Steady-State Pupil Size Varies with Circadian Phase and Sleep Homeostasis in Healthy Young Men
Abstract
1. Introduction
2. Results
3. Discussion
4. Methods
4.1. Participants
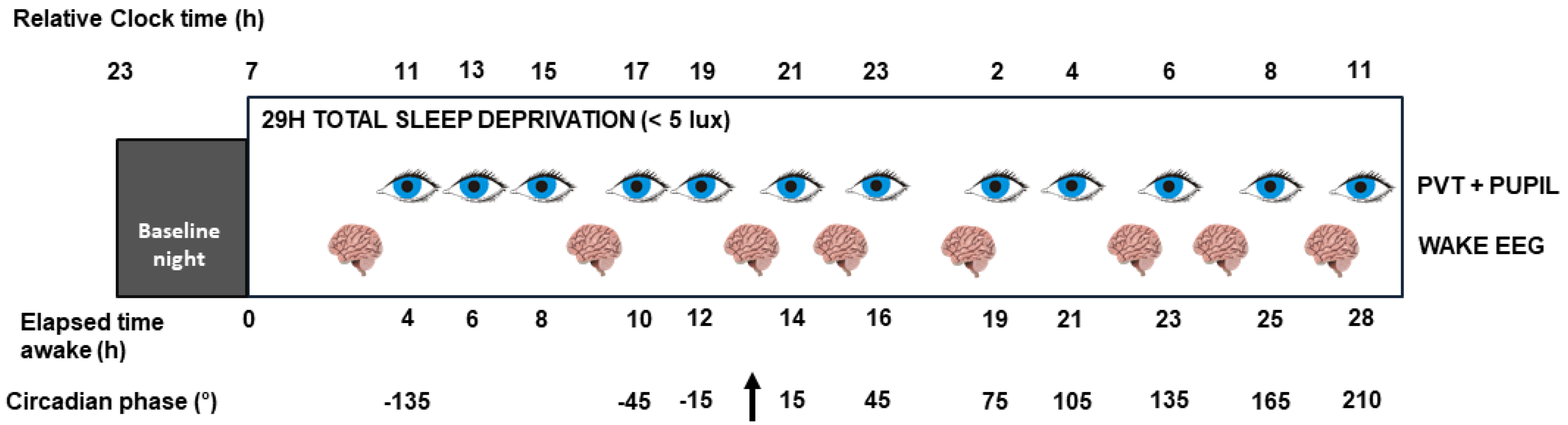
4.2. Experimental Protocol
4.3. Pupil Measures
4.4. Subjective Sleepiness or Fatigue
4.5. Subjective Affective Dimensions
4.6. Psychomotor Vigilance Task (PVT)
4.7. Spontaneous Waking EEG
4.8. Melatonin
4.9. Statistics
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wilhelm, H. The pupil. Curr. Opin. Neurol. 2008, 21, 36–42. [Google Scholar] [CrossRef]
- Kahneman, D.; Beatty, J. Pupil diameter and load on memory. Science 1966, 154, 1583–1585. [Google Scholar] [CrossRef] [PubMed]
- Hopstaken, J.F.; van der Linden, D.; Bakker, A.B.; Kompier, M.A.J. A multifaceted investigation of the link between mental fatigue and task disengagement. Psychophysiology 2015, 52, 305–315. [Google Scholar] [CrossRef]
- Einhauser, W.; Stout, J.; Koch, C.; Carter, O. Pupil dilation reflects perceptual selection and predicts subsequent stability in perceptual rivalry. Proc. Natl. Acad. Sci. USA 2008, 105, 1704–1709. [Google Scholar] [CrossRef] [PubMed]
- Naber, M.; Frässle, S.; Einhäuser, W. Perceptual rivalry: Reflexes reveal the gradual nature of visual awareness. PLoS ONE 2011, 6, e20910. [Google Scholar] [CrossRef]
- Hall, C.A.; Chilcott, R.P. Eyeing up the Future of the Pupillary Light Reflex in Neurodiagnostics. Diagnostics 2018, 8, 19. [Google Scholar] [CrossRef]
- Lykstad, J.; Hanna, A. Neuroanatomy, Pupillary Dilation Pathway; StatPearls Publishing: Treasure Island, CA, USA, 2018. [Google Scholar]
- Mather, M.; Harley, C.W. The Locus Coeruleus: Essential for Maintaining Cognitive Function and the Aging Brain. Trends Cogn. Sci. 2016, 20, 214–226. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.; Li, Y.; Kalwani, R.M.; Gold, J.I. Relationships between Pupil Diameter and Neuronal Activity in the Locus Coeruleus, Colliculi, and Cingulate Cortex. Neuron 2016, 89, 221–234. [Google Scholar] [CrossRef]
- Murphy, P.R.; O’Connell, R.G.; O’Sullivan, M.; Robertson, I.H.; Balsters, J.H. Pupil diameter covaries with BOLD activity in human locus coeruleus. Hum. Brain Mapp. 2014, 35, 4140–4154. [Google Scholar] [CrossRef]
- Lucas, R.J. Mammalian inner retinal photoreception. Curr. Biol. 2013, 23, R125–R133. [Google Scholar] [CrossRef] [PubMed]
- Bruijel, J.; van der Meijden, W.; Bijlenga, D.; Dorani, F.; Coppens, J.; te Lindert, B.; Kooij, J.; Van Someren, E. Individual Differences in the Post-Illumination Pupil Response to Blue Light: Assessment without Mydriatics. Biology 2016, 5, 34. [Google Scholar] [CrossRef]
