Diazonium Gold Salts as Novel Surface Modifiers: What Have We Learned So Far?
Abstract
1. Introduction
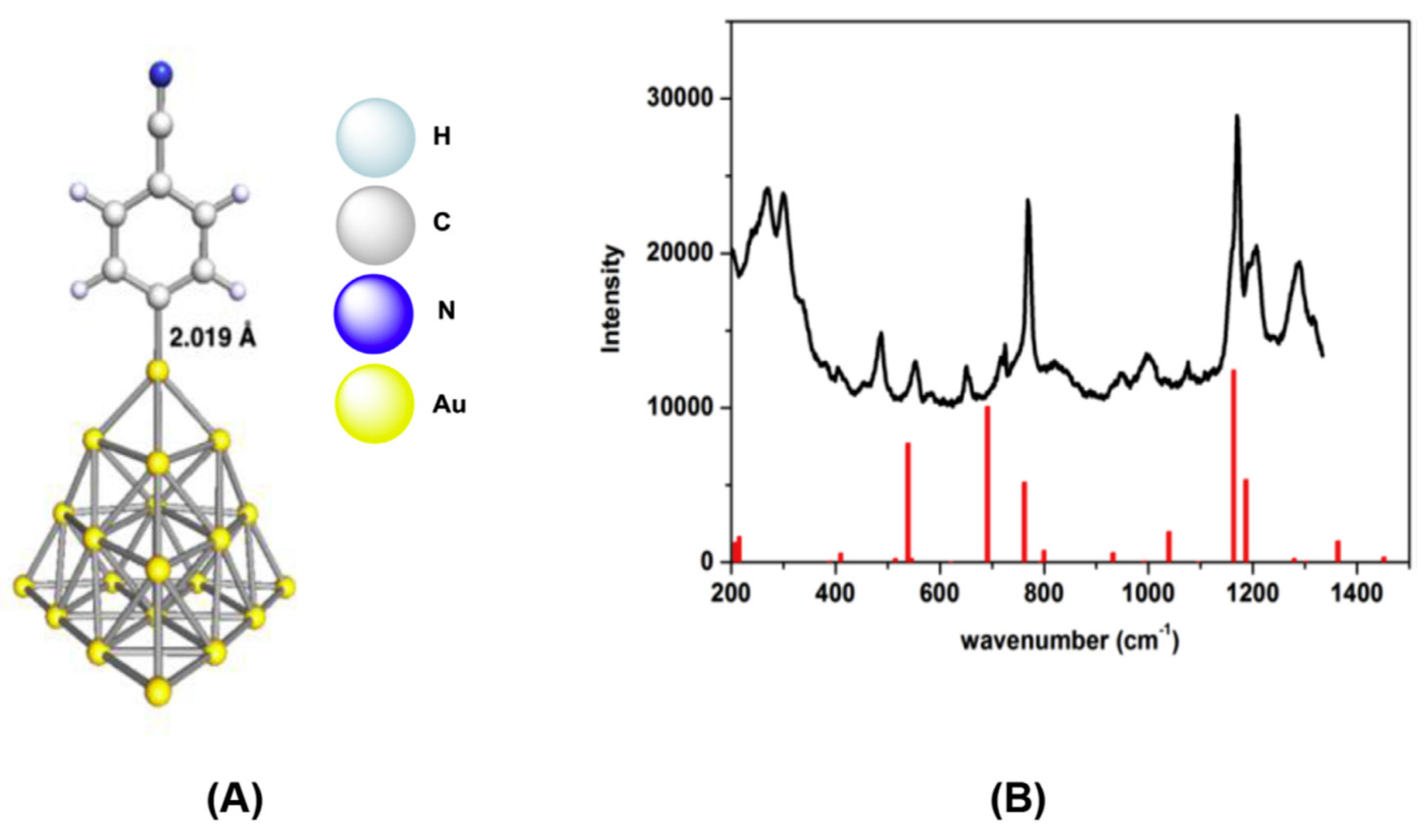
2. Metal Nanoparticles Modification with Aryls from Aryldiazonium Salts
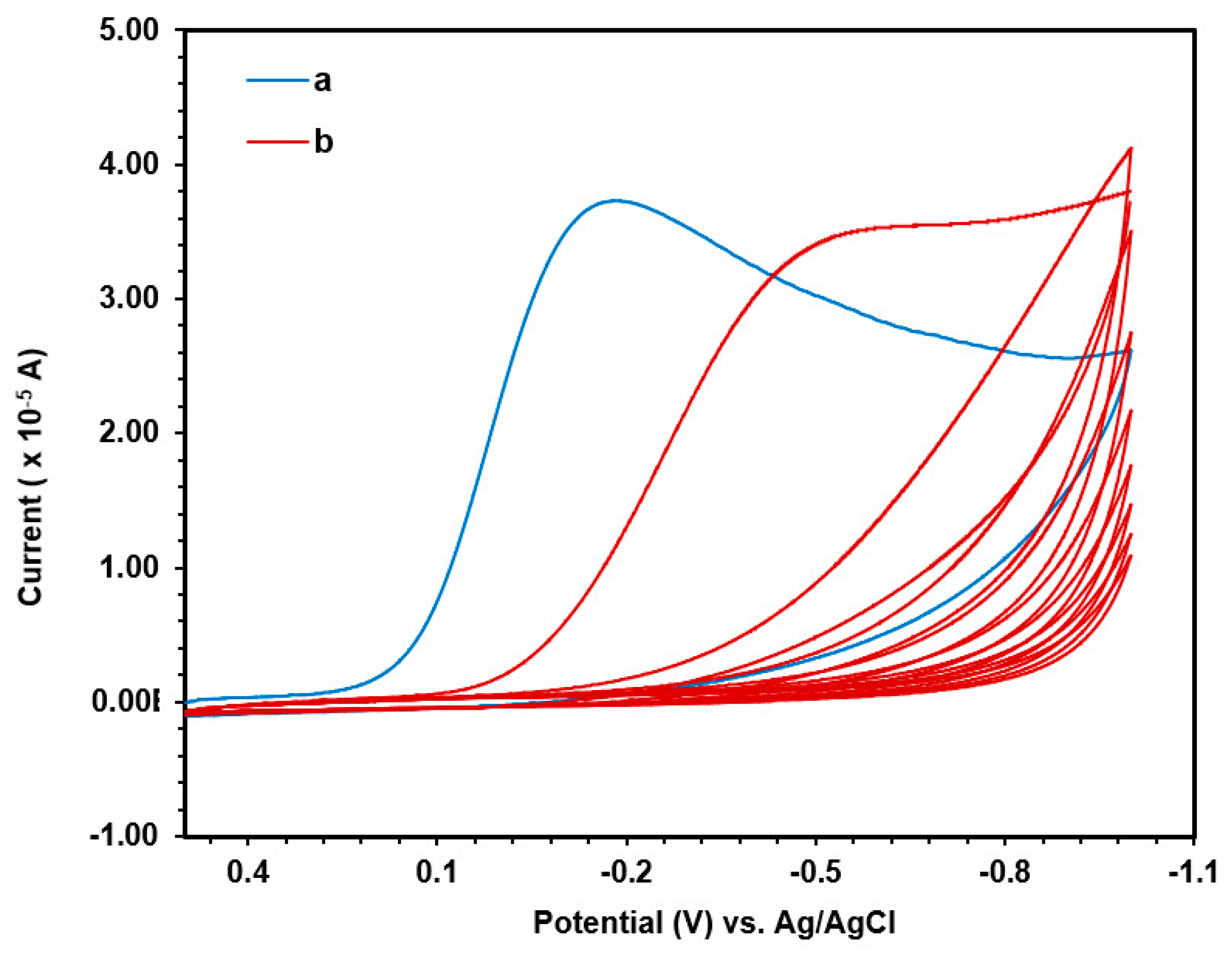
3. Aryldiazonium Gold Salts for Flat and Nanoparticulate Surface Modification
4. Applications
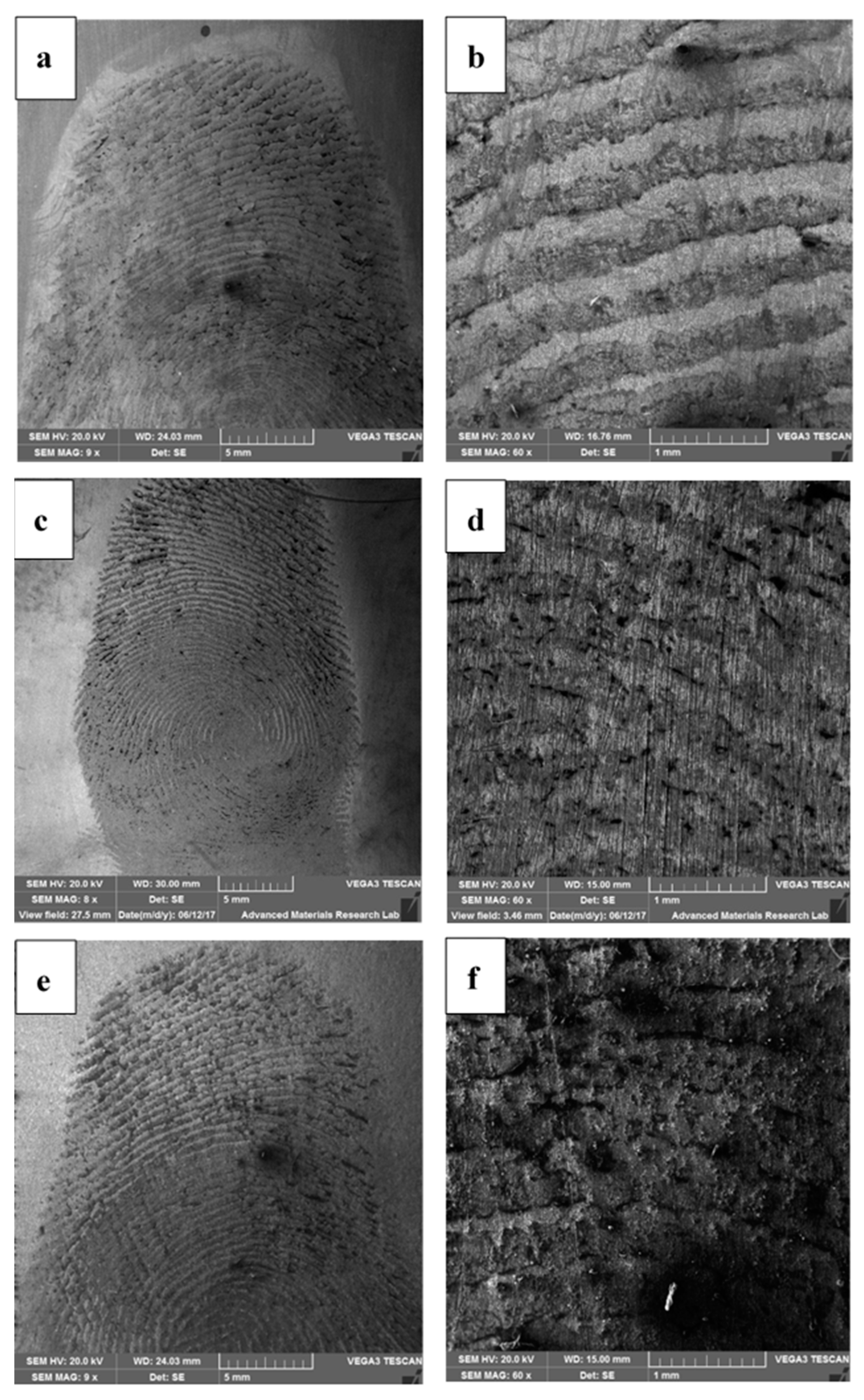
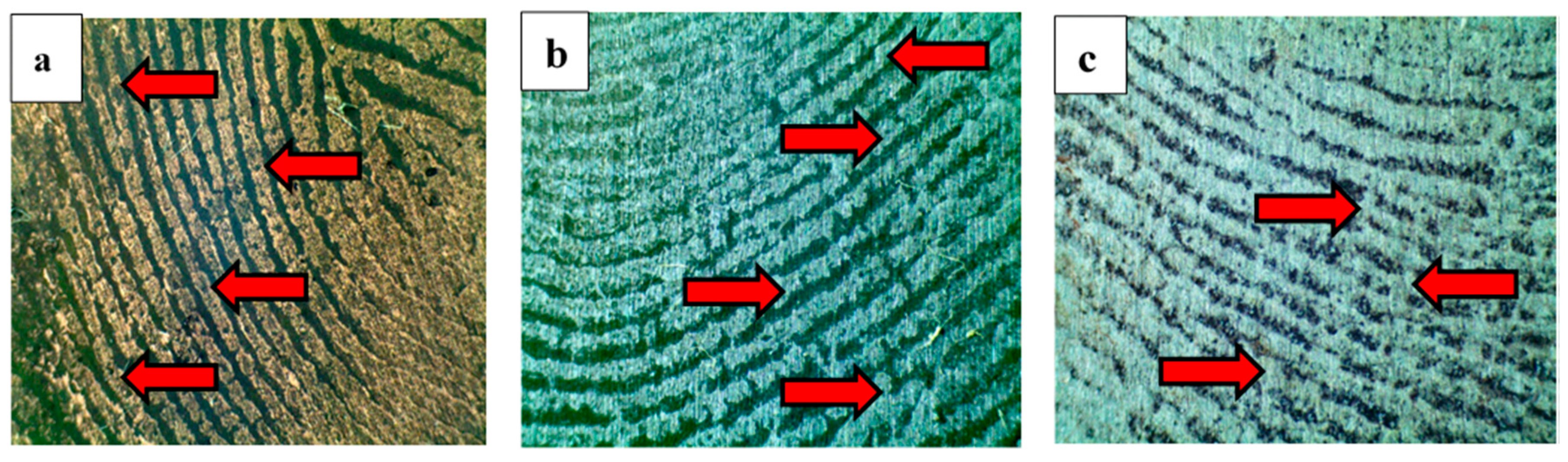
4.1. Forensic Nanotechnology
4.2. Catalysis
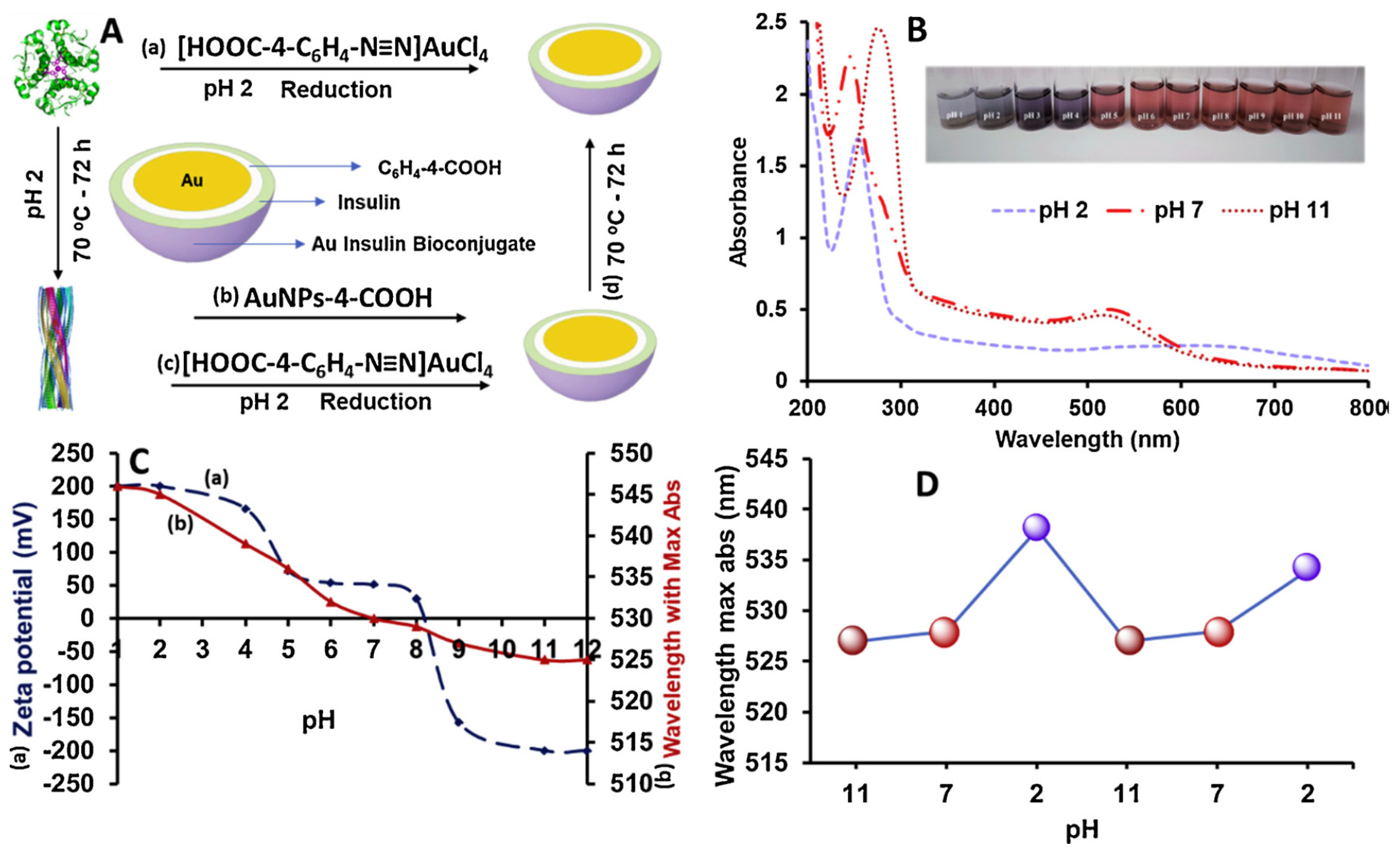
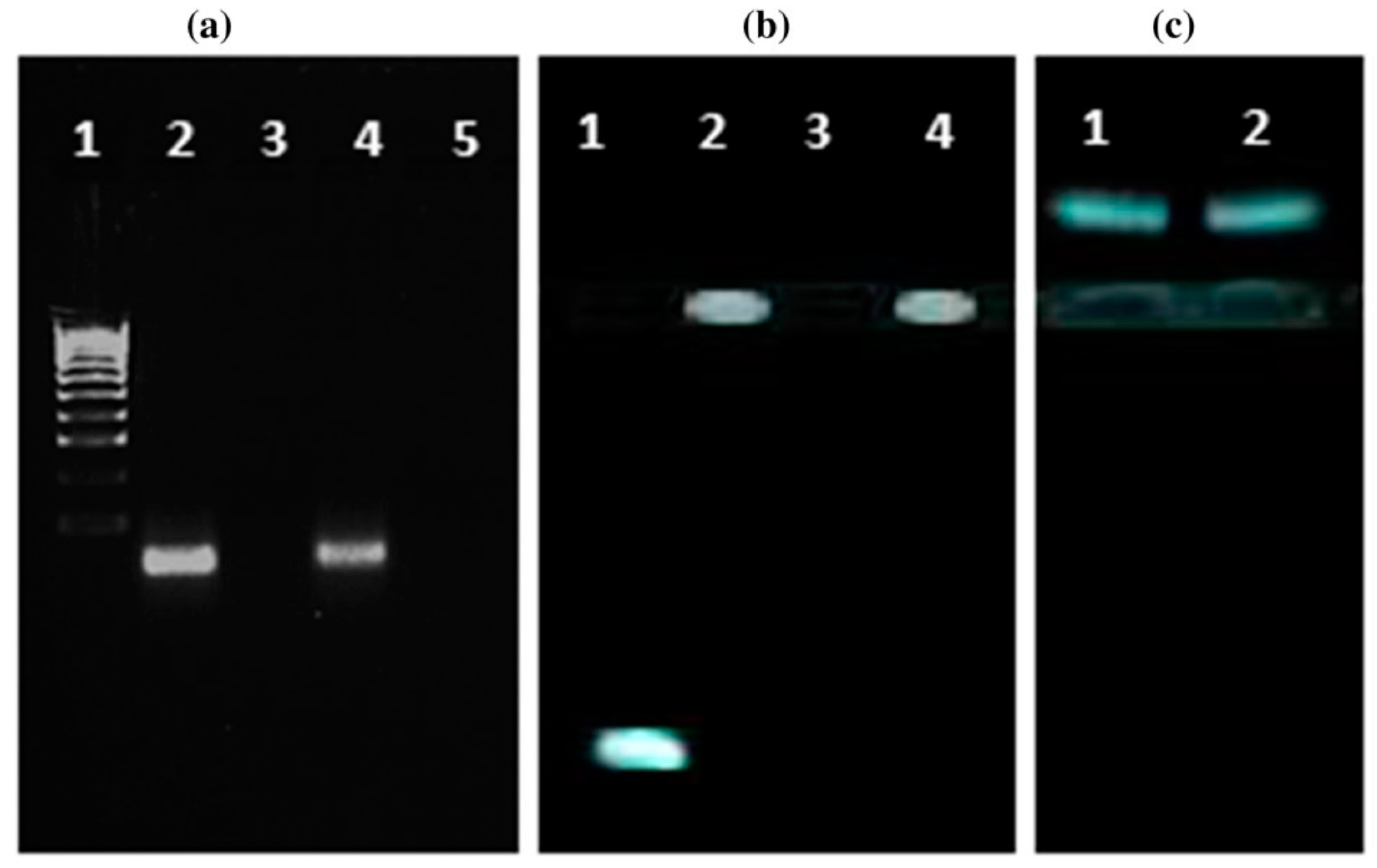
4.3. Nanomedicine Engineering
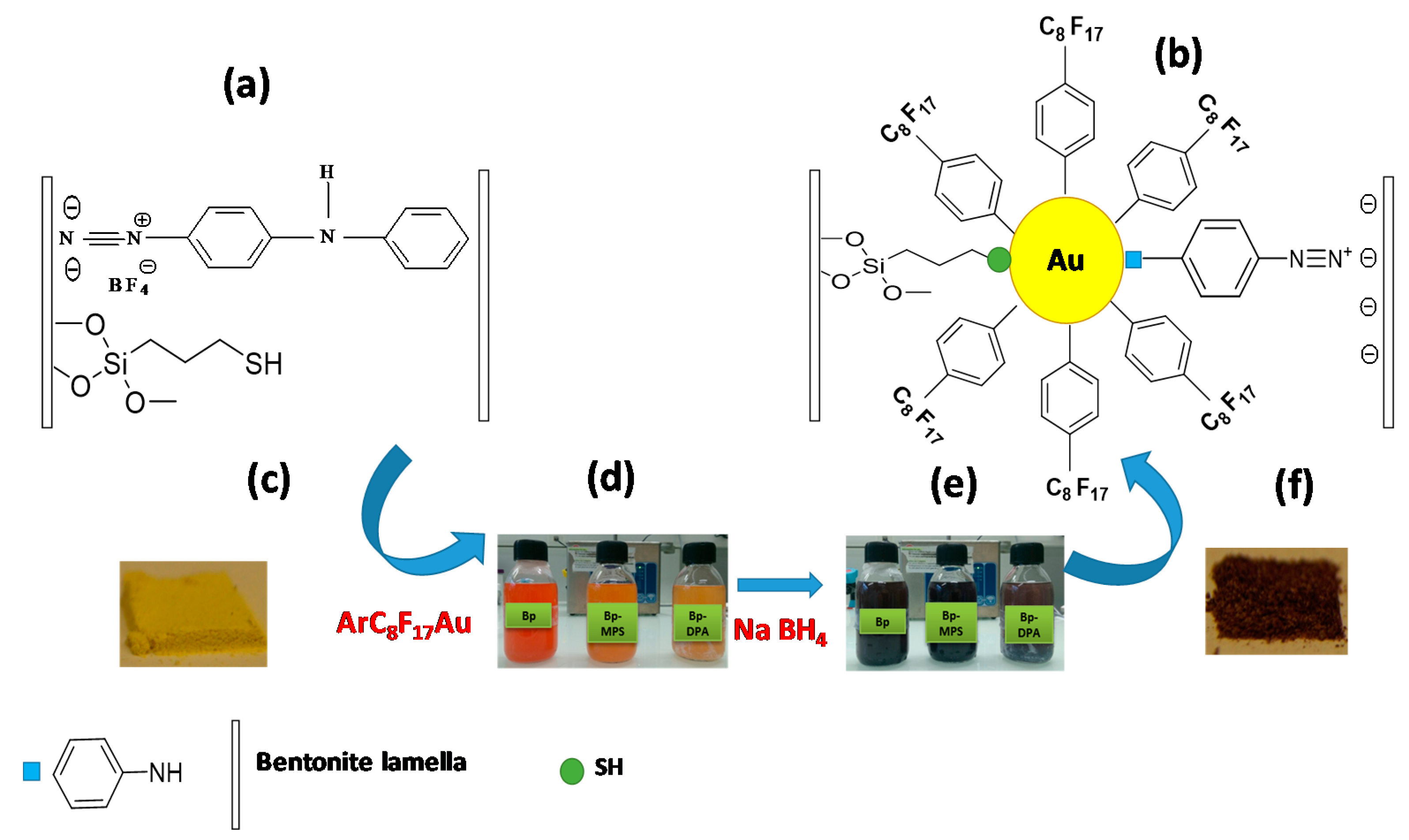
4.4. Energy Sector
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Delamar, M.; Hitmi, R.; Jean Pinson, J.; Saveant, J.M. Covalent modification of carbon surfaces by grafting of functionalized aryl radicals produced from electrochemical reduction of diazonium salts. J. Am. Chem. Soc. 1992, 114, 5883–5884. [Google Scholar] [CrossRef]
- Chehimi, M.M. Aryl Diazonium Salts: New Coupling Agents and Surface Science; Editorial Wiley-VCH: New York, NY, USA, 2012; ISBN 978-3-527-32998-4. [Google Scholar]
- Mohamed, A.A.; Salmi, Z.; Dahoumane, S.A.; Ahmed Mekki, A.; Benjamin Carbonnier, B.; Chehimi, M.M. Functionalization of nanomaterials with aryldiazonium salts. Adv. Colloid Interface Sci. 2015, 225, 16–36. [Google Scholar] [CrossRef] [PubMed]
- Doctorovich, F.; Escola, N.; Trapani, C.; Estrin, D.A.; Lebrero, M.C.G.; Turjanski, A.G. Stabilization of aliphatic and aromatic diazonium ions by coordination: An experimental and theoretical study. Organometallics 2000, 19, 3810–3817. [Google Scholar] [CrossRef]
