Tin(IV) Compounds as Photo-Stabilizers for Irradiated Surfaces of Poly(Vinyl Chloride) Films
Abstract
:1. Introduction
2. Experimental Methods
2.1. Materials and Devices
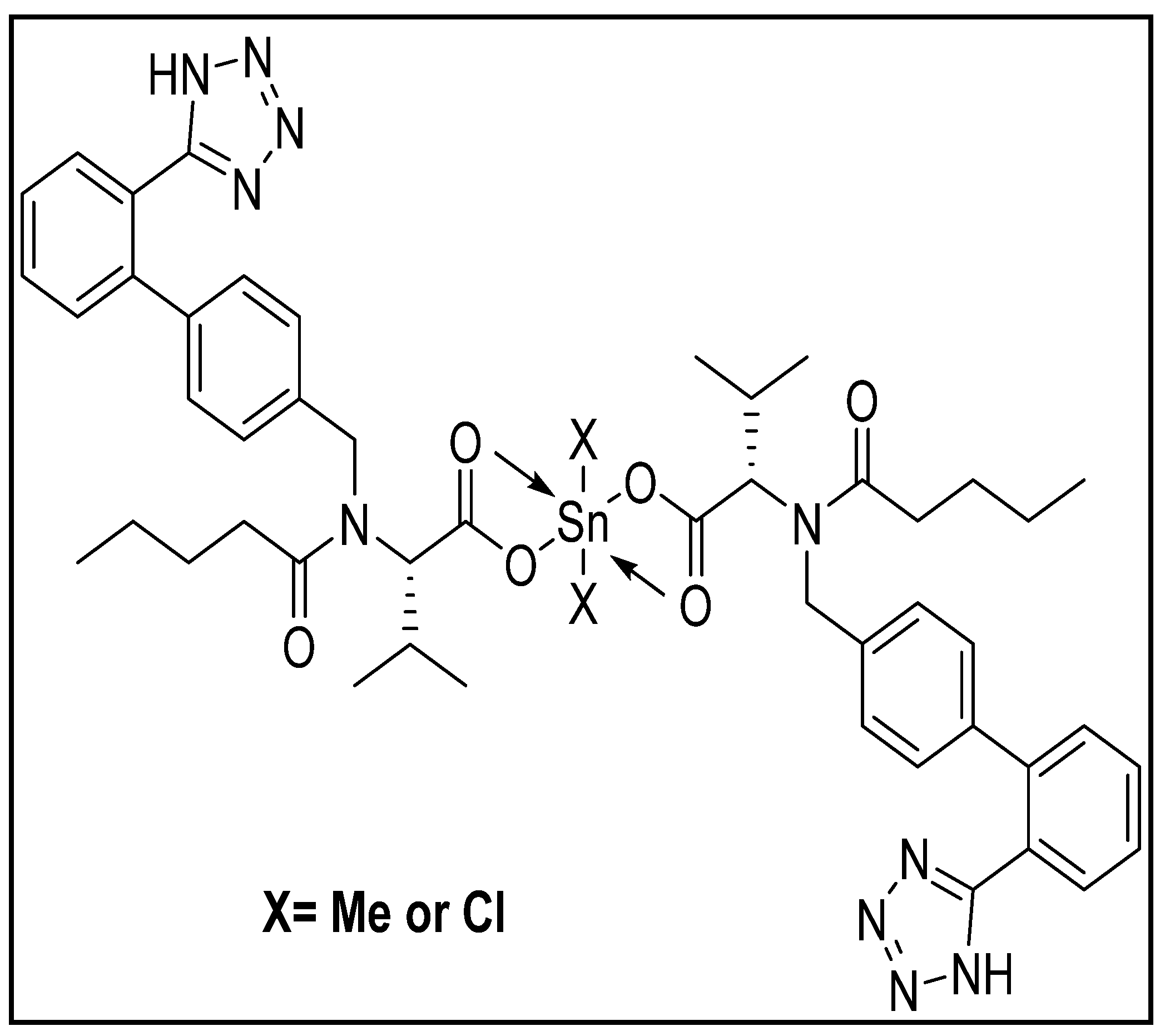
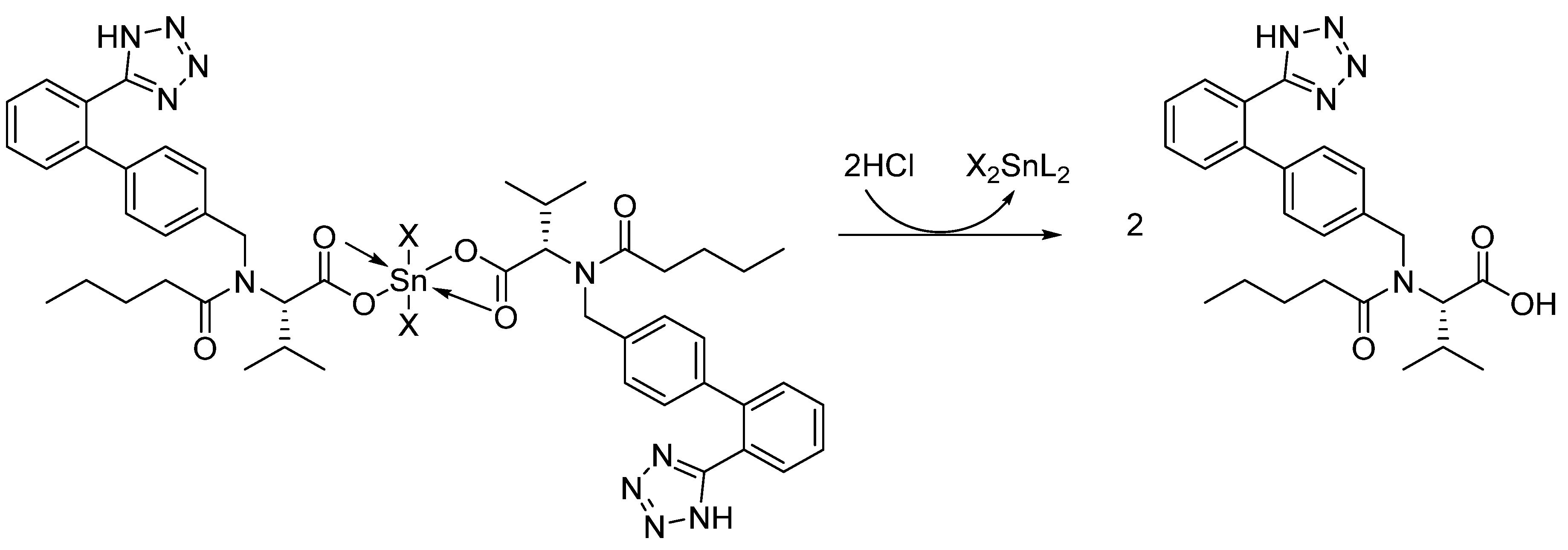
2.2. Preparation of Complexes
