Comparison between Electrooxidation of 1-Naphthol and 2-Naphthol in Different Non-Aqueous Solvents and Suppression of Layer Growth of Polymers
Abstract
1. Introduction
2. Results and Discussion
2.1. Studies with the Naphthol Isomers in Different Non-Aqueous Solvents
2.2. Effect of Dimethyl Formamide Solvent on Electrode Renewal
2.3. Suppression Ability of Naphthols in Electropolymerization of Selected Organic Monomers
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Abd El-Rahman, H.A. Stability and behaviour of poly-1-naphthol films towards charge transfer reactions. Thin Solid Films 1997, 310, 208–216. [Google Scholar] [CrossRef]
- Abdelaal, M.Y. Electrochemical polymerization of naphthols in aqueous medium. Int. J. Polym. Mater. 2005, 54, 151–159. [Google Scholar] [CrossRef]
- Meneguzzi, A.; Ferreira, C.A.; Pham, M.C.; Delamar, M.; Lacaze, P.C. Electrochemical synthesis and characterization of poly(5-amino-1-naphthol) on mild steel electrodes for corrosion protection. Electrochim. Acta 1999, 44, 2149–2156. [Google Scholar]
- Cinta, E.P.; Torresi, S.I.C. Resonant Raman spectroscopy as a tool for determining the formation of a ladder structure in electropolymerized poly(5-amino-1-naphthol). J. Electroanal. Chem. 2002, 518, 33–40. [Google Scholar] [CrossRef]
