Validation of an Automatic Arousal Detection Algorithm for Whole-Night Sleep EEG Recordings
Abstract
1. Introduction
2. Materials and Methods
2.1. Dataset
2.2. Human Arousals Scoring
2.3. Automatic Arousals Detection Algorithm
2.3.1. Preprocessing
2.3.2. Bad Channel Detection
2.3.3. Features Extraction
2.3.4. Arousal Detection
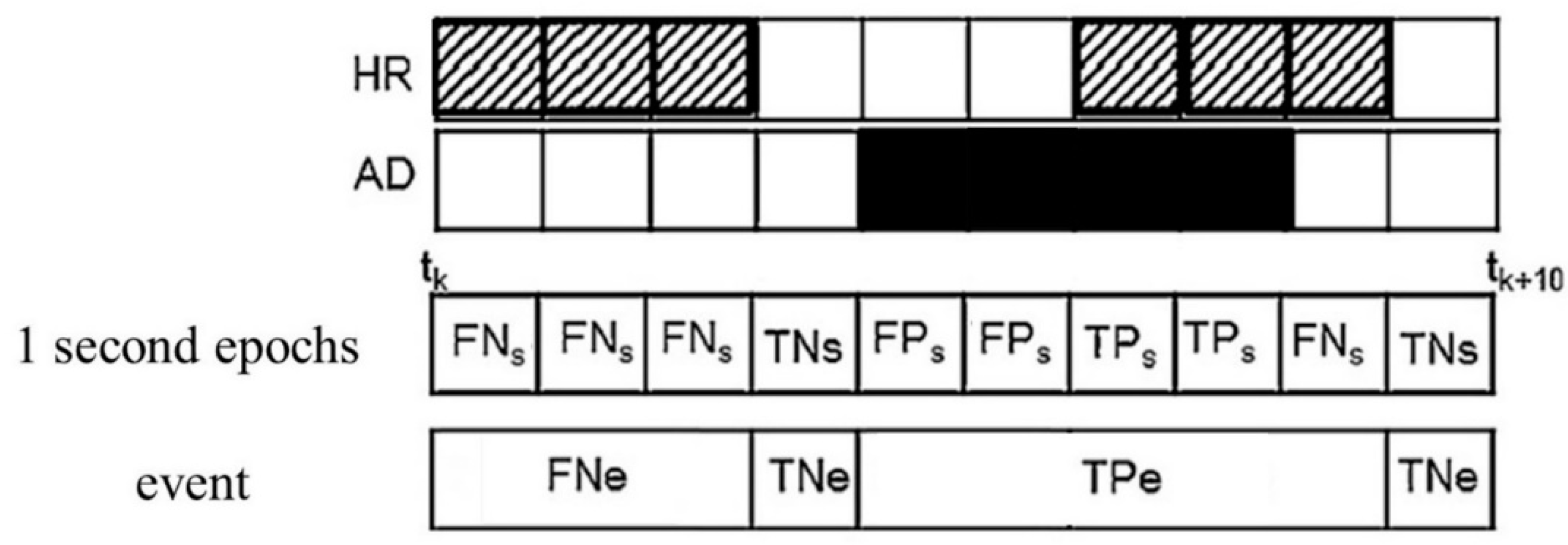
2.4. Comparison between Raters
2.4.1. Statistical Parameters
2.4.2. Time Frequency Analysis
2.4.3. Statistical Analyses
3. Results
3.1. Comparison of Human Raters (HR)
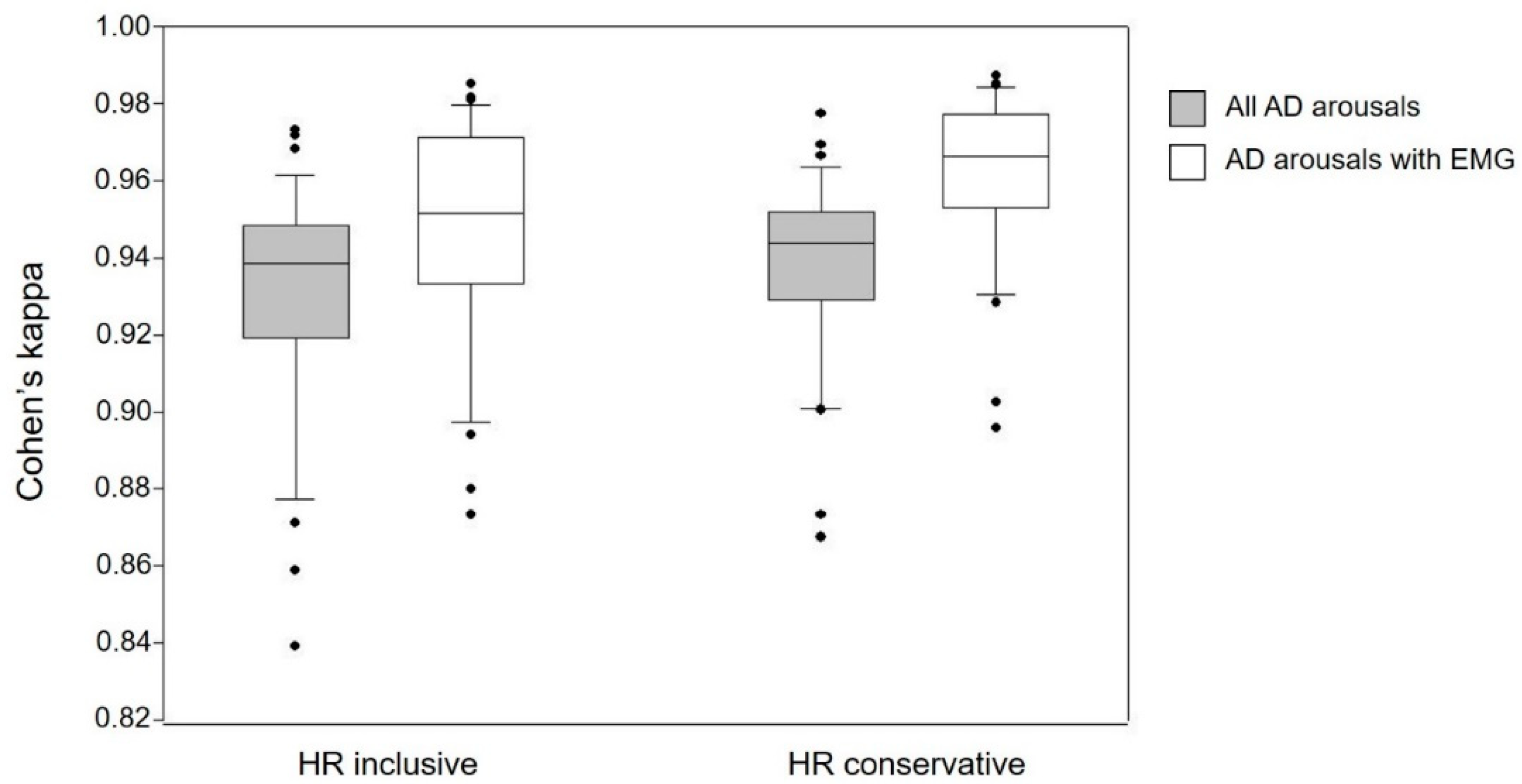
3.2. Automatic Arousal Detection (AD) vs. Human Raters
3.2.1. Impact of Age and Sex
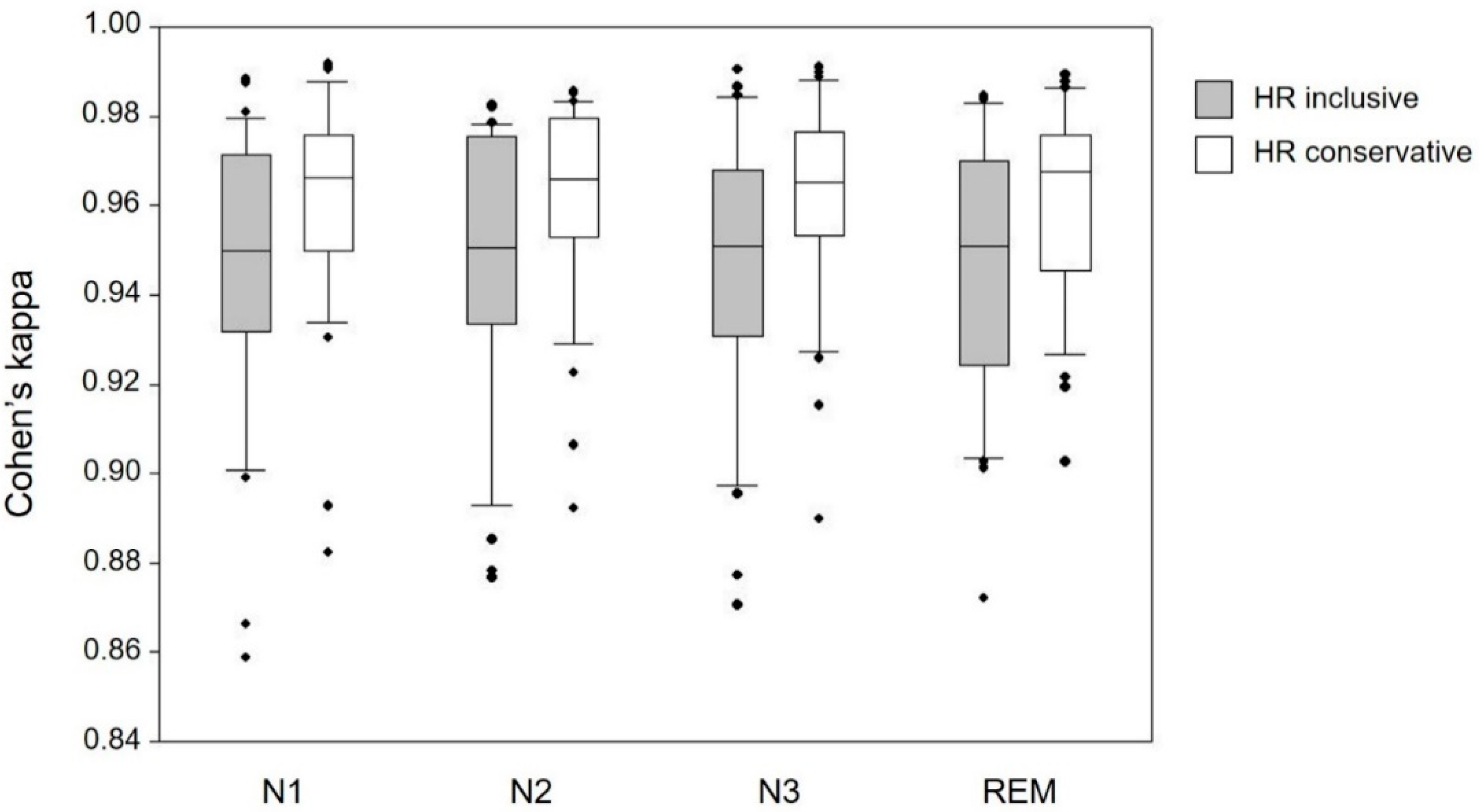
3.2.2. Impact of Sleep Stage
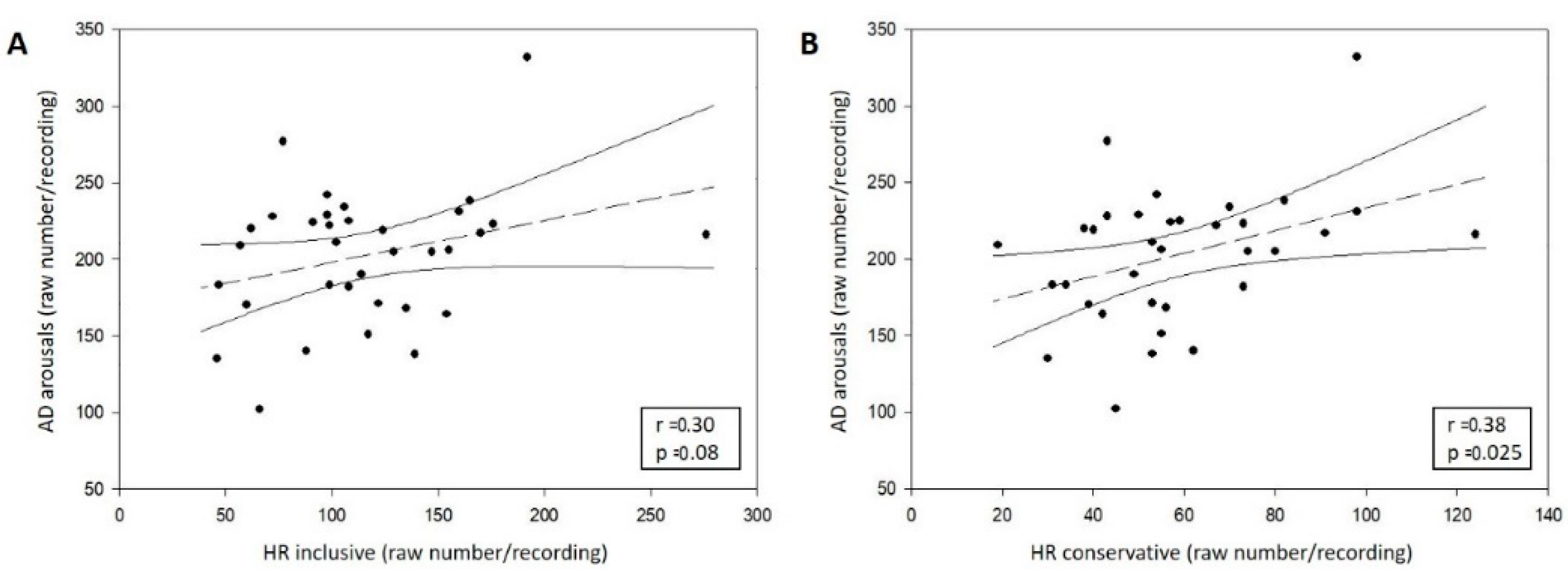
3.2.3. Correlation between AD and HR
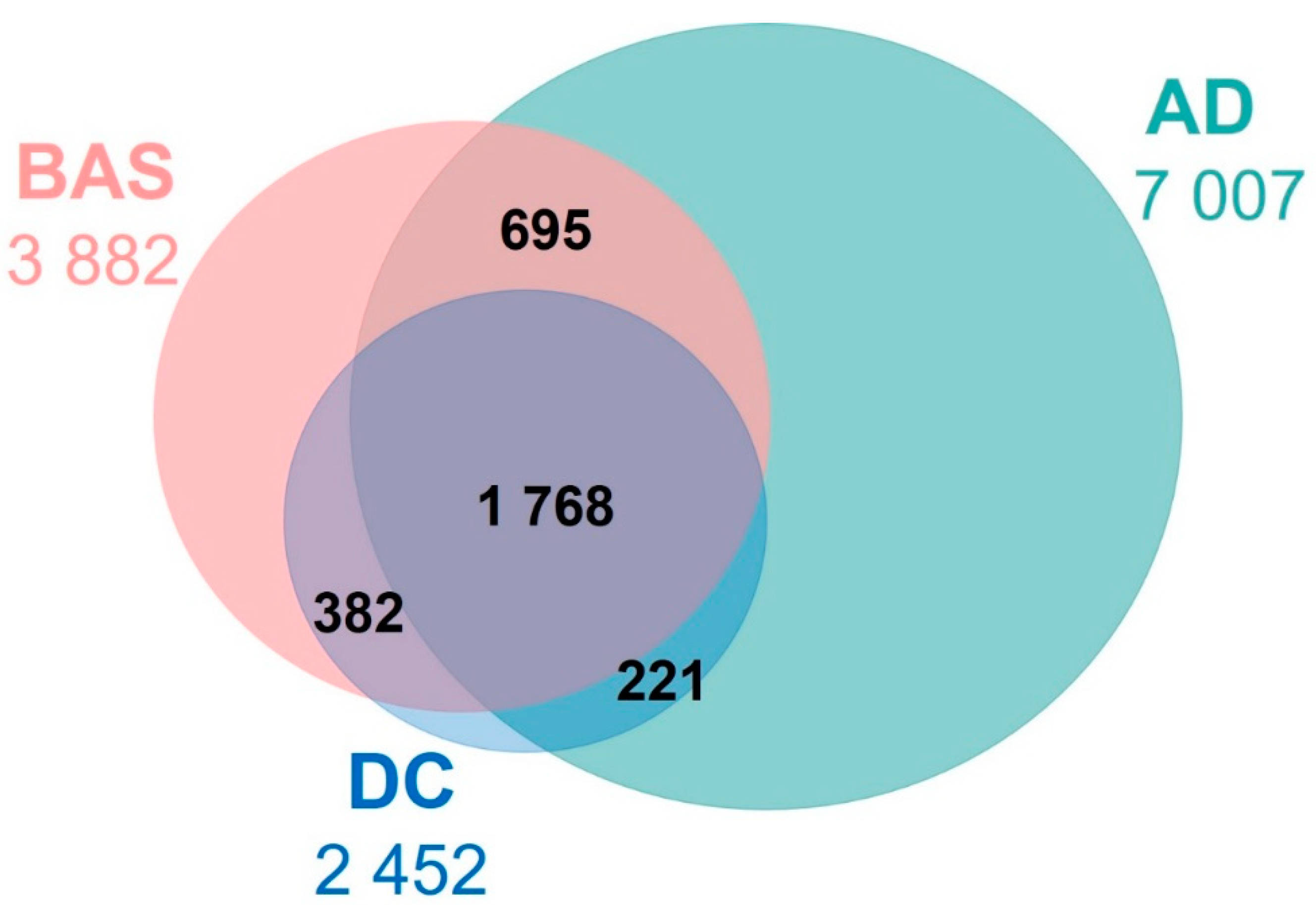
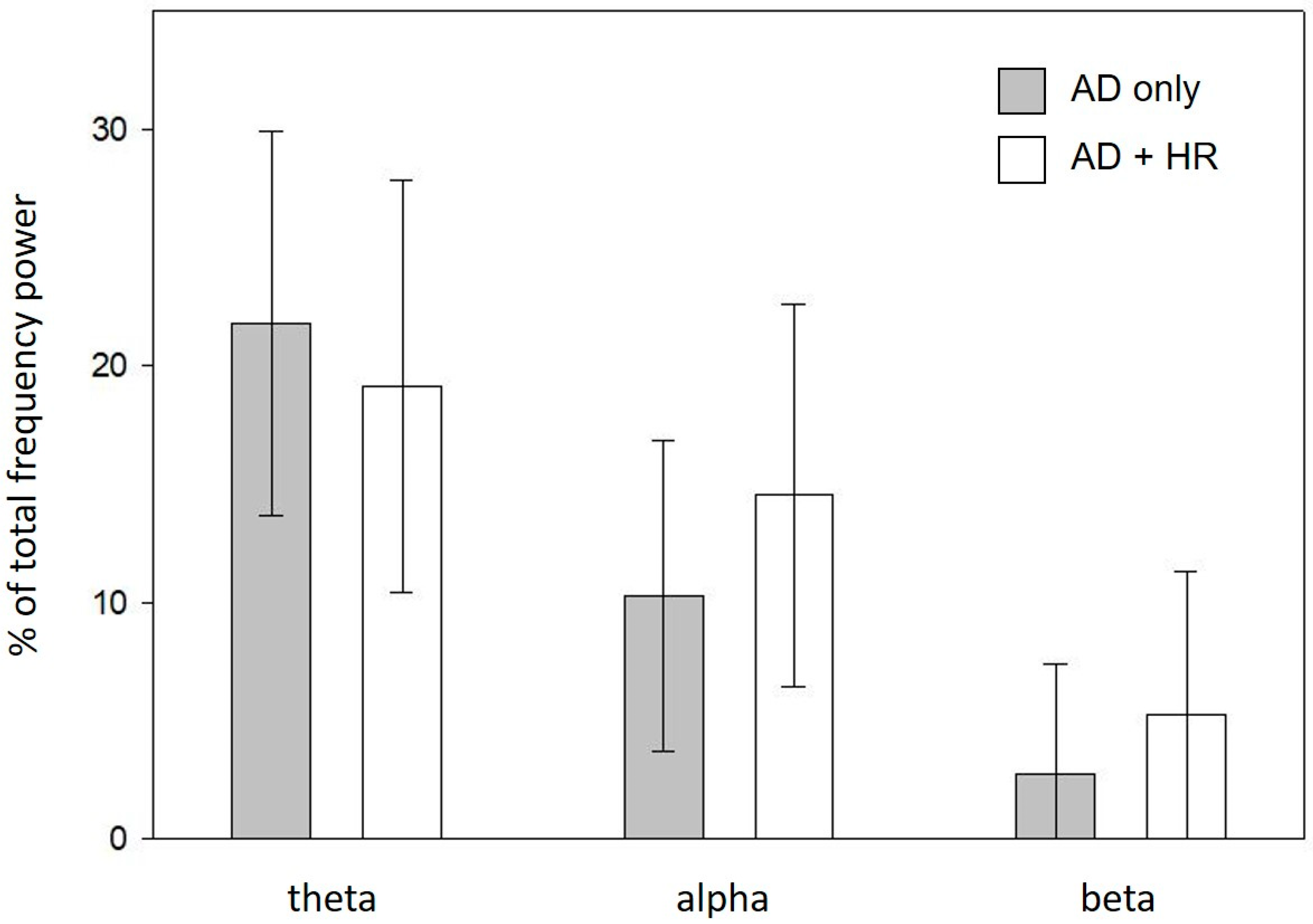
3.3. Characterisation of the Arousals Only Detected by AD
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Code Availability
Abbreviations
| AD | Automatic detection |
| EEG | Electroencephalogram |
| EMG | Electromyogram |
| FDR | False discovery ratio |
| HR | Human rater |
| SD | Standard deviation |
References
- Halász, P. Hierarchy of micro-arousals and the microstructure of sleep. Neurophysiol. Clin. 1998, 28, 461–475. [Google Scholar] [CrossRef]
- Halasz, P.; Terzano, M.; Parrino, L.; Bodizs, R. The nature of arousal in sleep. J. Sleep Res. 2004, 13, 1–23. [Google Scholar] [CrossRef]
- Malhotra, A.; Jordan, A. The importance of arousal in obstructive sleep apnea-updates from the American Thoracic Society 2016. J. Thorac. Dis. 2016, 8, S542–S544. [Google Scholar] [CrossRef]