2.3. Films Preparation
2.4. Films Irradiation
2.5. Estimating the Tin(IV) Compounds Activity as PVC Photo-Stabilizers
2.5.1. Using FTIR Spectrophotometry
2.5.2. Using Weight Loss
2.5.3. Measuring the Variation in Gel Content of PVC Films
2.5.4. Using Viscosity Average Molecular Weight (MV)
3. Results and Discussion
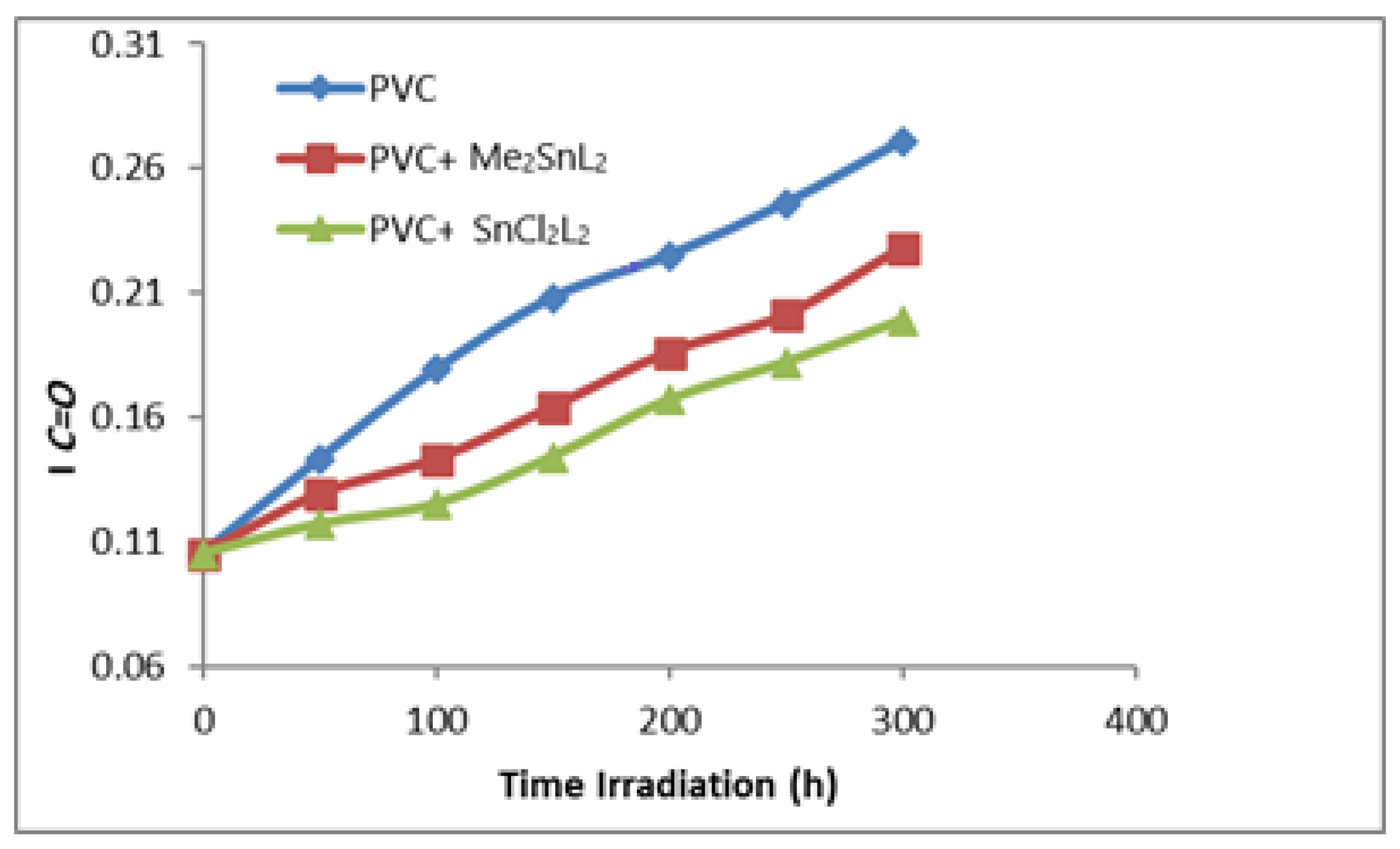
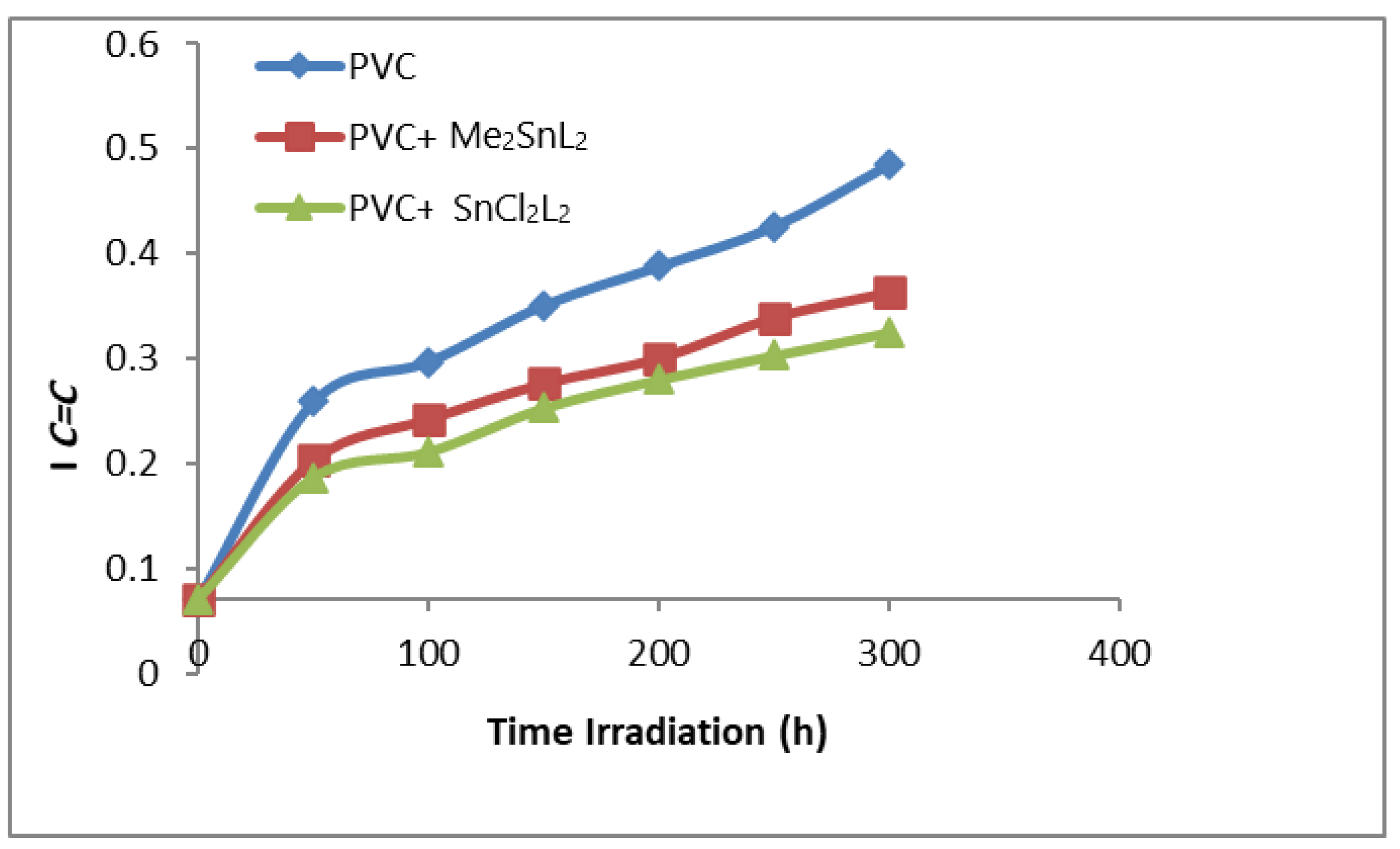
3.1. Stabilizing Appraisal of PVC by FTIR Spectroscopy
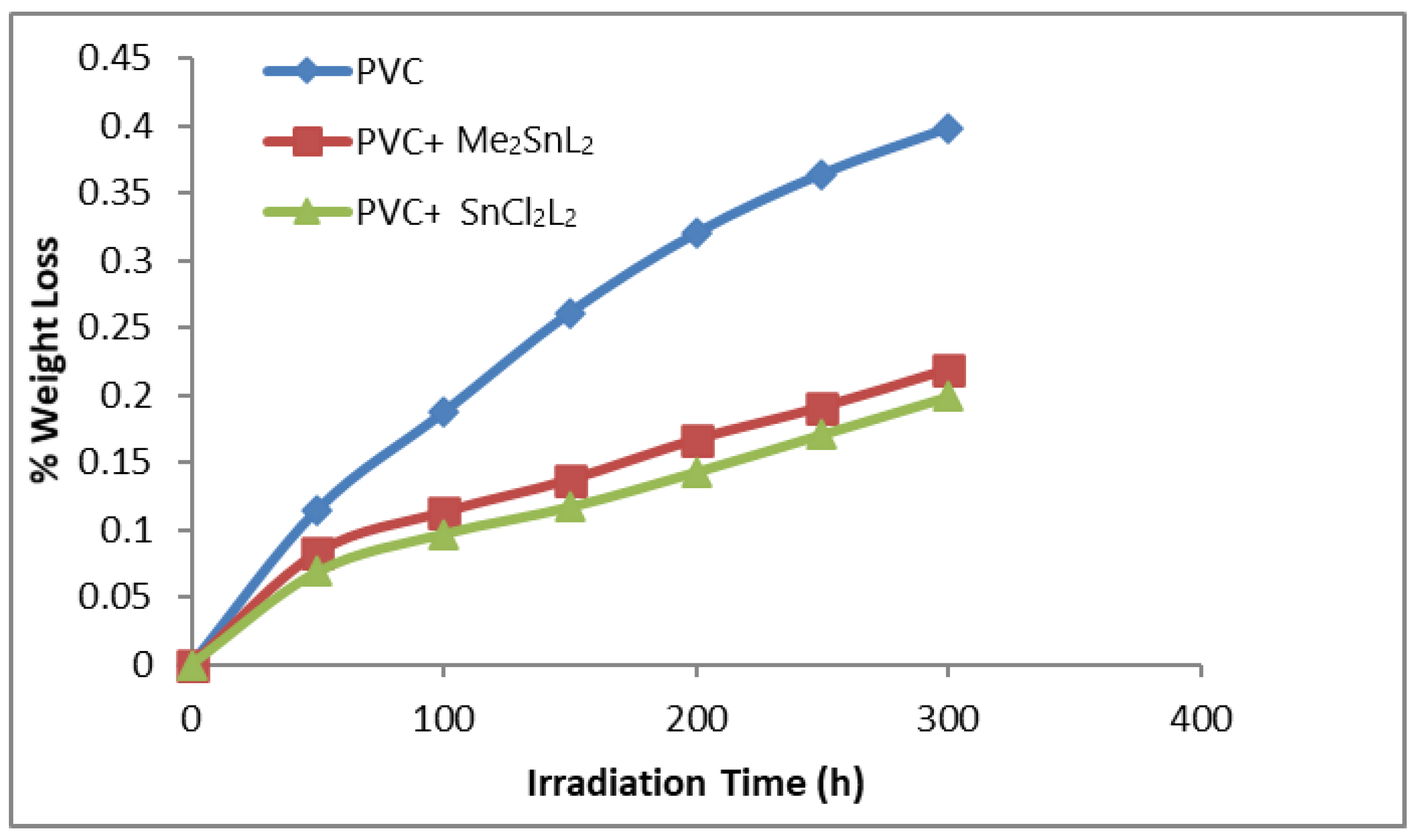
3.2. Stabilizing Appraisal of PVC by Weight Loss
3.3. Stabilizing Appraisal of PVC by Variation in Gel Content