- Kesavan, S.; John, S.A. Fabrication of aminotriazole grafted gold nanoparticles films on glassy carbon electrode and its application towards the simultaneous determination of theophylline and uric acid. Sens. Actuat. B-Chem. 2014, 205, 352–362. [Google Scholar] [CrossRef]
- Neal, S.N.; Orefuwa, S.A.; Overton, A.T.; Staples, R.J.; Mohamed, A.A. Synthesis of diazonium tetrachloroaurate(III) precursors for surface grafting. Inorganics 2013, 1, 70–84. [Google Scholar] [CrossRef]
- Filimonov, V.D.; Trusova, M.; Postnikov, P.; Krasnokutskaya, E.A.; Lee, Y.M.; Hwang, H.Y.; Kim, H.; Chi, K.W. Unusually stable, versatile, and pure arenediazonium tosylates: Their preparation, structures, and synthetic applicability. Org. Lett. 2008, 10, 3961–3964. [Google Scholar] [CrossRef]
- Overton, A.T.; Mohamed, A.A. Gold(III) diazonium complexes for electrochemical reductive grafting. Inorg. Chem. 2012, 51, 5500–5502. [Google Scholar] [CrossRef]
- Orefuwa, S.A.; Ravanbakhsh, M.; Sabine, N.; Neal, S.N.; Julie, B.; King, J.B.; Ahmed, A.; Mohamed, A.A. Robust organometallic gold nanoparticles. Organometallics 2014, 33, 439–442. [Google Scholar] [CrossRef]
- Enciso, A.E.; Doni, G.; Nifosì, R.; Palazzesi, F.; Gonzalez, R.; Ellsworth, A.A.; Coffer, J.L.; Walker, A.V.; Pavan, G.M.; Ahmed, A.; et al. Facile synthesis of stable, water soluble, dendron-coated gold nanoparticles. Nanoscale 2017, 9, 3128–3132. [Google Scholar] [CrossRef]
- Bell, K.J.; Brooksby, P.A.; Polson, M.I.J.; Downard, A.J. Evidence for covalent bonding of aryl groups to MnO2 nanorods from diazonium-based grafting. Chem. Commun. 2014, 50, 13687–13690. [Google Scholar] [CrossRef]
- Atmane, Y.A.; Sicard, L.; Lamouri, A.; Pinson, J.; Sicard, M.; Masson, C.; Nowak, S.; Decorse, P.; Piquemal, J.Y.; Galtayries, A.; et al. Functionalization of aluminum nanoparticles using a combination of aryl diazonium salt chemistry and iniferter method. J. Phys. Chem. C 2013, 117, 26000–26006. [Google Scholar] [CrossRef]
