Scale-Free Dynamics of the Mouse Wakefulness and Sleep Electroencephalogram Quantified Using Wavelet-Leaders
Abstract
:1. Introduction
2. Results
2.1. Scale-Invariance Measured Using the Multifractal Formalism
2.2. Scale-Invariance is More Anti-Persistent During Wakefulness
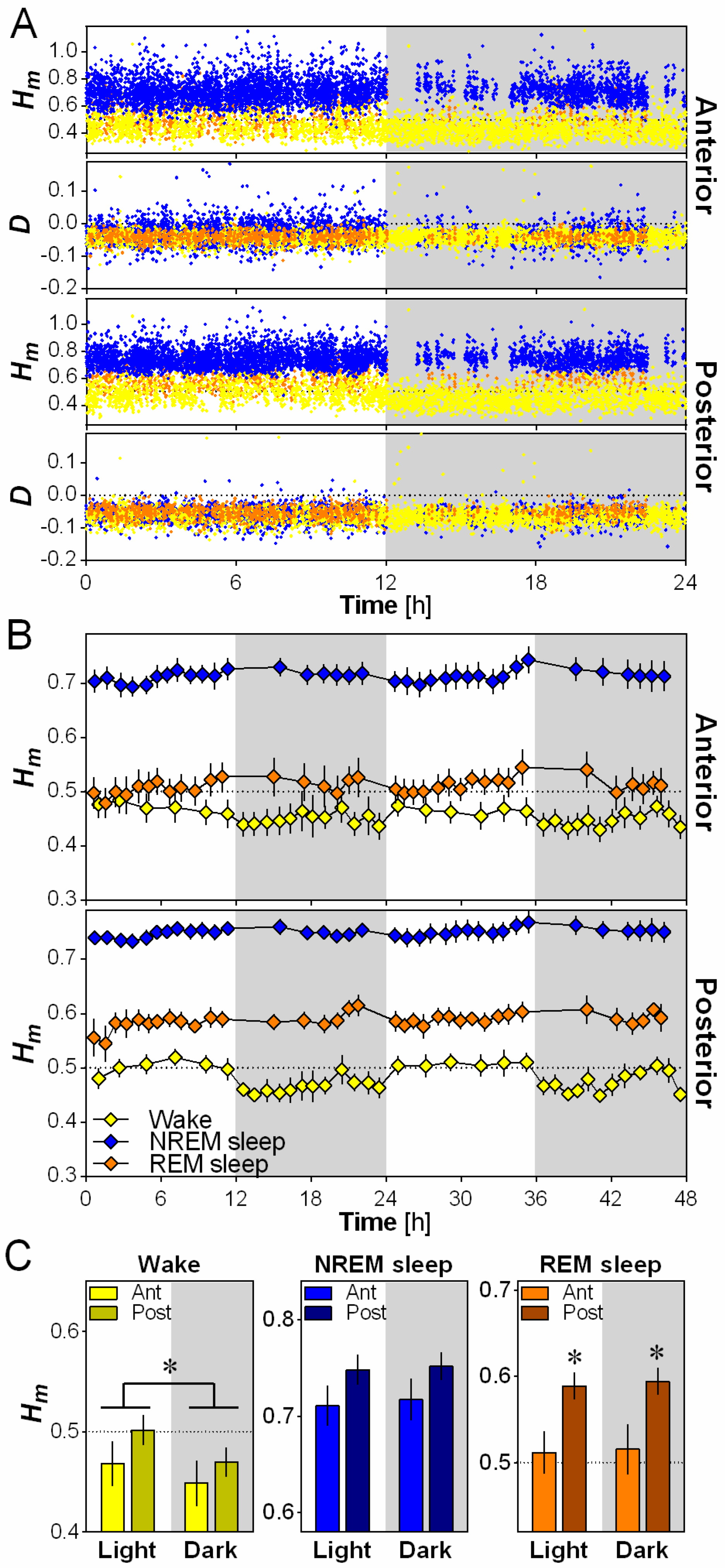
2.3. Scale-Invariance Varies with Electrode Location and Daytime
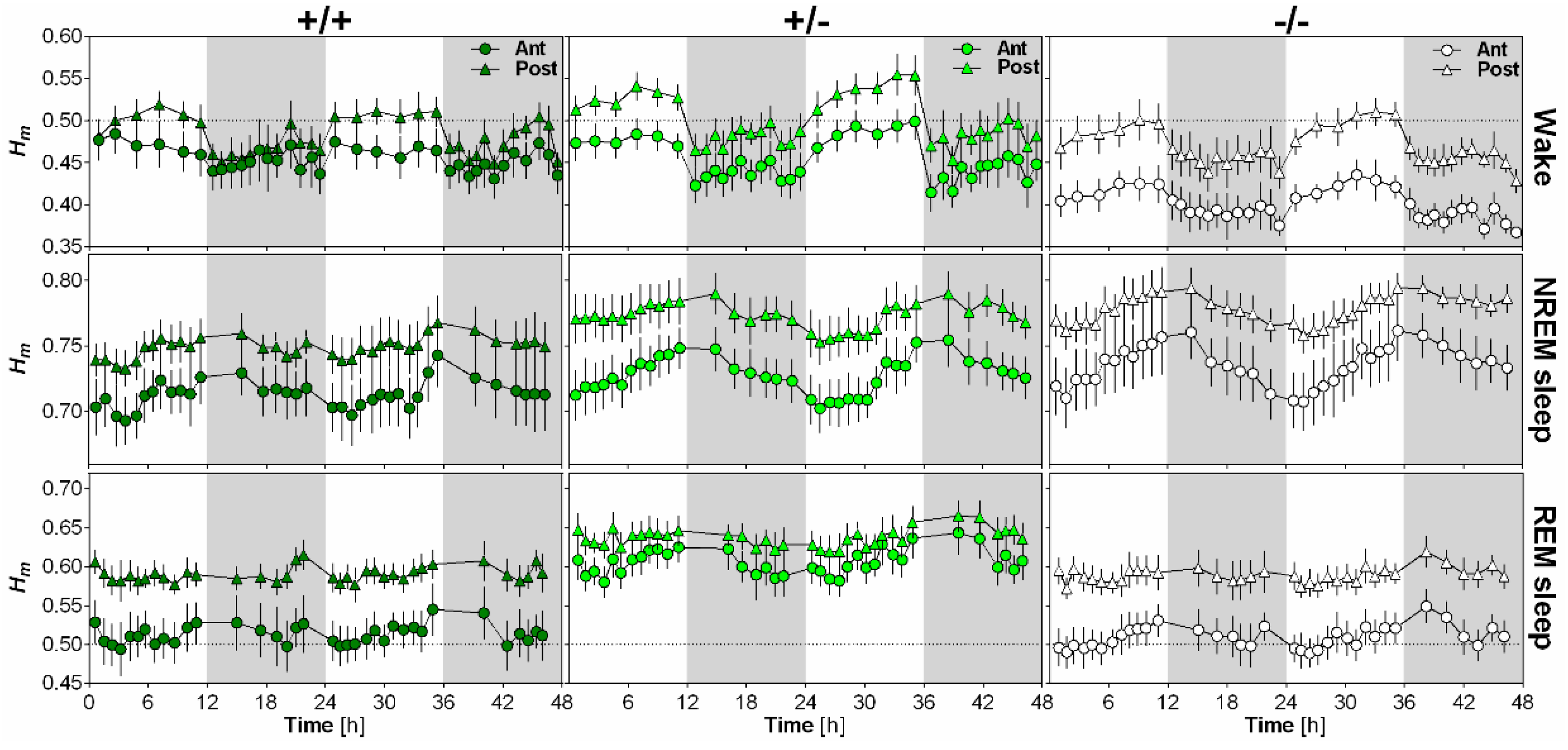
2.4. NLGN1 Absence Increases Scale-Free Anti-Persistence During Wake
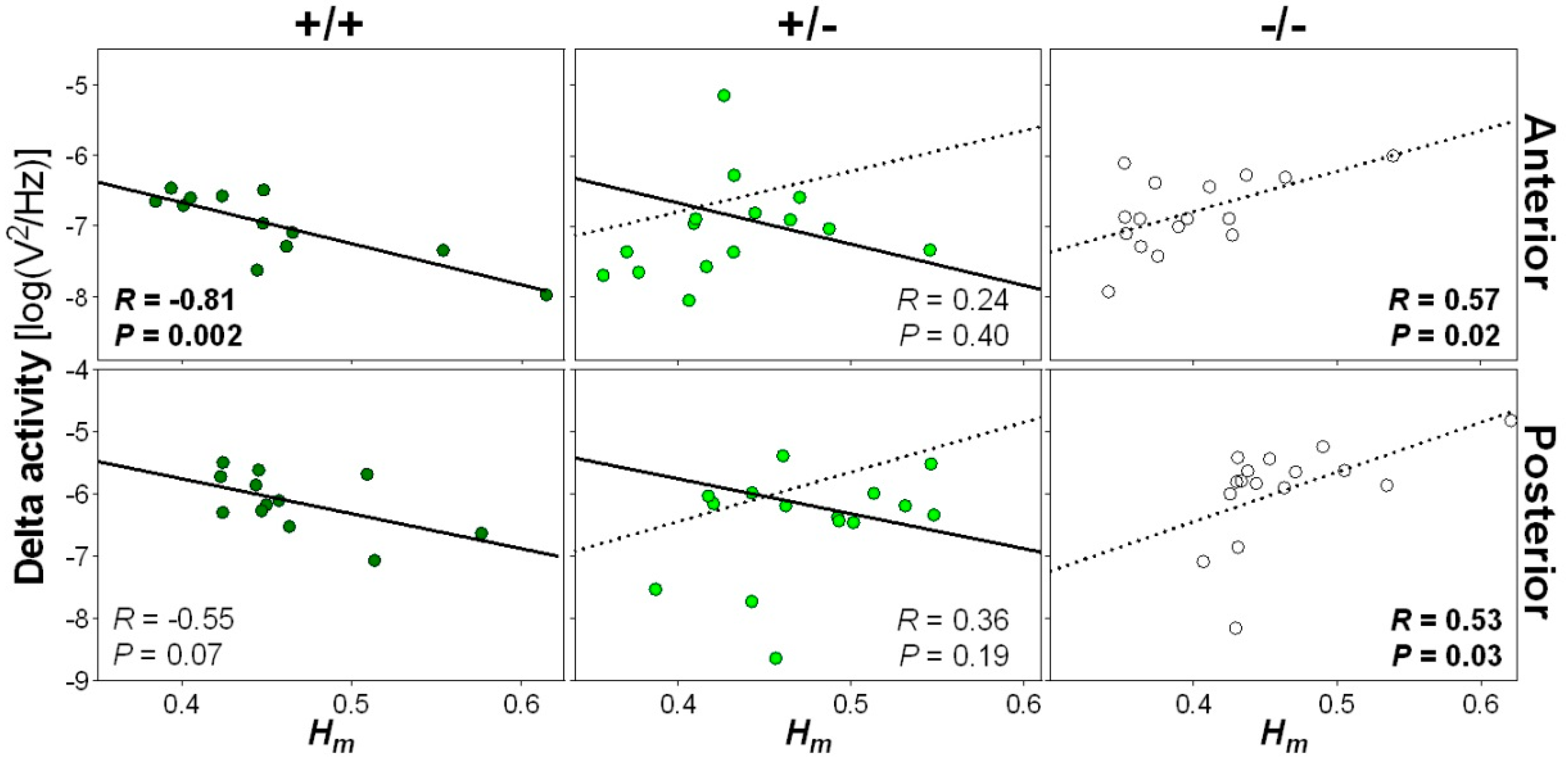
2.5. Opposite Relationship between Wake Scale-Invariance and NREM Sleep Delta Activity in Nlgn1 KO Mice
3. Discussion
4. Materials and Methods
4.1. Animals, Surgery and Protocol
4.2. Scale-Invariance and 1/f Power Spectrum
4.3. Wavelet Analysis of Scale-Invariance
4.4. The Wavelet Leaders Formalism
4.5. Multifractal Data Analysis and Spectral Analysis
4.6. Statistical Analyses
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Buzsáki, G.; Logothetis, N.; Singer, W. Scaling brain size, keeping timing: Evolutionary preservation of brain rhythms. Neuron 2013, 80, 751–764. [Google Scholar] [CrossRef] [PubMed]
- He, B.J.; Zempel, J.M.; Snyder, A.Z.; Raichle, M.E. The temporal structures and functional significance of scale-free brain activity. Neuron 2010, 66, 353–369. [Google Scholar] [CrossRef] [PubMed]
- Meisel, C.; Olbrich, E.; Shriki, O.; Achermann, P. Fading signatures of critical brain dynamics during sustained wakefulness in humans. J. Neurosci. 2013, 33, 17363–17372. [Google Scholar] [CrossRef] [PubMed]
- Thivierge, J.P.; Cisek, P. Nonperiodic synchronization in heterogeneous networks of spiking neurons. J. Neurosci. 2008, 28, 7968–7978. [Google Scholar] [CrossRef] [PubMed]
- Linkenkaer-Hansen, K.; Nikouline, V.V.; Palva, J.M.; Ilmoniemi, R.J. Long-range temporal correlations and scaling behavior in human brain oscillations. J. Neurosci. 2001, 21, 1370–1377. [Google Scholar] [CrossRef] [PubMed]
