obstructive sleep apnea patients. Medicine 2017, 96, e7917. [Google Scholar] [CrossRef]
- Santamaria-Martos, F.; Benítez, I.; Ortega, F.; Zapater, A.; Giron, C.; Pinilla, L.; Pascual, L.; Cortijo, A.; Dalmases, M.; Fernandez-Real, J.M.; et al. Circulating microRNA profile as a potential biomarker for obstructive sleep apnea diagnosis. Sci. Rep. 2019, 9, 13456. [Google Scholar] [CrossRef] [PubMed]
- Knarr, M.; Nagaraj, A.B.; Kwiatkowski, L.J.; DiFeo, A. miR-181a modulates circadian rhythm in immortalized bone marrow and adipose derived stromal cells and promotes differentiation through the regulation of PER3. Sci. Rep. 2019, 9, 307. [Google Scholar] [CrossRef] [PubMed]
- Khurana, S.; Waidha, K.; Guleria, R.; Sharda, S.; Bose, S. In-silico investigations of selective miRNA-gene targets and their validation studies in obstructive sleep apnea (OSA) patient cohorts. Comput. Biol. Chem. 2020, 87, 107264. [Google Scholar] [CrossRef] [PubMed]
- Kondratova, A.A.; Kondratov, R.V. The circadian clock and pathology of the ageing brain. Nat. Rev. Neurosci. 2012, 13, 325–335. [Google Scholar] [CrossRef]
- Videnovic, A.; Zee, P.C. Consequences of Circadian Disruption on Neurologic Health. Sleep Med. Clin. 2015, 10, 469–480. [Google Scholar] [CrossRef]
- Niedzielska, E.; Smaga, I.; Gawlik, M.; Moniczewski, A.; Stankowicz, P.; Pera, J.; Filip, M. Oxidative Stress in Neurodegenerative Diseases. Mol. Neurobiol. 2016, 53, 4094–4125. [Google Scholar] [CrossRef]
- Henchcliffe, C.; Beal, M.F. Mitochondrial biology and oxidative stress in Parkinson disease pathogenesis. Nat. Clin. Pract. Neurol. 2008, 4, 600–609. [Google Scholar] [CrossRef]
- Kinoshita, C.; Aoyama, K.; Nakaki, T. Neuroprotection afforded by circadian regulation of intracellular glutathione levels: A key role for miRNAs. Free Radic. Biol. Med. 2018, 119, 17–33. [Google Scholar] [CrossRef]
- Kondratov, R.V.; Kondratova, A.A.; Gorbacheva, V.Y.; Vykhovanets, O.V.; Antoch, M.P. Early aging and age-related pathologies in mice deficient in BMAL1, the core componentof the circadian clock. Genes Dev. 2006, 20, 1868–1873. [Google Scholar] [CrossRef]
- Renoux, A.J.; Todd, P.K. Neurodegeneration the RNA way. Prog. Neurobiol. 2012, 97, 173–189. [Google Scholar] [CrossRef]
- Stakos, D.A.; Stamatelopoulos, K.; Bampatsias, D.; Sachse, M.; Zormpas, E.; Vlachogiannis, N.I.; Tual-Chalot, S.; Stellos, K. The Alzheimer’s Disease Amyloid-Beta Hypothesis in Cardiovascular Aging and Disease. J. Am. Coll. Cardiol. 2020, 75, 952–967. [Google Scholar] [CrossRef] [PubMed]
- Brzecka, A.; Leszek, J.; Ashraf, G.M.; Ejma, M.; Ávila-Rodriguez, M.F.; Yarla, N.S.; Tarasov, V.V.; Chubarev, V.N.; Samsonova, A.N.; Barreto, G.E.; et al. Sleep Disorders Associated With Alzheimer’s Disease: A Perspective. Front. Neurosci. 2018, 12, 330. [Google Scholar] [CrossRef] [PubMed]
- Hood, S.; Amir, S. Neurodegeneration and the Circadian Clock. Front. Aging Neurosci. 2017, 9, 170. [Google Scholar] [CrossRef] [PubMed]
- Cronin, P.; McCarthy, M.J.; Lim, A.S.P.; Salmon, D.P.; Galasko, D.; Masliah, E.; De Jager, P.L.; Bennett, D.A.; Desplats, P. Circadian alterations during early stages of Alzheimer’s disease are associated with aberrant cycles of DNA methylation in BMAL1. Alzheimers Dement. 2017, 13, 689–700. [Google Scholar] [CrossRef]
- Cermakian, N.; Lamont, E.W.; Boudreau, P.; Boivin, D.B. Circadian clock gene expression in brain regions of Alzheimer ’s disease patients and control subjects. J. Biol. Rhythm. 2011, 26, 160–170. [Google Scholar] [CrossRef]
- Huang, Y.; Potter, R.; Sigurdson, W.; Santacruz, A.; Shih, S.; Ju, Y.-E.; Kasten, T.; Morris, J.C.; Mintun, M.; Duntley, S.; et al. Effects of age and amyloid deposition on Aβ dynamics in the human central nervous system. Arch. Neurol. 2012, 69, 51–58. [Google Scholar] [CrossRef]
- Kress, G.J.; Liao, F.; Dimitry, J.; Cedeno, M.R.; FitzGerald, G.A.; Holtzman, D.M.; Musiek, E.S. Regulation of amyloid-β dynamics and pathology by the circadian clock. J. Exp. Med. 2018, 215, 1059–1068. [Google Scholar] [CrossRef]