- Ohsaka, T.; Ohba, M.; Sato, M.; Oyama, N.; Tanaka, S.; Nakamura, S. Formation of a novel electroactive film by electropolymerization of 5-amino-1-naphthol. J. Electroanal. Chem. 1991, 300, 51–66. [Google Scholar] [CrossRef]
- Mostefai, M.; Pham, M.; Marsault, J.; Aubard, J.; Lacaze, P. Study of the redox process of poly(5-amino-1-naphthol) thin film by in situ Raman spectroscopy. J. Electrochem. Soc. 1996, 143, 2116–2119. [Google Scholar] [CrossRef]
- Pham, M.; Mostefai, M.; Simon, M.; Lacaze, P. Electrochemical synthesis and study of poly(5-amino-1-naphthol) film in aqueous and organic media. Synth. Met. 1994, 63, 7–15. [Google Scholar] [CrossRef]
- Chitravathi, S.; Swamy, B.E.K.; Mamatha, G.P.; Sherigara, B.S. Electrochemical behavior of poly(naphthol green B)-film modified carbon paste electrode and its application for the determination of dopamine and uric acid. J. Electroanal. Chem. 2012, 667, 66–75. [Google Scholar] [CrossRef]
- Kuskur, C.M.; Swamy, B.E.K.; Jayadevappa, H. Poly(naphthol green B) modified carbon paste electrode for the analysis of paracetamol and norepinephrine. Ionics 2019, 25, 1845–1855. [Google Scholar] [CrossRef]
- Kuskur, C.M.; Swamy, B.E.K.; Jayadevappa, H. Poly(naphthol green B) modified carbon paste electrode for catechol and hydroquinone. J. Electroanal. Chem. 2017, 804, 99–106. [Google Scholar] [CrossRef]