- Flandrin, P. Wavelet analysis and synthesis of fractional Brownian motion. IEEE Tras. Inf. Theory 1992, 38, 910–916. [Google Scholar] [CrossRef]
- Ma, Q.; Ning, X.; Wang, J.; Li, J. Sleep-stage characterization by nonlinear EEG analysis using Wavelet-based multifractal formalism. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2005, 5, 4526–4529. [Google Scholar] [PubMed]
- Jaffard, S.; Lashermes, B.; Abry, P. Wavelet leaders in multifractal analysis. In Wavelet Analysis and Applications; Qian, T., Vai, M.I., Yuesheng, X., Eds.; Birkhäuser: Basel, Switzerland, 2006; pp. 219–264. [Google Scholar]
- Zorick, T.; Mandelkern, M.A. Multifractal detrended fluctuation analysis of human EEG: Preliminary investigation and comparison with the wavelet transform modulus maxima technique. PLoS ONE 2013, 8, e68360. [Google Scholar] [CrossRef] [PubMed]
- Lavanga, M.; De Wel, O.; Caicedo, A.; Heremans, E.; Jansen, K.; Dereymaeker, A.; Naulaers, G.; Van Huffel, S. Automatic quiet sleep detection based on multifractality in preterm neonates: Effects of maturation. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2017, 2017, 2010–2013. [Google Scholar] [PubMed]
- Weiss, B.; Clemens, Z.; Bódizs, R.; Vágó, Z.; Halász, P. Spatio-temporal analysis of monofractal and multifractal properties of the human sleep EEG. J. Neurosci. Methods 2009, 185, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Wen, H.; Liu, Z. Separating fractal and oscillatory components in the power spectrum of neurophysiological signal. Brain Topogr. 2016, 29, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Diekelmann, S.; Born, J. The memory function of sleep. Nat. Rev. Neurosci. 2010, 11, 114–126. [Google Scholar] [CrossRef] [PubMed]
- Cedernaes, J.; Schiöth, H.B.; Benedict, C. Determinants of shortened, disrupted, and mistimed sleep and associated metabolic health consequences in healthy humans. Diabetes 2015, 64, 1073–1080. [Google Scholar] [CrossRef] [PubMed]