- Münch, M.; Léon, L.; Crippa, S.V.; Kawasaki, A. Circadian and wake-dependent effects on the pupil light reflex in response to narrow-bandwidth light pulses. Investig. Ophthalmol. Vis. Sci. 2012, 53, 4546–4555. [Google Scholar] [CrossRef] [PubMed]
- Zele, A.J.; Feigl, B.; Smith, S.S.; Markwell, E.L. The circadian response of intrinsically photosensitive retinal ganglion cells. PLoS ONE 2011, 6, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lowenstein, O.; Loewenfeld, I.E. The sleep-waking cycle and pupillary activity. Ann. N. Y. Acad. Sci. 1964, 117, 142–156. [Google Scholar] [CrossRef]
- Lowenstein, O.; Loewenfeld, I.E. Electronic pupillography; a new instrument and some clinical applications. AMA Arch. Ophthalmol. 1958, 59, 352–363. [Google Scholar] [CrossRef]
- Lowenstein, O. Pupillography; methods and diagnostic system. AMA Arch. Ophthalmol. 1956, 55, 565–571. [Google Scholar] [CrossRef]
- Lowenstein, O.; Loewenfeld, I.E. Types of central autonomic innervation and fatigue; pupillographic studies. AMA Arch. Neurol. Psychiatry 1951, 66, 580–599. [Google Scholar] [CrossRef]
- Yoss, R.E.; Moyer, N.J.; Hollenhorst, R.W. Pupil size and spontaneous pupillary waves associated with alertness, drowsiness, and sleep. Neurology 1970, 20, 545–554. [Google Scholar] [CrossRef]
- Massar, S.A.A.; Lim, J.; Sasmita, K.; Chee, M.W.L. Sleep deprivation increases the costs of attentional effort: Performance, preference and pupil size. Neuropsychologia 2019. [Google Scholar] [CrossRef]
- Daguet, I.; Bouhassira, D.; Gronfier, C. Baseline Pupil Diameter Is Not a Reliable Biomarker of Subjective Sleepiness. Front. Neurol. 2019, 10, 108. [Google Scholar] [CrossRef]
- Wilhelm, B.J.; Widmann, A.; Durst, W.; Heine, C.; Otto, G. Objective and quantitative analysis of daytime sleepiness in physicians after night duties. Int. J. Psychophysiol. 2009, 72, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, B.; Wilhelm, H.; Lüdtke, H.; Streicher, P.; Adler, M. Pupillographic assessment of sleepiness in sleep-deprived healthy subjects. Sleep 1998, 21, 258–265. [Google Scholar] [CrossRef]
- Lavie, P. Ultradian rhythms in alertness—A pupillometric study. Biol. Psychol. 1979, 9, 49–62. [Google Scholar] [CrossRef]
- Eggert, T.; Sauter, C.; Popp, R.; Zeitlhofer, J.; Danker-Hopfe, H. “Vigilance” of the German Society for Sleep Research and Sleep Medicine (DGSM) The Pupillographic SleepinessTest in adults: Effect of age, gender, and time of day on pupillometric variables. Am. J. Hum. Biol. 2012, 24, 820–828. [Google Scholar] [CrossRef]
- Wilhelm, H.; Lüdtke, H.; Wilhelm, B. Pupillographic sleepiness testing in hypersomniacs and normals. Graefes Arch. Clin. Exp. Ophthalmol. 1998, 236, 725–729. [Google Scholar] [CrossRef]
