Enhanced Circadian Entrainment in Mice and Its Utility under Human Shiftwork Schedules
Abstract
1. General Introduction
2. Study Results
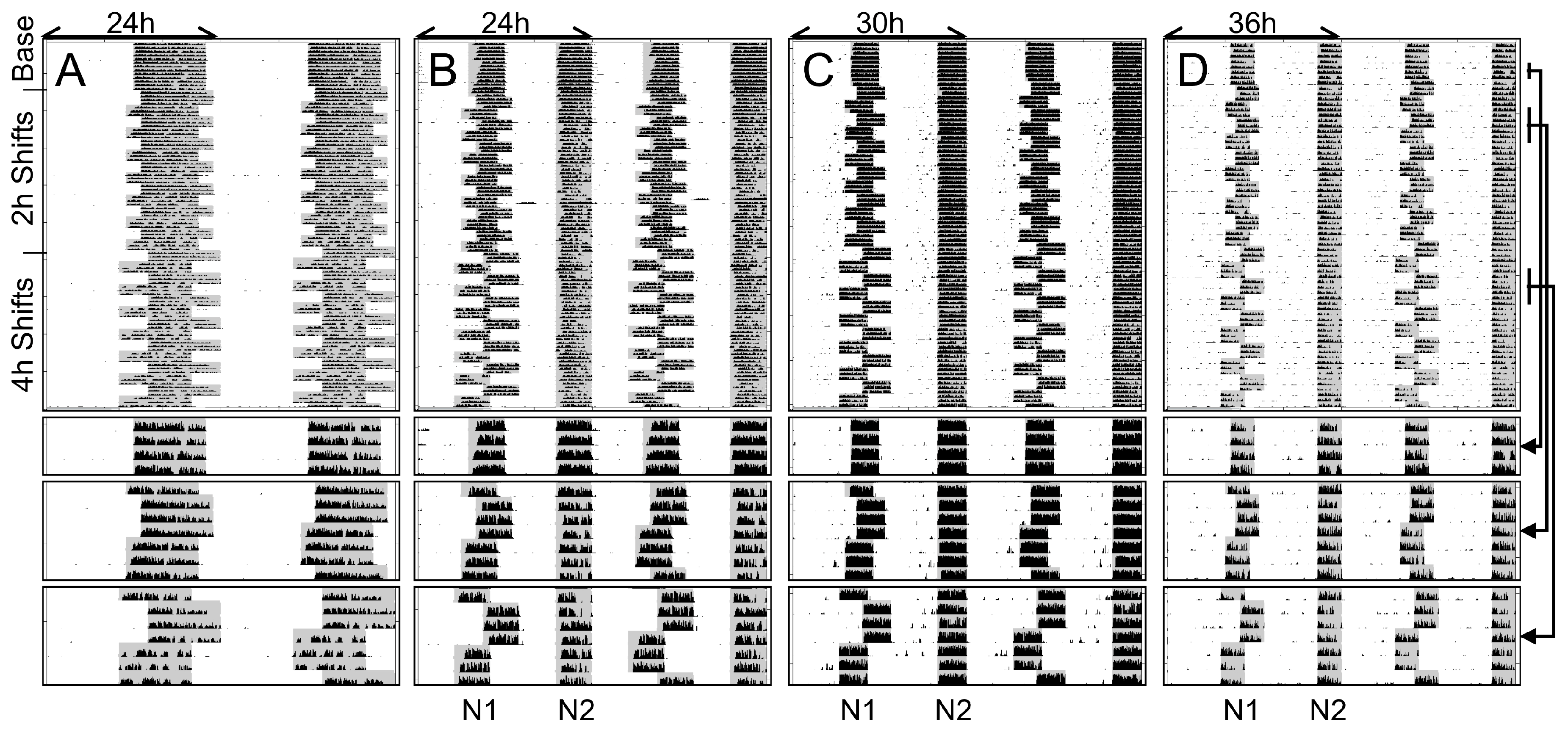
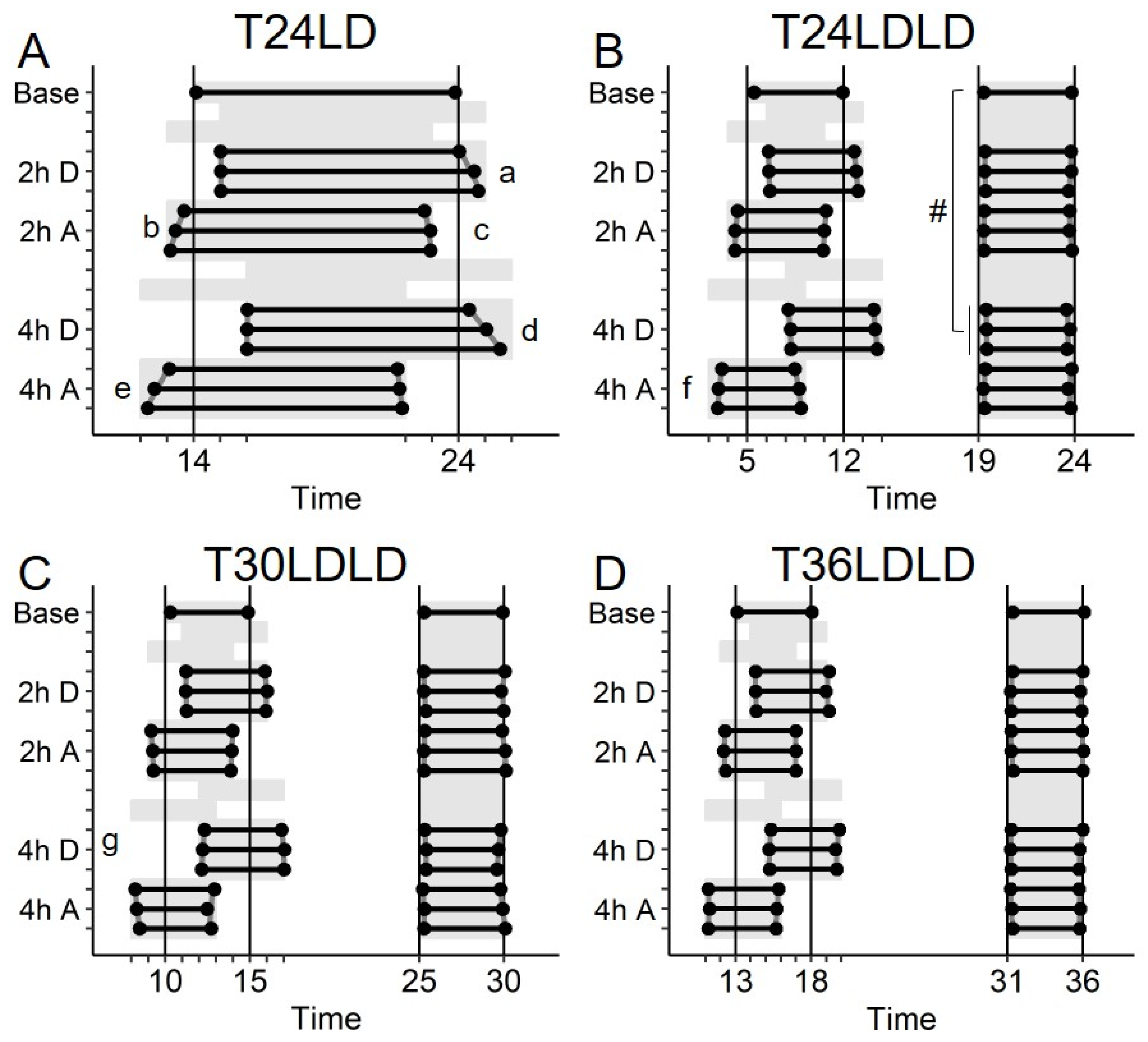
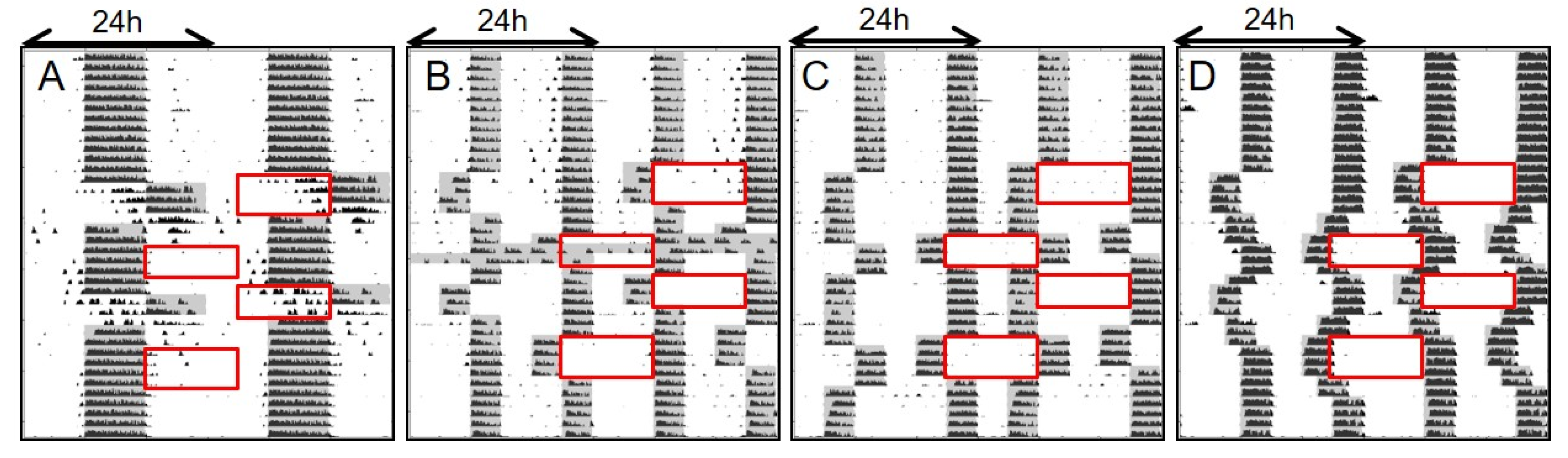
2.1. Study 1/Jitter—Nature of Entrainment to Bifurcated and Non-24 h Cycles
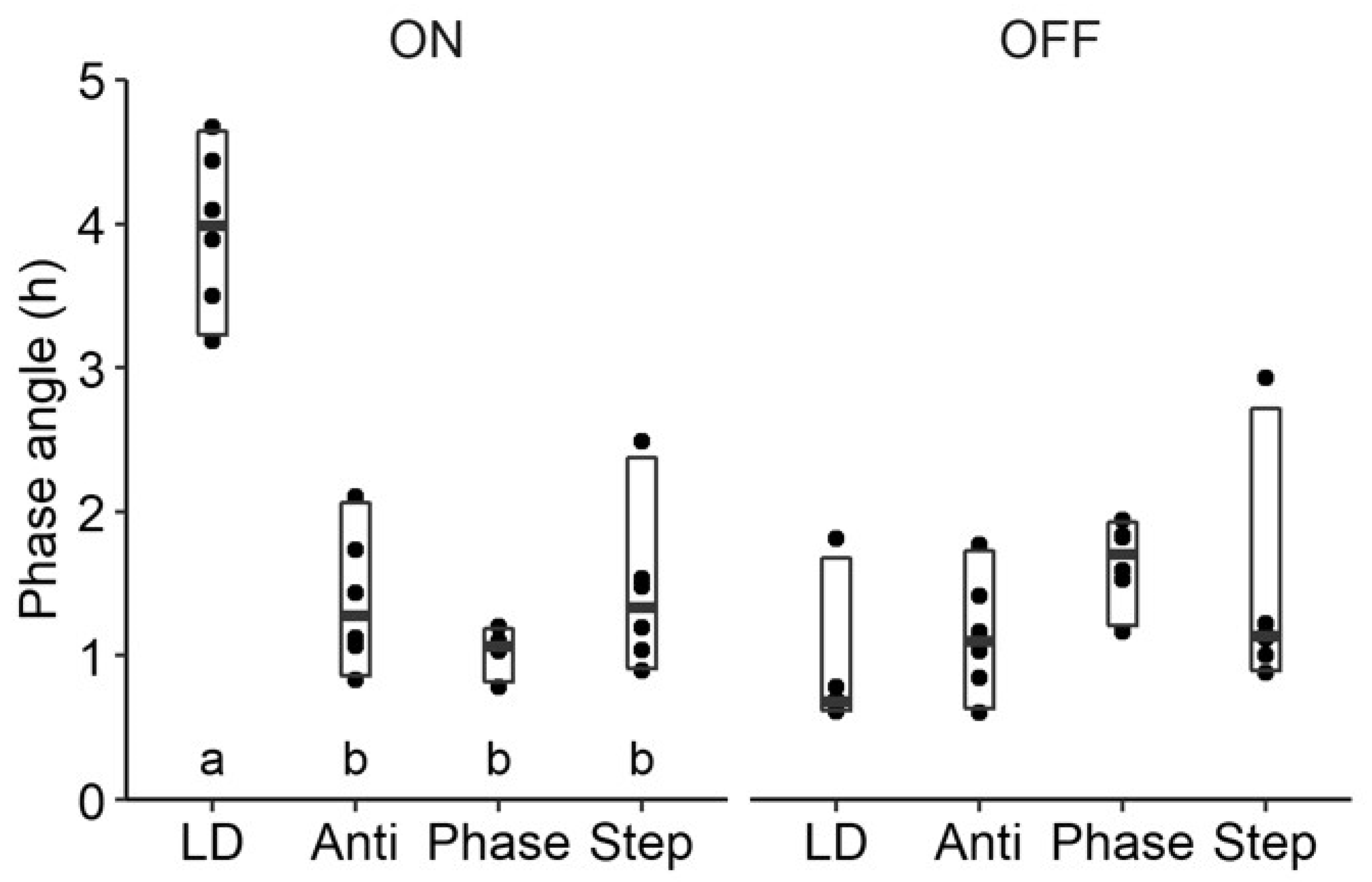
2.2. Study 1/Jitter—Results
2.3. Study 2/DuPont—DuPont Work Schedule with Bifurcation
2.4. Study 2/DuPont—Results
2.5. Study 3/Continental—Rotating Work Hours Using T-Cycles
2.6. Study 3/Continental—Results
3. Discussion
3.1. Summary
3.2. Building Rodent Shiftwork Models
3.3. Mechanisms of Behavioral Adaptation
4. Methods
4.1. Nomenclature
4.2. Housing and Lighting
4.3. Quantitative Assessments of Entrainment and Analyses
