Sleep Timing in Patients with Precocious and Delayed Pubertal Development
Abstract
:1. Introduction
2. Results
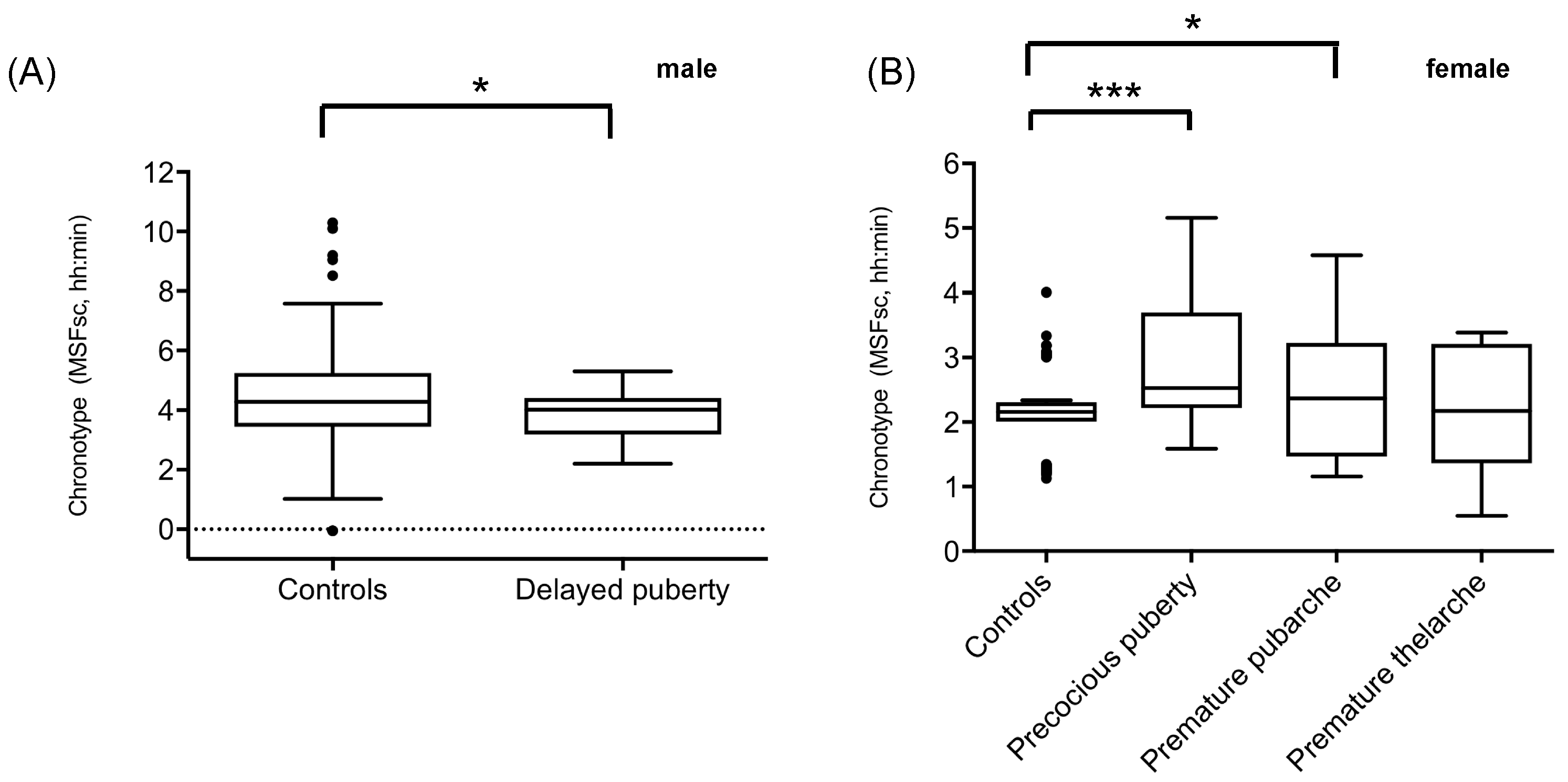
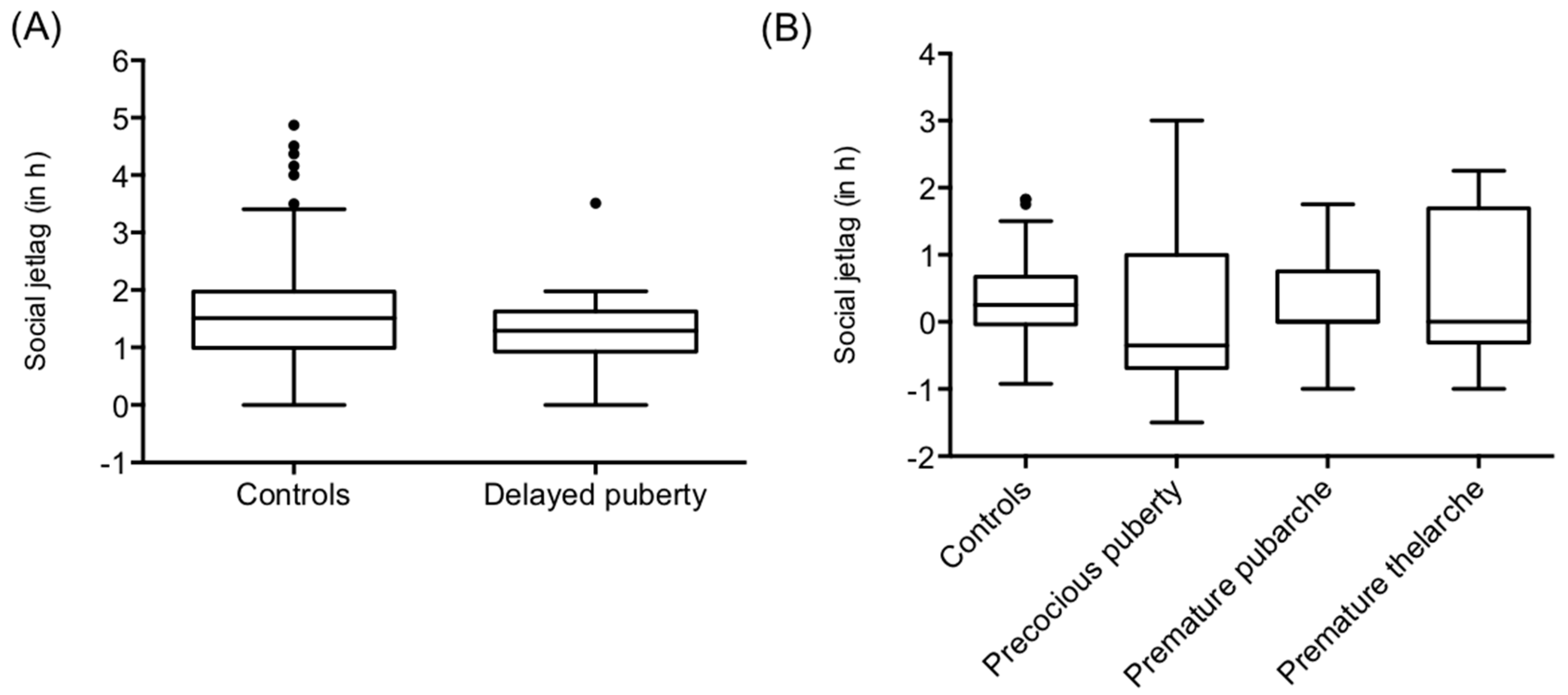
2.1. Sleep Timing in Patients with Precocious or Delayed Pubertal Development
2.2. Hormone Levels in Patients with Abnormal Pubertal Development
3. Discussion
4. Material and Methods
4.1. Patient Cohort
4.2. Database Control Cohorts
4.3. Sleep Assessments
4.4. Laboratory Measurements
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Roenneberg, T.; Kuehnle, T.; Juda, M.; Kantermann, T.; Allebrandt, K.; Gordijn, M.; Merrow, M. Epidemiology of the human circadian clock. Sleep Med. Rev. 2007, 11, 429–438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roenneberg, T. What is chronotype? Sleep Biol. Rhythm. 2012, 10, 75–76. [Google Scholar] [CrossRef]
- Borbely, A.A. A two process model of sleep regulation. Hum. Neurobiol. 1982, 1, 195–204. [Google Scholar] [PubMed]
- Jenni, O.G.; Achermann, P.; Carskadon, M.A. Homeostatic sleep regulation in adolescents. Sleep 2005, 28, 1446–1454. [Google Scholar] [CrossRef] [PubMed]
- Borbely, A.A.; Daan, S.; Wirz-Justice, A.; Deboer, T. The two-process model of sleep regulation: A reappraisal. J. Sleep Res. 2016, 25, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Daan, S.; Beersma, D.G.; Borbely, A.A. Timing of human sleep: Recovery process gated by a circadian pacemaker. Am. J. Physiol. 1984, 246, R161–R183. [Google Scholar] [CrossRef] [PubMed]
- Roenneberg, T.; Merrow, M. Entrainment of the human circadian clock. Cold Spring Harb Symp. Quant. Biol. 2007, 72, 293–299. [Google Scholar] [CrossRef]
- Carskadon, M.A.; Vieira, C.; Acebo, C. Association between puberty and delayed phase preference. Sleep 1993, 16, 258–262. [Google Scholar] [CrossRef]
- Carskadon, M.A.; Wolfson, A.R.; Acebo, C.; Tzischinsky, O.; Seifer, R. Adolescent sleep patterns, circadian timing, and sleepiness at a transition to early school days. Sleep 1998, 21, 871–881. [Google Scholar] [CrossRef]
- Crowley, S.J. Sleep behavior across the lifespan: How a model can expand our current understanding. Sleep Med. Rev. 2016, 28, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Crowley, S.J.; Acebo, C.; Carskadon, M.A. Sleep, circadian rhythms, and delayed phase in adolescence. Sleep Med. 2007, 8, 602–612. [Google Scholar] [CrossRef] [PubMed]