- Pressman, M.R.; Spielman, A.J.; Korczyn, A.D.; Rubenstein, A.E.; Pollak, C.P.; Weitzman, E.D. Patterns of daytime sleepiness in narcoleptics and normals: A pupillometric study. Electroencephalogr. Clin. Neurophysiol. 1984, 57, 129–133. [Google Scholar] [CrossRef]
- Kraemer, S.; Danker-Hopfe, H.; Dorn, H.; Schmidt, A.; Ehlert, I.; Herrmann, W.M. Time-of-day variations of indicators of attention: Performance, physiologic parameters, and self-assessment of sleepiness. Biol. Psychiatry 2000, 48, 1069–1080. [Google Scholar] [CrossRef]
- Noiman, R.; Korczyn, A.D. Circadian rhythm of the pupillary response to morphine and to naloxone. Chronobiol. Int. 1985, 2, 239–241. [Google Scholar] [CrossRef]
- Dijk, D.-J.; Duffy, J.F.; Czeisler, C.A. Circadian and sleep/wake dependent aspects of subjective alertness and cognitive performance. J. Sleep Res. 1992, 1, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Ranzijn, R.; Lack, L. The pupillary light reflex cannot be used to measure sleepiness. Psychophysiology 1997, 34, 17–22. [Google Scholar] [CrossRef]
- Loving, R.T.; Kripke, D.F.; Glazner, L.K. Circadian rhythms in the human pupil and eyelid. Am. J. Physiol. 1996, 271, R320–R324. [Google Scholar] [CrossRef]
- Beck, A.T.; Brown, G.; Epstein, N.; Steer, R.A. An Inventory for Measuring Clinical Anxiety: Psychometric Properties. J. Consult. Clin. Psychol. 1988, 56, 893–897. [Google Scholar] [CrossRef] [PubMed]
- Steer, R.A.; Ranieri, W.F.; Beck, A.T.; Clark, D.A. Further evidence for the validity of the beck anxiety inventory with psychiatric outpatients. J. Anxiety Disord. 1993, 7, 195–205. [Google Scholar] [CrossRef]
- Buysse, D.J.; Reynolds, C.F.; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Johns, M.W. A New Method for Measuring Daytime Sleepiness: The Epworth Sleepiness Scale. Sleep 1991, 14, 540–545. [Google Scholar] [CrossRef]
- Horne, J.A.; Ostberg, O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int. J. Chronobiol. 1976, 4, 97–110. [Google Scholar]
- Akerstedt, T.; Gillberg, M. Subjective and objective sleepiness in the active individual. Int. J. Neurosci. 1990, 52, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Basner, M.; Dinges, D.F. Maximizing sensitivity of the psychomotor vigilance test (PVT) to sleep loss. Sleep 2011, 34, 581–591. [Google Scholar] [CrossRef] [PubMed]
- Cajochen, C.; Wyatt, J.K.; Czeisler, C.A.; Dijk, D.J. Separation of circadian and wake duration-dependent modulation of EEG activation during wakefulness. Neuroscience 2002, 114, 1047–1060. [Google Scholar] [CrossRef]
- Jaeger, B.C.; Edwards, L.J.; Das, K.; Sen, P.K. An R 2 statistic for fixed effects in the generalized linear mixed model. J. Appl. Stat. 2017, 44, 1086–1105. [Google Scholar] [CrossRef]