4.4. Study 1/Jitter
4.4.1. Stable Entrainment
4.4.2. Repeated Phase Shifts
4.4.3. Exclusion of Non-Entrained Animals
4.4.4. Onset and Offsets/Activity in the Light
4.5. Study 2/DuPont
4.5.1. Stable Entrainment
4.5.2. Experimental Phase
4.5.3. Quantification of Adaptation
4.6. Study 3/Continental
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| LD | Light Dark |
| LDLD | Light Dark Light Dark |
| BSI | Bifurcation Symmetry Index |
| EQ | Entrainment Quotient |
| N1 | Night 1 |
| N2 | Night 2 |
| SCN | Suprachiasmatic nucleus |
References
- Moreno, C.R.C.; Marqueze, E.C.; Sargent, C.; Wright, J.K.P.; Ferguson, S.A.; Tucker, P. Working Time Society consensus statements: Evidence-based effects of shift work on physical and mental health. Ind. Health 2019, 57, 139–157. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.A.; Davidson, A.J. Health consequences of circadian disruption in humans and animal models. Prog. Mol. Biol. Transl. Sci. 2013, 119, 283–323. [Google Scholar] [PubMed]
- Straif, K.; Baan, R.; Grosse, Y.; Secretan, B.; El Ghissassi, F.; Bouvard, V.; Altieri, A.; Benbrahim-Tallaa, L.; Cogliano, V. WHO International Agency For Research on Cancer Monograph Working Group Carcinogenicity of shift-work, painting, and fire-fighting. Lancet. Oncol. 2007, 8, 1065–1066. [Google Scholar] [CrossRef]
- American College of Emergency Physicians (2017) Emergency Physician Shift Work. Available online: https://www.acep.org/patient-care/policy-statements/emergency-physician-shift-work/ (accessed on 21 August 2019).