- Roenneberg, T.; Allebrandt, K.V.; Merrow, M.; Vetter, C. Social jetlag and obesity. Curr. Biol. 2012, 22, 939–943. [Google Scholar] [CrossRef] [PubMed]
- Carskadon, M.A. Sleep in adolescents: The perfect storm. Pediatr. Clin. North. Am. 2011, 58, 637–647. [Google Scholar] [CrossRef] [PubMed]
- Wittmann, M.; Dinich, J.; Merrow, M.; Roenneberg, T. Social jetlag: Misalignment of biological and social time. Chronobiol. Int. 2006, 23, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Wong, P.M.; Hasler, B.P.; Kamarck, T.W.; Muldoon, M.F.; Manuck, S.B. Social Jetlag, Chronotype, and Cardiometabolic Risk. J. Clin. Endocrinol. Metab. 2015, 100, 4612–4620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van der Vinne, V.; Zerbini, G.; Siersema, A.; Pieper, A.; Merrow, M.; Hut, R.A.; Roenneberg, T.; Kantermann, T. Timing of examinations affects school performance differently in early and late chronotypes. J. Biol. Rhythms. 2015, 30, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Tonetti, L.; Natale, V.; Randler, C. Association between circadian preference and academic achievement: A systematic review and meta-analysis. Chronobiol. Int. 2015, 32, 792–801. [Google Scholar] [CrossRef]
- Tonetti, L.; Adan, A.; Di Milia, L.; Randler, C.; Natale, V. Measures of circadian preference in childhood and adolescence: A review. Eur. Psychiatry 2015, 30, 576–582. [Google Scholar] [CrossRef]
- Vetter, C.; Devore, E.E.; Ramin, C.A.; Speizer, F.E.; Willett, W.C.; Schernhammer, E.S. Mismatch of Sleep and Work Timing and Risk of Type 2 Diabetes. Diabetes Care 2015, 38, 1707–1713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mota, M.C.; Silva, C.M.; Balieiro, L.C.T.; Fahmy, W.M.; Crispim, C.A. Social jetlag and metabolic control in non-communicable chronic diseases: A study addressing different obesity statuses. Sci. Rep. 2017, 7, 6358. [Google Scholar] [CrossRef]
- Parsons, M.J.; Moffitt, T.E.; Gregory, A.M.; Goldman-Mellor, S.; Nolan, P.M.; Poulton, R.; Caspi, A. Social jetlag, obesity and metabolic disorder: Investigation in a cohort study. Int. J. Obes. (Lond.) 2015, 39, 842–848. [Google Scholar] [CrossRef] [PubMed]