- Boivin, D.B.; Czeisler, C.A.; Dijk, D.J.; Duffy, J.F.; Folkard, S.; Minors, D.S.; Totterdell, P.; Waterhouse, J.M. Complex interaction of the sleep-wake cycle and circadian phase modulates mood in healthy subjects. Arch. Gen. Psychiatry 1997, 54, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Meijer, J.H.; Michel, S.; VanderLeest, H.T.; Rohling, J.H.T. Daily and seasonal adaptation of the circadian clock requires plasticity of the SCN neuronal network. Eur. J. Neurosci. 2010, 32, 2143–2151. [Google Scholar] [CrossRef]
- Scammell, T.E.; Arrigoni, E.; Lipton, J.O. Neural Circuitry of Wakefulness and Sleep. Neuron 2017, 93, 747–765. [Google Scholar] [CrossRef] [PubMed]
- Guido, M.E.; Garbarino-Pico, E.; Contin, M.A.; Valdez, D.J.; Nieto, P.S.; Verra, D.M.; Acosta-Rodriguez, V.A.; de Zavalía, N.; Rosenstein, R.E. Inner retinal circadian clocks and non-visual photoreceptors: Novel players in the circadian system. Prog. Neurobiol. 2010, 92, 484–504. [Google Scholar] [CrossRef]
- Buijs, F.N.; León-Mercado, L.; Guzmán-Ruiz, M.; Guerrero-Vargas, N.N.; Romo-Nava, F.; Buijs, R.M. The Circadian System: A Regulatory Feedback Network of Periphery and Brain. Physiology 2016, 31, 170–181. [Google Scholar] [CrossRef] [PubMed]
- Aston-Jones, G.; Chen, S.; Zhu, Y.; Oshinsky, M.L. A neural circuit for circadian regulation of arousal. Nat. Neurosci. 2001, 4, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Daneault, V.; Dumont, M.; Massé, É.; Vandewalle, G.; Carrier, J. Light-sensitive brain pathways and aging. J. Physiol. Anthropol. 2016, 35. [Google Scholar] [CrossRef]
- Dash, M.B.; Douglas, C.L.; Vyazovskiy, V.V.; Cirelli, C.; Tononi, G. Long-Term Homeostasis of Extracellular Glutamate in the Rat Cerebral Cortex across Sleep and Waking States. J. Neurosci. 2009, 29, 620–629. [Google Scholar] [CrossRef] [PubMed]
- Retey, J.V.; Adam, M.; Gottselig, J.M.; Khatami, R.; Durr, R.; Achermann, P.; Landolt, H.-P. Adenosinergic Mechanisms Contribute to Individual Differences in Sleep Deprivation-Induced Changes in Neurobehavioral Function and Brain Rhythmic Activity. J. Neurosci. 2006, 26, 10472–10479. [Google Scholar] [CrossRef] [PubMed]
- Ding, F.; O’Donnell, J.; Xu, Q.; Kang, N.; Goldman, N.; Nedergaard, M. Changes in the composition of brain interstitial ions control the sleep-wake cycle. Science 2016, 352, 550–555. [Google Scholar] [CrossRef] [PubMed]
- Felder-Schmittbuhl, M.P.; Buhr, E.D.; Dkhissi-Benyahya, O.; Hicks, D.; Peirson, S.N.; Ribelayga, C.P.; Sandu, C.; Spessert, R.; Tosini, G. Ocular clocks: Adapting mechanisms for eye functions and health. Investig. Ophthalmol. Vis. Sci. 2018, 59, 4856–4870. [Google Scholar] [CrossRef]
- Strogatz, S.H.; Kronauer, R.E.; Czeisler, C.A. Circadian pacemaker interferes with sleep onset at specific times each day: Role in insomnia. Am. J. Physiol. 1987, 253, R172–R178. [Google Scholar] [CrossRef] [PubMed]