- Cai, C.X.; Xue, K.H. Electrochemical characterization of electropolymerized film of naphthol green B and its electrocatalytic activity toward NADH oxidation. Microchem. J. 1998, 58, 197–208. [Google Scholar] [CrossRef]
- Chandra, U.; Swamy, B.E.K.; Gilbert, O.; Sherigara, B.S. Poly(naphthol green B) film based sensor for resolution of dopamine in the presence of uric acid: A voltammetric study. Anal. Methods 2011, 3, 2068–2072. [Google Scholar] [CrossRef]
- Lu, B.; Liu, C.; Li, Y.; Xu, J.; Liu, G. Conducting polynaphthalenes from 1,1’-binaphthyl and 1,1’-bi-2-naphthol via electropolymerization. Synth. Met. 2011, 161, 188–195. [Google Scholar] [CrossRef]
- Lu, B.; Yan, J.; Xu, J.; Zhou, S.; Hu, X. Novel electroactive proton-doped conducting poly(aromatic ethers) with good fluorescence properties via electropolymerization. Macromolecules 2010, 43, 4599–4608. [Google Scholar] [CrossRef]
- Baibarac, M.; Daescu, M.; Socol, M.; Bartha, C.; Negrila, C.; Fejer, S.N. Influence of reduced graphene oxide on the electropolymerization of 5-amino-1-naphthol and the interaction of 1,4-phenylene diizothiocyanate with the poly(5-amino-1-naphthol)/reduced graphene oxide composite. Polymers 2020, 12, 1299. [Google Scholar] [CrossRef]