- Cain, N.; Gradisar, M. Electronic media use and sleep in school-aged children and adolescents: A review. Sleep Med. 2010, 11, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Van den Bulck, J. Television viewing, computer game playing, and Internet use and self-reported time to bed and time out of bed in secondary-school children. Sleep 2004, 27, 101–104. [Google Scholar] [CrossRef] [PubMed]
- Tarokh, L.; Saletin, J.M.; Carskadon, M.A. Sleep in adolescence: Physiology, cognition and mental health. Neurosci. Biobehav. Rev. 2016, 70, 182–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carskadon, M.A.; Acebo, C.; Jenni, O.G. Regulation of adolescent sleep: Implications for behavior. Ann. N. Y. Acad. Sci. 2004, 1021, 276–291. [Google Scholar] [CrossRef] [PubMed]
- McHill, A.W.; Klerman, E.B.; Slater, B.; Kangarloo, T.; Mankowski, P.W.; Shaw, N.D. The Relationship Between Estrogen and the Decline in Delta Power During Adolescence. Sleep 2017, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crowley, S.J.; Wolfson, A.R.; Tarokh, L.; Carskadon, M.A. An update on adolescent sleep: New evidence informing the perfect storm model. J. Adolesc. 2018, 67, 55–65. [Google Scholar] [CrossRef]
- Hagenauer, M.H.; King, A.F.; Possidente, B.; McGinnis, M.Y.; Lumia, A.R.; Peckham, E.M.; Lee, T.M. Changes in circadian rhythms during puberty in Rattus norvegicus: Developmental time course and gonadal dependency. Horm. Behav. 2011, 60, 46–57. [Google Scholar] [CrossRef] [Green Version]
- Hagenauer, M.H.; Ku, J.H.; Lee, T.M. Chronotype changes during puberty depend on gonadal hormones in the slow-developing rodent, Octodon degus. Horm. Behav. 2011, 60, 37–45. [Google Scholar] [CrossRef] [Green Version]
- Wollnik, F.; Dohler, K.D. Effects of adult or perinatal hormonal environment on ultradian rhythms in locomotor activity of laboratory LEW/Ztm rats. Physiol. Behav. 1986, 38, 229–240. [Google Scholar] [CrossRef] [Green Version]
- Foley, J.E.; Ram, N.; Susman, E.J.; Weinraub, M. Changes to sleep-wake behaviors are associated with trajectories of pubertal timing and tempo of secondary sex characteristics. J. Adolesc. 2018, 68, 171–186. [Google Scholar] [CrossRef] [PubMed]
