A Pilot Study on Circadian Activity Rhythm in Pediatric Attention-Deficit Hyperactivity Disorder
Abstract
:1. Introduction
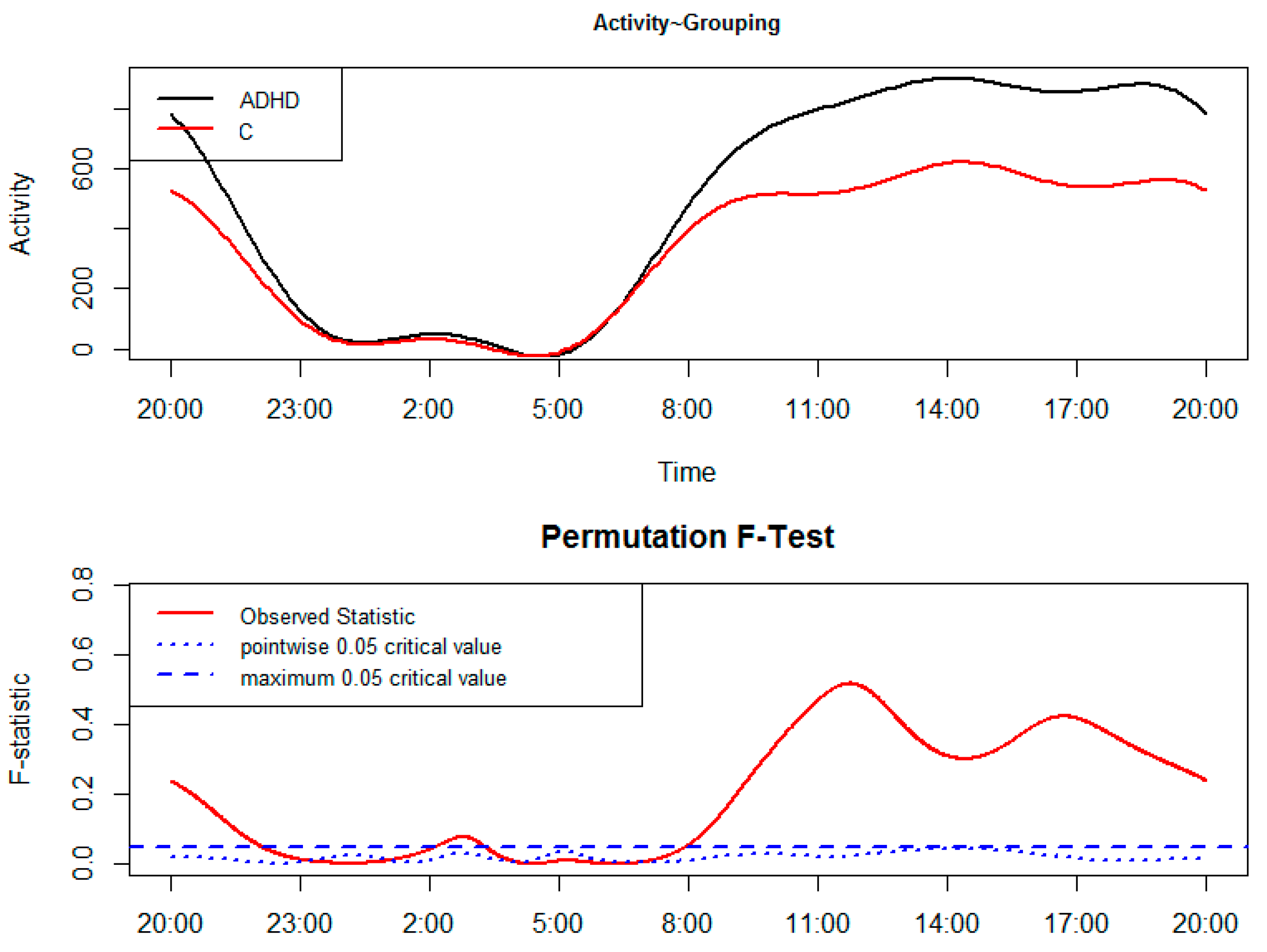
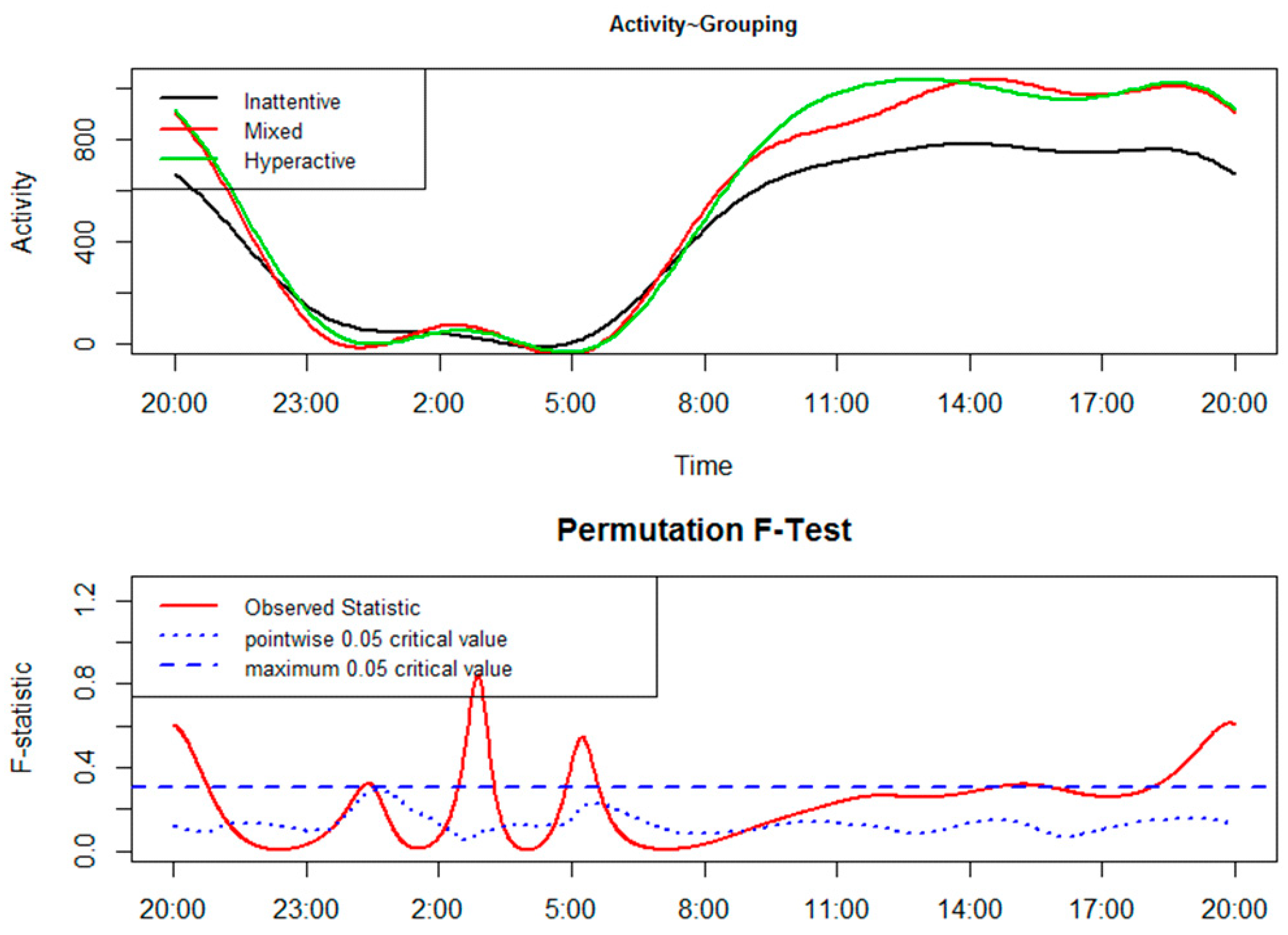
2. Results
3. Discussion
4. Materials and Methods
4.1. Participants
4.2. Actigraphy
4.3. Actigraphic Sleep Parameters
4.4. Procedure
4.5. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| FLM | Functional Linear Modeling |
| CAR | Circadian activity rhythm |
| ADHD | Attention-deficit hyperactivity disorder |
| C | Controls |
| WASO | Wake after sleep onset |
| SE | Sleep efficiency |
| M | Males |
| F | Females |
| BT | Bedtime |
| GUT | Get-up time |
| TIB | Time in bed |
| MS | Midpoint of sleep |
| TST | Total sleep time |
| SOL | Sleep onset latency |
| WB | Wake bouts |
| MAS | Mean activity score |
| IQ | Intelligence quotient |
| WISC-R | Wechsler Intelligence Scale for Children-revised |
| DSM-IV-TR | Diagnostic and Statistical Manual of Mental Disorders |
| SS | Sleep start |
| ANCOVA | Analysis of covariance |
References and Notes
- Xu, G.; Strathearn, L.; Liu, B.; Yang, B.; Bao, W. Twenty-Year Trends in Diagnosed Attention-Deficit/Hyperactivity Disorder Among US Children and Adolescents, 1997–2016. JAMA Netw. Open 2018, 1, e181471. [Google Scholar] [CrossRef] [PubMed]
- Barbaresi, W.J.; Weaver, A.L.; Voigt, R.G.; Killian, J.M.; Katusic, S.K. Comparing Methods to Determine Persistence of Childhood ADHD Into Adulthood: A Prospective, Population-Based Study. J. Atten. Disord. 2018, 22, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Ancoli-Israel, S.; Cole, R.; Alessi, C.; Chambers, M.; Moorcroft, W.; Pollak, C.P. The role of actigraphy in the study of sleep and circadian rhythms. Sleep 2003, 26, 342–392. [Google Scholar] [CrossRef] [PubMed]
- Coogan, A.N.; McGowan, N.M. A systematic review of circadian function, chronotype and chronotherapy in attention deficit hyperactivity disorder. Atten. Defic. Hyperact. Disord. 2017, 9, 129–147. [Google Scholar] [CrossRef] [PubMed]