- Chua, E.C.P.; Yeo, S.C.; Lee, I.T.G.; Tan, L.C.; Lau, P.; Tan, S.S.; Mien, I.H.; Gooley, J.J. Individual differences in physiologic measures are stable across repeated exposures to total sleep deprivation. Physiol. Rep. 2014, 2, e12129. [Google Scholar] [CrossRef]
- Vandewalle, G.; Archer, S.N.; Wuillaume, C.; Balteau, E.; Degueldre, C.; Luxen, A.; Dijk, D.J.; Maquet, P. Effects of light on cognitive brain responses depend on circadian phase and sleep homeostasis. J. Biol. Rhythms 2011, 26, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Chellappa, S.L.; Viola, A.U.; Schmidt, C.; Bachmann, V.; Gabel, V.; Maire, M.; Reichert, C.F.; Valomon, A.; Götz, T.; Landolt, H.P.; et al. Human melatonin and alerting response to blue-enriched light depend on a polymorphism in the clock gene PER3. J. Clin. Endocrinol. Metab. 2012, 97, E433–E437. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Dinges, D.F. Sleep deprivation and vigilant attention. Ann. N. Y. Acad. Sci. 2008, 1129, 305–322. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, B.; Giedke, H.; Lüdtke, H.; Bittner, E.; Hofmann, A.; Wilhelm, H. Daytime variations in central nervous system activation measured by a pupillographic sleepiness test. J. Sleep Res. 2001, 10, 1–7. [Google Scholar] [CrossRef]
- Wilhelm, B.; Bittner, E.; Hofmann, A.; Koerner, A.; Peters, T.; Lüdtke, H.; Wilhelm, H. Short-term reproducibility and variability of the pupillographic sleepiness test. Am. J. Hum. Biol. 2015, 27, 862–866. [Google Scholar] [CrossRef] [PubMed]
- Maire, M.; Reichert, C.F.; Gabel, V.; Viola, A.U.; Krebs, J.; Strobel, W.; Landolt, H.-P.; Bachmann, V.; Cajochen, C.; Schmidt, C. Time-on-task decrement in vigilance is modulated by inter-individual vulnerability to homeostatic sleep pressure manipulation. Front. Behav. Neurosci. 2014, 8, 1–10. [Google Scholar] [CrossRef][Green Version]
- Chellappa, S.L.; Steiner, R.; Oelhafen, P.; Cajochen, C. Sex differences in light sensitivity impact on brightness perception, vigilant attention and sleep in humans. Sci. Rep. 2017, 7, 14215. [Google Scholar] [CrossRef]
- Daneault, V.; Dumont, M.; Massé, É.; Forcier, P.; Boré, A.; Lina, J.-M.; Doyon, J.; Vandewalle, G.; Carrier, J. Plasticity in the Sensitivity to Light in Aging: Decreased Non-visual Impact of Light on Cognitive Brain Activity in Older Individuals but No Impact of Lens Replacement. Front. Physiol. 2018, 9, 1557. [Google Scholar] [CrossRef] [PubMed]
- McMorris, T.; Barwood, M.; Corbett, J. Central fatigue theory and endurance exercise: Toward an interoceptive model. Neurosci. Biobehav. Rev. 2018, 93, 93–107. [Google Scholar] [CrossRef] [PubMed]