- Meana-Esteban, B.; Kvarnström, C.; Geschke, B.; Heinze, J.; Ivaska, A. Electrochemical polymerization of 2-methoxynaphthalene. Synth. Met. 2003, 139, 133–143. [Google Scholar] [CrossRef]
- Richard, K.M.; Gewirth, A.A.J. Observation of electrode poisoning during the electro-oxidation of aromatic alcohols on (111)Au. Electrochem. Soc. 1996, 143, 2088–2092. [Google Scholar] [CrossRef]
- Ciriello, R.; Cataldi, T.R.I.; Centonze, D.; Guerrieri, A. Permselective behaviour of an electrosynthetized, nonconducting thin film of poly(2-naphthol) and its application to enzyme immobilization. Electroanalysis 2000, 12, 825–830. [Google Scholar] [CrossRef]
- Ciriello, R.; Guerrieri, A.; Pavese, F.; Salvi, M.A. Electrosynthetized, non-conducting films of poly(2-naphthol): Electrochemical and XPS investigations. Anal. Bioanal. Chem. 2008, 392, 913–926. [Google Scholar] [CrossRef] [PubMed]
- Guerrieri, A.; Lattanzio, V.; Palmisano, F.; Zambonin, P.G. Electrosynthesized poly(pyrrole)/poly(2-naphthol) bilayer membrane as an effective anti-interference layer for simultaneous determination of acethylcholine and choline by a dual electrode amperometric biosensor. Biosens. Bioelectron. 2006, 21, 1710–1718. [Google Scholar] [CrossRef]