- Griefahn, B.; Bröde, P.; Marks, A.; Basner, M. Autonomic arousals related to traffic noise during sleep. Sleep 2008, 31, 569–577. [Google Scholar] [CrossRef]
- McGuire, S.; Müller, U.; Elmenhorst, E.-M.; Basner, M. Inter-Individual differences in the effects of aircraft noise on sleep fragmentation. Sleep 2016, 39, 1107–1110. [Google Scholar] [CrossRef] [PubMed]
- Rudzik, F.; Thiesse, L.; Pieren, R.; Wunderli, J.M.; Brink, M.; Foraster, M.; Héritier, H.; Eze, I.C.; Garbazza, C.; Vienneau, D.; et al. Sleep spindle characteristics and arousability from nighttime transportation noise exposure in healthy young and older individuals. Sleep 2018, 41, 1–14. [Google Scholar] [CrossRef]
- Bonnet, M.H.; Arand, D.L. EEG arousal norms by age. J. Clin. Sleep Med. 2007, 3, 271–274. [Google Scholar] [CrossRef] [PubMed]
- Boselli, M.; Parrino, L.; Smerieri, A.; Terzano, M.G. Effect of age on EEG arousals in normal sleep. Sleep 1998, 21, 361–367. [Google Scholar]
- Bonnet, M.; Carley, D.; Carskadon, M.; Easton, P.; Guilleminault, C.; Harper, R.; Hayes, B.; Hirshkowitz, M.; Ktonas, P.; Keenan, S.; et al. ASDA report-EEG arousals: Scoring rules and examples. Sleep 1992, 15, 173–184. [Google Scholar]
- Iber, C.; Ancoli-Israel, S.; Chesson, A.; Quan, S. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications, 1st ed.; American Academy of Sleep Medicine: Wetchester, IL, USA, 2007. [Google Scholar]
- Bond, L.M. Updates to the scoring of sleep and associated events. A2Zzz 2014, 23, 20–26. [Google Scholar]
- Berry, R.B.; Brooks, R.; Gamaldo, C.; Harding, S.; Lloyd, R.; Quan, S.; Troester, M.; Vaughn, B. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminoloy and Technical Specifications. Version 2.4; American Academy of Sleep Medicine: Darien, IL, USA, 2017. [Google Scholar]