- Miyachi, M.; Yamamoto, Y.; Yamanoi, Y.; Minoda, A.; Oshima, S.; Kobori, Y.; Nishihara, H. Synthesis of diazenido-ligated vanadium nanoparticles. Langmuir 2013, 29, 5099–5103. [Google Scholar] [CrossRef] [PubMed]
- Griffete, N.; Herbst, F.; Pinson, J.; Ammar, S.; Mangeney, C. Preparation of water-soluble magnetic nanocrystals using aryl diazonium salt chemistry. J. Am. Chem. Soc. 2011, 133, 1646–1649. [Google Scholar] [CrossRef] [PubMed]
- Anariba, F.; DuVall, S.; McCreery, R. Mono- and multilayer formation by diazonium reduction on carbon surfaces monitored with atomic force microscopy “scratching”. Anal. Chem. 2003, 75, 3837–3844. [Google Scholar] [CrossRef] [PubMed]
- Allongue, P.; de Villeneuve, C.; Cherouvrier, G.; Cortes, R.; Bernard, M. Phenyl layers on H/Si(111) by electrochemical reduction of diazonium salts: Monolayer versus multilayer formation. J. Electroanal. Chem. 2003, 550–551, 161–174. [Google Scholar] [CrossRef]
- Kariuki, J.; McDermott, M. Formation of multilayers on glassy carbon electrodes via the reduction of diazonium salts. Langmuir 2001, 17, 5947–5951. [Google Scholar] [CrossRef]
- Brooksby, P.; Downard, A. Multilayer nitroazobenzene films covalently attached to carbon. An AFM and electrochemical study. J. Phys. Chem. B 2005, 109, 8791–8798. [Google Scholar] [CrossRef]
- Liu, G.; Bocking, T.; Gooding, J.J. Diazonium salts: Stable monolayers on gold electrodes for sensing applications. J. Electroanal. Chem. 2007, 600, 335–344. [Google Scholar] [CrossRef]
- Finklea, H.O.; Avery, S.; Lynch, M.; Furtsch, T. Blocking oriented monolayers of alkyl mercaptan on gold electrodes. Langmuir 1987, 3, 409–413. [Google Scholar] [CrossRef]