- Pham, M.C.; Moslih, J.; Chauveau, F.; Lacaze, P.C. In situ multiple internal reflection Fourier transform infrared spectroscopic (MIRFTIRS) study of the electrochemical immobilization of heteropolyanions in poly(1-naphthol) coated electrodes. J. Appl. Electrochem. 1991, 21, 902–909. [Google Scholar] [CrossRef]
- Rehan, H.H. Electrosynthesis and characterization of new conducting copolymer films from 1-naphthol and methyl naphthyl ether. Polym. Int. 2000, 49, 645–653. [Google Scholar] [CrossRef]
- Kiss, L.; Kunsági-Máté, S. Assessment of non-aqueous solvents in the electrooxidation of resorcinol, phloroglucinol, pyrogallol, and role of co-solvent in determination of pyrogallol with microelectrode voltammetry. Stud. Univ. Babes-Bolyai Chem. 2021, 66, 159–170. [Google Scholar]
- Gulnaz, R.N.; Vitaliy, V.Y.; Natalia, V.N.; Dmitry, E.K.; Albina, Y.Z.; Alexandr, I.K. Redox-switchable binding of ferrocyanide with tetra(viologen)calix [4] resorcine. J. Incl. Phenom. Macrocycl. Chem. 2012, 72, 299–308. [Google Scholar]
- Liska, A.; Rosenkranz, M.; Klíma, J.; Dunsch, L.; Lhoták, P.; Ludvík, J. Formation and proof of stable bi-, tri-, and tetraradical polyanions during the electrochemical reduction of cone-polynitrocalix[4]arenes. An ESR-UV-vis spectroelectrochemical study. Electrochim. Acta 2014, 140, 572–578. [Google Scholar] [CrossRef]