- Ma, Z.; Jiang, W.; Zhang, E.E. Orexin signaling regulates both the hippocampal clock and the circadian oscillation of Alzheimer’s disease-risk genes. Sci. Rep. 2016, 6, 36035. [Google Scholar] [CrossRef]
- Bélanger, V.; Picard, N.; Cermakian, N. The circadian regulation of Presenilin-2 gene expression. Chronobiol. Int. 2006, 23, 747–766. [Google Scholar] [CrossRef]
- Iitaka, C.; Miyazaki, K.; Akaike, T.; Ishida, N. A role for glycogen synthase kinase-3beta in the mammalian circadian clock. J. Biol. Chem. 2005, 280, 29397–29402. [Google Scholar] [CrossRef]
- Sahar, S.; Zocchi, L.; Kinoshita, C.; Borrelli, E.; Sassone-Corsi, P. Regulation of BMAL1 protein stability and circadian function by GSK3beta-mediated phosphorylation. PLoS ONE 2010, 5, e8561. [Google Scholar] [CrossRef] [PubMed]
- Harada, Y.; Sakai, M.; Kurabayashi, N.; Hirota, T.; Fukada, Y. Ser-557-phosphorylated mCRY2 is degraded upon synergistic phosphorylation by glycogen synthase kinase-3 beta. J. Biol. Chem. 2005, 280, 31714–31721. [Google Scholar] [CrossRef] [PubMed]
- Swarbrick, S.; Wragg, N.; Ghosh, S.; Stolzing, A. Systematic Review of miRNA as Biomarkers in Alzheimer’s Disease. Mol. Neurobiol. 2019, 56, 6156–6167. [Google Scholar] [CrossRef] [PubMed]
- Arnes, M.; Kim, Y.A.; Lannes, J.; Alaniz, M.E.; Cho, J.D.; McCabe, B.D.; Santa-Maria, I. MiR-219 deficiency in Alzheimer’s disease contributes to neurodegeneration and memory dysfunction through post-transcriptional regulation of tau-kinase network. bioRxiv 2019. [Google Scholar] [CrossRef]
- El Fatimy, R.; Li, S.; Chen, Z.; Mushannen, T.; Gongala, S.; Wei, Z.; Balu, D.T.; Rabinovsky, R.; Cantlon, A.; Elkhal, A.; et al. MicroRNA-132 provides neuroprotection for tauopathies via multiple signaling pathways. Acta Neuropathol. 2018, 136, 537–555. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.Y.; Hernandez-Rapp, J.; Jolivette, F.; Lecours, C.; Bisht, K.; Goupil, C.; Dorval, V.; Parsi, S.; Morin, F.; Planel, E.; et al. miR-132/212 deficiency impairs tau metabolism and promotes pathological aggregation in vivo. Hum. Mol. Genet. 2015, 24, 6721–6735. [Google Scholar] [CrossRef]
- Wennström, M.; Nielsen, H.M. Cell adhesion molecules in Alzheimer’s disease. Degener. Neurol. Neuromuscul. Dis. 2012, 2, 65–77. [Google Scholar] [CrossRef]
- Janelidze, S.; Mattsson, N.; Stomrud, E.; Lindberg, O.; Palmqvist, S.; Zetterberg, H.; Blennow, K.; Hansson, O. CSF biomarkers of neuroinflammation and cerebrovascular dysfunction in early Alzheimer disease. Neurology 2018, 91, e867–e877. [Google Scholar] [CrossRef]
- Nelson, P.T.; Wang, W.X. MiR-107 is reduced in Alzheimer’s disease brain neocortex: Validation study. J. Alzheimers Dis. JAD 2010, 21, 75–79. [Google Scholar] [CrossRef]
- Wang, W.X.; Rajeev, B.W.; Stromberg, A.J.; Ren, N.; Tang, G.; Huang, Q.; Rigoutsos, I.; Nelson, P.T. The expression of microRNA miR-107 decreases early in Alzheimer’s disease and may accelerate disease progression through regulation of beta-site amyloid precursor protein-cleaving enzyme 1. J. Neurosci. 2008, 28, 1213–1223. [Google Scholar] [CrossRef]
- Absalon, S.; Kochanek, D.M.; Raghavan, V.; Krichevsky, A.M. MiR-26b, upregulated in Alzheimer’s disease, activates cell cycle entry, tau-phosphorylation, and apoptosis in postmitotic neurons. J. Neurosci. 2013, 33, 14645–14659. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Jun, S.; Rellick, S.; Quintana, D.D.; Cavendish, J.Z.; Simpkins, J.W. Expression of microRNA-34a in Alzheimer’s disease brain targets genes linked to synaptic plasticity, energy metabolism, and resting state network activity. Brain Res. 2016, 1646, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Meng, F.; Venter, J.; Wu, N.; Wan, Y.; Standeford, H.; Francis, H.; Meininger, C.; Greene, J., Jr.; Trzeciakowski, J.P.; et al. miR-34a-dependent overexpression of Per1 decreases cholangiocarcinoma growth. J. Hepatol. 2016, 64, 1295–1304. [Google Scholar] [CrossRef]
- Sarkar, S.; Engler-Chiurazzi, E.B.; Cavendish, J.Z.; Povroznik, J.M.; Russell, A.E.; Quintana, D.D.; Mathers, P.H.; Simpkins, J.W. Over-expression of miR-34a induces rapid cognitive impairment and Alzheimer’s disease-like pathology. Brain Res. 2019, 1721, 146327. [Google Scholar] [CrossRef] [PubMed]