- Smith, M.R.; Eastman, C.I. Shift work: Health, performance and safety problems, traditional countermeasures, and innovative management strategies to reduce circadian misalignment. Nat. Sci. Sleep 2012, 4, 111–132. [Google Scholar] [PubMed]
- Kessler, E.J.; Sprouse, J.; Harrington, M.E. NAN-190 potentiates the circadian response to light and speeds re-entrainment to advanced light cycles. Neuroscience 2008, 154, 1187–1194. [Google Scholar] [CrossRef]
- An, S.; Harang, R.; Meeker, K.; Granados-Fuentes, D.; Tsai, C.A.; Mazuski, C.; Kim, J.; Doyle, F.J.; Petzold, L.R.; Herzog, E.D. A neuropeptide speeds circadian entrainment by reducing intercellular synchrony. Proc. Natl. Acad. Sci. USA 2013, 110, 4355–4361. [Google Scholar] [CrossRef]
- Kaur, G.; Thind, R.; Glass, J.D. Brief constant light accelerates serotonergic re-entrainment to large shifts of the daily light/dark cycle. Neuroscience 2009, 159, 1430–1440. [Google Scholar] [CrossRef][Green Version]
- Glickman, G.L.; Webb, I.C.; Elliott, J.; Baltazar, R.M.; Reale, M.E.; Lehman, M.N.; Gorman, M.R. Photic sensitivity for circadian response to light varies with photoperiod. J. Biol. Rhythms 2012, 27, 308–318. [Google Scholar] [CrossRef]
- Glickman, G.L.; Harrison, E.M.; Elliott, J.A.; Gorman, M.R. Increased photic sensitivity for phase resetting but not melatonin suppression in Siberian hamsters under short photoperiods. Horm. Behav. 2014, 65, 301–307. [Google Scholar] [CrossRef][Green Version]
- Ramkisoensing, A.; Gu, C.; van Engeldorp Gastelaars, H.M.D.; Michel, S.; Deboer, T.; Rohling, J.H.T.; Meijer, J.H. Enhanced phase resetting in the synchronized suprachiasmatic nucleus network. J. Biol. Rhythms 2014, 29, 4–15. [Google Scholar] [CrossRef]
- Evans, J.A.; Elliott, J.A.; Gorman, M.R. Dim nighttime illumination accelerates adjustment to timezone travel in an animal model. Curr. Biol. 2009, 19, 156–157. [Google Scholar] [CrossRef][Green Version]
- Gorman, M.R.; Elliott, J.A. Entrainment of 2 subjective nights by daily light:dark:light:dark cycles in 3 rodent species. J. Biol. Rhythms 2003, 18, 502–512. [Google Scholar] [CrossRef]
- Walbeek, T.J.; Gorman, M.R. Simple Lighting Manipulations Facilitate Behavioral Entrainment of Mice to 18-h Days. J. Biol. Rhythms 2017, 32, 309–322. [Google Scholar] [CrossRef]
- Gorman, M.R.; Kendall, M.; Elliott, J.A. Scotopic illumination enhances entrainment of circadian rhythms to lengthening light: dark cycles. J. Biol. Rhythms 2005, 20, 38–48. [Google Scholar] [CrossRef]
- Harrison, E.M.; Gorman, M.R. Rapid Adjustment of Circadian Clocks to Simulated Travel to Time Zones across the Globe. J. Biol. Rhythms 2015, 30, 557–562. [Google Scholar] [CrossRef]