- Werner, H.; Lebourgeois, M.K.; Geiger, A.; Jenni, O.G. Assessment of chronotype in four- to eleven-year-old children: Reliability and validity of the Children’s Chronotype Questionnaire (CCTQ). Chronobiol. Int. 2009, 26, 992–1014. [Google Scholar] [CrossRef] [PubMed]
- Roenneberg, T.; Kuehnle, T.; Pramstaller, P.P.; Ricken, J.; Havel, M.; Guth, A.; Merrow, M. A marker for the end of adolescence. Curr. Biol. 2004, 14, R1038–R1039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crowley, S.J.; Van Reen, E.; LeBourgeois, M.K.; Acebo, C.; Tarokh, L.; Seifer, R.; Barker, D.H.; Carskadon, M.A. A longitudinal assessment of sleep timing, circadian phase, and phase angle of entrainment across human adolescence. PLoS ONE 2014, 9, e112199. [Google Scholar] [CrossRef] [PubMed]
- Hagenauer, M.H.; Lee, T.M. The neuroendocrine control of the circadian system: Adolescent chronotype. Front. Neuroendocrinol. 2012, 33, 211–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juda, M.; Vetter, C.; Roenneberg, T. The Munich ChronoType Questionnaire for Shift-Workers (MCTQShift). J. Biol. Rhythms 2013, 28, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Bramswig, J.; Dubbers, A. Disorders of pubertal development. Dtsch. Arztebl. Int. 2009, 106, 295–303, quiz 304. [Google Scholar] [CrossRef]
- Crowley, S.J.; Suh, C.; Molina, T.A.; Fogg, L.F.; Sharkey, K.M.; Carskadon, M.A. Estimating the dim light melatonin onset of adolescents within a 6-h sampling window: The impact of sampling rate and threshold method. Sleep Med. 2016, 20, 59–66. [Google Scholar] [CrossRef]
- Pinto, F.T.; Golombek, D.A. Neuroactive steroids alter the circadian system of the Syrian hamster in a phase-dependent manner. Life Sci. 1999, 65, 2497–2504. [Google Scholar] [CrossRef]
- Brockman, R.; Bunick, D.; Mahoney, M.M. Estradiol deficiency during development modulates the expression of circadian and daily rhythms in male and female aromatase knockout mice. Horm. Behav. 2011, 60, 439–447. [Google Scholar] [CrossRef]
- Albers, H.E. Gonadal hormones organize and modulate the circadian system of the rat. Am. J. Physiol. 1981, 241, R62–R66. [Google Scholar] [CrossRef] [PubMed]