- Silber, M.H.; Ancoli-Israel, S.; Bonnet, M.H.; Chokroverty, S.; Grigg-Damberger, M.M.; Hirshkowitz, M.; Kapen, S.; Keenan, S.A.; Kryger, M.H.; Penzel, T.; et al. The visual scoring of sleep in adults. J. Clin. Sleep Med. 2007, 3, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Wendt, S.L.; Welinder, P.; Sorensen, H.B.D.; Peppard, P.E.; Jennum, P.; Perona, P.; Mignot, E.; Warby, S.C. Inter-expert and intra-expert reliability in sleep spindle scoring. Clin. Neurophysiol. 2015, 126, 1548–1556. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, M.H.; Doghramji, K.; Roehrs, T.; Stepanski, E.J.; Sheldon, S.H.; Walters, A.S.; Wise, M.; Chesson, A.L. The scoring of arousal in sleep: Reliability, validity, and alternatives. J. Clin. Sleep Med. 2007, 3, 133–145. [Google Scholar] [CrossRef]
- Loredo, J.S.; Clausen, J.L.; Ancoli-Israel, S.; Dimsdale, J.E. Night-to-night arousal variability and interscorer reliability of arousal measurements. Sleep 1999, 22, 916–920. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.K.; Galster, P.; Lau, T.S.; Lutz, J.M.; Marcus, C.L. Reliability of scoring arousals in normal children and children with obstructive sleep apnea syndrome. Sleep 2004, 27, 1139–1145. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.J. Arousals in sleep-disordered breathing: Patterns and implications. Sleep 2003, 26, 1042–1047. [Google Scholar] [CrossRef]
- Crowell, D.H.; Kulp, T.D.; Kapuniai, L.E.; Hunt, C.E.; Brooks, L.J.; Weese-Mayer, D.E.; Silvestri, J.; Ward, S.D.; Corwin, M.; Tinsley, L.; et al. Infant polysomnography: Reliability and validity of infant arousal assessment. J. Clin. Neurophysiol. 2002, 19, 469–483. [Google Scholar] [CrossRef]