- Harrison, E.M.; Walbeek, T.J.; Sun, J.; Johnson, J.; Poonawala, Q.; Gorman, M.R. Extraordinary behavioral entrainment following circadian rhythm bifurcation in mice. Sci. Rep. 2016, 6, 38479. [Google Scholar] [CrossRef]
- Gorman, M.R.; Elliott, J.A. Exceptional Entrainment of Circadian Activity Rhythms with Manipulations of Rhythm Waveform in Male Syrian Hamsters. Yale J. Biol. Med. 2019, 92, 187–199. [Google Scholar]
- Harrison, E.M.; Gorman, M.R. Changing the waveform of circadian rhythms: Considerations for shift-work. Front. Neurol. 2012, 3, 72. [Google Scholar] [CrossRef]
- Sun, J.; Joye, D.A.M.; Farkas, A.H.; Gorman, M.R. Photoperiodic Requirements for Induction and Maintenance of Rhythm Bifurcation and Extraordinary Entrainment in Male Mice. Clocks Sleep 2019, 1, 290–305. [Google Scholar] [CrossRef]
- Rosenthal, S.L.; Vakili, M.M.; Evans, J.A.; Elliott, J.A.; Gorman, M.R. Influence of photoperiod and running wheel access on the entrainment of split circadian rhythms in hamsters. BMC Neurosci. 2005, 6, 41. [Google Scholar] [CrossRef]
- Noguchi, T.; Harrison, E.M.; Sun, J.; May, D.; Ng, A.; Welsh, D.K.; Gorman, M.R. Circadian rhythm bifurcation induces flexible phase resetting by reducing circadian amplitude. Eur. J. Neurosci. 2018. [Google Scholar] [CrossRef]
- Raiewski, E.E.; Elliott, J.A.; Evans, J.A.; Glickman, G.L.; Gorman, M.R. Twice daily melatonin peaks in Siberian but not Syrian hamsters under 24 h light: dark: light: dark cycles. Chronobiol. Int. 2012, 29, 1206–1215. [Google Scholar] [CrossRef]
- Gorman, M.R.; Yellon, S.M.; Lee, T.M. Temporal reorganization of the suprachiasmatic nuclei in hamsters with split circadian rhythms. J. Biol. Rhythms 2001, 16, 552–563. [Google Scholar] [CrossRef]
- Yan, L.; Silver, R.; Gorman, M.R. Reorganization of suprachiasmatic nucleus networks under 24-h LDLD conditions. J. Biol. Rhythm. 2010, 25, 19–27. [Google Scholar] [CrossRef]
- Granada, A.E.; Bordyugov, G.; Kramer, A.; Herzel, H. Human chronotypes from a theoretical perspective. PLoS ONE 2013, 8, e59464. [Google Scholar] [CrossRef]
- Pittendrigh, C.S.; Daan, S. A functional analysis of circadian pacemakers in nocturnal rodents. J. Comp. Physiol. A 1976, 106, 223–252. [Google Scholar] [CrossRef]
- Gorman, M.R.; Steele, N.A. Phase angle difference alters coupling relations of functionally distinct circadian oscillators revealed by rhythm splitting. J. Biol. Rhythm. 2006, 21, 195–205. [Google Scholar] [CrossRef]
- Brown, L.A.; Fisk, A.S.; Pothecary, C.A.; Peirson, S.N. Telling the Time with a Broken Clock: Quantifying Circadian Disruption in Animal Models. Biology 2019, 8, 18. [Google Scholar] [CrossRef]