- Watts, M.E.; Williams, S.M.; Nithianantharajah, J.; Claudianos, C. Hypoxia-Induced MicroRNA-210 Targets Neurodegenerative Pathways. Noncoding RNA 2018, 4, 10. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Liu, Z.; Nian, X.; Xu, X.; Zhang, Y. miR-210 controls the evening phase of circadian locomotor rhythms through repression of Fasciclin 2. PLoS Genet. 2019, 15, e1007655. [Google Scholar] [CrossRef]
- Weigelt, C.M.; Hahn, O.; Arlt, K.; Gruhn, M.; Jahn, A.J.; Eßer, J.; Werner, J.A.; Klein, C.; Büschges, A.; Grönke, S.; et al. Loss of miR-210 leads to progressive retinal degeneration in Drosophila melanogaster. Life Sci. Alliance 2019, 2, e201800149. [Google Scholar] [CrossRef]
- Liu, E.Y.; Cali, C.P.; Lee, E.B. RNA metabolism in neurodegenerative disease. Dis. Model. Mech. 2017, 10, 509–518. [Google Scholar] [CrossRef]
- Nagaraj, S.; Zoltowska, K.M.; Laskowska-Kaszub, K.; Wojda, U. microRNA diagnostic panel for Alzheimer’s disease and epigenetic trade-off between neurodegeneration and cancer. Ageing Res. Rev. 2019, 49, 125–143. [Google Scholar] [CrossRef]
- Fransquet, P.D.; Ryan, J. Micro RNA as a potential blood-based epigenetic biomarker for Alzheimer’s disease. Clin. Biochem. 2018, 58, 5–14. [Google Scholar] [CrossRef]
- Lobentanzer, S.; Hanin, G.; Klein, J.; Soreq, H. Sex-related perturbations in schizophrenia and bipolar disorder brains reflect microRNA-mediated cholinergic/neurokine interactions. bioRxiv 2019, 600932. [Google Scholar] [CrossRef]
- Jin, Y.; Tu, Q.; Liu, M. MicroRNA-125b regulates Alzheimer’s disease through SphK1 regulation. Mol. Med. Rep. 2018, 18, 2373–2380. [Google Scholar] [CrossRef]
- Hébert, S.S.; Horré, K.; Nicolaï, L.; Papadopoulou, A.S.; Mandemakers, W.; Silahtaroglu, A.N.; Kauppinen, S.; Delacourte, A.; De Strooper, B. Loss of microRNA cluster miR-29a/b-1 in sporadic Alzheimer’s disease correlates with increased BACE1/beta-secretase expression. Proc. Natl. Acad. Sci. USA 2008, 105, 6415–6420. [Google Scholar] [CrossRef]
- Hayes, M.T. Parkinson’s Disease and Parkinsonism. Am. J. Med. 2019, 132, 802–807. [Google Scholar] [CrossRef]
- Ascherio, A.; Schwarzschild, M.A. The epidemiology of Parkinson’s disease: Risk factors and prevention. Lancet Neurol. 2016, 15, 1257–1272. [Google Scholar] [CrossRef]
- Kafka, M.S.; Benedito, M.A.; Roth, R.H.; Steele, L.K.; Wolfe, W.W.; Catravas, G.N. Circadian rhythms in catecholamine metabolites and cyclic nucleotide production. Chronobiol. Int. 1986, 3, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Sleipness, E.P.; Sorg, B.A.; Jansen, H.T. Diurnal differences in dopamine transporter and tyrosine hydroxylase levels in rat brain: Dependence on the suprachiasmatic nucleus. Brain Res. 2007, 1129, 34–42. [Google Scholar] [CrossRef]
- Mendoza, J.; Challet, E. Circadian insights into dopamine mechanisms. Neuroscience 2014, 282, 230–242. [Google Scholar] [CrossRef]
- Witkovsky, P. Dopamine and retinal function. Doc. Ophthalmol. 2004, 108, 17–40. [Google Scholar] [CrossRef]
- Loddo, G.; Calandra-Buonaura, G.; Sambati, L.; Giannini, G.; Cecere, A.; Cortelli, P.; Provini, F. The Treatment of Sleep Disorders in Parkinson’s Disease: From Research to Clinical Practice. Front. Neurol. 2017, 8, 42. [Google Scholar] [CrossRef]
- Comella, C.L. Sleep disorders in Parkinson’s disease: An overview. Mov. Disord. 2007, 22, S367–S373. [Google Scholar] [CrossRef]
- Hartmann, A.; Veldhuis, J.D.; Deuschle, M.; Standhardt, H.; Heuser, I. Twenty-four hour cortisol release profiles in patients with Alzheimer’s and Parkinson’s disease compared to normal controls: Ultradian secretory pulsatility and diurnal variation. Neurobiol. Aging 1997, 18, 285–289. [Google Scholar] [CrossRef]
- Cai, Y.; Liu, S.; Sothern, R.B.; Xu, S.; Chan, P. Expression of clock genes Per1 and Bmal1 in total leukocytes in health and Parkinson’s disease. Eur. J. Neurol. 2010, 17, 550–554. [Google Scholar] [CrossRef]