- Drinnan, M.J.; Murray, A.; Griffiths, C.J.; Gibson, G.J. Interobserver variability in recognizing arousal in respiratory sleep disorders. Am. J. Respir. Crit. Care Med. 1998, 158, 358–362. [Google Scholar] [CrossRef]
- Faust, O.; Razaghi, H.; Barika, R.; Ciaccio, E.J.; Acharya, U.R. A review of automated sleep stage scoring based on physiological signals for the new millennia. Comput. Methods Program Biomed. 2019, 176, 81–91. [Google Scholar] [CrossRef]
- Berthomier, C.; Muto, V.; Schmidt, C.; Vandewalle, G.; Jaspar, M.; Devillers, J.; Gaggioni, G.; Chellappa, S.L.; Meyer, C.; Phillips, C.; et al. Exploring scoring methods for research studies: Accuracy and variability of visual and automated sleep scoring. J. Sleep Res. 2020, e12994. [Google Scholar] [CrossRef]
- Agarwal, R. Automatic detection of micro-arousals. Annu. Int. Conf. IEEE Eng. Med. Biol. Proc. 2005, 7, 1158–1161. [Google Scholar]
- Cho, S.P.; Lee, J.; Park, H.D.; Lee, K.J. Detection of arousals in patients with respiratory sleep disorders using a single channel EEG. Annu. Int. Conf. IEEE Eng. Med. Biol. Proc. 2005, 7, 2733–2735. [Google Scholar]
- De Carli, F.; Nobili, L.; Gelcich, P.; Ferrillo, F. A method for the automatic detection of arousals during sleep. Sleep 1999, 22, 561–572. [Google Scholar] [CrossRef] [PubMed]
- De Carli, F.; Nobili, L.; Beelke, M.; Watanabe, T.; Smerieri, A.; Parrino, L.; Terzano, M.G.; Ferrillo, F. Quantitative analysis of sleep EEG microstructure in the time-frequency domain. Brain Res. Bull. 2004, 63, 399–405. [Google Scholar] [CrossRef]