- Isah, S.; Workie, B.; Marcano, A.; Panicker, S.; Mohammed, A. Electrochemical reductive grafting and photothermal properties studies of bis(diazonium) gold(III) salts, Abstract of Papers. In Proceedings of the American Chemical Society 256th National Meeting, Boston, MA, USA, 19–23 August 2018. [Google Scholar]
- Workie, B.; Mohammed, A. Electrochemical Reductive Grafting of studies of diazonium Gold(III) salts on glassy carbon electrodes, Abstract of Papers. In Proceedings of the American Chemical Society 254th National Meeting, Washington, DC, USA, 20–24 August 2017. [Google Scholar]
- Neal, S.; Workie, B.; McCandless, B.; Mohammed, A. Surface morphology of the grafted perfluorinated gold-organic Film, Abstract of Papers. In Proceedings of the American Chemical Society 252nd National Meeting, Philadelphia, PA, USA, 21–25 August 2016. [Google Scholar]
- Neal, S.; Workie, B.; McCandless, B.; Mohamed, A.A. Gold-organic thin films from the reductive grafting of diazonium gold(III) salts. J. Electroanal. Chem. 2015, 757, 73–79. [Google Scholar] [CrossRef]
- Fernandez-Bravo, A.; Sivakumar, P.; Melikechi, N.; Mohamed, A.A. Femtosecond laser ablation synthesis of aryl functional group substituted gold nanoparticles. J. Nanosci. Nanotechnol. 2017, 17, 2852–2856. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.A.; Neal, S.N.; Atallah, B.; AlBab, N.D.; Alawadhi, H.A.; Pajouhafsar, Y.; Abdou, H.E.; Workie, B.; Sahle-Demessie, E.; Han, C.; et al. Synthesis of gold organometallics at the nanoscale. J. Organomet. Chem. 2018, 877, 1–11. [Google Scholar] [CrossRef]
- Brunelle, E.; Huynh, C.; Le, A.M.; Halamkova, L.; Agudelo, J.; Halamek, J. New horizons for ninhydrin: Colorimetric determination of gender from fingerprints. Anal. Chem. 2016, 88, 2413–2420. [Google Scholar] [CrossRef] [PubMed]