- Terrinoni, A.; Calabrese, C.; Basso, D.; Aita, A.; Caporali, S.; Plebani, M.; Bernardini, S. The circulating miRNAs as diagnostic and prognostic markers. Clin. Chem. Lab. Med. 2019, 57, 932–953. [Google Scholar] [CrossRef]
- Serafin, A.; Foco, L.; Zanigni, S.; Blankenburg, H.; Picard, A.; Zanon, A.; Giannini, G.; Pichler, I.; Facheris, M.F.; Cortelli, P.; et al. Overexpression of blood microRNAs 103a, 30b, and 29a in l-dopa–treated patients with PD. Neurology 2015, 84, 645–653. [Google Scholar] [CrossRef]
- Wang, R.; Yang, Y.; Wang, H.; He, Y.; Li, C. MiR-29c protects against inflammation and apoptosis in Parkinson’s disease model in vivo and in vitro by targeting SP1. Clin. Exp. Pharmacol. Physiol. 2020, 47, 372–382. [Google Scholar] [CrossRef]
- Figueredo Dde, S.; Barbosa, M.R.; Gitaí, D.L.; de Andrade, T.G. Predicted microRNAs for mammalian circadian rhythms. J. Biol. Rhythm. 2013, 28, 107–116. [Google Scholar] [CrossRef]
- Roser, A.E.; Caldi Gomes, L.; Schünemann, J.; Maass, F.; Lingor, P. Circulating miRNAs as Diagnostic Biomarkers for Parkinson’s Disease. Front. Neurosci. 2018, 12. [Google Scholar] [CrossRef] [PubMed]
- Weigend, S.; Holst, S.C.; Meier, J.; Brock, M.; Kohler, M.; Landolt, H.-P. Prolonged Waking and Recovery Sleep Affect the Serum MicroRNA Expression Profile in Humans. Clocks Sleep 2019, 1, 75–86. [Google Scholar] [CrossRef]
- Vallelunga, A.; Iannitti, T.; Dati, G.; Capece, S.; Maugeri, M.; Tocci, E.; Picillo, M.; Volpe, G.; Cozzolino, A.; Squillante, M.; et al. Serum miR-30c-5p is a potential biomarker for multiple system atrophy. Mol. Biol. Rep. 2019, 46, 1661–1666. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Geng, L.; Chen, Y. MiR-19b alleviates MPP+-induced neuronal cytotoxicity via targeting the HAPLN4/MAPK pathway in SH-SY5Y cells. RSC Adv. 2018, 8, 10706–10714. [Google Scholar] [CrossRef]
- Chen, L.; Gan, L.; Zhou, H.-Y.; Liang, J.-H. Protective effects of miR-19b in Parkinson’s disease by inhibiting the activation of iNOS through negative regulation of p38 signaling pathways. Int. J. Clin. Exp. Med. 2019, 12, 4735–4744. [Google Scholar]
- Fernández-Santiago, R.; Iranzo, A.; Gaig, C.; Serradell, M.; Fernández, M.; Tolosa, E.; Santamaría, J.; Ezquerra, M. MicroRNA association with synucleinopathy conversion in rapid eye movement behavior disorder. Ann. Neurol. 2015, 77, 895–901. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Xu, J.; Wu, M.; Hu, J.M. Protective role of microRNA-221 in Parkinson’s disease. Bratisl. Lek. Listy 2018, 119, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Hicks, S.D.; Khurana, N.; Williams, J.; Dowd Greene, C.; Uhlig, R.; Middleton, F.A. Diurnal oscillations in human salivary microRNA and microbial transcription: Implications for human health and disease. PLoS ONE 2018, 13, e0198288. [Google Scholar] [CrossRef]
- Kim, W.; Lee, Y.; McKenna, N.D.; Yi, M.; Simunovic, F.; Wang, Y.; Kong, B.; Rooney, R.J.; Seo, H.; Stephens, R.M.; et al. miR-126 contributes to Parkinson’s disease by dysregulating the insulin-like growth factor/phosphoinositide 3-kinase signaling. Neurobiol. Aging 2014, 35, 1712–1721. [Google Scholar] [CrossRef]
- Qu, M.J.; Pan, J.J.; Shi, X.J.; Zhang, Z.J.; Tang, Y.H.; Yang, G.Y. MicroRNA-126 is a prospective target for vascular disease. Neuroimmunol. Neuroinflamm. 2018, 5, 10. [Google Scholar] [CrossRef]
- Gordon, P.H. Amyotrophic Lateral Sclerosis: An update for 2013 Clinical Features, Pathophysiology, Management and Therapeutic Trials. Aging Dis. 2013, 4, 295–310. [Google Scholar] [CrossRef]
- Patacchioli, F.R.; Monnazzi, P.; Scontrini, A.; Tremante, E.; Caridi, I.; Brunetti, E.; Buttarelli, F.R.; Pontieri, F.E. Adrenal dysregulation in amyotrophic lateral sclerosis. J. Endocrinol. Investig. 2003, 26, RC23–RC25. [Google Scholar] [CrossRef]
- Ahmed, R.M.; Newcombe, R.E.; Piper, A.J.; Lewis, S.J.; Yee, B.J.; Kiernan, M.C.; Grunstein, R.R. Sleep disorders and respiratory function in amyotrophic lateral sclerosis. Sleep Med. Rev. 2016, 26, 33–42. [Google Scholar] [CrossRef]