- Alvarez-Estevez, D.; Fernández-Varela, I. Large-scale validation of an automatic EEG arousal detection algorithm using different heterogeneous databases. Sleep Med. 2019, 57, 6–14. [Google Scholar] [CrossRef]
- Ugur, T.K.; Erdamar, A. An efficient automatic arousals detection algorithm in single channel EEG. Comput. Methods Programs Biomed. 2019, 173, 131–138. [Google Scholar] [CrossRef]
- Miller, D.; Ward, A.; Bambos, N. Automatic sleep arousal identification from physiological waveforms using deep learning. Comput. Cardiol. 2018, 1–4. [Google Scholar] [CrossRef]
- Zabihi, M.; Rad, A.B.; Sarkka, S.; Kiranyaz, S.; Katsaggelos, A.K.; Gabbouj, M. Automatic sleep arousal detection using state distance analysis in phase space. Comput. Cardiol. 2018, 1–4. [Google Scholar] [CrossRef]
- Crespo-Garcia, M.; Atienza, M.; Cantero, J.L. Muscle artifact removal from human sleep EEG by using independent component analysis. Ann. Biomed. Eng. 2008, 36, 467–475. [Google Scholar] [CrossRef]
- Coppieters ’t Wallant, D.; Maquet, P.; Phillips, C. Sleep spindles as an electrographic element: Description and automatic detection methods. Neural Plast. 2016, 2016. [Google Scholar] [CrossRef]
- Warby, S.C.; Wendt, S.L.; Welinder, P.; Munk, E.G.S.; Carrillo, O.; Sorensen, H.B.D.; Jennum, P.; Peppard, P.E.; Perona, P.; Mignot, E. Sleep spindle detection: Crowdsourcing and evaluating performance of experts, non-experts, and automated methods. Dev. Psychopathol. 2015, 27, 1251–1265. [Google Scholar] [CrossRef] [PubMed]