- Peng, D.; Wu, X.; Liu, X.; Huang, M.; Wang, D.; Liu, R. Color-tunable binuclear (Eu, Tb) nanocomposite powder for the enhanced development of latent fingerprints based on electrostatic interactions. ACS Appl. Mater. Interfaces 2018, 10, 32859–32866. [Google Scholar] [CrossRef]
- Chen, Y.; Kuo, S.; Tsai, W.; Ke, C.; Liao, C.; Chen, C.; Wang, Y.; Chen, H.; Chan, Y. Dual colorimetric and fluorescent imaging of latent fingerprints on both porous and nonporous surfaces with near-infrared fluorescent semiconducting polymer dots. Anal. Chem. 2016, 88, 11616–11623. [Google Scholar] [CrossRef]
- Yoon, J.; Jin, Y.; Sakaguchi, T.; Kwak, G. Visualization of sweat fingerprints on various surfaces using a conjugated polyelectrolyte. ACS Appl. Mater. Interfaces 2016, 8, 24025–24029. [Google Scholar] [CrossRef]
- Xie, H.; Wen, Q.; Huang, H.; Sun, T.; Li, P.; Li, Y.; Yu, X.; Wang, Q. Synthesis of bright up conversion submicrocrystals for high-contrast imaging of latent-fingerprints with cyanoacrylate fuming. RSC Adv. 2015, 5, 79525–79531. [Google Scholar] [CrossRef]
- Williams, G.; McMurray, N. Latent fingermark visualisation using a scanning Kelvin probe. Forensic Sci. Int. 2007, 167, 102–109. [Google Scholar] [CrossRef]
- Davis, L.W.L.; Kelly, P.F.; King, R.S.P.; Bleay, S.M. Visualisation of latent fingermarks on polymer banknotes using copper vacuum metal deposition: A preliminary study. Forensic Sci. Int. 2016, 266, e86–e92. [Google Scholar] [CrossRef]
- Bond, J.W.; Phil, D. Visualization of latent fingerprint corrosion of metallic surfaces. J. Forensic Sci. 2008, 53, 812–822. [Google Scholar] [CrossRef]