3.4. Stabilizing Appraisal of PVC by Variation in Viscosity Average Molecular Weight
3.5. Surface Analysis
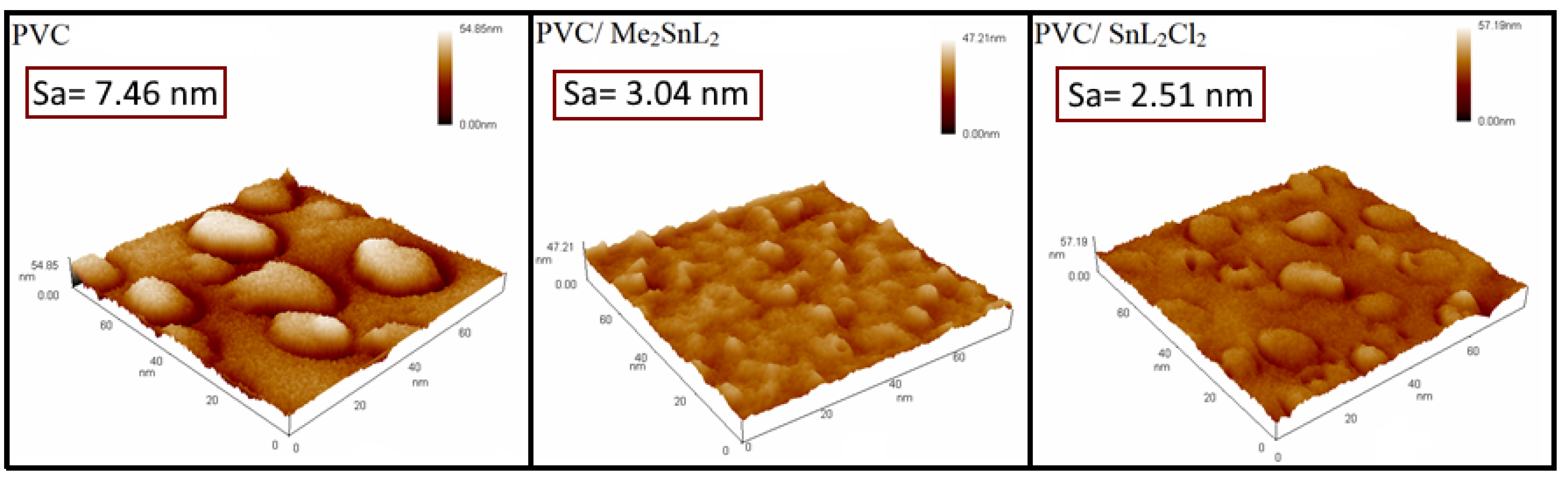
3.5.1. Stabilizing Appraisal of PVC by Atomic Force Microscope
3.5.2. Stabilizing Appraisal of PVC by Scanning Electron Microscopy