- Eastman, C.I. Circadian rhythms and bright light: Recommendations for shift work. Work Stress 1990, 4, 245–260. [Google Scholar] [CrossRef]
- Saderi, N.; Báez-Ruiz, A.; Azuara-Álvarez, L.E.; Escobar, C.; Salgado-Delgado, R.C. Differential Recovery Speed of Activity and Metabolic Rhythms in Rats After an Experimental Protocol of Shift-Work. J. Biol. Rhythm. 2019, 34, 154–166. [Google Scholar] [CrossRef]
- Oike, H.; Sakurai, M.; Ippoushi, K.; Kobori, M. Time-fixed feeding prevents obesity induced by chronic advances of light/dark cycles in mouse models of jet-lag/shift work. Biochem. Biophys. Res. Commun. 2015, 465, 556–561. [Google Scholar] [CrossRef]
- Karatsoreos, I.N.; Bhagat, S.; Bloss, E.B.; Morrison, J.H.; McEwen, B.S. Disruption of circadian clocks has ramifications for metabolism, brain, and behavior. Proc. Natl. Acad. Sci. USA 2011, 108, 1657–1662. [Google Scholar] [CrossRef]
- McGowan, N.M.; Coogan, A.N. Circadian and behavioural responses to shift work-like schedules of light/dark in the mouse. J. Mol. Psychiatry 2013, 1, 7. [Google Scholar] [CrossRef]
- Akerstedt, T. Shift work and disturbed sleep/wakefulness. Occup. Med. 2003, 53, 89–94. [Google Scholar] [CrossRef]
- Gamble, K.L.; Motsinger-Reif, A.A.; Hida, A.; Borsetti, H.M.; Servick, S.V.; Ciarleglio, C.M.; Robbins, S.; Hicks, J.; Carver, K.; Hamilton, N.; et al. Shift Work in Nurses: Contribution of Phenotypes and Genotypes to Adaptation. PLoS ONE 2011, 6, e18395. [Google Scholar] [CrossRef]
- Petrov, M.E.; Clark, C.B.; Molzof, H.E.; Johnson, R.L.; Cropsey, K.L.; Gamble, K.L. Sleep Strategies of Night-Shift Nurses on Days Off: Which Ones are Most Adaptive? Front. Neurol. 2014, 5, 277. [Google Scholar] [CrossRef]
- Patton, D.F.; Mistlberger, R.E. Circadian adaptations to meal timing: Neuroendocrine mechanisms. Front. Neurosci. 2013, 7, 185. [Google Scholar] [CrossRef]
- Edgar, D.M.; Dement, W.C. Regularly scheduled voluntary exercise synchronizes the mouse circadian clock. Am. J. Physiol. 1991, 261, 928–933. [Google Scholar] [CrossRef]
- Walbeek, T.J.; Joye, D.A.M.; Mishra, I.; Gorman, M.R. Physiological, behavioral and environmental factors influence bifurcated circadian entrainment in mice. Physiol. Behav. 2019, 210, 112625. [Google Scholar] [CrossRef]
- Evans, J.A.; Elliott, J.A.; Gorman, M.R. Individual Differences in Circadian Waveform of Siberian Hamsters under Multiple Lighting Conditions. J. Biol. Rhythm. 2012, 27, 410–419. [Google Scholar] [CrossRef]