- Mesnage, A.; Lefèvre, X.; Jégou, P.; Deniau, G.; Palacin, S. Spontaneous grafting of diazonium salts: Chemical mechanism on metallic surfaces. Langmuir 2012, 28, 11767–11778. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.A.L.; Alawadhi, A.H.; Park, J.; Abdou, H.E.; Mohamed, A.A. Evaluation of diazonium gold(III) salts in forensic chemistry: Latent fingerprint development on metal surfaces. Forensic Chem. 2019, 13, 100144. [Google Scholar] [CrossRef]
- Beresford, A.L.; Hillman, A.R. Electrochromic enhancement of latent fingerprints on stainless steel surfaces. Anal. Chem. 2010, 82, 483–486. [Google Scholar] [CrossRef] [PubMed]
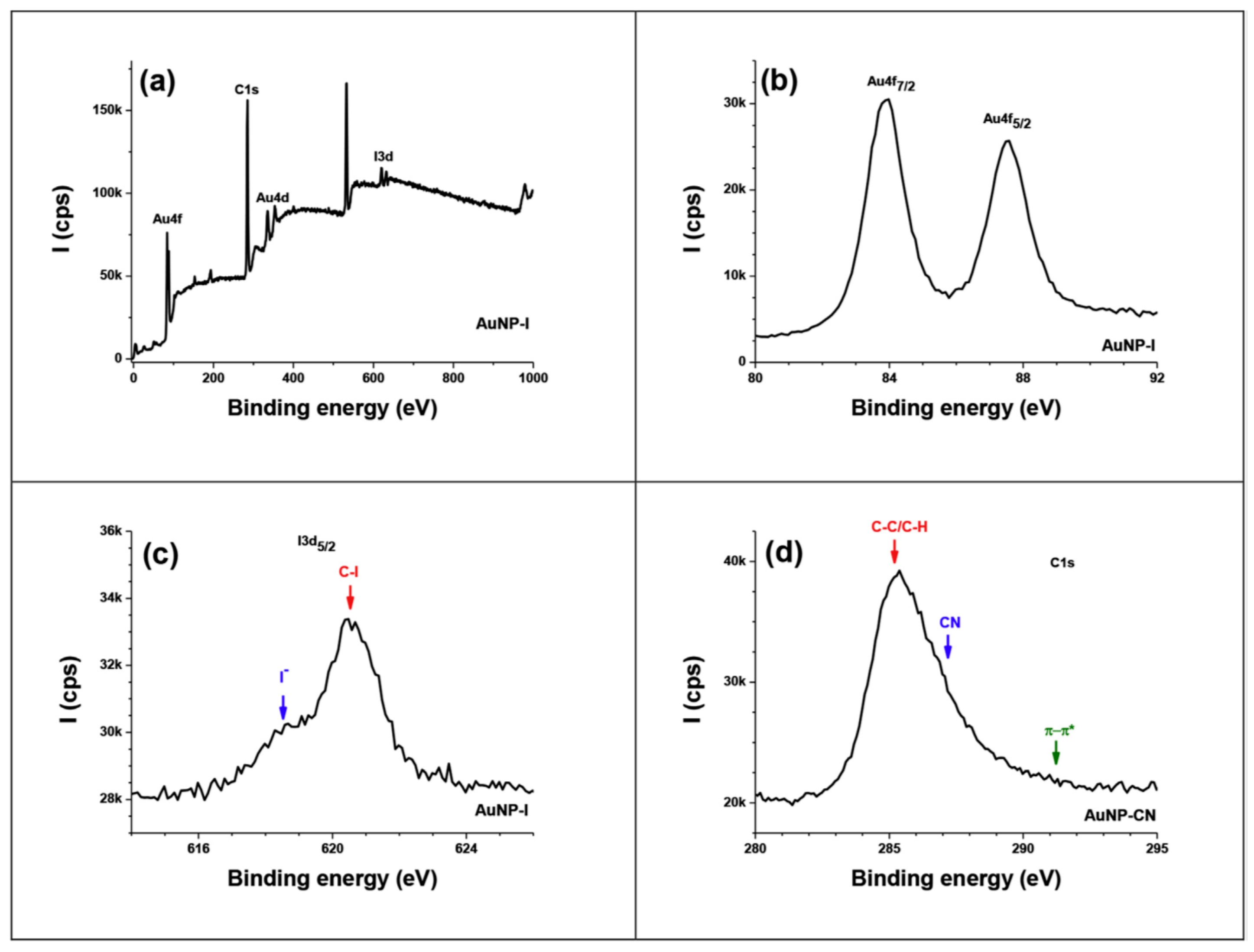
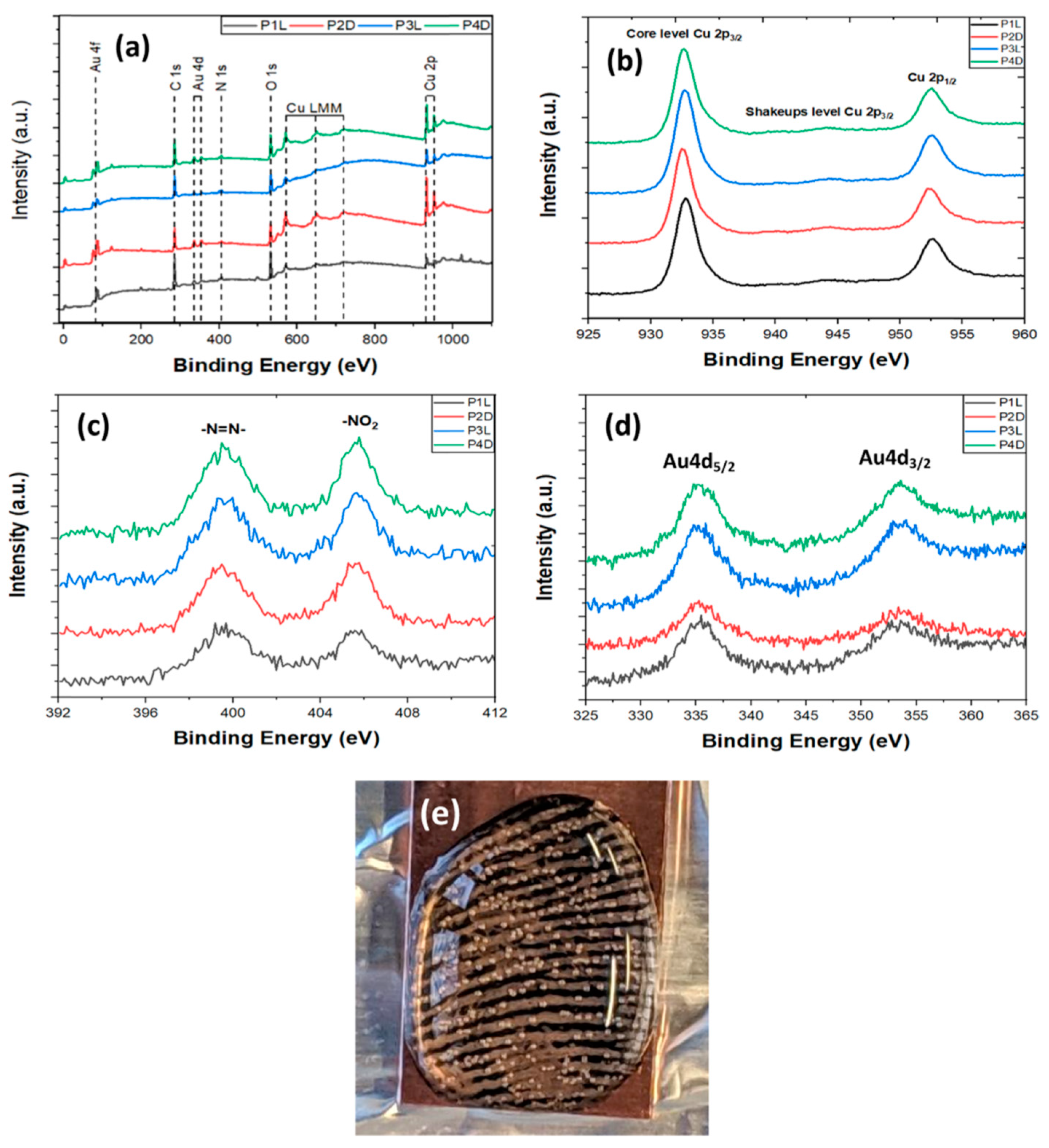
- Almheiri, S.; Ahmad, A.A.L.; Droumaguet, B.L.; Pires, R.; Mohamed, A.A.; Chehimi, M.M. Development of latent fingerprints via aryldiazonium tetrachloroaurate salts on copper surfaces: An XPS study. Langmuir 2020, 36, 74–83. [Google Scholar] [CrossRef]
- Lopes, T.S.; Alves, G.G.; Pereira, M.R.; Granjeiro, J.M.; Leite, P.E.C. Advances and potential application of gold nanoparticles in nanomedicine. J. Cell. Biochem. 2019, 120, 16370–16378. [Google Scholar] [CrossRef]
- Thompson, D.T. Using gold nanoparticles for catalysis. Nanotoday 2007, 4, 40–43. [Google Scholar] [CrossRef]
- Wu, Y.Y.; Mashayekhi, N.A.; Kung, H.H. Au–metal oxide support interface as catalytic active sites. Catal. Sci. Technol. 2013, 3, 2881–2891. [Google Scholar] [CrossRef]
- Jlassi, K.; Zavahir, S.; Kasak, P.; Krupa, I.; Mohamed, A.A.; Chehimi, M.M. Emerging clay-aryl-gold nanohybrids for efficient electrocatalytic proton reduction. Energy Convers. Manag. 2018, 168, 170–177. [Google Scholar] [CrossRef]
- Chaudhuri, R.G.; Paria, S. Core/shell nanoparticles: Classes, properties, synthesis mechanisms, characterization, and applications. Chem. Rev. 2012, 112, 2373–2433. [Google Scholar] [CrossRef]