- Imeraj, L.; Antrop, I.; Roeyers, H.; Deschepper, E.; Bal, S.; Deboutte, D. Diurnal variations in arousal: A naturalistic heart rate study in children with ADHD. Eur. Child Adolesc. Psychiatry 2011, 20, 381–392. [Google Scholar] [CrossRef] [PubMed]
- Dane, A.V.; Schachar, R.J.; Tannock, R. Does actigraphy differentiate ADHD subtypes in a clinical research setting? J. Am. Acad. Child Adolesc. Psychiatry 2000, 39, 752–760. [Google Scholar] [CrossRef] [PubMed]
- Tonetti, L.; Conca, A.; Giupponi, G.; Filardi, M.; Natale, V. Circadian activity rhythm in adult attention-deficit hyperactivity disorder. J. Psychiatr. Res. 2018, 103, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xian, H.; Licis, A.; Deych, E.; Ding, J.; McLelande, J.; Toedebusch, C.; Li, T.; Duntley, S.; Shannon, W. Measuring the impact of apnea and obesity on circadian activity patterns using functional linear modeling of actigraphy data. J. Circadian Rhythms 2011, 9, 11. [Google Scholar] [CrossRef] [PubMed]
- Filardi, M.; Pizza, F.; Bruni, O.; Natale, V.; Plazzi, G. Circadian rest-activity rhythm in pediatric type 1 narcolepsy. Sleep 2016, 39, 1241–1247. [Google Scholar] [CrossRef] [PubMed]
- Banihashemi, N.; Robillard, R.; Yang, J.; Carpenter, J.S.; Hermens, D.F.; Naismith, S.L.; Terpening, Z.; White, D.; Scott, E.M.; Hickie, I.B. Quantifying the effect of body mass index, age, and depression severity on 24-h activity patterns in persons with a lifetime history of affective disorders. BMC Psychiatry 2016, 16, 317. [Google Scholar] [CrossRef] [PubMed]
- De Crescenzo, F.; Licchelli, S.; Ciabattini, M.; Menghini, D.; Armando, M.; Alfieri, P.; Mazzone, L.; Pontrelli, G.; Livadiotti, S.; Foti, F.; et al. The use of actigraphy in the monitoring of sleep and activity in ADHD: A meta-analysis. Sleep Med. Rev. 2016, 26, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Melegari, M.G.; Vittori, E.; Mallia, L.; Devoto, A.; Lucidi, F.; Ferri, R.; Bruni, O. Actigraphic sleep pattern of preschoolers with ADHD. J. Atten. Disord. 2016. [Google Scholar] [CrossRef] [PubMed]
