Low-Temperature Synthesis Strategy for MoS2 Slabs Supported on TiO2(110)
Abstract
1. Introduction
2. Materials and Methods
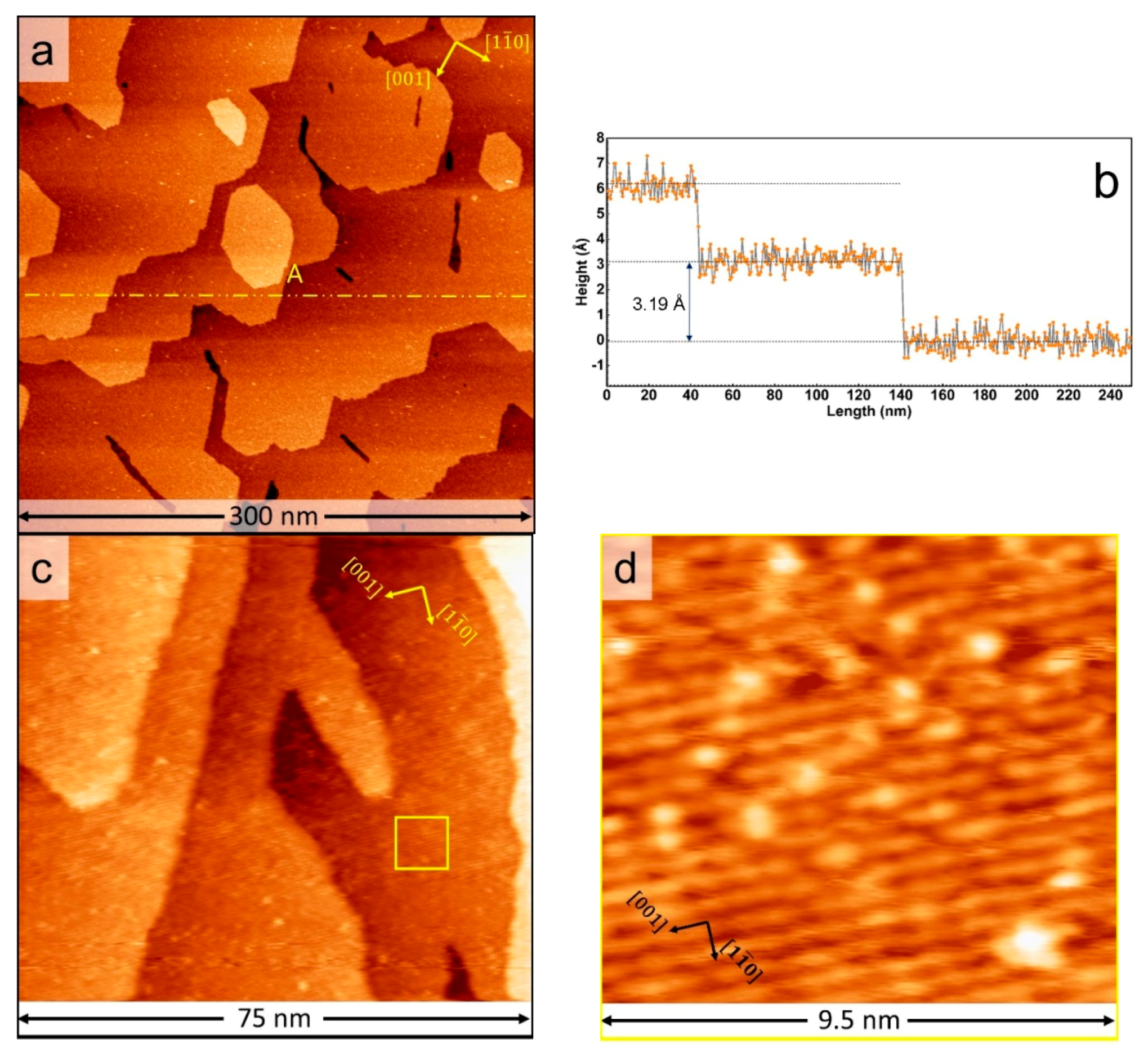
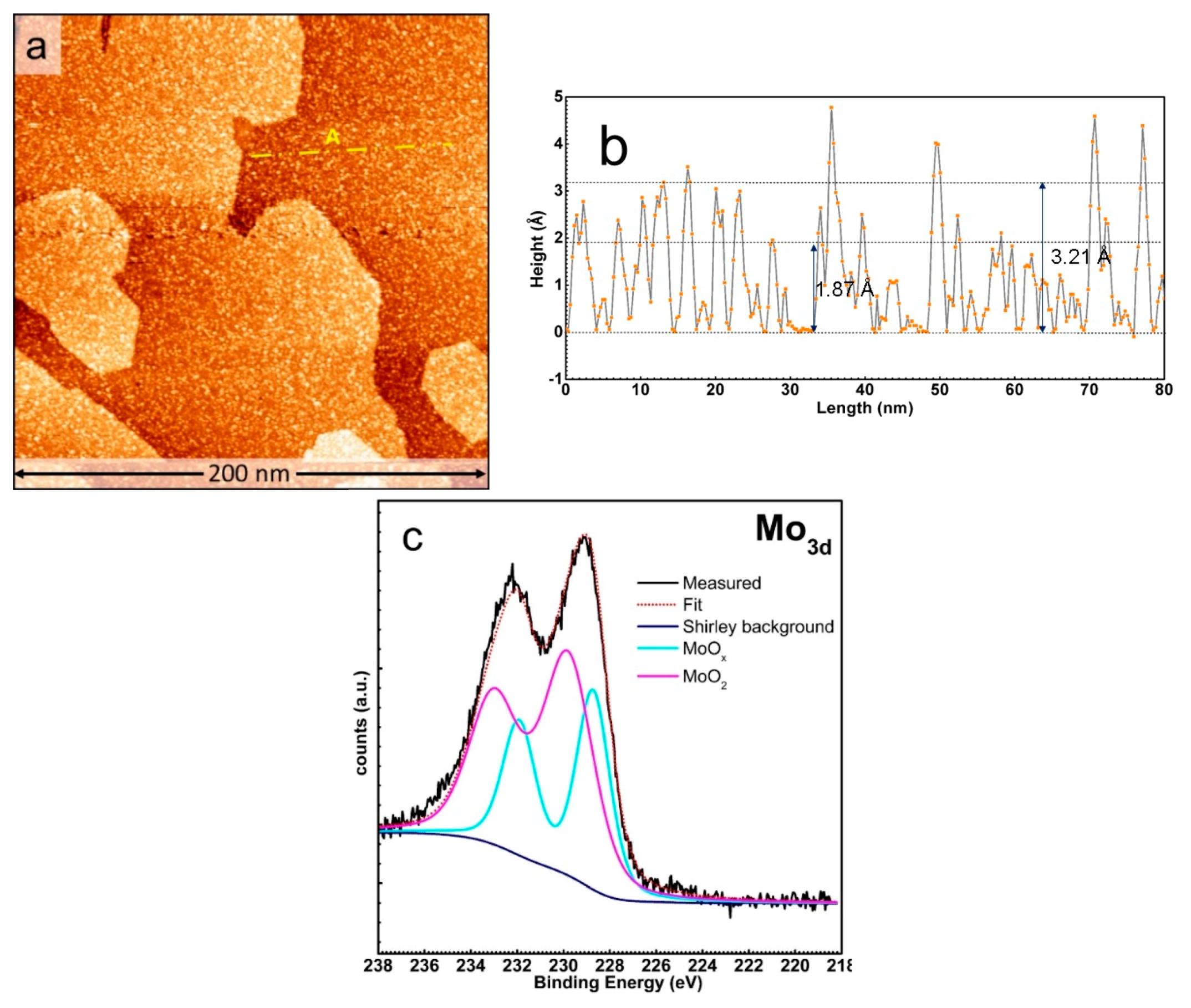
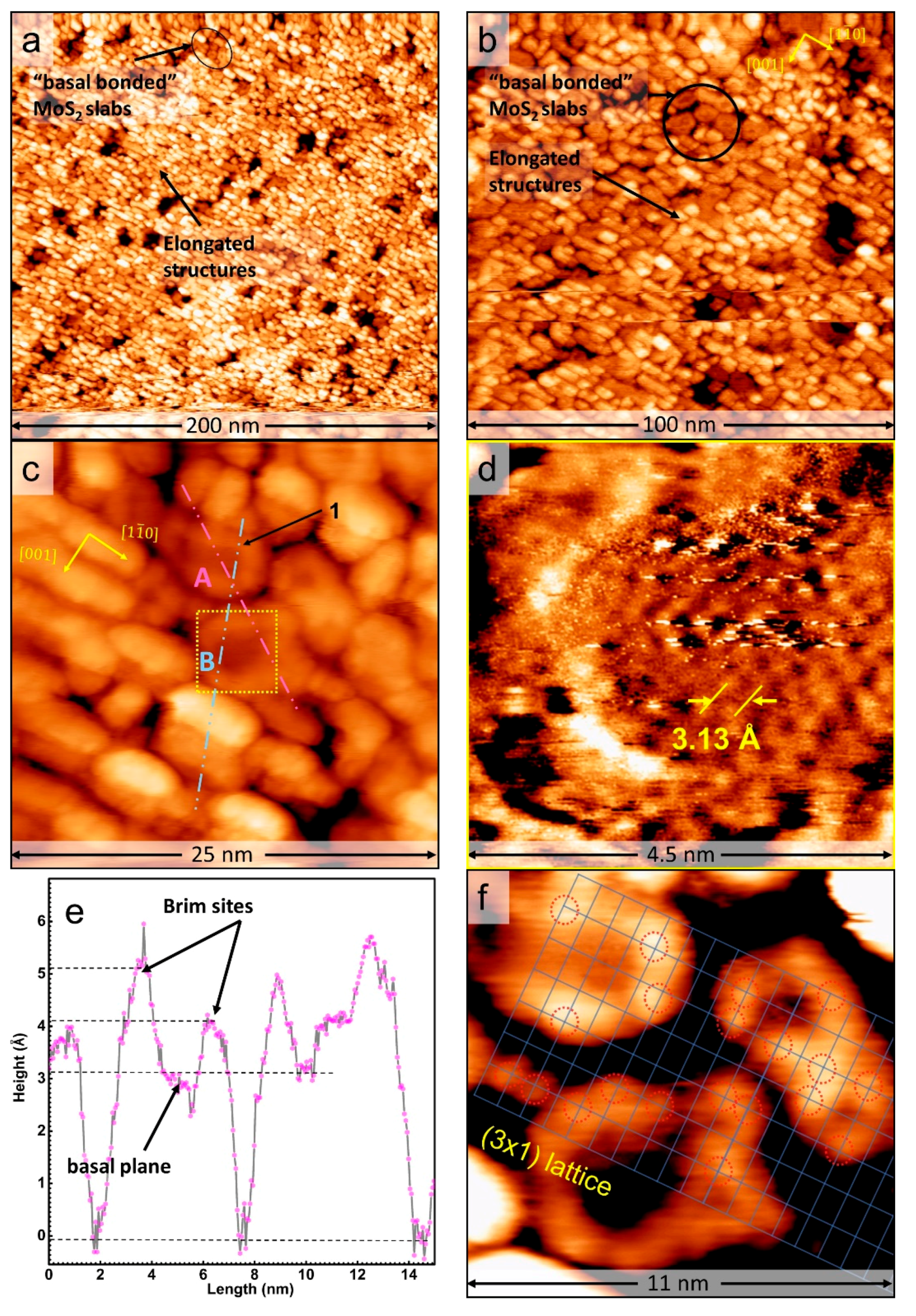
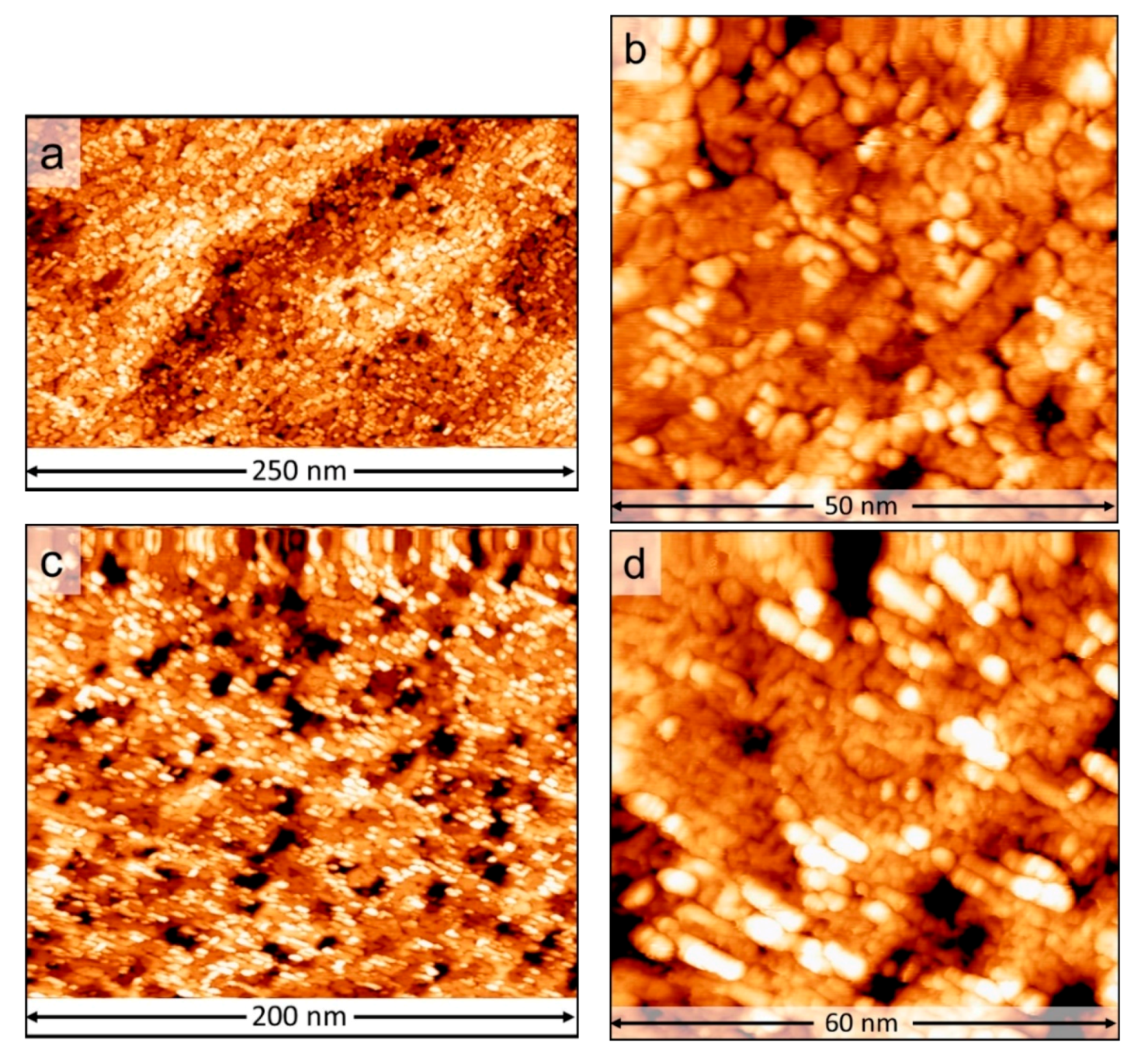
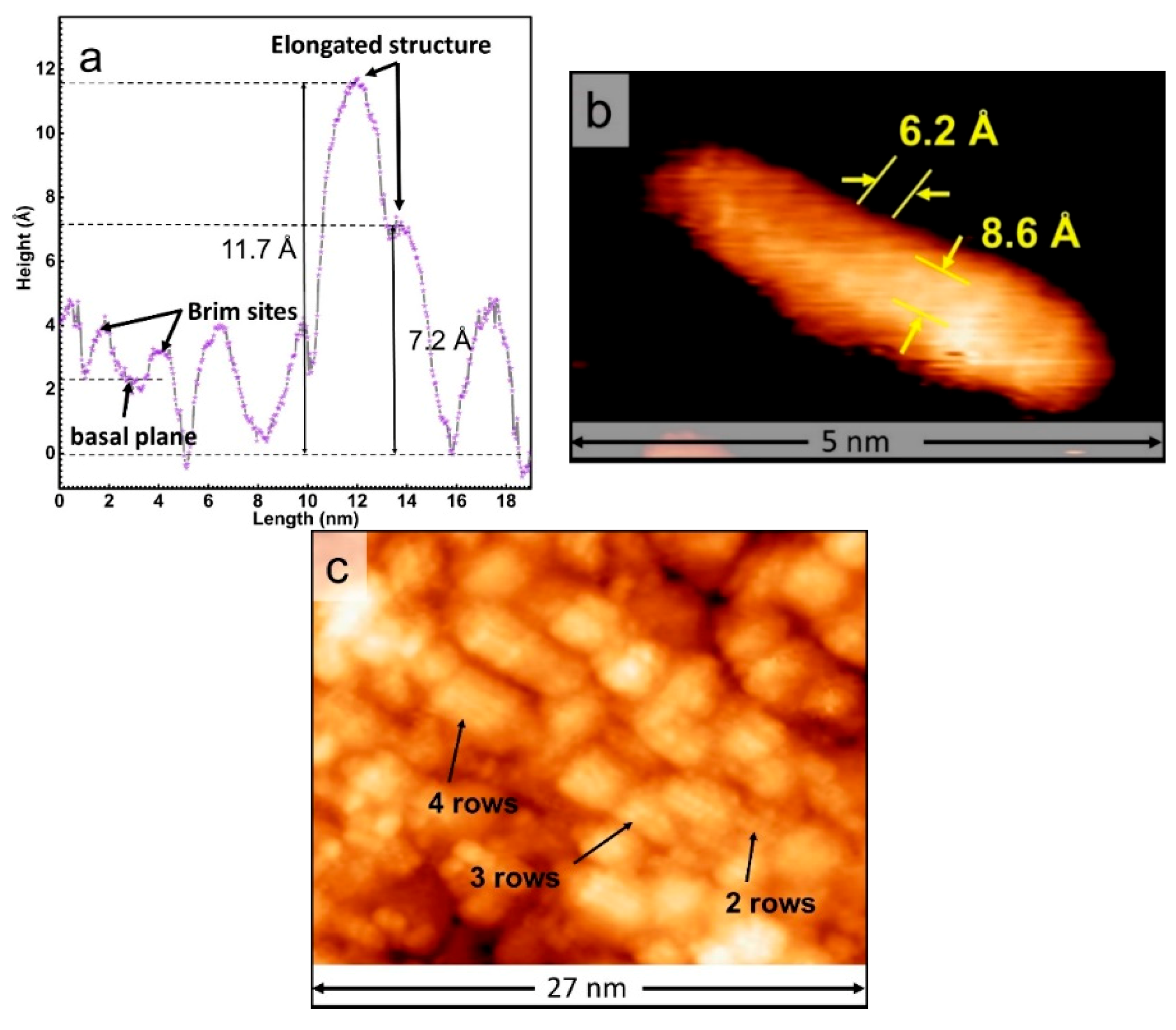
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zeng, H.; Dai, J.; Yao, W.; Xiao, D.; Cui, X. Valley polarization in MoS 2 Monolayers by optical pumping. Nat. Nanotechnol. 2012, 7, 490–493. [Google Scholar] [CrossRef]
- Chhowalla, M.; Shin, H.S.; Eda, G.; Li, L.J.; Loh, K.P.; Zhang, H. The chemistry of two-dimensional layered transition metal dichalcogenide nanosheets. Nat. Chem. 2013, 5, 263–275. [Google Scholar] [CrossRef]
- Jariwala, D.; Sangwan, V.K.; Lauhon, L.J.; Marks, T.J.; Hersam, M.C. Emerging device applications for semiconducting two-dimensional transition metal dichalcogenides. ACS Nano 2014, 8, 1102–1120. [Google Scholar] [CrossRef]
- Silambarasan, K.; Archana, J.; Harish, S.; Navaneethan, M.; Ganesh, R.S.; Ponnusamy, S.; Muthamizhchelvan, C.; Hara, K. One-Step Fabrication of Ultrathin Layered 1T@2H Phase MoS2 with High Catalytic Activity Based Counter Electrode for Photovoltaic Devices. J. Mater. Sci. Technol. 2020, 51, 94–101. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, H.; Qu, J.; Li, J. Two-dimensional layered MoS2: Rational design, properties and electrochemical applications. Energy Environ. Sci. 2016, 9, 1190–1209. [Google Scholar] [CrossRef]
- Kibsgaard, J.; Clausen, B.S.; Topsøe, H.; Lægsgaard, E.; Lauritsen, J.V.; Besenbacher, F. Scanning tunneling microscopy studies of TiO2-supported hydrotreating catalysts: Anisotropic particle shapes by edge-specific MoS2-support bonding. J. Catal. 2009, 263, 98–103. [Google Scholar] [CrossRef]
- Brunet, S.; Mey, D.; Pérot, G.; Bouchy, C.; Diehl, F. On the hydrodesulfurization of FCC gasoline: A review. Appl. Catal. A Gen. 2005, 278, 143–172. [Google Scholar] [CrossRef]