- Shahid, A.; Shen, J.; Shapiro, C.M. Measurements of sleepiness and fatigue. J. Psychosom. Res. 2010, 69, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Aston-Jones, G.; Cohen, J.D. An integrative theory of locus coeruleus-norepinephrine function: Adaptive gain and optimal performance. Annu. Rev. Neurosci. 2005, 28, 403–450. [Google Scholar] [CrossRef] [PubMed]
- Hull, J.T.; Wright, K.P.; Czeisler, C.A. The influence of subjective alertness and motivation on human performance independent of circadian and homeostatic regulation. J. Biol. Rhythms 2003, 18, 329–338. [Google Scholar] [CrossRef]
- Chellappa, S.L.; Gaggioni, G.; Ly, J.Q.M.; Papachilleos, S.; Borsu, C.; Brzozowski, A.; Rosanova, M.; Sarasso, S.; Luxen, A.; Middleton, B.; et al. Circadian dynamics in measures of cortical excitation and inhibition balance. Sci. Rep. 2016, 6, 33661. [Google Scholar] [CrossRef]
- Ly, J.Q.M.; Gaggioni, G.; Chellappa, S.L.; Papachilleos, S.; Brzozowski, A.; Borsu, C.; Rosanova, M.; Sarasso, S.; Middleton, B.; Luxen, A.; et al. Circadian regulation of human cortical excitability. Nat. Commun. 2016, 7, 11828. [Google Scholar] [CrossRef]
- Gaggioni, G.; Ly, J.Q.M.; Chellappa, S.L.; Coppieters ‘t Wallant, D.; Rosanova, M.; Sarasso, S.; Luxen, A.; Salmon, E.; Middleton, B.; Massimini, M.; et al. Human fronto-parietal response scattering subserves vigilance at night. Neuroimage 2018, 175, 354–364. [Google Scholar] [CrossRef]
- Duffy, J.E.; Dijk, D.J. Getting through to circadian oscillators: Why use constant routines? J. Biol. Rhythms 2002, 17, 4–13. [Google Scholar] [CrossRef]
- Silva, E.J.; Wang, W.; Ronda, J.M.; Wyatt, J.K.; Duffy, J.F. Circadian and wake-dependent influences on subjective sleepiness, cognitive throughput, and reaction time performance in older and young adults. Sleep 2010, 33, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, C.; Collette, F.; Reichert, C.F.; Maire, M.; Vandewalle, G.; Peigneux, P.; Cajochen, C. Pushing the limits: Chronotype and time of day modulate working memory-dependent cerebral activity. Front. Neurol. 2015, 6. [Google Scholar] [CrossRef]
- Graw, P.; Kräuchi, K.; Knoblauch, V.; Wirz-Justice, A.; Cajochen, C. Circadian and wake-dependent modulation of fastest and slowest reaction times during the psychomotor vigilance task. Physiol. Behav. 2004, 80, 695–701. [Google Scholar] [CrossRef]
- Jung, C.M.; Ronda, J.M.; Czeisler, C.A.; Wright, K.P. Comparison of sustained attention assessed by auditory and visual psychomotor vigilance tasks prior to and during sleep deprivation. J. Sleep Res. 2011, 20, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Roach, G.D.; Dawson, D.; Lamond, N. Can a shorter psychomotor vigilance task be used as a reasonable substitute for the ten-minute psychomotor vigilance task? Chronobiol. Int. 2006, 23, 1379–1387. [Google Scholar] [CrossRef] [PubMed]