- Borbély, A.A.; Daan, S.; Wirz-Justice, A.; Deboer, T. The two-process model of sleep regulation: A reappraisal. J. Sleep Res. 2016, 25, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Tonetti, L.; Zoppello, M.; Termine, C.; Lanzi, G.; Fazzi, E. Applicazione dell’attigrafia per la valutazione del Disturbo da Deficit di Attenzione/Iperattività (DDAI) [Application of actigraphy to the assessment of attention-deficit hyperactivity disorder (ADHD)]. In Promuovere Benessere con Persone Gruppi Comunità [Promoting well-being with individuals, groups, communities], Proceedings of the VII National Congress of Health Psychology, Cesena, Italy, 28–30 September 2006; Cicognani, E., Palestini, L., Eds.; Società Editrice “Il Ponte Vecchio”: Cesena, Italy, 2006; pp. 231–232. (In Italian) [Google Scholar]
- Natale, V.; Zoppello, M.; Tonetti, L.; Termine, C.; Rossi, G.; Fazzi, E. Disturbo da Deficit dell’Attenzione/Iperattività e qualità del sonno: Uno studio attigrafico [Attention-deficit hyperactivity disorder and sleep quality: An actigraphic study]. In Proceedings of the XVI National Congress of the Italian Sleep Medicine Association (Associazione Italiana di Medicina del Sonno, AIMS), Milan, Italy, 12–15 November 2006; Associazione Italiana di Medicina del Sonno (AIMS): Milan, Italy, 2006; p. 60. (In Italian). [Google Scholar]
- Wechsler, D. WISC-R: Scala di Intelligenza Wechsler per Bambini Riveduta: Manuale [WISC-R: Wechsler Intelligence Scale for Children-Revised: Manual]; Organizzazioni Speciali: Florence, Italy, 1998; p. 224. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th ed.; American Psychiatric Association: Washington, DC, USA, 2000. [Google Scholar]
- Please note that in Italy the administration of methylphenidate and atomoxetine to treat ADHD in children was approved by the Agenzia Italiana del Farmaco (Rome, Italy) in 2007.