- Staresina, B.P.; Cooper, E.; Henson, R.N. Reversible information flow across the medial temporal lobe: The hippocampus links cortical modules during memory retrieval. J. Neurosci. 2013, 33, 14184–14192. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, K.M.; Lu, L.; Colgin, L.L.; Moser, M.B.; Moser, E.I. Coordination of entorhinal-hippocampal ensemble activity during associative learning. Nature 2014, 510, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, H.C.; Lyons, L.C. Synchrony and desynchrony in circadian clocks: Impacts on learning and memory. Learn. Mem. 2015, 22, 426–437. [Google Scholar] [CrossRef] [PubMed]
- Südhof, T.C. Neuroligins and neurexins link synaptic function to cognitive disease. Nature 2008, 455, 903–911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chubykin, A.A.; Atasoy, D.; Etherton, M.R.; Brose, N.; Kavalali, E.T.; Gibson, J.R.; Südhof, T.C. Activity-dependent validation of excitatory versus inhibitory synapses by neuroligin-1 versus neuroligin-2. Neuron 2007, 54, 919–931. [Google Scholar] [CrossRef] [PubMed]
- Budreck, E.C.; Kwon, O.B.; Jung, J.H.; Baudouin, S.; Thommen, A.; Kim, H.S.; Fukazawa, Y.; Harada, H.; Tabuchi, K.; Shigemoto, R.; et al. Neuroligin-1 controls synaptic abundance of NMDA-type glutamate receptors through extracellular coupling. Proc. Natl. Acad. Sci. USA 2013, 110, 725–730. [Google Scholar] [CrossRef] [PubMed]
- Blundell, J.; Blaiss, C.A.; Etherton, M.R.; Espinosa, F.; Tabuchi, K.; Walz, C.; Bolliger, M.F.; Südhof, T.C.; Powell, C.M. Neuroligin-1 deletion results in impaired spatial memory and increased repetitive behavior. J. Neurosci. 