- Dinges, D.F.; Powell, J.W. Microcomputer analyses of performance on a portable, simple visual RT task during sustained operations. Behav. Res. Methods Instrum. Comput. 1985, 17, 625–655. [Google Scholar] [CrossRef]
- Welch, P.D. The Use of Fast Fourier Transform for the Estimation of Power Spectra: A Method Based on Time Averaging Over Short, Modified Periodograms. IEEE Trans. Audio Electroacoust. 1967, 15, 70–73. [Google Scholar] [CrossRef]
- English, J.; Middleton, B.A.; Arendt, J.; Wirz-Justice, A. Rapid direct measurement of melatonin in saliva using an iodinated tracer and solid phase second antibody. Ann. Clin. Biochem. 1993, 30 Pt 4, 415–416. [Google Scholar] [CrossRef]
- Klerman, H.; St. Hilaire, M.A.; Kronauer, R.E.; Gooley, J.J.; Gronfier, C.; Hull, J.T.; Lockley, S.W.; Santhi, N.; Wang, W.; Klerman, E.B.; et al. Analysis Method and Experimental Conditions Affect Computed Circadian Phase from Melatonin Data. PLoS ONE 2012, 7, e33836. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, S.; Schielzeth, H. A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol. Evol. 2013, 4, 133–142. [Google Scholar] [CrossRef]
- La Morgia, C.; Carelli, V.; Carbonelli, M. Melanopsin Retinal Ganglion Cells and Pupil: Clinical Implications for Neuro-Ophthalmology. Front. Neurol. 2018, 9, 1047. [Google Scholar] [CrossRef]





| N | 20 |
| Age (y) | 22.9 (2.7) |
| Ethnicity | Caucasian |
| BMI (kg/m2) | 22.1 (2.2) |
| Anxiety level (BAI) | 1.4 (2.2) |
| Mood (BDI-II) | 1.1 (1.9) |
| Caffeine (cups/day) | 0.4 (0.5) |
| Alcohol (doses/week) | 3.2 (3) |
| Sleep quality (PSQI) | 3.8 (0.7) |
| Daytime propensity to fall asleep (ESS) | 3.5 (2.9) |
| Chronotype (HO) | 52.9 (5.1) |
| Sleep time (hh:min, sleep diary) | 23:37 (44 min) |
| Wake time (hh:min, sleep diary) | 7:33 (45 min) |
| Sleep time (hh:min, actigraphy) | 23:31 (46 min) |
| Wake time (hh:min, actigraphy) Average clock time of dim-light melatonin onset (hh:mm) | 7:31 (47 min) 21:07 (77 min) |
| Steady-State Pupil Size | Circadian Phase | Steady-State Pupil Size x Circadian Phase | ||
| Dependent variables | Subjective sleepiness | F1,199.7 = 0.03 p = 0.87 | F12,158 = 2.65 p = 0.03 R2β* = 0.17 | F12,157 = 1.23 p = 0.27 |
| PVT mean reaction times | F1,178.8 = 5.69 p = 0.02 R2β* = 0.03 | F12,165.1 = 0.98 p = 0.47 | F12,165.1 = 0.65 p = 0.8 | |
| Relative theta power | F1,147.4 = 1.19 p = 0.28 | F12,143.7 = 1.31 p = 0.22 | F12,143 = 1.00 p = 0.46 | |
| Fatigue | F1,196.4 = 0.03 p = 0.86 | F12,165.2 = 2.93 p = 0.001 R2β* = 0.18 | F12,164.8 = 1.14 p = 0.33 | |
| Pupil size variability | Circadian phase | Pupil size variability x circadian phase | ||
| Dependent variables | Subjective sleepiness | F1,187.6 = 2.75 p = 0.099 | F12, 159.1 = 3.58 p < 0.001 R2β* = 0.21 | F12,157.1 = 0.99 p = 0.46 |