- Martoni, M.; Carissimi, A.; Fabbri, M.; Filardi, M.; Tonetti, L.; Natale, V. 24-h actigraphic monitoring of motor activity, sleeping and eating behaviors in underweight, normal weight, overweight and obese children. Eat. Weight Disord.-Stud. Anorex. 2016, 21, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Oakley, N.R. Validation with Polysomnography of the Sleepwatch Sleep/Wake Scoring Algorithm Used by the Actiwatch Activity Monitoring System; Technical Report; Mini-Mitter: Bend, OR, USA, 1997. [Google Scholar]
- Tonetti, L.; Pasquini, F.; Fabbri, M.; Belluzzi, M.; Natale, V. Comparison of two different actigraphs with polysomnography in healthy young subjects. Chronobiol. Int. 2008, 25, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Natale, V.; Léger, D.; Martoni, M.; Bayon, V.; Erbacci, A. The role of actigraphy in the assessment of primary insomnia: A retrospective study. Sleep Med. 2014, 15, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Acebo, C.; Sadeh, A.; Seifer, R.; Tzischinsky, O.; Wolfson, A.R.; Hafer, A.; Carskadon, M.A. Estimating sleep patterns with activity monitoring in children and adolescents: How many nights are necessary for reliable measures? Sleep 1999, 22, 95–103. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing. 2017. Available online: https://www.r-project.org/ (accessed on 10 September 2018).
| Actigraphic Sleep Parameter | ADHD Children | C | Mean by Gender | Statistics a | |
|---|---|---|---|---|---|
| BT | M | 22:13 ± 0:11 | 22:17 ± 0:06 | 22:15 ± 0:06 | Gender: F1,126 = 0.46; p = 0.50 |
| F | 21:53 ± 0:22 | 22:21 ± 0:05 | 22:07 ± 0:11 | Group: F1,126 = 1.29; p = 0.26 | |
| Mean by group | 22:03 ± 0:13 | 22:19 ± 0:04 | Interaction: F1,126 = 1.08; p = 0.30 | ||
| GUT | M | 07:34 ± 0:08 | 07:30 ± 0:05 | 07:32 ± 0:05 | Gender: F1,126 = 0.02; p = 0.88 |
| F | 07:33 ± 0:17 | 07:34 ± 0:04 | 07:34 ± 0:08 | Group: F1,126 = 0.01; p = 0.92 | |
| Mean by group | 07:34 ± 0:10 | 07:32 ± 0:04 | Interaction: F1,126 = 0.08; p = 0.77 | ||
| TIB | M | 560.59 ± 9.23 | 553.70 ± 5.34 | 557.14 ± 5.01 | Gender: F1,126 = 0.70; p = 0.40 |
| F | 579.99 ± 18.20 | 551.49 ± 4.63 | 565.74 ± 9.30 | Group: F1,126 = 2.27; p = 0.13 | |
| Mean by group | 570.29 ± 10.71 | 552.60 ± 3.61 | Interaction: F1,126 = 1.11; p = 0.30 | ||
| MS | M | 02:54 ± 0:08 | 02:53 ± 0:05 | 02:53 ± 0:05 | Gender: F1,126 = 0.13; p = 0.72 |
| F | 02:43 ± 0:17 | 02:58 ± 0:04 | 02:50 ± 0:08 | Group: F1,126 = 0.45; p = 0.50 | |
| Mean by group | 02:48 ± 0:10 | 02:56 ± 0:04 | Interaction: F1,126 = 0.64; p = 0.42 | ||
| TST | M | 475.30 ± 8.56 | 485.73 ± 4.95 | 480.52 ± 4.64 | Gender: F1,126 = 1.47; p = 0.23 |
| F | 487.90 ± 16.87 | 496.23 ± 4.29 | 492.07 ± 8.62 | Group: F1,126 = 0.74; p = 0.39 | |