2010, 30, 2115–2129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.; Jung, S.Y.; Lee, Y.K.; Park, S.; Choi, J.S.; Lee, C.J.; Kim, H.S.; Choi, Y.B.; Scheiffele, P.; Bailey, C.H.; et al. Neuroligin-1 is required for normal expression of LTP and associative fear memory in the amygdala of adult animals. Proc. Natl. Acad. Sci. USA 2008, 105, 9087–9092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Zhou, Z.; Zhang, X.; Tong, H.; Li, P.; Zhang, Z.C.; Jia, Z.; Xie, W.; Han, J. Drosophila neuroligin 4 regulates sleep through modulating GABA transmission. J. Neurosci. 2013, 33, 15545–15554. [Google Scholar] [CrossRef] [PubMed]
- El Helou, J.; Bélanger-Nelson, E.; Freyburger, M.; Dorsaz, S.; Curie, T.; La Spada, F.; Gaudreault, P.O.; Beaumont, É.; Pouliot, P.; Lesage, F.; et al. Neuroligin-1 links neuronal activity to sleep-wake regulation. Proc. Natl. Acad. Sci. USA 2013, 110, 9974–9979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Massart, R.; Freyburger, M.; Suderman, M.; Paquet, J.; El Helou, J.; Belanger-Nelson, E.; Rachalski, A.; Koumar, O.C.; Carrier, J.; Szyf, M.; et al. The genome-wide landscape of DNA methylation and hydroxymethylation in response to sleep deprivation impacts on synaptic plasticity genes. Transl. Psychiatry 2014, 4, e347. [Google Scholar] [CrossRef] [PubMed]
- Ciuciu, P.; Abry, P.; Rabrait, C.; Wendt, H. Log-wavelet Leaders cumulant based multifractal analysis of EVI fMRI time series: Evidence of scaling in ongoing and evoked brain activity. IEEE J. Sel. Top. Signal Process. 2008, 2, 929–943. [Google Scholar] [CrossRef]
- Wendt, H.; Roux, S.G.; Abry, P.; Jaffard, S. Wavelet-leaders and bootstrap for multifractal analysis of images. Signal Process. 2009, 89, 1100–1114. [Google Scholar] [CrossRef]
- Muzy, J.F.; Bacry, E.; Arneodo, A. The multifractal formalism revisited with wavelet. Int. J. Bifurc. Chaos 1994, 4, 245–302. [Google Scholar] [CrossRef]
- McGinley, M.J.; Vinck, M.; Reimer, J.; Batista-Brito, R.; Zagha, E.; Cadwell, C.R.; Tolias, A.S.; Cardin, J.A.; McCormick, D.A. Waking state: Rapid variations modulate neural and behavioral responses. Neuron 2015, 87, 1143–1161. [Google Scholar] [CrossRef] [PubMed]