- Liska, A.; Vojtísek, P.; Fry, A.J.; Ludvík, J. Electrochemical and quantum chemical investigation of tetranitrocalix[4]arenes: Molecules with multiple redox centers. J. Org. Chem. 2013, 78, 10651–10656. [Google Scholar] [CrossRef]
- Liska, A.; Flídrová, K.; Lhoták, P.; Ludvík, J. Influence of structure on electrochemical reduction of isomeric mono- and di-, nitro- or nitrosocalix[4]arenes. Monatsch. Chem. 2015, 146, 857–862. [Google Scholar] [CrossRef]
- Vitaly, V.Y.; Gulnaz, R.N.; Natalya, V.N.; Albina, Y.Z.; Dmitry, E.K.; Yulia, S.S.; Margit, G.; Wolf, D.H.; Andrey, A.K.; Alexandr, I.K. Electrodriven molecular system based on tetraviologen calix[4]resorcine and dianion 1,5-bis(n-sulfonatophenyl)-3,7-diphenyl-1,5-diaza-3,7-diphosphacyclooctane. Electrochim. Acta 2013, 111, 466–473. [Google Scholar]
- Kiss, L.; Kovács, F.; Kunsági-Máté, S. Role of allyl alcohol and sodium 4-vinylbenzenesulphonate in the electrooxidation of phenol. Chem. Phys. Lett. 2021, 764, 138270. [Google Scholar] [CrossRef]
- Kiss, L.; Kovács, F.; Li, H.; Kiss, A.; Kunsági-Máté, S. Electrochemical polymerization of phenol on platinum and glassy carbon electrodes in mesityl oxide. Chem. Phys. Lett. 2020, 754, 137642. [Google Scholar] [CrossRef]
- Kiss, L.; Kovács, F.; Kunsági-Máté, S. Investigation of anodic behaviour of phenylethers in non-aqueous solvents on platinum and glassy carbon electrodes. J. Iran. Chem. Soc. 2021, 18, 1677–1687. [Google Scholar] [CrossRef]