- Abizaid, A.; Mezei, G.; Horvath, T.L. Estradiol enhances light-induced expression of transcription factors in the SCN. Brain Res. 2004, 1010, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Karatsoreos, I.N.; Butler, M.P.; Lesauter, J.; Silver, R. Androgens modulate structure and function of the suprachiasmatic nucleus brain clock. Endocrinology 2011, 152, 1970–1978. [Google Scholar] [CrossRef] [PubMed]
- Morin, L.P.; Cummings, L.A. Effect of surgical or photoperiodic castration, testosterone replacement or pinealectomy on male hamster running rhythmicity. Physiol. Behav. 1981, 26, 825–838. [Google Scholar] [CrossRef]
- Thomas, E.M.; Armstrong, S.M. Effect of ovariectomy and estradiol on unity of female rat circadian rhythms. Am. J. Physiol. 1989, 257, R1241–R1250. [Google Scholar] [CrossRef] [PubMed]
- Berberoglu, M. Precocious puberty and normal variant puberty: Definition, etiology, diagnosis and current management. J. Clin. Res. Pediatr. Endocrinol. 2009, 1, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Iglowstein, I.; Jenni, O.G.; Molinari, L.; Largo, R.H. Sleep duration from infancy to adolescence: Reference values and generational trends. Pediatrics 2003, 111, 302–307. [Google Scholar] [CrossRef]
- Benjamini, Y.H.Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. 1995, 57, 289–300. [Google Scholar] [CrossRef]

| Delayed Puberty | Precocious Central Puberty | Premature Pubarche | Premature Thelarche | Database Control Group, Adolescents (Roenneberg et al.) | Database Control Group, Children (Werner et al.) | |
|---|---|---|---|---|---|---|
| Samples size | 19 boys | 13 girls | 19 girls | 10 girls | 240 | 69 |
| Age (years) | 15.7 ± 1.1 | 8.0 ± 1.3 | 6.6 ± 1.3 | 5 ± 2.6 | 15.1 ± 1.2 | 6.4 ± 1.2 |
| Sex (female/male) | 0/19 | 13/0 | 19/0 | 10/0 | 0/240 | 69/0 |
| Tanner stage (breast) | n/a | 2.9 ± 0.9 | 1.3 ± 0.8 | 2.2 ± 0.4 | n/a | n/a |
| Tanner stage (genitalia) | 2.0 ± 1 | n/a | n/a | n/a | n/a | n/a |
| Tanner stage (pubic hair) | 2.0 ± 1 | 2.5 ± 1.3 | 2.2 ± 0.9 | 1.0 ± 0 | n/a | n/a |
| Delayed Puberty | Precocious Central Puberty | Premature Pubarche | Premature Thelarche | Database Control Group, Adolescents (Roenneberg et al.) | Database Control Group, Children (Werner et al.) | Statistical Test | |