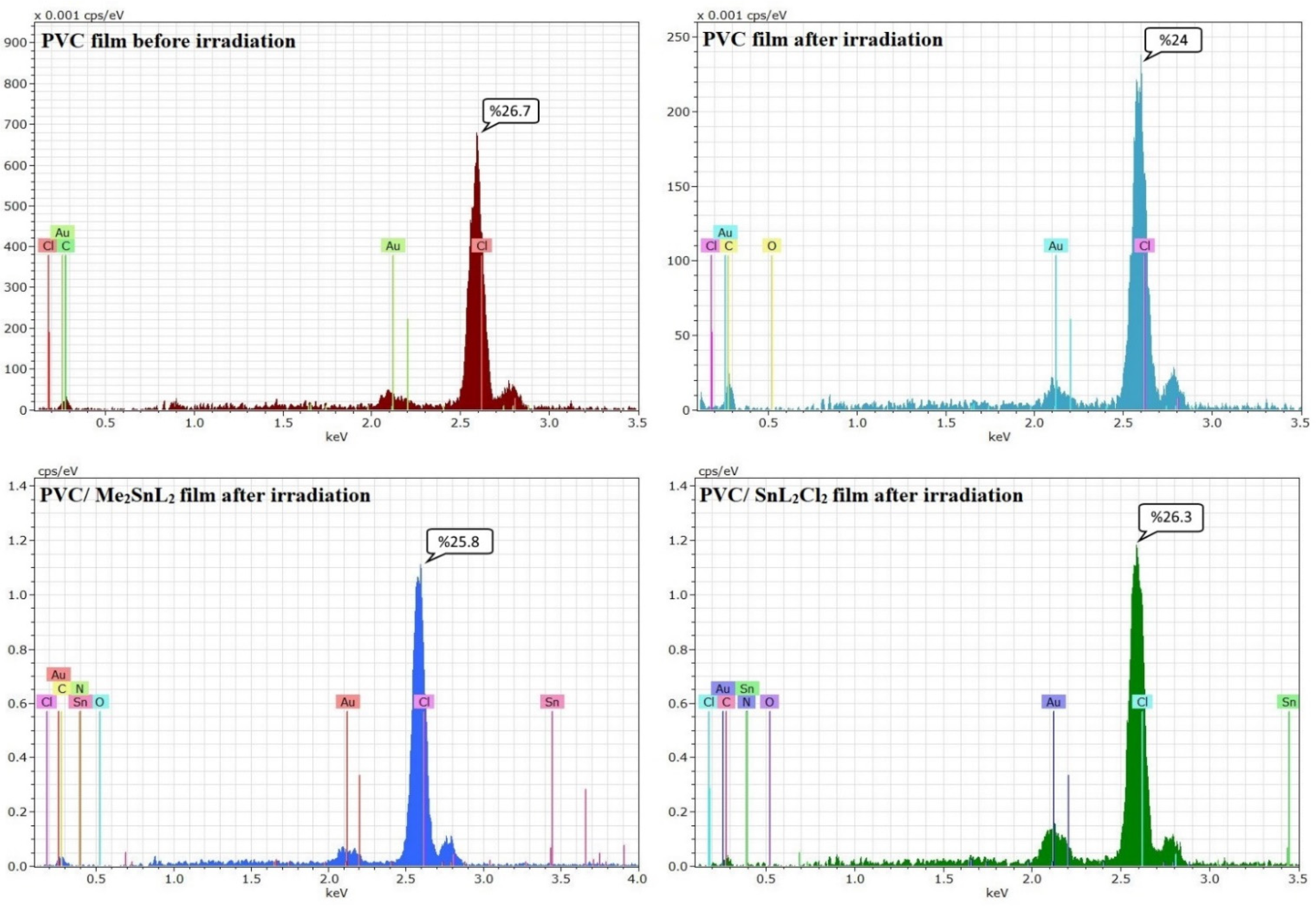
3.5.3. Assessment of Photodegradation of PVC Using Energy Dispersive X-ray (EDX) Mapping
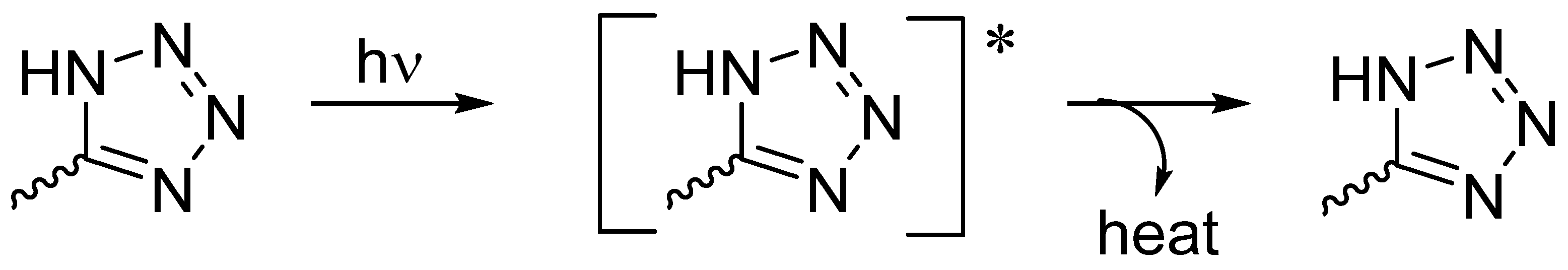
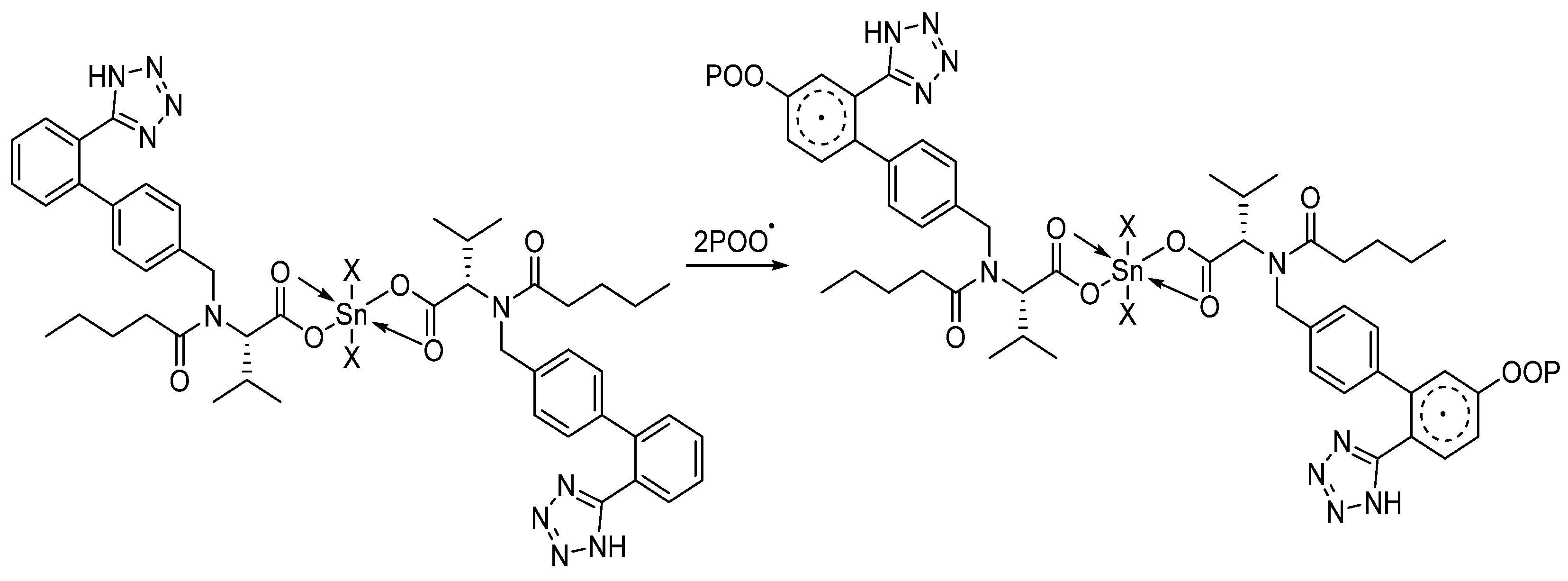
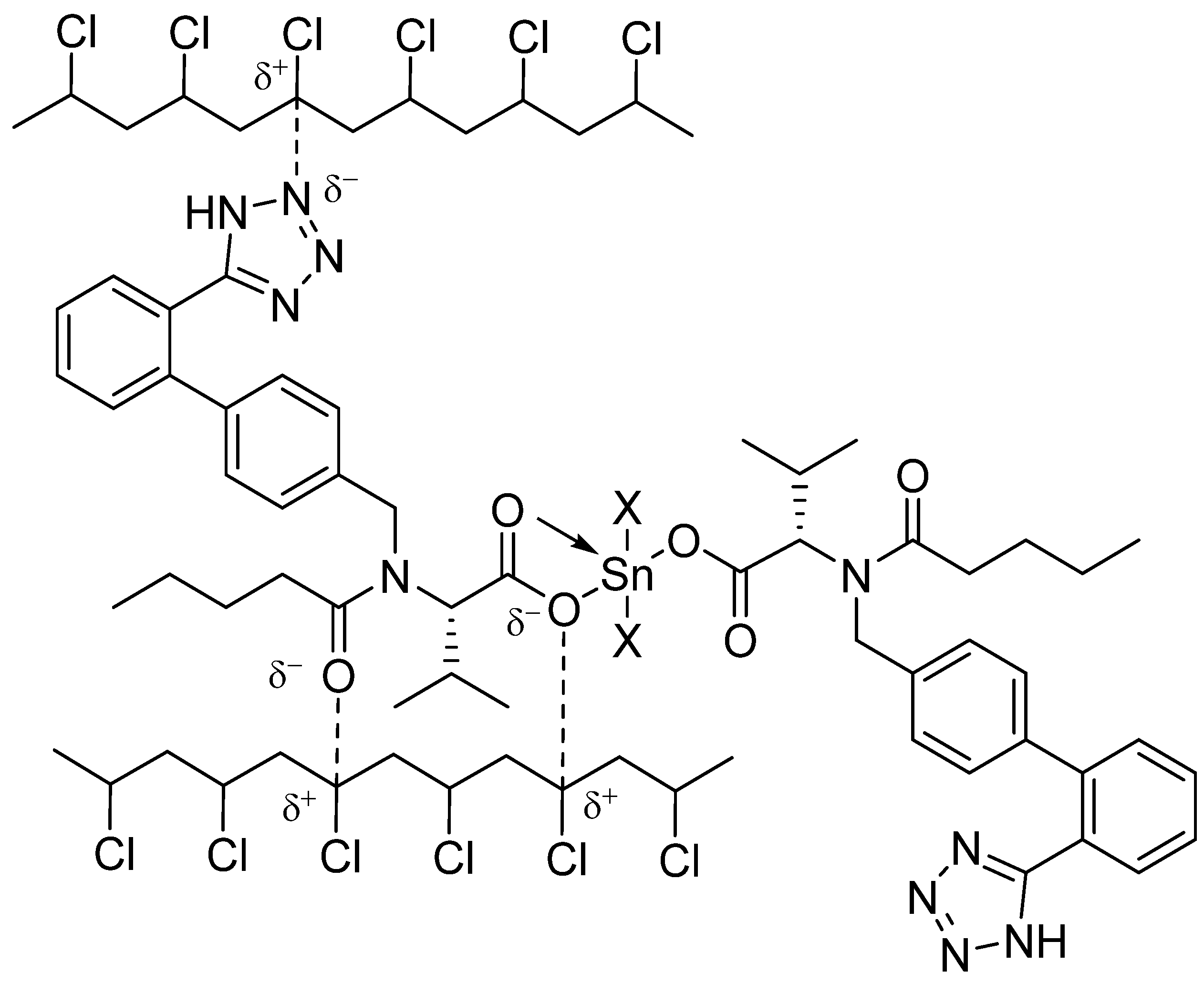
3.6. Suggested Mechanisms of Organotin Complexes Efficiency
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Burgess, R.H. Manufacture and Processing of PVC; CRC Press: Boca Raton, FL, USA, 1982; pp. xi–xii. [Google Scholar]
- Yngve, V. Stabilized vinyl resins. U.S. Patent 2219463, 1941, 35, 1145.