- Steriade, M.; McCarley, R.W. Brainstem Control of Wakefulness and Sleep; Plenum Press: New York, NY, USA, 1990. [Google Scholar]
- Steriade, M.; McCormick, D.A.; Sejnowski, T.J. Thalamocortical oscillations in the sleeping and aroused brain. Science 1993, 262, 679–685. [Google Scholar] [CrossRef] [PubMed]
- De Gennaro, L.; Ferrara, M.; Curcio, G.; Cristiani, R. Antero-posterior EEG changes during the wakefulness-sleep transition. Clin. Neurophysiol. 2001, 112, 1901–1911. [Google Scholar] [CrossRef]
- Kätzel, D.; Zemelman, B.V.; Buetfering, C.; Wölfel, M.; Miesenböck, G. The columnar and laminar organization of inhibitory connections to neocortical excitatory cells. Nat. Neurosci. 2011, 14, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Jackson, J.; Goutagny, R.; Williams, S. Fast and slow γ rhythms are intrinsically and independently generated in the subiculum. J. Neurosci. 2011, 31, 12104–12117. [Google Scholar] [CrossRef] [PubMed]
- Moroni, F.; Nobili, L.; Curcio, G.; De Carli, F.; Fratello, F.; Marzano, C.; De Gennaro, L.; Ferrillo, F.; Cossu, M.; Francione, S.; et al. Sleep in the human hippocampus: A stereo-EEG study. PLoS ONE 2007, 2, e867. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ouyang, G.; Usami, A.; Ikegaya, Y.; Sik, A. Scale-free topology of the CA3 hippocampal network: A novel method to analyze functional neuronal assemblies. Biophys. J. 2010, 98, 1733–1741. [Google Scholar] [CrossRef] [PubMed]
- Tsai, J.W.; Hannibal, J.; Hagiwara, G.; Colas, D.; Ruppert, E.; Ruby, N.F.; Heller, H.C.; Franken, P.; Bourgin, P. Melanopsin as a sleep modulator: Circadian gating of the direct effects of light on sleep and altered sleep homeostasis in Opn4(−/−) mice. PLoS Biol. 2009, 7, e1000125. [Google Scholar] [CrossRef] [PubMed]