|---|---|---|---|---|---|---|---|
| Chronotype (MSFsc) | 3.8 ± 0.8 p = 0.039 FDR cor. p = 0.1248 d = 0.23 | 3.0 ± 1.1 p < 0.001 FDR cor. p = 0.008 d = 0.34 | 2.58 ± 1.0 p = 0.03 FDR cor. p = 0.1248 d = 0.24 | 2.2 ± 1.0 p = 0.7314 FDR cor. p = 0.7802 | 4.46 ± 1.4 | 2.1 ± 0.6 | - T-test (one sided) - Effect size (Cohen’s d) |
| Sleep duration on school days (h) | 8.0 ± 1.5 p < 0.001 FDR cor. p = 0.008 d = 0.55 | 10.1 ± 0.8 p = 0.2088 FDR cor. p = 0.4176 | 10.04 ± 0.7 p = 0.0643 FDR cor. p = 0.1715 | 10.12 ± 1.0 p = 0.3144 FDR cor. p = 0.4895 | 7.3 ± 1.0 | 10.4 ± 0.6 | - T-test (one sided) - Effect size (Cohen’s d) |
| Sleep duration on free days (h) | 8.3 ± 1.5 p = 0.47 FDR cor. p = 0.5785 | 10.19 ± 1.0 p = 0.052 FDR cor. p = 0.1248 | 10.43 ± 0.9 p = 0.2014 FDR cor. p = 0.4176 | 10.65 ± 1.6 p = 0.9191 FDR cor. p = 0.9191 | 8.4 ± 1.3 | 10.68 ± 0.7 | - T-test (one sided) |
| Social jetlag (h) | 1.32 ± 0.74 p = 0.3255 FDR cor. p = 0.4895 | 0.09 ± 1.28 p = 0.3365 FDR cor. p = 0.4895 | 0.39 ± 0.74 p = 0.6642 FDR cor. p = 0.7591 | 0.52 ± 1.15 p = 0.3792 FDR cor. p = 0.5056 | 1.52 ± 0.87 | 0.318 ± 0.61 | - T-test (one sided) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jessen, E.; Vetter, C.; Roenneberg, T.; Liesenkötter, K.-P.; Werner, H.; Jenni, O.G.; Lankes, E.; Blankenstein, O.; Neumann, U.; Köhler, B.; et al. Sleep Timing in Patients with Precocious and Delayed Pubertal Development. Clocks & Sleep 2019, 1, 140-150. https://doi.org/10.3390/clockssleep1010013
Jessen E, Vetter C, Roenneberg T, Liesenkötter K-P, Werner H, Jenni OG, Lankes E, Blankenstein O, Neumann U, Köhler B, et al. Sleep Timing in Patients with Precocious and Delayed Pubertal Development. Clocks & Sleep. 2019; 1(1):140-150. https://doi.org/10.3390/clockssleep1010013
Chicago/Turabian StyleJessen, Elena, Celine Vetter, Till Roenneberg, Klaus-Peter Liesenkötter, Helene Werner, Oskar G. Jenni, Erwin Lankes, Oliver Blankenstein, Uta Neumann, Birgit Köhler, and et al. 2019. "Sleep Timing in Patients with Precocious and Delayed Pubertal Development" Clocks & Sleep 1, no. 1: 140-150. https://doi.org/10.3390/clockssleep1010013