- Taylor, J.P.; Brown, R.H., Jr.; Cleveland, D.W. Decoding ALS: From genes to mechanism. Nature 2016, 539, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.S.; Lee, M.H.; Lee, S.H.; Bae, K. Cu/Zn superoxide dismutase is differentially regulated in period gene-mutant mice. Biochem. Biophys. Res. Commun. 2011, 409, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Hirano, A.; Nakagawa, T.; Yoshitane, H.; Oyama, M.; Kozuka-Hata, H.; Lanjakornsiripan, D.; Fukada, Y. USP7 and TDP-43: Pleiotropic Regulation of Cryptochrome Protein Stability Paces the Oscillation of the Mammalian Circadian Clock. PLoS ONE 2016, 11, e0154263. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Zhang, T.; Wang, H.; Wang, T.; Qin, M.; Bao, P.; Wang, R.; Liu, Y.; Chang, H.C.; Yan, J.; et al. Neurodegeneration-associated FUS is a novel regulator of circadian gene expression. Transl. Neurodegener. 2018, 7, 24. [Google Scholar] [CrossRef] [PubMed]
- Debray, S.; Race, V.; Crabbé, V.; Herdewyn, S.; Matthijs, G.; Goris, A.; Dubois, B.; Thijs, V.; Robberecht, W.; Van Damme, P. Frequency of C9orf72 repeat expansions in amyotrophic lateral sclerosis: A Belgian cohort study. Neurobiol. Aging 2013, 34, 2890-e7. [Google Scholar] [CrossRef] [PubMed]
- Dedeene, L.; Van Schoor, E.; Vandenberghe, R.; Van Damme, P.; Poesen, K.; Thal, D.R. Circadian sleep/wake-associated cells show dipeptide repeat protein aggregates in C9orf72-related ALS and FTLD cases. Acta Neuropathol. Commun. 2019, 7, 189. [Google Scholar] [CrossRef]
- Ricci, C.; Marzocchi, C.; Battistini, S. MicroRNAs as Biomarkers in Amyotrophic Lateral Sclerosis. Cells 2018, 7, 219. [Google Scholar] [CrossRef]
- Ma, G.; Wang, Y.; Li, Y.; Cui, L.; Zhao, Y.; Zhao, B.; Li, K. MiR-206, a key modulator of skeletal muscle development and disease. Int. J. Biol. Sci. 2015, 11, 345–352. [Google Scholar] [CrossRef]
- Zhou, W.; Li, Y.; Wang, X.; Wu, L.; Wang, Y. MiR-206-mediated dynamic mechanism of the mammalian circadian clock. BMC Syst. Biol. 2011, 5, 141. [Google Scholar] [CrossRef]
- Tasca, E.; Pegoraro, V.; Merico, A.; Angelini, C. Circulating microRNAs as biomarkers of muscle differentiation and atrophy in ALS. Clin. Neuropathol. 2016, 35, 22–30. [Google Scholar] [CrossRef]
- Raheja, R.; Regev, K.; Healy, B.C.; Mazzola, M.A.; Beynon, V.; Von Glehn, F.; Paul, A.; Diaz-Cruz, C.; Gholipour, T.; Glanz, B.I.; et al. Correlating serum micrornas and clinical parameters in amyotrophic lateral sclerosis. Muscle Nerve 2018, 58, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Figueroa-Romero, C.; Hur, J.; Lunn, J.S.; Paez-Colasante, X.; Bender, D.E.; Yung, R.; Sakowski, S.A.; Feldman, E.L. Expression of microRNAs in human post-mortem amyotrophic lateral sclerosis spinal cords provides insight into disease mechanisms. Mol. Cell Neurosci. 2016, 71, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Fu, X.; Wu, B.; Zhu, J.; Zhao, Z. Circadian regulation of microRNA-target chimeras in Drosophila. bioRxiv 2019, 622183. [Google Scholar] [CrossRef]
- Matamala, J.M.; Arias-Carrasco, R.; Sanchez, C.; Uhrig, M.; Bargsted, L.; Matus, S.; Maracaja-Coutinho, V.; Abarzua, S.; van Zundert, B.; Verdugo, R.; et al. Genome-wide circulating microRNA expression profiling reveals potential biomarkers for amyotrophic lateral sclerosis. Neurobiol. Aging 2018, 64, 123–138. [Google Scholar] [CrossRef]
- Nguyen, T.; Nioi, P.; Pickett, C.B. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J. Biol. Chem. 2009, 284, 13291–13295. [Google Scholar] [CrossRef]
- Datta Chaudhuri, A.; Yelamanchili, S.V.; Fox, H.S. MicroRNA-142 Reduces Monoamine Oxidase A Expression and Activity in Neuronal Cells by Downregulating SIRT1. PLoS ONE 2013, 8, e79579. [Google Scholar] [CrossRef][Green Version]
- Nakahata, Y.; Kaluzova, M.; Grimaldi, B.; Sahar, S.; Hirayama, J.; Chen, D.; Guarente, L.P.; Sassone-Corsi, P. The NAD+-dependent deacetylase SIRT1 modulates CLOCK-mediated chromatin remodeling and circadian control. Cell 2008, 134, 329–340. [Google Scholar] [CrossRef]
- Singh, C.K.; Chhabra, G.; Ndiaye, M.A.; Garcia-Peterson, L.M.; Mack, N.J.; Ahmad, N. The Role of Sirtuins in Antioxidant and Redox Signaling. Antioxid. Redox Signal. 2018, 28, 643–661. [Google Scholar] [CrossRef]