- Cao, C.; Zhang, Y.; Jiang, C.; Qi, M.; Liu, G. Advances on aryldiazonium salt chemistry based interfacial fabrication for sensing applications. ACS Appl. Mater. Interfaces 2017, 9, 5031–5049. [Google Scholar] [CrossRef]
- Mirkhalaf, F.; Paprotny, J.; Schiffrin, D.J. Synthesis of metal nanoparticles stabilized by metal-carbon Bonds. J. Am. Chem. Soc. 2006, 128, 7400–7401. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Widawsky, J.R.; Vázquez, H.; Schneebeli, S.T.; Hybertsen, M.S.; Breslow, R.; Venkataraman, L. Highly conducting π-conjugated molecular junctions covalently bonded to gold electrodes. J. Am. Chem. Soc. 2011, 133, 17160–17163. [Google Scholar] [CrossRef] [PubMed]
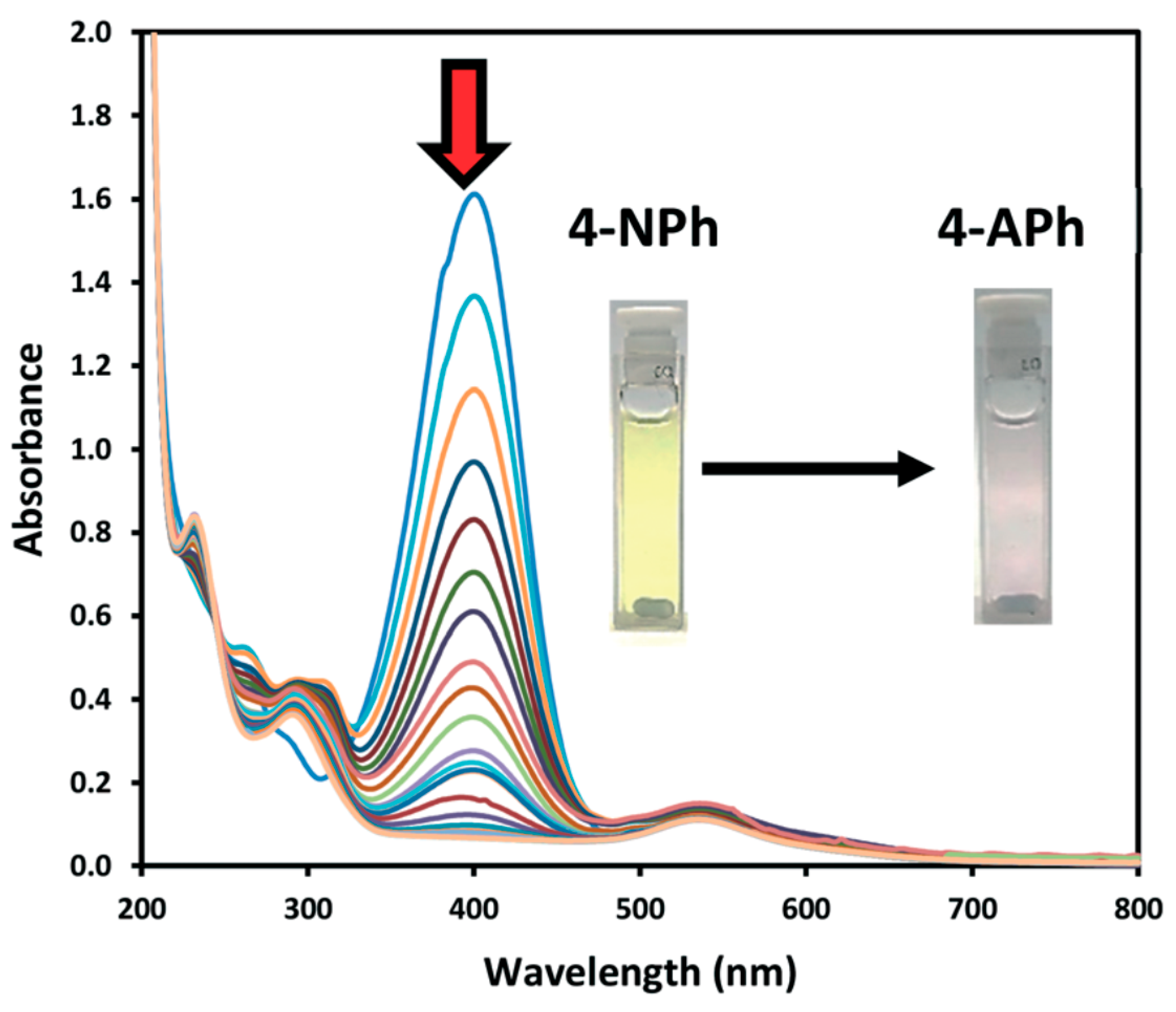
- Gu, S.; Wunder, S.; Lu, Y.; Ballauff, M.; Fenger, R.; Rademann, K.; Jaquet, B.; Zaccone, A. Kinetic analysis of the catalytic reduction of 4-nitrophenol by metallic nanoparticles. J. Phys. Chem. C 2014, 118, 18618–18625. [Google Scholar] [CrossRef]
- Ahmad, A.A.L.; Panicker, S.; Chehimi, M.M.; Monge, M.; Lopez-de-Luzuriaga, J.M.; Mohamed, A.A.; Bruce, A.E.; Bruce, M.R.M. Synthesis of water-soluble gold–aryl nanoparticles with distinct catalytic performance in the reduction of the environmental pollutant 4-nitrophenol. Catal. Sci. Technol. 2019, 9, 6059–6071. [Google Scholar] [CrossRef]
- Ridgway, Z.; Zhang, X.; Wong, A.G.; Abedini, A.; Schmidt, A.M.; Raleigh, D.P. Analysis of the role of the conserved disulfide in amyloid formation by human islet amyloid polypeptide in homogeneous and heterogeneous environments. Biochemistry 2018, 57, 3065–3074. [Google Scholar] [CrossRef]
- AlBab, N.D.; Hameed, M.K.; Maresova, A.; Ahmady, I.M.; Arooj, M.; Han, C.; Workie, B.; Chehimi, M.; Mohamed, A.A. Inhibition of amyloid fibrillation, enzymatic degradation and cytotoxicity of insulin at carboxyl tailored gold-aryl nanoparticles surface. Colloids Surf. A Physicochem. Eng. Asp. 2020, 586, 124279. [Google Scholar] [CrossRef]