- Song, C.; Ma, X. New Design approaches to ultra-clean diesel fuels by deep desulfurization and deep dearomatization. Appl. Catal. B Environ. 2003, 41, 207–238. [Google Scholar] [CrossRef]
- Somorjai, G.A.; De Beer, V.H.J. Structure and Function of The Catalyst and The Promoter In Co—Mo Hydrodesuifurization Catalysts. Catal. Rev. 1989, 31, 1–41. [Google Scholar] [CrossRef]
- Liu, C.; Wang, L.; Tang, Y.; Luo, S.; Liu, Y.; Zhang, S.; Zeng, Y.; Xu, Y. Vertical single or few-layer MoS2 nanosheets rooting into TiO2 Nanofibers for highly efficient photocatalytic hydrogen evolution. Appl. Catal. B Environ. 2015, 164, 1–9. [Google Scholar] [CrossRef]
- Kooyman, P.J.; Hensen, E.J.M.; De Jong, A.M.; Niemantsverdriet, J.W.; Van Veen, J.A.R. The observation of nanometer-sized entities in sulphided mo-based catalysts on various supports. Catal. Lett. 2001, 74, 49–53. [Google Scholar] [CrossRef]
- Shido, T.; Prins, R. Why EXAFS Underestimated the size of small supported MoS2 particles. J. Phys. Chem. B 1998, 102, 8426–8435. [Google Scholar] [CrossRef]
- Galhenage, R.P.; Yan, H.; Rawal, T.B.; Le, D.; Brandt, A.J.; Maddumapatabandi, T.D.; Nguyen, N.; Rahman, T.S.; Chen, D.A. MoS2 nanoclusters grown on TiO2: Evidence for new adsorption sites at edges and sulfur vacancies. J. Phys. Chem. C 2019, 123, 7185–7201. [Google Scholar] [CrossRef]
- Liu, H.; Li, Y.; Xiang, M.; Zeng, H.; Shao, X. Single-layered MoS2 directly grown on rutile TiO2(110) for enhanced interfacial charge transfer. ACS Nano 2019, 13, 6083–6089. [Google Scholar] [CrossRef]
- Coulier, L.; De Beer, V.H.J.; Van Veen, J.A.R.; Niemantsverdriet, J.W. On the formation of cobalt-molybdenum sulfides in silica-supported hydrotreating model catalysts. Top. Catal. 2000, 13, 99–108. [Google Scholar] [CrossRef]
- Sanders, A.F.H.; De Jong, A.M.; De Beer, V.H.J.; Van Veen, J.A.R.; Niemantsverdriet, J.W. Formation of cobalt-molybdenum sulfides in hydrotreating catalysts: A surface science approach. Appl. Surf. Sci. 1999, 144, 380–384. [Google Scholar] [CrossRef]