- Emde, A.; Eitan, C.; Liou, L.-L.; Libby, R.T.; Rivkin, N.; Magen, I.; Reichenstein, I.; Oppenheim, H.; Eilam, R.; Silvestroni, A.; et al. Dysregulated miRNA biogenesis downstream of cellular stress and ALS-causing mutations: A new mechanism for ALS. EMBO J. 2015, 34, 2633–2651. [Google Scholar] [CrossRef]
- Kovanda, A.; Leonardis, L.; Zidar, J.; Koritnik, B.; Dolenc-Groselj, L.; Ristic Kovacic, S.; Curk, T.; Rogelj, B. Differential expression of microRNAs and other small RNAs in muscle tissue of patients with ALS and healthy age-matched controls. Sci. Rep. 2018, 8, 5609. [Google Scholar] [CrossRef]
- Kawahara, Y.; Mieda-Sato, A. TDP-43 promotes microRNA biogenesis as a component of the Drosha and Dicer complexes. Proc. Natl. Acad. Sci. USA 2012, 109, 3347–3352. [Google Scholar] [CrossRef] [PubMed]
- Morlando, M.; Dini Modigliani, S.; Torrelli, G.; Rosa, A.; Di Carlo, V.; Caffarelli, E.; Bozzoni, I. FUS stimulates microRNA biogenesis by facilitating co-transcriptional Drosha recruitment. EMBO J. 2012, 31, 4502–4510. [Google Scholar] [CrossRef] [PubMed]
- Aten, S.; Hansen, K.F.; Snider, K.; Wheaton, K.; Kalidindi, A.; Garcia, A.; Alzate-Correa, D.; Hoyt, K.R.; Obrietan, K. miR-132 couples the circadian clock to daily rhythms of neuronal plasticity and cognition. Learn. Mem. 2018, 25, 214–229. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Sanchez, M.; Licitra, F.; Underwood, B.R.; Rubinsztein, D.C. Huntington’s Disease: Mechanisms of Pathogenesis and Therapeutic Strategies. Cold Spring Harb. Perspect. Med. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Herzog-Krzywoszanska, R.; Krzywoszanski, L. Sleep Disorders in Huntington’s Disease. Front. Psychiatry 2019, 10, 221. [Google Scholar] [CrossRef]
- Faragó, A.; Zsindely, N.; Bodai, L. Mutant huntingtin disturbs circadian clock gene expression and sleep patterns in Drosophila. Sci. Rep. 2019, 9, 7174. [Google Scholar] [CrossRef]
- Morton, A.J.; Wood, N.I.; Hastings, M.H.; Hurelbrink, C.; Barker, R.A.; Maywood, E.S. Disintegration of the sleep-wake cycle and circadian timing in Huntington’s disease. J. Neurosci. 2005, 25, 157–163. [Google Scholar] [CrossRef]
- Dong, X.; Cong, S. The Emerging Role of microRNAs in Polyglutamine Diseases. Front. Mol. Neurosci. 2019, 12. [Google Scholar] [CrossRef]
- Hoss, A.G.; Labadorf, A.; Latourelle, J.C.; Kartha, V.K.; Hadzi, T.C.; Gusella, J.F.; MacDonald, M.E.; Chen, J.-F.; Akbarian, S.; Weng, Z.; et al. miR-10b-5p expression in Huntington’s disease brain relates to age of onset and the extent of striatal involvement. BMC Med. Genom. 2015, 8, 10. [Google Scholar] [CrossRef]
- Konovalova, J.; Gerasymchuk, D.; Parkkinen, I.; Chmielarz, P.; Domanskyi, A. Interplay between MicroRNAs and Oxidative Stress in Neurodegenerative Diseases. Int. J. Mol. Sci. 2019, 20, 6055. [Google Scholar] [CrossRef]
- Liu, T.; Im, W.; Mook-Jung, I.; Kim, M. MicroRNA-124 slows down the progression of Huntington’s disease by promoting neurogenesis in the striatum. Neural Regen. Res. 2015, 10, 786–791. [Google Scholar] [CrossRef]
- Xue, Y.; Zhang, Y. Emerging roles for microRNA in the regulation of Drosophila circadian clock. BMC Neurosci. 2018, 19, 1. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lamba, P.; Guo, P.; Emery, P. miR-124 Regulates the Phase of Drosophila Circadian Locomotor Behavior. J. Neurosci. 2016, 36, 2007–2013. [Google Scholar] [CrossRef] [PubMed]
- Martí, E.; Pantano, L.; Bañez-Coronel, M.; Llorens, F.; Miñones-Moyano, E.; Porta, S.; Sumoy, L.; Ferrer, I.; Estivill, X. A myriad of miRNA variants in control and Huntington’s disease brain regions detected by massively parallel sequencing. Nucleic Acids Res. 2010, 38, 7219–7235. [Google Scholar] [CrossRef] [PubMed]
- Sinha, M.; Ghose, J.; Bhattarcharyya, N.P. Micro RNA -214,-150,-146a and-125b target Huntingtin gene. RNA Biol. 2011, 8, 1005–1021. [Google Scholar] [CrossRef] [PubMed]
- Mészáros, L.; Hoffmann, A.; Wihan, J.; Winkler, J. Current Symptomatic and Disease-Modifying Treatments in Multiple System Atrophy. Int. J. Mol. Sci. 2020, 21, 2775. [Google Scholar] [CrossRef] [PubMed]