| PVT mean reaction times | F1,10,65 = 0.2 p = 0.66 | F12,163.1 = 0.9 p = 0.55 | F12,150.3 = 1.64 p = 0.085 | |
| Relative theta power | F1,173.9 = 1.22 p = 0.27 | F12,144.4 = 1.80 p = 0.059 | F12,142 = 1.21 p = 0.29 | |
| Fatigue | F1,183.7 = 1.61 p = 0.21 | F12,167.6 = 8.49 p < 0.0001 R2β* = 0.38 | F12,166.1 = 2.54 p = 0.004 R2β* = 0.16 | |
| Steady-State Pupil Size | Circadian Phase | Steady-State Pupil Size x Circadian Phase | ||
| Dependent variables | Motivation | F1,196.7 = 4.46 p = 0.04 R2β* = 0.022 | F12,162.2 = 2.79 p = 0.002 R2β* = 0.17 | F12,161.7 = 1.62 p = 0.09 |
| Joy | F1,194.8 = 2.81 p = 0.095 | F12,164.5 = 1.90 p = 0.04 R2β* = 0.12 | F12,164 = 1.24 p = 0.26 | |
| Stress | F1,172.9 = 1.20 p = 0.27 | F12,160.5 = 1.60 p = 0.096 | F12,160.4 = 1.00 p = 0.45 | |
| Anguish | F1,199.5 = 0.87 p = 0.35 | F12,162.4 = 1.61 p = 0.094 | F12,161.5 = 1.48 p = 0.14 | |
| Sociability | F1,198.4 = 0.68 p = 0.41 | F12,161.2 = 2.19 p = 0.01 R2β* = 0.14 | F12,160.2 = 1.52 p = 0.12 | |
| Pupil size variability | Circadian phase | Pupil size variability x circadian phase | ||
| Dependent variables | Motivation | F1,185.5 = 0.91 p = 0.34 | F12,165.2 = 1.99 p = 0.03 R2β* = 0.13 | F12,163.2 = 0.77 p = 0.68 |
| Joy | F1,182.6 = 2.36 p = 0.13 | F12,165.2 = 3.34 p = 0.0002 R2β* = 0.2 | F12,163.1 = 1.23 p = 0.27 | |
| Stress | F1,161.1 = 0.71 p = 0.40 | F12,161.9 = 0.76 p = 0.69 | F12,161.3 = 0.30 p = 0.99 | |
| Anguish | F1,193.9 = 4.81 p = 0.03 R2β* = 0.024 | F12,165.2 = 1.90 p = 0.04 R2β* = 0.12 | F12,162.4 = 1.68 p = 0.076 | |
| Sociability | F1,189.6 = 4.02 p = 0.046 R2β* = 0.021 | F12,165.2 = 1.29 p = 0.23 | F12,162.6 = 0.84 p = 0.61 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Van Egroo, M.; Gaggioni, G.; Cespedes-Ortiz, C.; Ly, J.Q.M.; Vandewalle, G. Steady-State Pupil Size Varies with Circadian Phase and Sleep Homeostasis in Healthy Young Men. Clocks & Sleep 2019, 1, 240-258. https://doi.org/10.3390/clockssleep1020021
Van Egroo M, Gaggioni G, Cespedes-Ortiz C, Ly JQM, Vandewalle G. Steady-State Pupil Size Varies with Circadian Phase and Sleep Homeostasis in Healthy Young Men. Clocks & Sleep. 2019; 1(2):240-258. https://doi.org/10.3390/clockssleep1020021
Chicago/Turabian StyleVan Egroo, Maxime, Giulia Gaggioni, Cristian Cespedes-Ortiz, Julien Q. M. Ly, and Gilles Vandewalle. 2019. "Steady-State Pupil Size Varies with Circadian Phase and Sleep Homeostasis in Healthy Young Men" Clocks & Sleep 1, no. 2: 240-258. https://doi.org/10.3390/clockssleep1020021
APA StyleVan Egroo, M., Gaggioni, G., Cespedes-Ortiz, C., Ly, J. Q. M., & Vandewalle, G. (2019). Steady-State Pupil Size Varies with Circadian Phase and Sleep Homeostasis in Healthy Young Men. Clocks & Sleep, 1(2), 240-258. https://doi.org/10.3390/clockssleep1020021