- Escobar, J.; Toledo, J.A.; Cortés, M.A.; Mosqueira, M.L.; Pérez, V.; Ferrat, G.; López-Salinas, E.; Torres-García, E. Highly active sulfided CoMo catalyst on nano-structured TiO2. Catal. Today 2005, 106, 222–226. [Google Scholar] [CrossRef]
- Uetsuka, H.; Onishi, H.; Harada, Y.; Sakama, H.; Sakashita, Y. Microscope observation of MoS2 nanoparticles synthesized on rutile TiO2 single crystals. e-J. Surf. Sci. Nanotechnol. 2004, 2, 32–37. [Google Scholar] [CrossRef][Green Version]
- Uetsuka, H.; Onishi, H.; Ikeda, S.; Harada, Y.; Sakama, H.; Sakashita, Y. Atomic force microscope observation of MoS2 particles synthesized on mica, MoS2, and graphite. e-J. Surf. Sci. Nanotechnol. 2003, 1, 80–83. [Google Scholar] [CrossRef][Green Version]
- Leliveld, R.G.; Van Dillen, A.J.; Geus, J.W.; Koningsberger, D.C. A Mo-K edge XAFS study of the metal sulfide-support interaction in (Co)Mo supported alumina and titania catalysts. J. Catal. 1997, 165, 184–196. [Google Scholar] [CrossRef]
- Leliveld, R.G.; Van Dillen, A.J.; Geus, J.W.; Koningsberger, D.C. The sulfidation of γ-Alumina and titania supported (cobalt)molybdenum oxide catalysts monitored by EXAFS. J. Catal. 1997, 171, 115–129. [Google Scholar] [CrossRef]
- Hebenstreit, E.L.D.; Hebenstreit, W.; Diebold, U. Structures of sulfur on TiO2(1 1 0) determined by scanning tunneling microscopy, X-ray photoelectron spectroscopy and low-energy electron diffraction. Surf. Sci. 2001, 470, 347–360. [Google Scholar] [CrossRef]
- Hebenstreit, E.L.; Hebenstreit, W.; Geisler, H.; Thornburg, S.N.; Ventrice, C.A.; Hite, D.A.; Sprunger, P.T. Sulfur on TiO2(110) studied with resonant photoemission. Phys. Rev. B Condens. Matter Mater. Phys. 2000, 461, 87–97. [Google Scholar]
- Hebenstreit, E.L.D.; Hebenstreit, W.; Diebold, U. Adsorption of sulfur on TiO2(110) studied with STM, LEED and XPS: Temperature-dependent change of adsorption site combined with O-S exchange. Surf. Sci. 2000, 461, 87–97. [Google Scholar] [CrossRef]