- Yin, S.X.; Lu, W.T.; Guo, C.Y. Enhancing thermoelectric performance of polyaniline/single-walled carbon nanotube composites via dimethyl sulfoxide-mediated electropolymerization. ACS Appl. Mater. Interfaces 2021, 13, 3930–3936. [Google Scholar] [CrossRef] [PubMed]
- Kiss, L. Coexistence of substituted phenols reduces the fouling ability of phenol in non-aqueous solvents. Period. Polytech-Chem. 2022, 66, 409–413. [Google Scholar] [CrossRef]
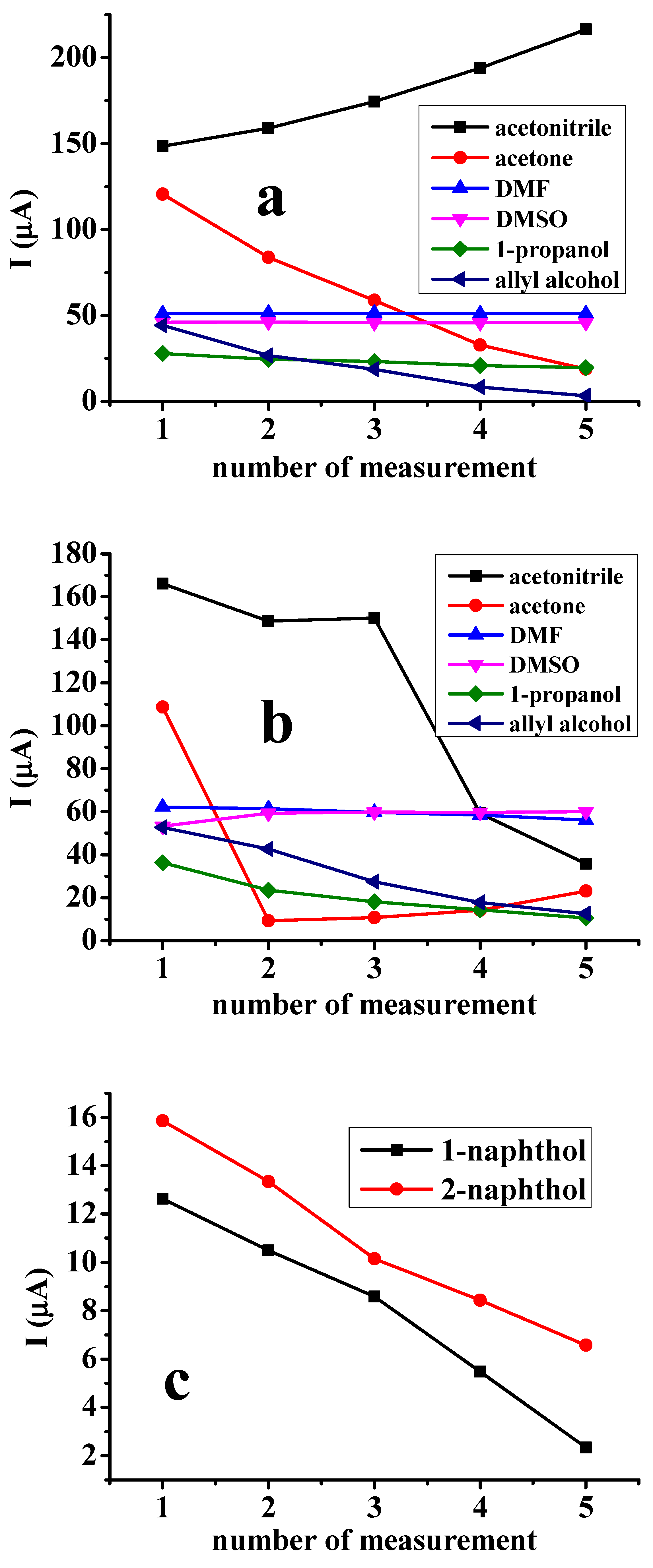
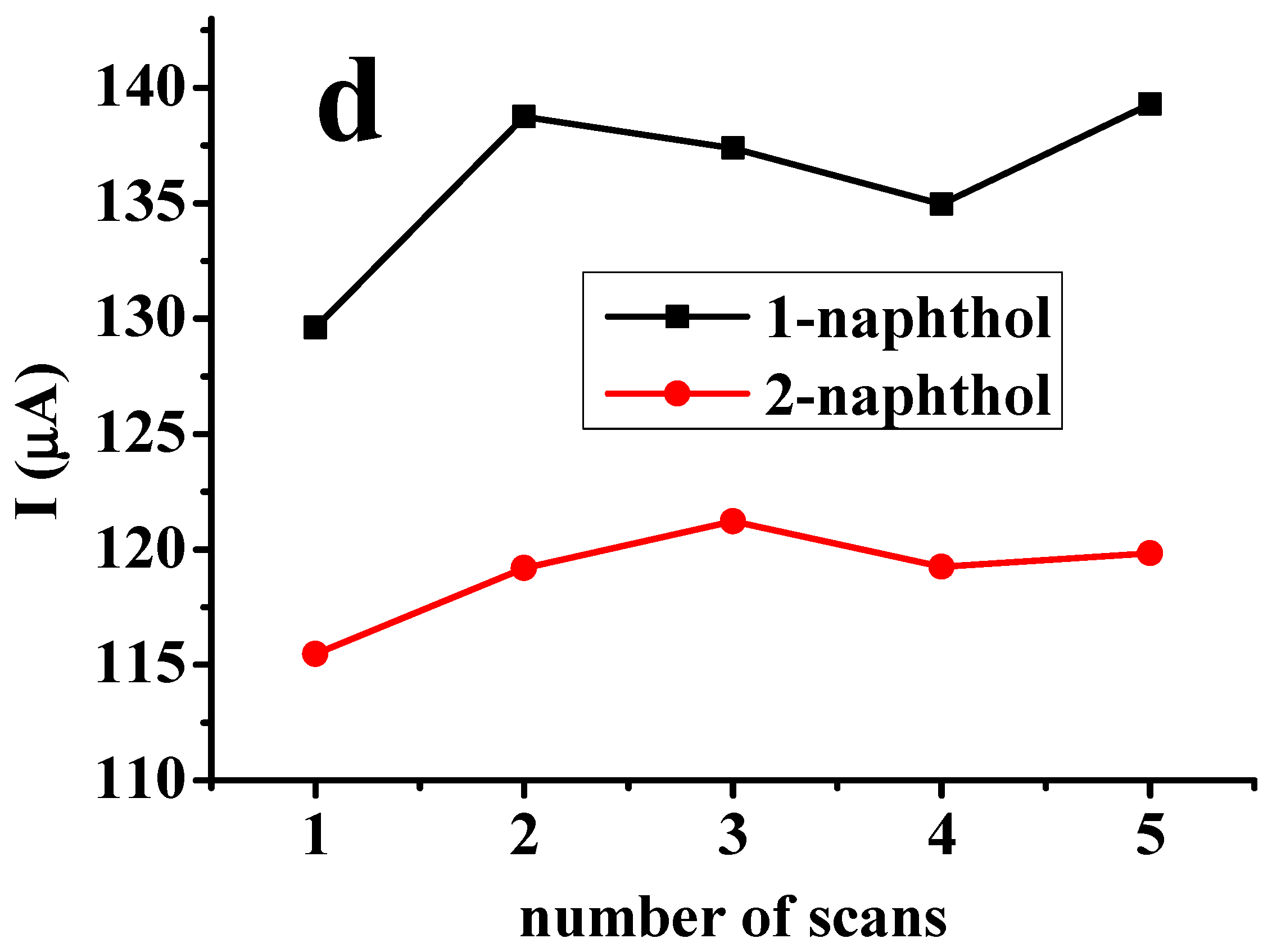

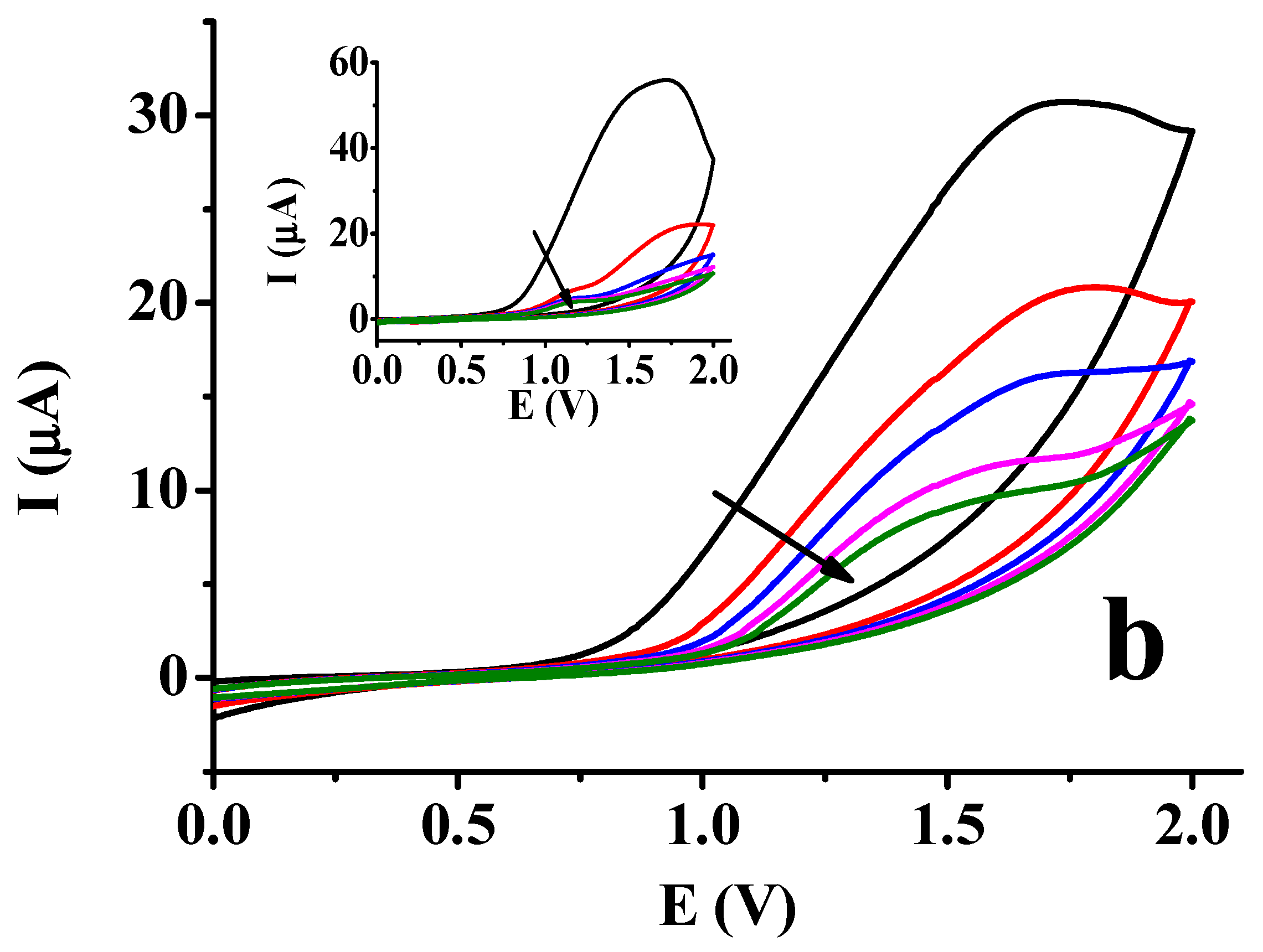
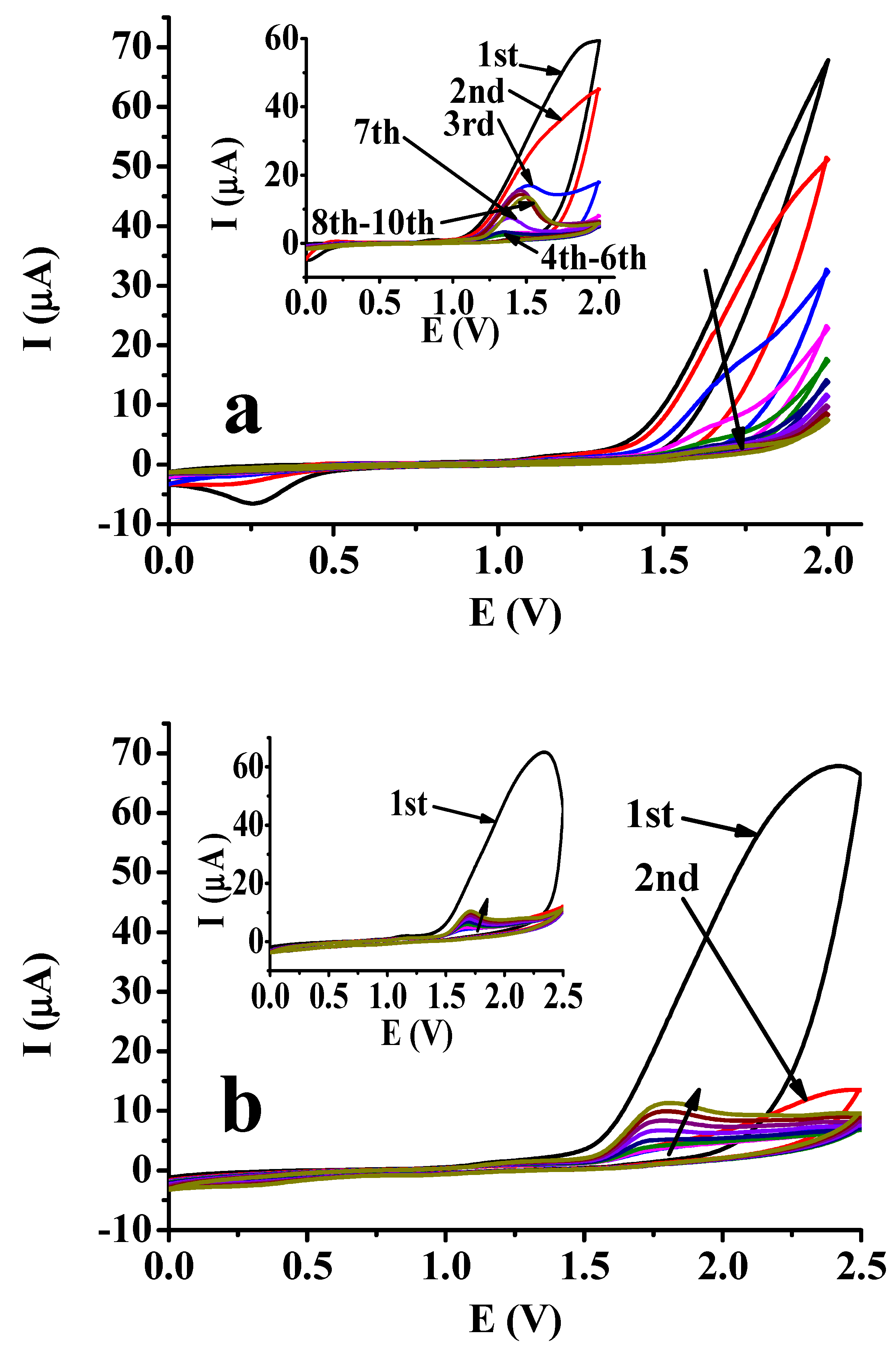
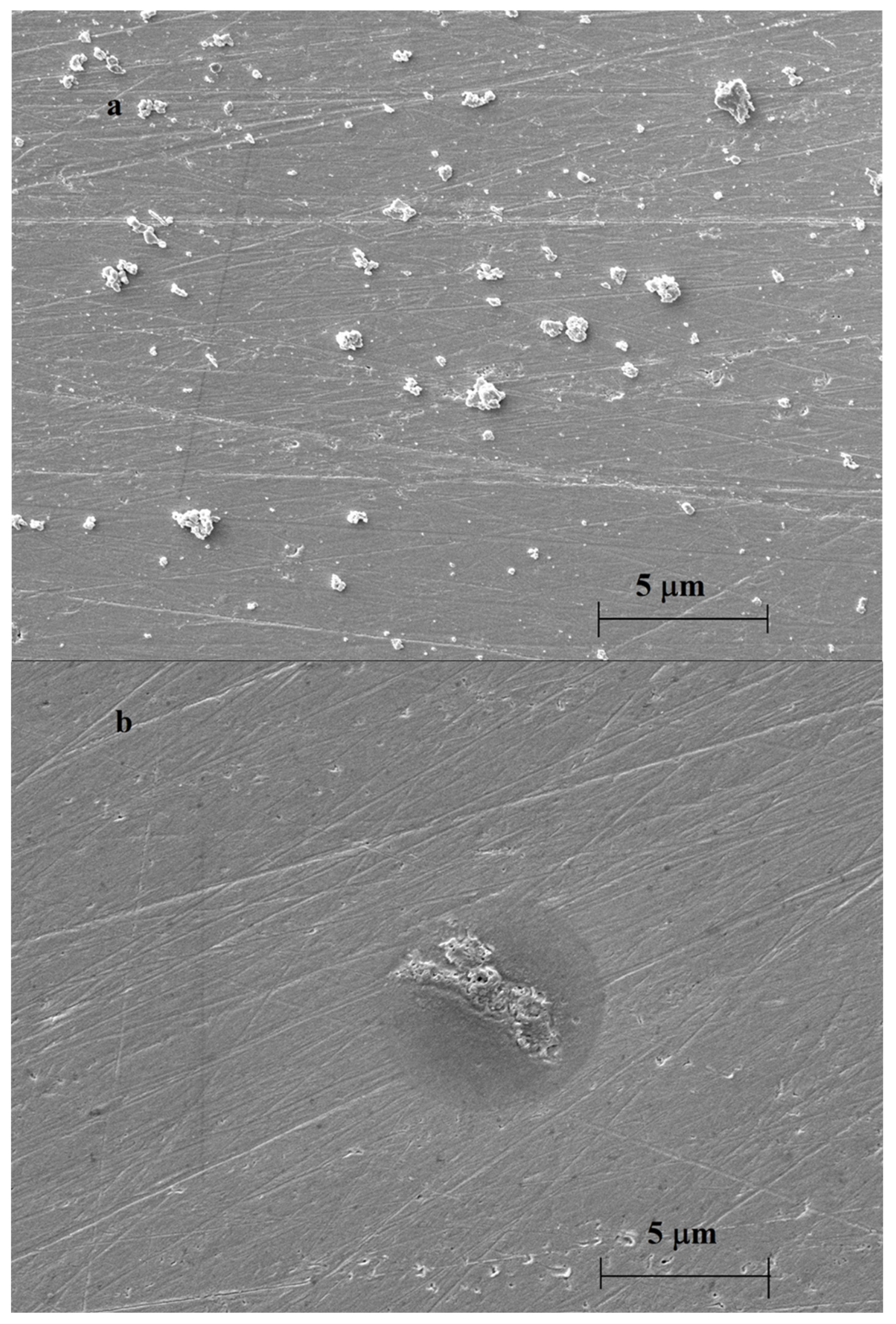
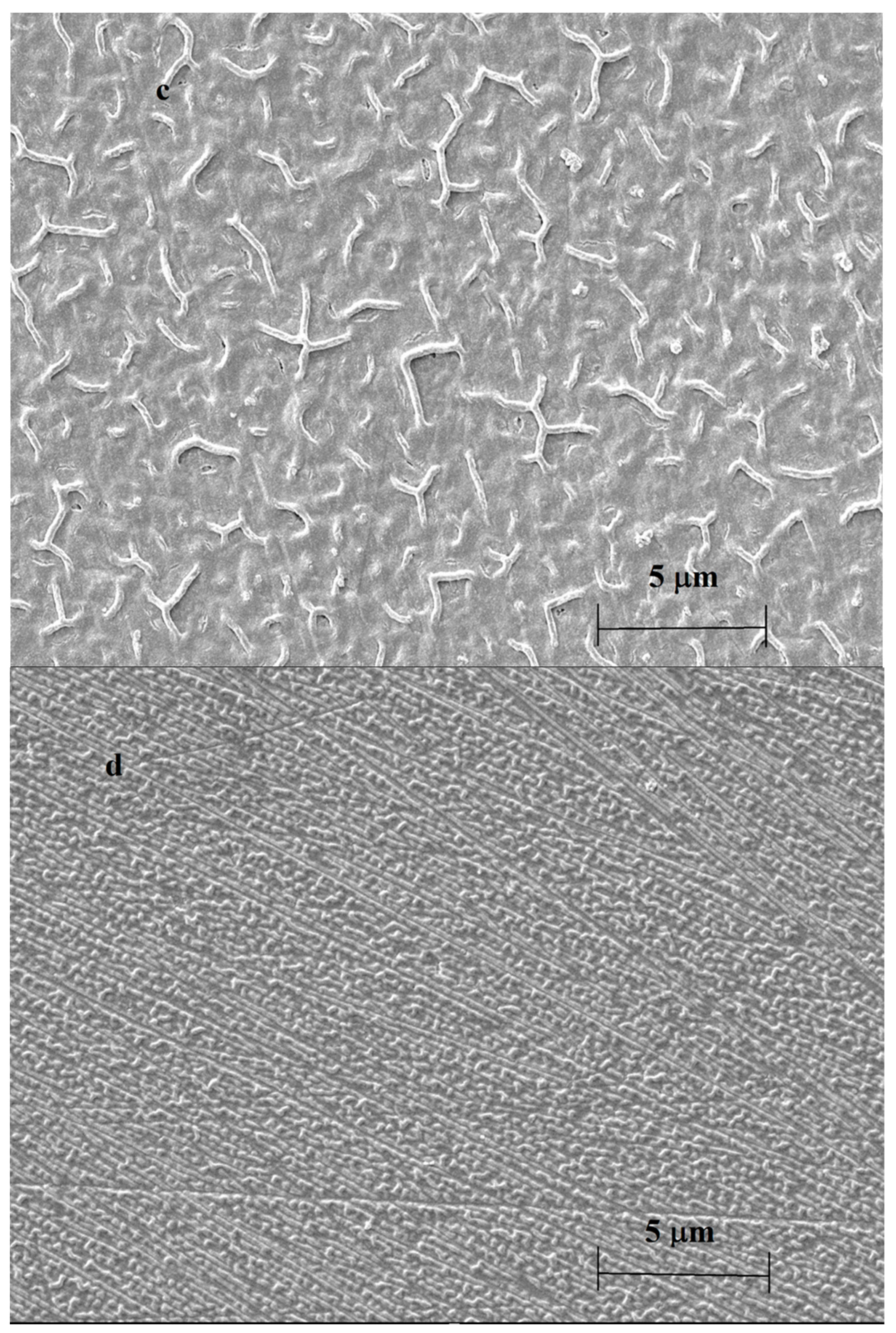

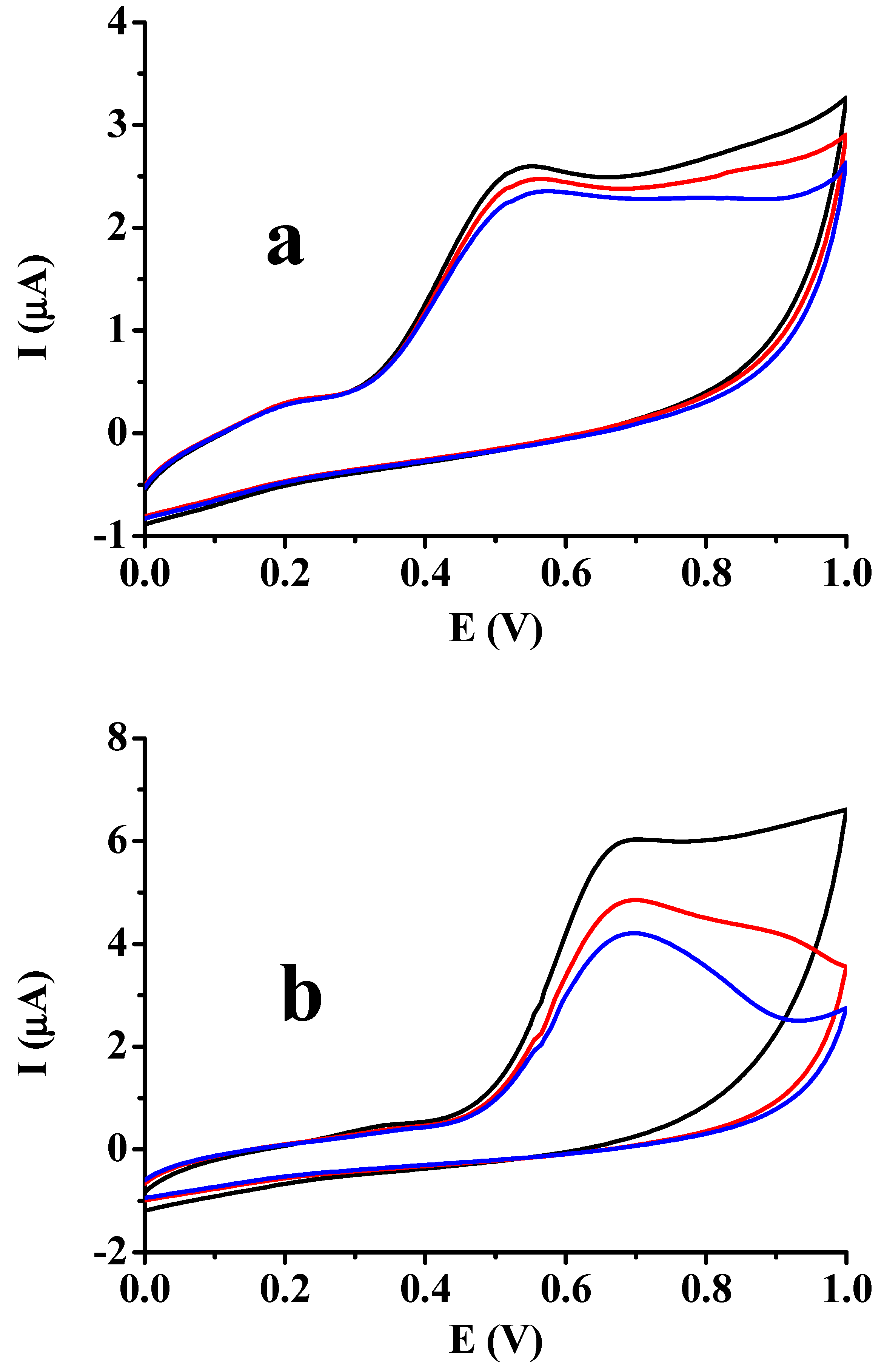
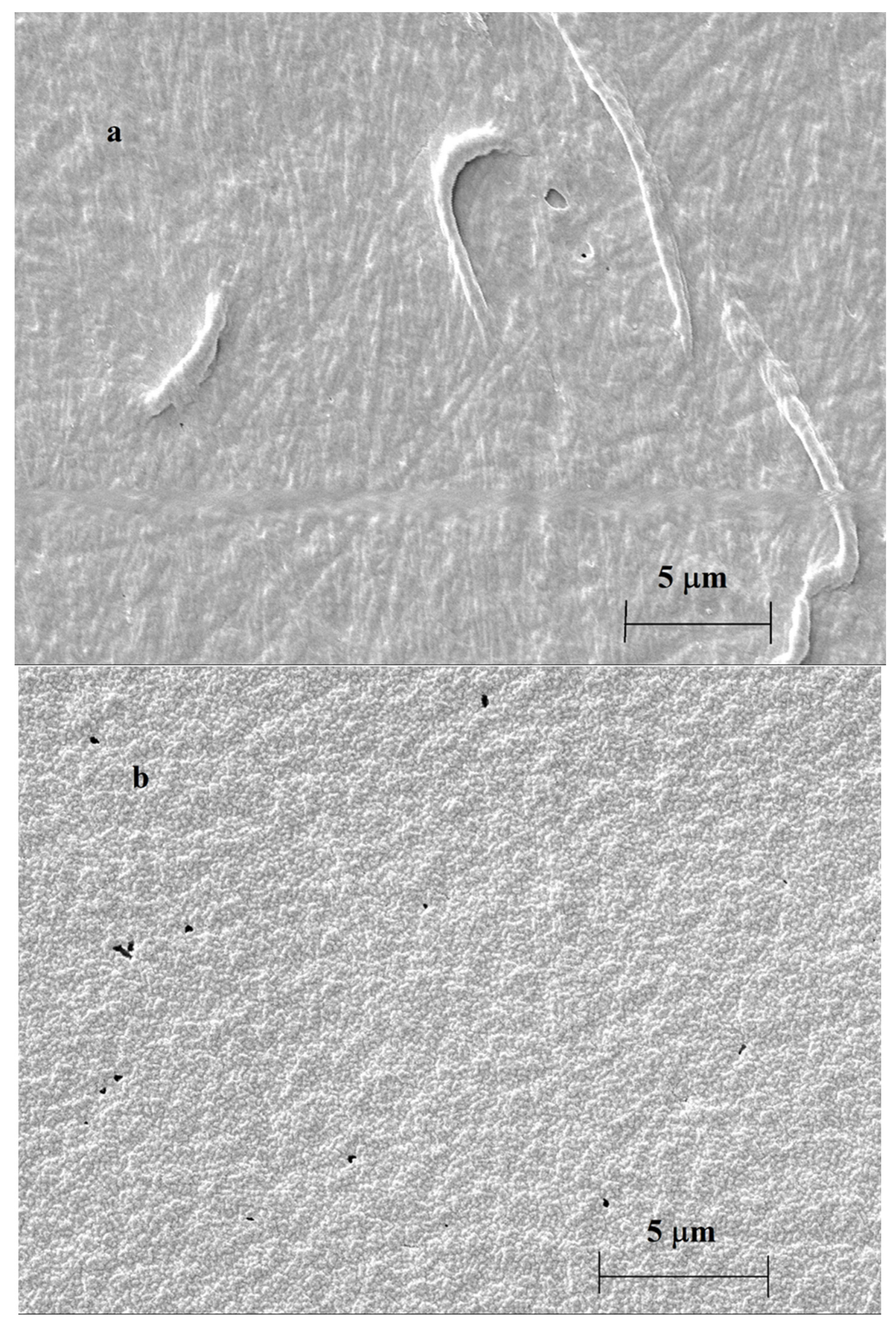

| Deposit | 1st CV | 2nd CV | 3rd CV | 10th CV | ||||
|---|---|---|---|---|---|---|---|---|
| Org. | Aq. | Org. | Aq. | Org. | Aq. | Org. | Aq. | |
| 1-NP MIBK | 107.6 | 89.2 | 107.7 | 81.1 | 105.8 | 54.5 | 104 | 36.3 |
| 2-NP MIBK | 96 | 52.4 | 94.1 | 49.4 | 94 | 47 | 94.4 | 46.4 |
| 1-NP MZO | 109 | 86.3 | 111.1 | 78.9 | 108.7 | 45.4 | 100.5 | 41.4 |
| 2-NP MZO | 92.6 | 60 | 91.6 | 49.6 | 91.5 | 46.6 | 99 | 42.2 |