- Santhi, N.; Lazar, A.S.; McCabe, P.J.; Lo, J.C.; Groeger, J.A.; Dijk, D.J. Sex differences in the circadian regulation of sleep and waking cognition in humans. Proc. Natl. Acad. Sci. USA 2016, 113, E2730–E2739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jedlicka, P.; Vnencak, M.; Krueger, D.D.; Jungenitz, T.; Brose, N.; Schwarzacher, S.W. Neuroligin-1 regulates excitatory synaptic transmission, LTP and EPSP-spike coupling in the dentate gyrus in vivo. Brain Struct. Funct. 2015, 220, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Peixoto, R.T.; Kunz, P.A.; Kwon, H.; Mabb, A.M.; Sabatini, B.L.; Philpot, B.D.; Ehlers, M.D. Transsynaptic signaling by activity-dependent cleavage of neuroligin-1. Neuron 2012, 76, 396–409. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.B.; Kozorovitskiy, Y.; Oh, W.J.; Peixoto, R.T.; Akhtar, N.; Saulnier, J.L.; Gu, C.; Sabatini, B.L. Neuroligin-1-dependent competition regulates cortical synaptogenesis and synapse number. Nat. Neurosci. 2012, 15, 1667–1674. [Google Scholar] [CrossRef] [PubMed]
- Boucetta, S.; Cissé, Y.; Mainville, L.; Morales, M.; Jones, B.E. Discharge profiles across the sleep-waking cycle of identified cholinergic, GABAergic, and glutamatergic neurons in the pontomesencephalic tegmentum of the rat. J. Neurosci. 2014, 34, 4708–4727. [Google Scholar] [CrossRef] [PubMed]
- Conroy, W.G.; Nai, Q.; Ross, B.; Naughton, G.; Berg, D.K. Postsynaptic neuroligin enhances presynaptic inputs at neuronal nicotinic synapses. Dev. Biol. 2007, 307, 79–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dijk, D.J.; Lockley, S.W. Integration of human sleep-wake regulation and circadian rhythmicity. J. Appl. Physiol. 2002, 92, 852–862. [Google Scholar] [CrossRef] [PubMed]
- Gkogkas, C.G.; Khoutorsky, A.; Ran, I.; Rampakakis, E.; Nevarko, T.; Weatherill, D.B.; Vasuta, C.; Yee, S.; Truitt, M.; Dallaire, P.; et al. Autism-related deficits via dysregulated eIF4E-dependent translational control. Nature 2013, 493, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Varoqueaux, F.; Aramuni, G.; Rawson, R.L.; Mohrmann, R.; Missler, M.; Gottmann, K.; Zhang, W.; Sudhof, T.C.; Brose, N. Neuroligins determine synapse maturation and function. Neuron 2006, 51, 741–754. [Google Scholar] [CrossRef] [PubMed]