- Saksvik, I.B.; Bjorvatn, B.; Hetland, H.; Sandal, G.M.; Pallesen, S. Individual differences in tolerance to shift work—A systematic review. Sleep Med. Rev. 2011, 15, 221–235. [Google Scholar] [CrossRef]
- Nachreiner, F. Individual and social determinants of shiftwork tolerance. Scand. J. Work. Environ. Health 1998, 24, 35–42. [Google Scholar]
- Ritonja, J.; Aronson, K.J.; Matthews, R.W.; Boivin, D.B.; Kantermann, T. Working Time Society consensus statements: Individual differences in shift work tolerance and recommendations for research and practice. Ind. Health 2019, 57, 201–212. [Google Scholar] [CrossRef]
- Evans, J.A.; Gorman, M.R. Split circadian rhythms of female Syrian hamsters and their offspring. Physiol. Behav. 2002, 76, 469–478. [Google Scholar] [CrossRef]
- Harrison, E.; Carmack, S.; Block, C.; Sun, J.; Anagnostaras, S.; Gorman, M.R. Circadian waveform bifurcation, but not phase-shifting, leaves cued fear memory intact. Physiol. Behav. 2017, 169, 106–113. [Google Scholar] [CrossRef]
- Daan, S.; Aschoff, J. The Entrainment of Circadian Rhythm. In Handbook of Behavioral Neurobiology; Volume 12—Circadian, Clocks; Takahashi, J.S., Turek, F.W., Moore, R.Y., Eds.; Springer: New York, NY, USA, 2001; pp. 7–43. ISBN 978-0-306-46504-8. [Google Scholar]
- Evans, J.A.; Gorman, M.R. In synch but not in step: Circadian clock circuits regulating plasticity in daily rhythms. Neuroscience 2016, 320, 259–280. [Google Scholar] [CrossRef]
- Gorman, M.R.; Harrison, E.M.; Evans, J.A. Circadian Waveform and Its Significance for Clock Organization and Plasticity. In Biological Timekeeping: Clocks, Rhythms and Behaviour; Springer India: New Delhi, India, 2017; pp. 59–79. [Google Scholar]
- Watanabe, T.; Naito, E.; Nakao, N.; Tei, H.; Yoshimura, T.; Ebihara, S. Bimodal clock gene expression in mouse suprachiasmatic nucleus and peripheral tissues under a 7-hour light and 5-hour dark schedule. J. Biol. Rhythm. 2007, 22, 58–68. [Google Scholar] [CrossRef]
- De la Iglesia, H.O.; Cambras, T.; Schwartz, W.J.; Díez-Noguera, A. Forced desynchronization of dual circadian oscillators within the rat suprachiasmatic nucleus. Curr. Biol. 2004, 14, 796–800. [Google Scholar] [CrossRef]
- Anglès-Pujolràs, M.; Díez-Noguera, A.; Cambras, T. Exposure to T-cycles of 22 and 23 h during lactation modifies the later dissociation of motor activity and temperature circadian rhythms in rats. Chronobiol. Int. 2007, 24, 1049–1064. [Google Scholar] [CrossRef]
- Casiraghi, L.P.; Oda, G.A.; Chiesa, J.J.; Friesen, W.O.; Golombek, D.A. Forced Desynchronization of Activity Rhythms in a Model of Chronic Jet Lag in Mice. J. Biol. Rhythm. 2012, 27, 59–69. [Google Scholar] [CrossRef]