- Hrbek, J.; Rodriguez, J.A.; Dvorak, J.; Jirsak, T. Sulfur adsorption and reaction with a Tio2(110) surface: O↔s exchange and sulfide formation. Collect. Czechoslov. Chem. Commun. 2001, 66, 1149–1163. [Google Scholar] [CrossRef]
- Herbschleb, C.T.; Van Der Tuijn, P.C.; Roobol, S.B.; Navarro, V.; Bakker, J.W.; Liu, Q.; Stoltz, D.; Cañas-Ventura, M.E.; Verdoes, G.; Van Spronsen, M.A.; et al. The ReactorSTM: Atomically resolved scanning tunneling microscopy under high-pressure, high-temperature catalytic reaction conditions. Rev. Sci. Instrum. 2014, 85, 83703. [Google Scholar] [CrossRef]
- Wagner, C.D. Sensitivity factors for XPS analysis of surface atoms. J. Electron Spectros. Relat. Phenom. 1983, 32, 99–102. [Google Scholar] [CrossRef]
- Rost, M.J.; Crama, L.; Schakel, P.; Van Tol, E.; Van Velzen-Williams, G.B.E.M.; Overgauw, C.F.; Ter Horst, H.; Dekker, H.; Okhuijsen, B.; Seynen, M.; et al. Scanning probe microscopes go video rate and beyond. Rev. Sci. Instrum. 2005, 76, 053710. [Google Scholar] [CrossRef]
- Rost, M.J.; van Baarle, G.J.C.; Katan, A.J.; van Spengen, W.M.; Schakel, P.; van Loo, W.A.; Oosterkamp, T.H.; Frenken, J.W.M. Video-rate scanning probe control challenges: Setting the stage for a microscopy revolution. Asian J. Control 2009, 11, 110–129. [Google Scholar] [CrossRef]
- Horcas, I.; Fernández, R.; Gómez-Rodríguez, J.M.; Colchero, J.; Gómez-Herrero, J.; Baro, A.M. WSXM: A software for scanning probe microscopy and a tool for nanotechnology. Rev. Sci. Instrum. 2007, 78, 013705. [Google Scholar] [CrossRef]
- Atuchin, V.V.; Kesler, V.G.; Pervukhina, N.V.; Zhang, Z. Ti 2p and O 1s core levels and chemical bonding in titanium-bearing oxides. J. Electron Spectros. Relat. Phenom. 2006, 152, 18–24. [Google Scholar] [CrossRef]
- Domenichini, B.; Pétigny, S.; Blondeau-Patissier, V.; Steinbrunn, A.; Bourgeois, S. Effect of the surface stoichiometry on the interaction of Mo with TiO2 (110). Surf. Sci. 2000, 468, 192–202. [Google Scholar] [CrossRef]