- Titow, W.V. PVC Plastics Properties, Processing, and Applications; Elsevier: Amsterdam, The Netherlands, 1990; p. 787. [Google Scholar]
- Carroll, W.F.; Johnson, R.W.; Moore, S.S.; Paradis, R.A. Applied Plastics Engineering Handbook; Elsevier: Amsterdam, The Netherlands, 2011; pp. 61–76. [Google Scholar]
- Titow, W.T. PVC Technology, 4th ed.; Elsevier: Amsterdam, The Netherlands, 1984; pp. 207–208. [Google Scholar]
- Nass, L.I.; Heiberger, C.A. Encyclopedia of PVC, 2nd ed.; Marcel Dekker: New York, NY, USA, 1986; p. 397. [Google Scholar]
- Akovali, G. Plastic materials: Polyvinyl chloride (PVC). In Toxicity of Building Materials; Woodhead Publishing Limited: Sawston, UK, 2012; pp. 23–53. [Google Scholar]
- Cadogan, D.F.; Howick, C.J. Plasticizers. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, Germany, 2000. [Google Scholar]
- Gao, A.X.; Bolt, J.D.; Feng, A.A. Role of titanium dioxide pigments in outdoor weathering of rigid PVC. Plast. Rubber Compos. 2008, 37, 397–402. [Google Scholar] [CrossRef]
- Chai, R.D.; Zhang, J. Synergistic effect of hindered amine light stabilizers/ultraviolet absorbers on the polyvinyl chloride/powder nitrile rubber blends during photodegradation. Polym. Eng. Sci. 2013, 53, 1760–1769. [Google Scholar] [CrossRef]
- Watheq, B.; Yousif, E.; Al-Mashhadani, M.H.; Mohammed, A.; Ahmed, D.S.; Kadhom, M.; Jawad, A.H. A Surface Morphological Study, Poly (Vinyl Chloride) Photo-Stabilizers Utilizing Ibuprofen Tin Complexes against Ultraviolet Radiation. Surfaces 2020, 3, 579–593. [Google Scholar] [CrossRef]
- Abed, R.N.; Kadhom, M.; Ahmed, D.S.; Hadawey, A.; Yousif, E. Enhancing Optical Properties of Modified PVC and Cr2O3 Nanocomposite. Trans. Electr. Electron. Mater. 2021, 22, 317–327. [Google Scholar] [CrossRef]
- Ahmed, D.S.; Kadhom, M.; Hadi, A.G.; Bufaroosha, M.; Salih, N.; Al-Dahhan, W.H.; Yousif, E. Tetra Schiff Bases as Polyvinyl Chloride Thermal Stabilizers. Chemistry 2021, 3, 288–295. [Google Scholar] [CrossRef]
- Holleman, A.F.; Wiberg, E. Inorganic Chemistry; Academic Press: Cambridge, MA, USA, 2001; pp. 903–909. [Google Scholar]
- Levy, J. Tin, 1st ed.; The Risen Publishing Group, Inc.: New York, NY, USA, 2009; pp. 27–29. [Google Scholar]
- Alaa Mohammed, A.; Makia, R.; Ali, M.; Raheem, R.; Yousif, Y. Cytotoxic Effects of Valsartan Organotin(IV) Complexes on Human Lung Cancer Cells. Biointerface Res. Appl. Chem. 2020, 11, 8156–8164. [Google Scholar]
- Mohammed, A.; Yousif, E.; El-Hiti, G.A. Synthesis and Use of Valsartan Metal Complexes as Media for Carbon Dioxide Storage. Materials 2020, 13, 1183. [Google Scholar] [CrossRef] [Green Version]
- Mohammed, A.; El-Hiti, G.A.; Yousif, E.; Ahmed, A.A.; Ahmed, D.S.; Alotaibi, M.H. Protection of Poly(Vinyl Chloride) Films against Photodegradation Using Various Valsartan Tin Complexes. Polymers 2020, 12, 969. [Google Scholar] [CrossRef] [Green Version]
- Gaumet, S.; Gardette, J. Photo-oxidation of poly(vinyl chloride): Part 2-A comparative study of the carbonylated products in photo-chemical and thermal oxidations. Polym. Degrad. Stab. 1991, 33, 17–34. [Google Scholar] [CrossRef]
- Hadi, A.G.; Jawad, K.; El-Hiti, G.A.; Alotaibi, M.H.; Ahmed, A.A.; Ahmed, D.S.; Yousif, E. Photostabilization of poly(vinyl chloride) by organotin(IV) compounds against photodegradation. Molecules 2019, 24, 3557. [Google Scholar] [CrossRef] [Green Version]
- Sabaa, M.W.; Oraby, E.H.; Abdul Naby, A.S.; Mohamed, R.R. N-Phenyl-3-substituted-5-pyrazolone derivatives as organic stabilizer for rigid PVC against photodegradation. J. Appl. Polym. Sci. 2005, 101, 1543–1555. [Google Scholar] [CrossRef]
- Mark, J.E. Physical Properties of Polymers Handbook, 2nd ed.; Springer: New York, NY, USA, 2007. [Google Scholar]
- Gardette, J.L.; Gaumet, S.; Lemaire, J. Photooxidation of poly(viny1 chloride). 1. A re-examination of the mechanism. Macromolecules 1989, 22, 2576–2581. [Google Scholar] [CrossRef]
- Yassin, A.A.; Sabaa, M.W. Degradation and stabilization of poly(vinyl chloride). J. Macromol. Sci. Part C Polym. Rev. 1990, 30, 491–558. [Google Scholar] [CrossRef]
- Bacaloglu, R.; Fisch, M. Degradation and stabilization of poly(vinyl chloride). V. Reaction mechanism of poly(vinyl chloride) degradation. Polym. Degrad. Stab. 1995, 47, 33–57. [Google Scholar] [CrossRef]