- Cochen De Cock, V. Sleep Abnormalities in Multiple System Atrophy. Curr. Treat. Options Neurol. 2018, 20, 16. [Google Scholar] [CrossRef]
- Benarroch, E.E.; Schmeichel, A.M.; Sandroni, P.; Low, P.A.; Parisi, J.E. Differential involvement of hypothalamic vasopressin neurons in multiple system atrophy. Brain 2006, 129, 2688–2696. [Google Scholar] [CrossRef][Green Version]
- Ozawa, T.; Oyanagi, K.; Tanaka, H.; Horikawa, Y.; Takahashi, H.; Morita, T.; Tsuji, S. Suprachiasmatic nucleus in a patient with multiple system atrophy with abnormal circadian rhythm of arginine-vasopressin secretion into plasma. J. Neurol. Sci. 1998, 154, 116–121. [Google Scholar] [CrossRef]
- Ozawa, T.; Tanaka, H.; Nakano, R.; Sato, M.; Inuzuka, T.; Soma, Y.; Yoshimura, N.; Fukuhara, N.; Tsuji, S. Nocturnal decrease in vasopressin secretion into plasma in patients with multiple system atrophy. J. Neurol. Neurosurg. Psychiatry 1999, 67, 542–545. [Google Scholar] [CrossRef]
- Kume, K.; Iwama, H.; Deguchi, K.; Ikeda, K.; Takata, T.; Kokudo, Y.; Kamada, M.; Fujikawa, K.; Hirose, K.; Masugata, H.; et al. Serum microRNA expression profiling in patients with multiple system atrophy. Mol. Med. Rep. 2018, 17, 852–860. [Google Scholar] [CrossRef] [PubMed]
- Uwatoko, H.; Hama, Y.; Iwata, I.T.; Shirai, S.; Matsushima, M.; Yabe, I.; Utsumi, J.; Sasaki, H. Identification of plasma microRNA expression changes in multiple system atrophy and Parkinson’s disease. Mol. Brain 2019, 12, 49. [Google Scholar] [CrossRef] [PubMed]
- Ubhi, K.; Rockenstein, E.; Kragh, C.; Inglis, C.; Spencer, B.; Michael, S.; Mante, M.; Adame, A.; Galasko, D.; Masliah, E. Widespread microRNA dysregulation in multiple system atrophy-disease-related alteration in miR-96. Eur. J. Neurosci. 2014, 39, 1026–1041. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, C.; Aoyama, K.; Matsumura, N.; Kikuchi-Utsumi, K.; Watabe, M.; Nakaki, T. Rhythmic oscillations of the microRNA miR-96-5p play a neuroprotective role by indirectly regulating glutathione levels. Nat. Commun. 2014, 5, 3823. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.T.; Chu, K.; Jung, K.H.; Ban, J.J.; Im, W.S.; Jo, H.Y.; Park, J.H.; Lim, J.Y.; Shin, J.W.; Moon, J.; et al. Altered Expression of miR-202 in Cerebellum of Multiple-System Atrophy. Mol. Neurobiol. 2015, 51, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Schafferer, S.; Khurana, R.; Refolo, V.; Venezia, S.; Sturm, E.; Piatti, P.; Hechenberger, C.; Hackl, H.; Kessler, R.; Willi, M.; et al. Changes in the miRNA-mRNA Regulatory Network Precede Motor Symptoms in a Mouse Model of Multiple System Atrophy: Clinical Implications. PLoS ONE 2016, 11, e0150705. [Google Scholar] [CrossRef]
- Smith, S.S.; Dole, N.S.; Franceschetti, T.; Hrdlicka, H.C.; Delany, A.M. MicroRNA-433 Dampens Glucocorticoid Receptor Signaling, Impacting Circadian Rhythm and Osteoblastic Gene Expression. J. Biol. Chem. 2016, 291, 21717–21728. [Google Scholar] [CrossRef]

| Disease | miRNA | Target Clock Gene | Rhythmicity | Regulation | Predicted Disease Mechanism | Related Sleep Disorder | Reference |
|---|---|---|---|---|---|---|---|
| Alzheimer’s disease | miR-107 | Clock | rhythmic | temporal cortex ↓ | Increased BACE1 expression | obstructive sleep apnea | [40,91,114,120,121] |
| whole blood ↓ | |||||||
| plasma ↓ | |||||||
| miR-125b | Clock | n.d. | hippocampus↑ | Increased BACE1, APP and Tau protein expression | n.d. | [114,129,130] | |
| serum ↓ | |||||||
| miR-132 | n.d. | rhythmic | temporal cortex ↓ | Pathological aggregation of tau protein | n.d. | [9,116,117] | |
| serum ↑ | |||||||
| exsome ↓ | |||||||
| miR-146a | n.d. | rhythmic | frontal cortex↑ | Increased tau hyperphosphorylation | chronic short sleep | [62,64,66,79,114,118,119] | |
| hippocampus↑ | fatal familial insomnia | ||||||
| plasma ↓ | |||||||
| serum ↓ | |||||||
| miR-210 | Per (Drosophila) | n.d. | hippocampus↓ | Dysregulation of hypoxic stress pathway | n.d. | [114,126] | |
| CSF ↓ | |||||||
| serum ↓ | |||||||
| plasma ↑ | |||||||
| miR-219 | n.d. | rhythmic | entorhinal cortex↓ | Accumulation of insoluble tau | n.d. | [115] | |