- Coppieters ’t Wallant, D.; Muto, V.; Gaggioni, G.; Jaspar, M.; Chellappa, S.; Meyer, C.; Vandewalle, G.; Maquet, P.; Phillips, C. Automatic artifacts and arousals detection in whole-night sleep EEG recordings. J. Neurosci. Methods 2016, 258, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Buysse, D.J.; Reynolds, C.F.; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Johns, M.W. A new method for measuring daytime sleepiness: The Epworth sleepiness scale. Sleep 1991, 14, 540–545. [Google Scholar] [CrossRef] [PubMed]
- Richard, B.B.; Rita, B.; Charlene, E.G.; Susan, M.H.; Robin, M.L. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology, and Technical Specifications, Version 2.2; American Academy of Sleep Medicine: Darien, IL, USA, 2015; Volume 28, pp. 391–397. [Google Scholar]
- Landis, J.R.; Koch, G.G. The measurement of observer agreement for categorical data. J. Biom. 1977, 33, 159–174. [Google Scholar] [CrossRef]
- Tallon-Baudry, C.; Bertrand, O. Oscillatory gamma activity in humans and its role in object representation. Trends Cogn. Sci. 1999, 3, 151–162. [Google Scholar] [CrossRef]
- Richard, B.B.; Rita, B.; Gamaldo, C.E.; Harding, S.M.; Lloyd, R.M.; Marcus, C.L.; Vaughn, B.V. Should the arousal scoring rule be changed? J. Clin. Sleep Med. 2015, 11, 497–499. [Google Scholar]
| SCORER | YOUNG | OLDER | |||||
|---|---|---|---|---|---|---|---|
| F | M | Total | F | M | Total | ||
| BAS1 | 3 | 3 | 6 | 4 | 5 | 9 | 15 |
| BAS2 | 0 | 4 | 4 | 2 | 2 | 4 | 8 |
| BAS3 | 2 | 2 | 4 | 0 | 1 | 1 | 5 |
| BAS4 | 2 | 2 | 4 | 2 | 1 | 3 | 7 |
| TOTAL | 7 | 11 | 18 | 8 | 9 | 17 | 35 |
| Kappa Value | Interpretation |
|---|---|
| <0.00 | Poor |
| 0.00–0.20 | Slight |
| 0.21–40 | Fair |
| 41–0.60 | Moderate |
| 0.61–0.80 | Substantial |
| 0.81–1.00 | Almost perfect |
| Gold Standard | Compared | Ss | κs | See | Cs | FDRe |