- Freyburger, M.; Pierre, A.; Paquette, G.; Bélanger-Nelson, E.; Bedont, J.; Gaudreault, P.O.; Drolet, G.; Laforest, S.; Blackshaw, S.; Cermakian, N.; et al. EphA4 is involved in sleep regulation but not in the electrophysiological response to sleep deprivation. Sleep 2016, 39, 613–624. [Google Scholar] [CrossRef] [PubMed]
- Mallat, S. A Wavelet Tour of Signal Processing; Academic Press: San Diego, CA, USA, 1998. [Google Scholar]
- Jaffard, S. Wavelet techniques in multifractal analysis. In Fractal Geometry and Applications. Proceedings of Symposia in Pure Mathematics; Lapidus, M., van Frankenhuijsen, M., Eds.; AMS: Providence, RI, USA, 2004; Volume 72, pp. 91–152. [Google Scholar]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lina, J.-M.; O’Callaghan, E.K.; Mongrain, V. Scale-Free Dynamics of the Mouse Wakefulness and Sleep Electroencephalogram Quantified Using Wavelet-Leaders. Clocks & Sleep 2019, 1, 50-64. https://doi.org/10.3390/clockssleep1010006
Lina J-M, O’Callaghan EK, Mongrain V. Scale-Free Dynamics of the Mouse Wakefulness and Sleep Electroencephalogram Quantified Using Wavelet-Leaders. Clocks & Sleep. 2019; 1(1):50-64. https://doi.org/10.3390/clockssleep1010006
Chicago/Turabian StyleLina, Jean-Marc, Emma Kate O’Callaghan, and Valérie Mongrain. 2019. "Scale-Free Dynamics of the Mouse Wakefulness and Sleep Electroencephalogram Quantified Using Wavelet-Leaders" Clocks & Sleep 1, no. 1: 50-64. https://doi.org/10.3390/clockssleep1010006
APA StyleLina, J.-M., O’Callaghan, E. K., & Mongrain, V. (2019). Scale-Free Dynamics of the Mouse Wakefulness and Sleep Electroencephalogram Quantified Using Wavelet-Leaders. Clocks & Sleep, 1(1), 50-64. https://doi.org/10.3390/clockssleep1010006