- Blondeau-Patissier, V.; Lian, G.D.; Domenichini, B.; Steinbrunn, A.; Bourgeois, S.; Dickey, E.C. Molybdenum thin-film growth on rutile titanium dioxide (1 1 0). Surf. Sci. 2002, 506, 119–128. [Google Scholar] [CrossRef]
- Prunier, J.; Domenichini, B.; Li, Z.; Møller, P.J.; Bourgeois, S. A photoemission study of molybdenum hexacarbonyl adsorption and decomposition on TiO2(1 1 0) surface. Surf. Sci. 2007, 601, 1144–1152. [Google Scholar] [CrossRef]
- Domenichini, B.; Petukhov, M.; Rizzi, G.A.; Sambi, M.; Bourgeois, S.; Granozzi, G. Epitaxial growth of molybdenum on TiO2(1 1 0). Surf. Sci. 2003, 544, 135–146. [Google Scholar] [CrossRef]
- Bruix, A.; Füchtbauer, H.G.; Tuxen, A.K.; Walton, A.S.; Andersen, M.; Porsgaard, S.; Besenbacher, F.; Hammer, B.; Lauritsen, J.V. In situ detection of active edge sites in single-layer MoS2 catalysts. ACS Nano 2015, 9, 9322–9330. [Google Scholar] [CrossRef]
- Weber, T.; Muijsers, J.C.; Van Wolput, J.H.M.C.; Verhagen, C.P.J.; Niemantsverdriet, J.W. Basic reaction steps in the sulfidation of crystalline MoO3 to MoS2, as studied by X-ray photoelectron and infrared emission spectroscopy. J. Phys. Chem. 1996, 100, 14144–14150. [Google Scholar] [CrossRef]
- Bremmer, G.M.; Van Haandel, L.; Hensen, E.J.M.; Frenken, J.W.M.; Kooyman, P.J. Instability of NiMoS2 and CoMoS2 Hydrodesulfurization catalysts at ambient conditions: A Quasi in situ high-resolution transmission electron microscopy and X-ray photoelectron spectroscopy study. J. Phys. Chem. C 2016, 120, 19204–19211. [Google Scholar] [CrossRef]
- Bremmer, G.M.; van Haandel, L.; Hensen, E.J.M.; Frenken, J.W.M.; Kooyman, P.J. The effect of oxidation and resulfidation on (Ni/Co)MoS2 hydrodesulfurisation catalysts. Appl. Catal. B Environ. 2019, 243, 145–150. [Google Scholar] [CrossRef]
- Diebold, U. Structure and properties of TiO2 Surfaces: A brief review. Appl. Phys. A Mater. Sci. Process. 2003, 76, 681–687. [Google Scholar] [CrossRef]