- Hameed, M.K.; Ahmady, I.M.; Alawadhi, H.; Workie, B.; Sahle-Demessie, E.; Changseok Han, C.; Chehimi, M.M.; Mohamed, A.A. Gold-carbon nanoparticles mediated delivery of BSA: Remarkable robustness and hemocompatibility. Colloids Surf. A Physicochem. Eng. Asp. 2018, 558, 351–358. [Google Scholar] [CrossRef]
- Panicker, S.; Ahmady, I.M.; Almehdi, A.M.; Workie, B.; Sahle-Demessie, E.; Han, C.; Chehimi, M.M.; Mohamed, A.A. Gold-Aryl nanoparticles coated with polyelectrolytes for adsorption and protection of DNA against nuclease degradation. Appl. Organomet. Chem. 2019, 33, e4803. [Google Scholar] [CrossRef]
- Mirkhalaf, F.; Graves, J.E. Nanostructured electrocatalysts immobilised on electrode surfaces and organic film templates. Chem. Pap. 2012, 66, 472–483. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, A.A.L.; Workie, B.; Mohamed, A.A. Diazonium Gold Salts as Novel Surface Modifiers: What Have We Learned So Far? Surfaces 2020, 3, 182-196. https://doi.org/10.3390/surfaces3020014
Ahmad AAL, Workie B, Mohamed AA. Diazonium Gold Salts as Novel Surface Modifiers: What Have We Learned So Far? Surfaces. 2020; 3(2):182-196. https://doi.org/10.3390/surfaces3020014
Chicago/Turabian StyleAhmad, Ahmad A. L., Bizuneh Workie, and Ahmed A. Mohamed. 2020. "Diazonium Gold Salts as Novel Surface Modifiers: What Have We Learned So Far?" Surfaces 3, no. 2: 182-196. https://doi.org/10.3390/surfaces3020014
APA StyleAhmad, A. A. L., Workie, B., & Mohamed, A. A. (2020). Diazonium Gold Salts as Novel Surface Modifiers: What Have We Learned So Far? Surfaces, 3(2), 182-196. https://doi.org/10.3390/surfaces3020014