| miR-26b | n.d. | rhythmic | temporal cortex ↓ | Induced tau hyperphosphorylation | n.d. | [22,23,114,122] | |
| whole blood ↓ | Aβ accumulation | ||||||
| serum ↓ | |||||||
| miR-29a/b | Per1 | rhythmic | cortex↓ | Increased BACE1 expression | [129,130,131,134] | ||
| Per2 | (primary transcript) | whole blood ↓ | n.d. | ||||
| Per3 | serum ↓ | ||||||
| plasma exosome ↓ | |||||||
| blood mononuclear cells ↓ | |||||||
| miR-34a | Per1 | rhythmic | temporal cortex↓ | Accumulation of intraneuronal Aβ | n.d. | [27,114,124,125,129,130,131] | |
| Per2 | hippocampus↑ | Induced tau hyperphosphorylation | |||||
| frontal cortex↑ | |||||||
| plasma ↓ | |||||||
| blood mononuclear cells ↑ | |||||||
| Parkinson’s disease | miR-126 | Dbp | n.d. | dopaminergic neurons↑ | Dysregulation of trophic support in DA neurons | chronic short sleep | [64,149,157,158] |
| blood mononuclear cells ↓ | |||||||
| miR-19b | Clock | n.d. | CSF↓ | Promotion of cell apoptosis | idiopathic REM sleep | [48,149,152,153,154] | |
| Rora | serum ↓ | behavior syndrome | |||||
| blood mononuclear cells ↓ | |||||||
| miR-221 | n.d. | rhythmic | serum ↓ | Inhibition of cell proliferation | n.d. | [155,156] | |
| Promotion of apoptosis | |||||||
| miR-29a/c | Per1 | rhythmic | serum ↓ | Doperminergic neuron loss | n.d. | [21,145,146,147,148,149] | |
| Per2 | (primary transcript) | blood mononuclear cells ↓ | α-synuclein accumulation | ||||
| Per3 | |||||||
| miR-30c | n.d. | n.d. | serum ↓ | Progression of α-synucleinopathies? | narcolepsy | [148,149,151] | |
| blood mononuclear cells ↓ | (Predicted by computational analysis of gene network) | ||||||
| Amyotrophic lateral sclerosis | miR-132 | n.d. | rhythmic | muscle ↑ | Inhibition of neurite outgrowth | n.d. | [175,181,182,183,184] |
| miR-133a/b | n.d. | n.d. | spinal cord↓ | Involved in muscle proliferation and regeneration | n.d. | [171,172,173,174] | |
| serum ↑ | |||||||
| miR-142 | Bmal1 | rhythmic | spinal cord↑ | Promotion of ALS pathogenesis | n.d. | [173,175,176,177,178] | |
| serum ↑ | |||||||
| miR-206 | Clock | rhythmic | serum ↑ | Involved in reinnervation process | n.d. | [169,170] | |
| Huntington’s disease | miR-124 | Clock | n.d. | leukocytes ↓ | Disease progression | n.d. | [189,192,193,194] |
| miR-132 | n.d. | rhythmic | frontal cortex↓ | Enhancement of oxidative stress | n.d. | [9,189,190] | |
| miR-146a | n.d. | rhythmic | frontal cortex↑ | Targeting Huntingtin gene | chronic short sleep | [64,66,78,195,196] | |
| striatum↑ | fatal familial insomnia | ||||||
| Multiple system atrophy | miR-24 | Per2 | rhythmic | CSF↓ | Involved in cerebellar degeneration | disordered sleep patterns | [156,202,203] |
| serum ↓ | (autistic children) | ||||||
| plasma ↓ | |||||||
| miR-433 | Per2 | rhythmic | cerebellum↓ | Involved in formation of glial cytoplasmic inclusions | n.d. | [206,207,208] | |
| miR-96 | n.d. | rhythmic | frontal cortex↑ | Inhibition of transporters involved in antioxidant defense | n.d. | [204,205] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kinoshita, C.; Okamoto, Y.; Aoyama, K.; Nakaki, T. MicroRNA: A Key Player for the Interplay of Circadian Rhythm Abnormalities, Sleep Disorders and Neurodegenerative Diseases. Clocks & Sleep 2020, 2, 282-307. https://doi.org/10.3390/clockssleep2030022
Kinoshita C, Okamoto Y, Aoyama K, Nakaki T. MicroRNA: A Key Player for the Interplay of Circadian Rhythm Abnormalities, Sleep Disorders and Neurodegenerative Diseases. Clocks & Sleep. 2020; 2(3):282-307. https://doi.org/10.3390/clockssleep2030022
Chicago/Turabian StyleKinoshita, Chisato, Yayoi Okamoto, Koji Aoyama, and Toshio Nakaki. 2020. "MicroRNA: A Key Player for the Interplay of Circadian Rhythm Abnormalities, Sleep Disorders and Neurodegenerative Diseases" Clocks & Sleep 2, no. 3: 282-307. https://doi.org/10.3390/clockssleep2030022
APA StyleKinoshita, C., Okamoto, Y., Aoyama, K., & Nakaki, T. (2020). MicroRNA: A Key Player for the Interplay of Circadian Rhythm Abnormalities, Sleep Disorders and Neurodegenerative Diseases. Clocks & Sleep, 2(3), 282-307. https://doi.org/10.3390/clockssleep2030022