- Diebold, U.; Li, M.; Dulub, O.; Hebenstreit, E.L.D.; Hebenstreit, W. The relationship between bulk and surface properties of rutile TiO2(110). Surf. Rev. Lett. 2000, 7, 613–617. [Google Scholar] [CrossRef]
- Diebold, U.; Lehman, J.; Mahmoud, T.; Kuhn, M.; Leonardelli, G.; Hebenstreit, W.; Schmid, M.; Varga, P. Intrinsic defects on a TiO2(110)(1 × 1) surface and their reaction with oxygen: A scanning tunneling microscopy study. Surf. Sci. 1998, 411, 137–153. [Google Scholar] [CrossRef]
- Diebold, U.; Anderson, J.F.; Ng, K.O.; Vanderbilt, D. Evidence for the Tunneling site on transition-metal oxides: TiO2(110). Phys. Rev. Lett. 1996, 77, 1322–1325. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Hebenstreit, W.; Diebold, U.; Tyryshkin, A.M.; Bowman, M.K.; Dunham, G.G.; Henderson, M.A. The influence of the bulk reduction state on the surface structure and morphology of rutile TiO2(110) single crystals. J. Phys. Chem. B 2000, 104, 4944–4950. [Google Scholar] [CrossRef]
- Berkó, A.; Magony, A.; Szökõ, J. Characterization of Mo deposited on a TiO2(110) surface by scanning tunneling microscopy and auger electron spectroscopy. Langmuir 2005, 21, 4562–4570. [Google Scholar] [CrossRef]
- Kitchin, J.R.; Barteau, M.A.; Chen, J.G. A comparison of gold and molybdenum nanoparticles on TiO2(1 1 0) 1 × 2 reconstructed single crystal surfaces. Surf. Sci. 2003, 526, 323–331. [Google Scholar] [CrossRef]
- Campbell, C.T. Metal films and particles on oxide surfaces: Structural, electronic and chemisorptive properties. J. Chem. Soc. Faraday Trans. 1996, 92, 1435–1445. [Google Scholar] [CrossRef]
- Diebold, U.; Pan, J.M.; Madey, T.E. Ultrathin metal films on TiO2(110): Metal overlayer spreading and surface reactivity. Surf. Sci. 1993, 287, 896–900. [Google Scholar] [CrossRef]
- Diebold, U.; Pan, J.M.; Madey, T.E. Ultrathin metal film growth on TiO2(110): An overview. Surf. Sci. 1995, 331, 845–854. [Google Scholar] [CrossRef]