| FLO | 81.9 | 89.9 | 91.7 | 89.4 | 58.6 | 77.4 | 48 | 58.2 |
| FLO+1-NP | 107 | 96.9 | 100 | 95.2 | 97 | 63.6 | 60.3 | 55.5 |
| FLO+2-NP | 101.6 | 94.9 | 106.6 | 81.4 | 104 | 63.9 | 87 | 52.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiss, L.; Szabó, P.; Kunsági-Máté, S. Comparison between Electrooxidation of 1-Naphthol and 2-Naphthol in Different Non-Aqueous Solvents and Suppression of Layer Growth of Polymers. Surfaces 2024, 7, 164-180. https://doi.org/10.3390/surfaces7010011
Kiss L, Szabó P, Kunsági-Máté S. Comparison between Electrooxidation of 1-Naphthol and 2-Naphthol in Different Non-Aqueous Solvents and Suppression of Layer Growth of Polymers. Surfaces. 2024; 7(1):164-180. https://doi.org/10.3390/surfaces7010011
Chicago/Turabian StyleKiss, László, Péter Szabó, and Sándor Kunsági-Máté. 2024. "Comparison between Electrooxidation of 1-Naphthol and 2-Naphthol in Different Non-Aqueous Solvents and Suppression of Layer Growth of Polymers" Surfaces 7, no. 1: 164-180. https://doi.org/10.3390/surfaces7010011
APA StyleKiss, L., Szabó, P., & Kunsági-Máté, S. (2024). Comparison between Electrooxidation of 1-Naphthol and 2-Naphthol in Different Non-Aqueous Solvents and Suppression of Layer Growth of Polymers. Surfaces, 7(1), 164-180. https://doi.org/10.3390/surfaces7010011