| Mean by group | 481.60 ± 9.93 | 490.98 ± 3.35 | Interaction: F1,126 = 0.01; p = 0.91 | ||
| SE | M | 84.88 ± 0.96 | 87.67 ± 0.56 | 86.28 ± 0.52 | Gender: F1,126 = 0.18; p = 0.68 |
| F | 83.68 ± 1.90 | 89.77 ± 0.48 | 86.73 ± 0.97 | Group: F1,126 = 13.19; p < 0.001 | |
| Mean by group | 84.28 ± 1.12 | 88.72 ± 0.38 | Interaction: F1,126 = 2.37; p = 0.13 | ||
| SOL | M | 22.21 ± 2.79 | 17.92 ± 1.61 | 20.06 ± 1.51 | Gender: F1,126 = 0.75; p = 0.39 |
| F | 22.58 ± 5.50 | 12.16 ± 1.40 | 17.37 ± 2.81 | Group: F1,126 = 4.30; p = 0.04 | |
| Mean by group | 22.39 ± 3.24 | 15.04 ± 1.09 | Interaction: F1,126 = 0.98; p = 0.33 | ||
| WB | M | 27.35 ± 1.65 | 24.92 ± 0.95 | 26.13 ± 0.89 | Gender: F1,126 = 0.89; p = 0.35 |
| F | 31.89 ± 3.25 | 23.84 ± 0.83 | 27.86 ± 1.66 | Group: F1,126 = 6.25; p = 0.01 | |
| Mean by group | 29.62 ± 1.91 | 24.38 ± 0.64 | Interaction: F1,126 = 2.35; p = 0.13 | ||
| MAS | M | 24.44 ± 2.66 | 16.57 ± 1.54 | 20.51 ± 1.44 | Gender: F1,126 = 0.24; p = 0.62 |
| F | 24.11 ± 5.25 | 13.99 ± 1.33 | 19.05 ± 2.68 | Group: F1,126 = 7.06; p = 0.009 | |
| Mean by group | 24.28 ± 3.09 | 15.28 ± 1.04 | Interaction: F1,126 = 0.14; p = 0.71 | ||
| WASO | M | 52.51 ± 4.11 | 43.30 ± 2.37 | 47.91 ± 2.23 | Gender: F1,126 = 0.26; p = 0.61 |
| F | 61.79 ± 8.09 | 38.67 ± 2.06 | 50.23 ± 4.13 | Group: F1,126 = 9.58; p < 0.005 | |
| Mean by group | 57.15 ± 4.76 | 40.99 ± 1.61 | Interaction: F1,126 = 2.32; p = 0.13 | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tonetti, L.; Zoppello, M.; Rossi, G.; Balottin, U.; Fabbri, M.; Filardi, M.; Martoni, M.; Natale, V. A Pilot Study on Circadian Activity Rhythm in Pediatric Attention-Deficit Hyperactivity Disorder. Clocks & Sleep 2019, 1, 385-393. https://doi.org/10.3390/clockssleep1030031
Tonetti L, Zoppello M, Rossi G, Balottin U, Fabbri M, Filardi M, Martoni M, Natale V. A Pilot Study on Circadian Activity Rhythm in Pediatric Attention-Deficit Hyperactivity Disorder. Clocks & Sleep. 2019; 1(3):385-393. https://doi.org/10.3390/clockssleep1030031
Chicago/Turabian StyleTonetti, Lorenzo, Marina Zoppello, Giorgio Rossi, Umberto Balottin, Marco Fabbri, Marco Filardi, Monica Martoni, and Vincenzo Natale. 2019. "A Pilot Study on Circadian Activity Rhythm in Pediatric Attention-Deficit Hyperactivity Disorder" Clocks & Sleep 1, no. 3: 385-393. https://doi.org/10.3390/clockssleep1030031
APA StyleTonetti, L., Zoppello, M., Rossi, G., Balottin, U., Fabbri, M., Filardi, M., Martoni, M., & Natale, V. (2019). A Pilot Study on Circadian Activity Rhythm in Pediatric Attention-Deficit Hyperactivity Disorder. Clocks & Sleep, 1(3), 385-393. https://doi.org/10.3390/clockssleep1030031