- Rodriguez, J.A.; Hrbek, J.; Chang, Z.; Dvorak, J.; Jirsak, T.; Maiti, A. Importance of O vacancies in the behavior of oxide surfaces: Adsorption of sulfur on (formula presented). Phys. Rev. B Condens. Matter Mater. Phys. 2002, 65. [Google Scholar] [CrossRef]
- Helveg, S.; Lauritsen, J.V.; Lægsgaard, E.; Stensgaard, I.; Nørskov, J.K.; Clausen, B.S.; Topsøe, H.; Besenbacher, F. Atomic-scale structure of single-layer MoS2 nanoclusters. Phys. Rev. Lett. 2000, 84, 951–954. [Google Scholar] [CrossRef]
- Lauritsen, J.V.; Kibsgaard, J.; Olesen, G.H.; Moses, P.G.; Hinnemann, B.; Helveg, S.; Nørskov, J.K.; Clausen, B.S.; Topsøe, H.; Lægsgaard, E.; et al. Location and Coordination of promoter atoms in Co- and Ni-promoted MoS2-based hydrotreating catalysts. J. Catal. 2007, 249, 220–233. [Google Scholar] [CrossRef]
- Han, X.; Tong, X.; Liu, X.; Chen, A.; Wen, X.; Yang, N.; Guo, X.Y. Hydrogen evolution reaction on hybrid catalysts of vertical MoS2 nanosheets and hydrogenated graphene. ACS Catal. 2018, 8, 1828–1836. [Google Scholar] [CrossRef]
- Rodriguez, J.A.; Chaturvedi, S.; Kuhn, M.; Hrbek, J. Reaction of H2S and S2 with metal/oxide surfaces: Band-gap size and chemical reactivity. J. Phys. Chem. B 1998, 102, 5511–5519. [Google Scholar] [CrossRef]
- Hartmann, N.; Biener, J.; Madix, R.J. Monitoring the interaction of sulfur dioxide with a TiO2(110) surface at 300 K by scanning tunneling microscopy. Surf. Sci. 2002, 505, 81–92. [Google Scholar] [CrossRef]
- Le, D.; Sun, D.; Lu, W.; Aminpour, M.; Wang, C.; Ma, Q.; Rahman, T.S.; Bartels, L. Growth of aligned Mo6S6 nanowires on Cu(111). Surf. Sci. 2013, 611, 1–4. [Google Scholar] [CrossRef]
- Tiwari, R.K.; Yang, J.; Saeys, M.; Joachim, C. Surface Reconstruction of MoS2 to Mo2S3. Surf. Sci. 2008, 602, 2628–2633. [Google Scholar] [CrossRef]
- Sun, D.; Lu, W.; Le, D.; Ma, Q.; Aminpour, M.; Alcántara Ortigoza, M.; Bobek, S.; Mann, J.; Wyrick, J.; Rahman, T.S.; et al. An MoS x structure with high affinity for adsorbate interaction. Angew. Chem. Int. Ed. 2012, 51, 10284–10288. [Google Scholar] [CrossRef]