- Leise, T.L.; Goldberg, A.; Michael, J.; Montoya, G.; Solow, S.; Molyneux, P.; Vetrivelan, R.; Harrington, M.E. Recurring circadian disruption alters circadian clock sensitivity to resetting. Eur. J. Neurosci. 2018. [Google Scholar] [CrossRef]
- Akerstedt, T. Shift work and disturbed sleep/wakefulness. Sleep Med. Rev. 1998, 2, 117–128. [Google Scholar] [CrossRef]
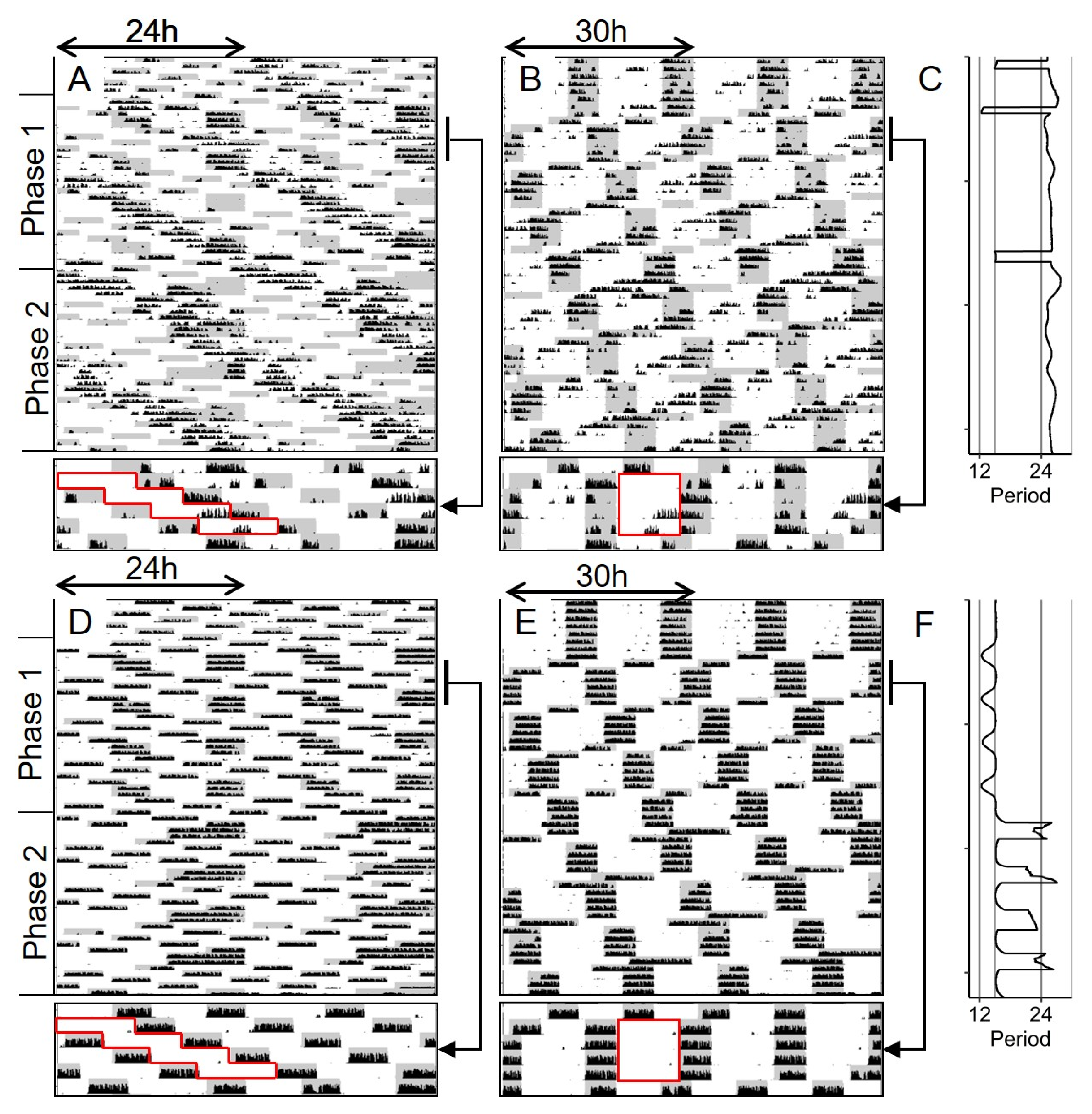
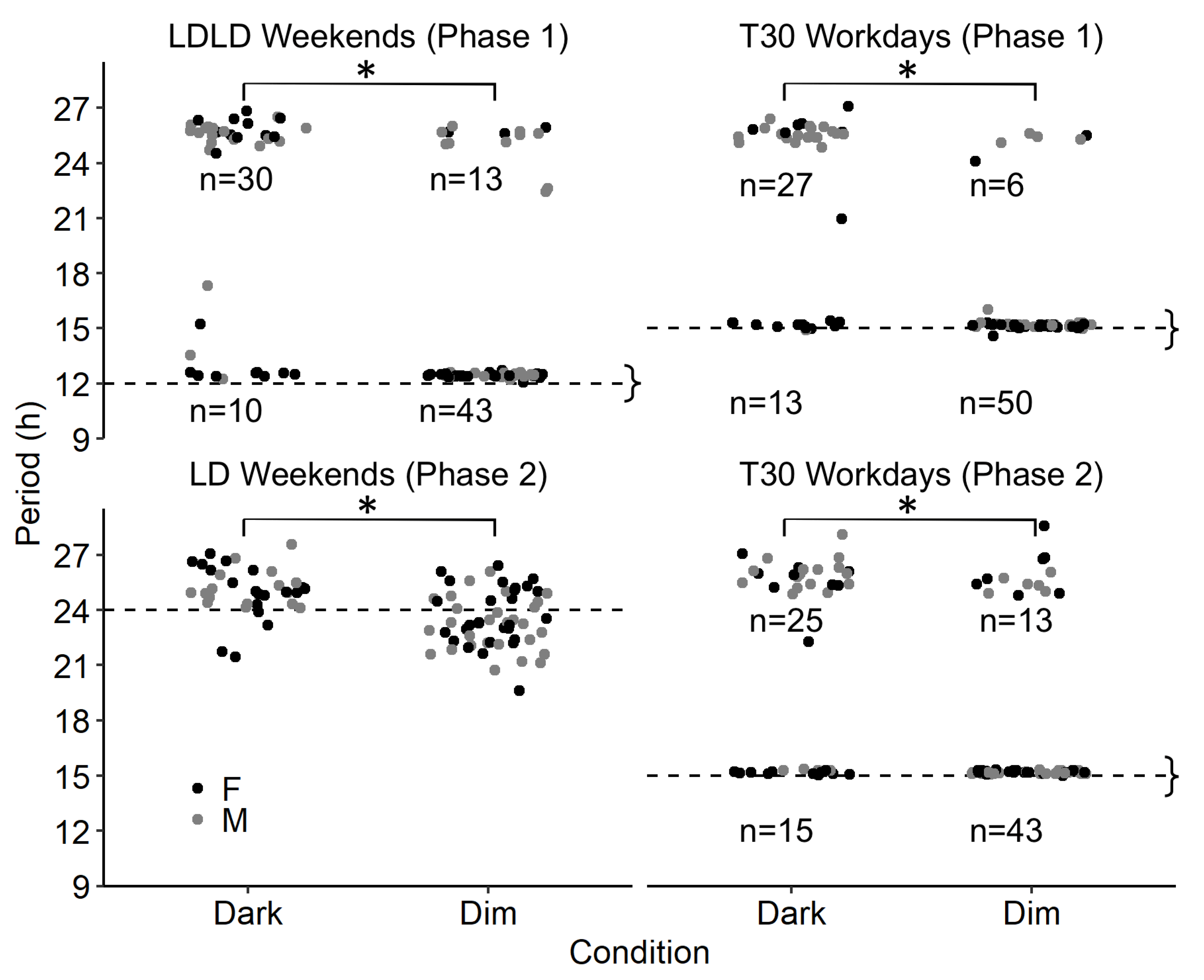

| Phase 0: Stable Entrainment | Phase 1: 2 h-Phase Shifts | Phase 2: 4 h-Phase Shifts | ||
|---|---|---|---|---|
| LD (n = 10) | 28 cycles: LDim 14:10 | 7 × 6 cycles: LDim 14:10 | 7 × 6 cycles: LDim 14:10 | |
| Bifurcation (n = 14) | 28 cycles: LDimLDim 7:5:7:5 | 7 × 6 cycles: LDimLDim 8:5:6:5/6:5:8:5 | 7 × 6 cycles: LDimLDim 9:5:5:5/5:5:9:5 | |
| T30 (n = 16) | 14 cycles:LDimLDim 7:5:7:5 | 11 cycles:LDimLDim 10:5:10:5 | 6 × 6 cycles: LDimLDim 11:5:9:5/9:5:11:5 | 6 × 6 cycles: LDimLDim 12:5:8:5/8:5:12:5 |
| T36 (n = 16) | 14 cycles: LDimLDim 7:5:7:5 | 10 cycles: LDimLDim 13:5:13:5 | 5 × 6 cycles: LDimLDim 14:5:12:5/12:5:14:5 | 5 × 6 cycles: LDimLDim 15:5:11:5/11:5:15:5 |
| Phase 0: Stable Entrainment | Phase 1: Bifurcated Weekends | Phase 2: Non-Bifurcated Weekend | ||
|---|---|---|---|---|
| Dark (n = 10) | 14 cycles: LDark 14:10 | Six cycles: LDarkLDark 10:5:10:5 | Four repeats of: 4× LDarkLDark 10:5:10:5, 2× LDarkLDark 7:5:7:5 | Four repeats of: 4× LDarkLDark 10:5:10:5, 2× LDark 14:10 |
| Dim (n = 14) | 14 cycles: LDimLDim 7:5:7:5 | Six cycles: LDimLDim 10:5:10:5 | Four repeats of: 4× LDimLDim 10:5:10:5, 2× LDimLDim 7:5:7:5 | Four repeats of: 4× LDimLDim 10:5:10:5, 2× LDim 14:10 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Walbeek, T.J.; Harrison, E.M.; Soler, R.R.; Gorman, M.R. Enhanced Circadian Entrainment in Mice and Its Utility under Human Shiftwork Schedules. Clocks & Sleep 2019, 1, 394-413. https://doi.org/10.3390/clockssleep1030032
Walbeek TJ, Harrison EM, Soler RR, Gorman MR. Enhanced Circadian Entrainment in Mice and Its Utility under Human Shiftwork Schedules. Clocks & Sleep. 2019; 1(3):394-413. https://doi.org/10.3390/clockssleep1030032
Chicago/Turabian StyleWalbeek, Thijs J., Elizabeth M. Harrison, Robert R. Soler, and Michael R. Gorman. 2019. "Enhanced Circadian Entrainment in Mice and Its Utility under Human Shiftwork Schedules" Clocks & Sleep 1, no. 3: 394-413. https://doi.org/10.3390/clockssleep1030032
APA StyleWalbeek, T. J., Harrison, E. M., Soler, R. R., & Gorman, M. R. (2019). Enhanced Circadian Entrainment in Mice and Its Utility under Human Shiftwork Schedules. Clocks & Sleep, 1(3), 394-413. https://doi.org/10.3390/clockssleep1030032