|---|---|---|---|---|---|---|
| BAS | DC | 94 ± 3% | 0.97 ± 0.02 | 58 ± 16% | 72 ± 7% | 36 ± 12% |
| DC | BAS | 89 ± 4% | 0.94 ± 0.02 | 81 ± 26% | 78 ± 12% | 78 ± 9% |
| Gold Standard | Ss | κs | See | Cs | FDRe |
|---|---|---|---|---|---|
| HR inclusive EMG | 86 ± 6% 90 ± 6% | 0.93 ± 0.03 0.95 ± 0.03 | 67 ± 23% 45 ± 23% | 59 ± 13% 61 ± 15% | 61 ± 16% 28 ± 25% |
| HR conservative EMG | 88 ± 4% 92 ± 4% | 0.94 ± 0.02 0.96 ± 0.02 | 83 ± 26% 64 ± 27% | 58 ± 14% 60 ± 15% | 74 ± 12% 41 ± 23% |
| DC | BAS * | Inclusive | Conservative | AD | AD EMG | |
|---|---|---|---|---|---|---|
| Young | 63 ± 31 | 84 ± 30 | 93 ± 37 | 51 ± 19 | 208 ± 48 | 68 ± 22 |
| Old | 79 ± 34 | 141 ± 53 | 142 ± 46 | 67 ± 23 | 193 ± 39 | 70 ± 25 |
| Gold Standard | AD Arousals | Ss | κs | See | Cs | FDRe | |
|---|---|---|---|---|---|---|---|
| HR inclusive | All | Age Sex | p = 0.10 | p = 0.12 | p = 0.01 | p = 0.24 | p = 0.05 |
| F = 2.83 | F = 2.49 | F = 7.53 | F = 1.42 | F = 4.23 | |||
| p = 0.10 | p = 0.10 | p = 0.16 | p = 0.21 | p = 0.49 | |||
| F = 2.91 | F = 2.83 | F = 2.12 | F = 1.63 | F = 0.48 | |||
| EMG | Age Sex | p = 0.002 * | p = 0.002 * | p = 0.01 | p = 0.41 | p = 0.61 | |
| F = 11.48 | F = 11.03 | F = 6.89 | F = 0.71 | F = 0.26 | |||
| p = 0.07 | p = 0.07 | p = 0.78 | p = 0.13 | p = 0.70 | |||
| F = 3.49 | F = 3.48 | F = 0.08 | F = 2.41 | F = 0.15 | |||
| HR conservative | All | Age Sex | p = 0.96 | p = 0.91 | p = 0.33 | p = 0.86 | p = 0.07 |
| F = 0.00 | F = 0.01 | F = 0.99 | F = 0.03 | F = 3.52 | |||
| p = 0.21 | p = 0.21 | p = 0.12 | p = 0.27 | p = 0.37 | |||
| F = 1.64 | F = 1.62 | F = 2.49 | F = 1.24 | F = 0.82 | |||
| EMG | Age Sex | p = 0.09 | p = 0.10 | p = 0.05 | p = 0.97 | p = 0.88 | |