- Kibsgaard, J.; Tuxen, A.; Levisen, M.; Lægsgaard, E.; Gemming, S.; Seifert, G.; Lauritsen, J.V.; Besenbacher, F. Atomic-scale structure of Mo6S6 nanowires. Nano Lett. 2008, 8, 3928–3931. [Google Scholar] [CrossRef]
- Bao, Y.; Yang, M.; Tan, S.J.R.; Liu, Y.P.; Xu, H.; Liu, W.; Nai, C.T.; Feng, Y.P.; Lu, J.; Loh, K.P. Substoichiometric molybdenum sulfide phases with catalytically active basal planes. J. Am. Chem. Soc. 2016, 138, 14121–14128. [Google Scholar] [CrossRef]
- Chen, G.; Song, X.; Guan, L.; Cha, J.; Zhang, H.; Wang, S.; Pan, J.; Tao, J. Defect assisted coupling of a MoS2/TiO2 interface and tuning of its electronic structure. Nanotechnology 2016, 27, 355203. [Google Scholar] [CrossRef]
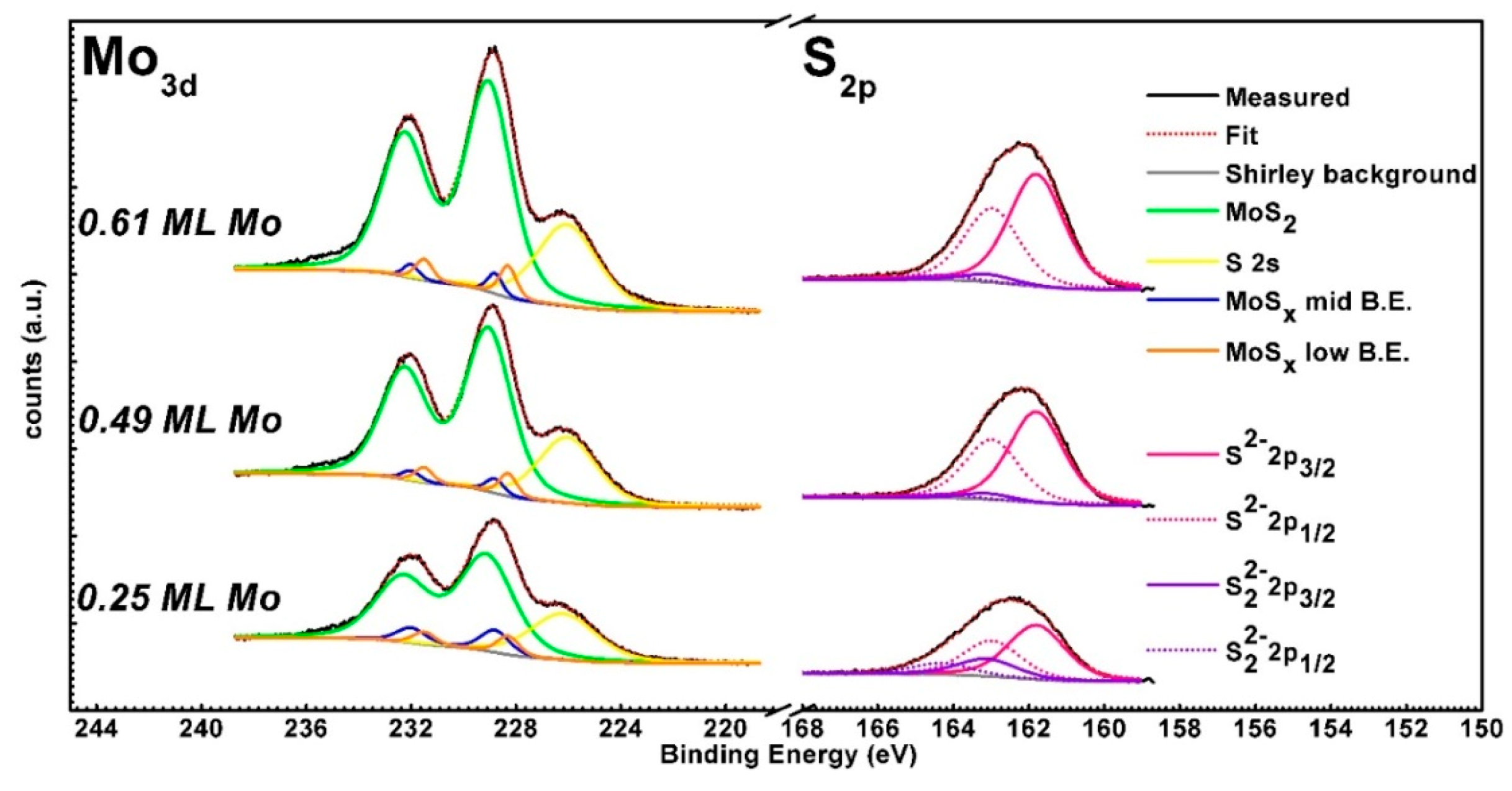

| Components | Mo Metal | MoOx | MoO2 | MoS2 |
|---|---|---|---|---|
| Binding energy (eV) | 228.0 | 228.7 | 229.8 | 229.2 |
| ΔBE * (eV) | 3.15 | 3.15 | 3.15 | 3.15 |
| Components | MoSx | S 2s | S2− 2p | S22− 2p |
| Binding energy (eV) | 228.3, 228.8 | 226.2 | 161.8 | 163.1 |
| ΔBE * (eV) | 3.15 | 1.16 | 1.16 |
| Mo Coverage (ML) | Mo:S | MoS2:MoSx | S2−:S22− |
|---|---|---|---|
| 0.25 | 1:2.31 | 1:0.241 | 1:0.329 |
| 0.49 | 1:2.22 | 1:0.096 | 1:0.056 |
| 0.61 | 1:2.24 | 1:0.093 | 1:0.062 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prabhu, M.K.; Groot, I.M.N. Low-Temperature Synthesis Strategy for MoS2 Slabs Supported on TiO2(110). Surfaces 2020, 3, 605-621. https://doi.org/10.3390/surfaces3040041
Prabhu MK, Groot IMN. Low-Temperature Synthesis Strategy for MoS2 Slabs Supported on TiO2(110). Surfaces. 2020; 3(4):605-621. https://doi.org/10.3390/surfaces3040041
Chicago/Turabian StylePrabhu, Mahesh K., and Irene M. N. Groot. 2020. "Low-Temperature Synthesis Strategy for MoS2 Slabs Supported on TiO2(110)" Surfaces 3, no. 4: 605-621. https://doi.org/10.3390/surfaces3040041
APA StylePrabhu, M. K., & Groot, I. M. N. (2020). Low-Temperature Synthesis Strategy for MoS2 Slabs Supported on TiO2(110). Surfaces, 3(4), 605-621. https://doi.org/10.3390/surfaces3040041