- Jiménez, A.; López, J.; Vilaplana, H.; Dussel, H.-J. Thermal degradation of plastisols. Effect of some additives on the evolution of gaseous products. J. Anal. Appl. Pyrol. 1997, 40, 201–215. [Google Scholar] [CrossRef]
- Blazsó, M.; Jakab, E. Effect of metals, metal oxides, and carboxylates on the thermal decomposition processes of poly(vinyl chloride). J. Anal. Appl. Pyrol. 1999, 49, 125–143. [Google Scholar] [CrossRef]
- Jafari, A.J.; Donaldson, J.D. Determination of HCl and VOC emission from thermal degradation of PVC in the absence and presence of copper, copper(II) Oxide and copper(II) chloride. Eur. J. Chem. 2009, 6, 685–692. [Google Scholar] [CrossRef] [Green Version]
- Allcock, H.; Lampe, F.; Mark, J.E. Contemporary Polymer Chemistry, 3rd ed.; Pearson Prentice-Hall: Hoboken, NJ, USA, 2003. [Google Scholar]
- Pospíšil, J.; Klemchuk, P.P. Oxidation Inhibition in Organic Materials; CRC Press: Boca Raton, FL, USA, 1989; pp. 48–49. [Google Scholar]
- Zheng, X.; Tang, L.; Zhang, N.; Gao, Q.; Zhang, C.; Zhu, Z. Dehydrochlorination of PVC materials at high temperature. Energy Fuels 2003, 17, 896–900. [Google Scholar] [CrossRef]
- Mehmood, N.; Andreasson, E.; Kao-Walter, S. SEM observations of a metal foil laminated with a polymer film. Procedia Mater. Sci. 2014, 3, 1435–1440. [Google Scholar] [CrossRef] [Green Version]
- Nikafshar, S.; Zabihi, O.; Ahmadi, M.; Mirmohseni, A.; Taseidifar, M.; Naebe, M. The effects of UV light on the chemical and mechanical properties of a transparent epoxy-diamine system in the presence of an organic UV absorber. Materials 2017, 10, 180. [Google Scholar] [CrossRef]
- Alotaibi, M.H.; El-Hiti, G.A.; Hashim, H.; Hameed, A.S.; Ahmed, D.S.; Yousif, E. SEM analysis of the tunable honeycomb structure of irradiated poly(vinyl chloride) films doped with polyphosphate. Heliyon 2018, 4, e01013. [Google Scholar] [CrossRef] [Green Version]
- Mohamed, S.H.; Hameed, A.S.; El-Hiti, G.A.; Ahmed, D.S.; Kadhom, M.; Baashen, M.A.; Bufaroosha, M.; Ahmed, A.A.; Yousif, E. A process for the synthesis and use of highly aromatic organosilanes as additives for poly (vinyl chloride) films. Processes 2021, 9, 91. [Google Scholar] [CrossRef]
- Alotaibi, M.H.; El-Hiti, G.A.; Yousif, E.; Ahmed, D.S.; Hashim, H.; Hameed, A.S.; Ahmed, A. Evaluation of the use of polyphosphates as photostabilizers and in the formation of ball-like polystyrene materials. J. Polym. Res. 2019, 26, 161. [Google Scholar] [CrossRef]
- Shi, W.; Zhang, J.; Shi, X.; Jiang, G. Different photodegradation processes of PVC with different average degrees of polymerization. J. Appl. Polym. Sci. 2008, 107, 528–540. [Google Scholar] [CrossRef]
- Farjamia, M.; Vahid, V.; Moghadassi, A. Fabrication of a new emulsion polyvinyl chloride(EPVC) nanocomposite ultrafiltration membrane modified by para-hydroxybenzoate alumoxane (PHBA) additive to improve permeability and antifouling performance. Chem Eng. Res. Des. 2020, 153, 8–20. [Google Scholar] [CrossRef]
- Ali, M.M.; El-Hiti, G.A.; Yousif, E. Photostabilizing efficiency of poly(vinyl chloride) in the presence of organotin(IV) complexes as photostabilizers. Molecules 2016, 21, 1151. [Google Scholar] [CrossRef] [Green Version]
- Mohammed, R.; El-Hiti, G.A.; Ahmed, A.; Yousif, E. Poly(vinyl chloride) doped by 2-(4-isobutylphenyl)propanoate metal complexes: Enhanced resistance to UV irradiation. Arab. J. Sci. Eng. 2017, 42, 4307–4315. [Google Scholar] [CrossRef]
- Shyichuk, A.V.; White, J.R. Analysis of chain-scission and crosslinking rates on the photooxidation of polystyrene. J. Appl. Polym. Sci. 2000, 77, 3015–3023. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohammed, A.; Kadhom, M.; Yousif, E. Tin(IV) Compounds as Photo-Stabilizers for Irradiated Surfaces of Poly(Vinyl Chloride) Films. Surfaces 2021, 4, 279-292. https://doi.org/10.3390/surfaces4040023
Mohammed A, Kadhom M, Yousif E. Tin(IV) Compounds as Photo-Stabilizers for Irradiated Surfaces of Poly(Vinyl Chloride) Films. Surfaces. 2021; 4(4):279-292. https://doi.org/10.3390/surfaces4040023
Chicago/Turabian StyleMohammed, Alaa, Mohammed Kadhom, and Emad Yousif. 2021. "Tin(IV) Compounds as Photo-Stabilizers for Irradiated Surfaces of Poly(Vinyl Chloride) Films" Surfaces 4, no. 4: 279-292. https://doi.org/10.3390/surfaces4040023
APA StyleMohammed, A., Kadhom, M., & Yousif, E. (2021). Tin(IV) Compounds as Photo-Stabilizers for Irradiated Surfaces of Poly(Vinyl Chloride) Films. Surfaces, 4(4), 279-292. https://doi.org/10.3390/surfaces4040023