| F = 3.05 | F = 2.87 | F = 4.21 | F = 0.00 | F = 0.02 | |||
| p = 0.04 | p = 0.04 | p = 0.45 | p = 0.21 | p = 0.56 | |||
| F = 4.68 | F = 4.71 | F = 0.58 | F = 1.63 | F = 0.35 | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chylinski, D.; Rudzik, F.; Coppieters ‘t Wallant, D.; Grignard, M.; Vandeleene, N.; Van Egroo, M.; Thiesse, L.; Solbach, S.; Maquet, P.; Phillips, C.; et al. Validation of an Automatic Arousal Detection Algorithm for Whole-Night Sleep EEG Recordings. Clocks & Sleep 2020, 2, 258-272. https://doi.org/10.3390/clockssleep2030020
Chylinski D, Rudzik F, Coppieters ‘t Wallant D, Grignard M, Vandeleene N, Van Egroo M, Thiesse L, Solbach S, Maquet P, Phillips C, et al. Validation of an Automatic Arousal Detection Algorithm for Whole-Night Sleep EEG Recordings. Clocks & Sleep. 2020; 2(3):258-272. https://doi.org/10.3390/clockssleep2030020
Chicago/Turabian StyleChylinski, Daphne, Franziska Rudzik, Dorothée Coppieters ‘t Wallant, Martin Grignard, Nora Vandeleene, Maxime Van Egroo, Laurie Thiesse, Stig Solbach, Pierre Maquet, Christophe Phillips, and et al. 2020. "Validation of an Automatic Arousal Detection Algorithm for Whole-Night Sleep EEG Recordings" Clocks & Sleep 2, no. 3: 258-272. https://doi.org/10.3390/clockssleep2030020
APA StyleChylinski, D., Rudzik, F., Coppieters ‘t Wallant, D., Grignard, M., Vandeleene, N., Van Egroo, M., Thiesse, L., Solbach, S., Maquet, P., Phillips, C., Vandewalle, G., Cajochen, C., & Muto, V. (2020). Validation of an Automatic Arousal Detection Algorithm for Whole-Night Sleep EEG Recordings. Clocks & Sleep, 2(3), 258-272. https://doi.org/10.3390/clockssleep2030020







