A Simple Method for the Quantification of Free Isocyanates on the Surface of Cellulose Nanocrystals upon Carbamation using Toluene Diisocyanate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
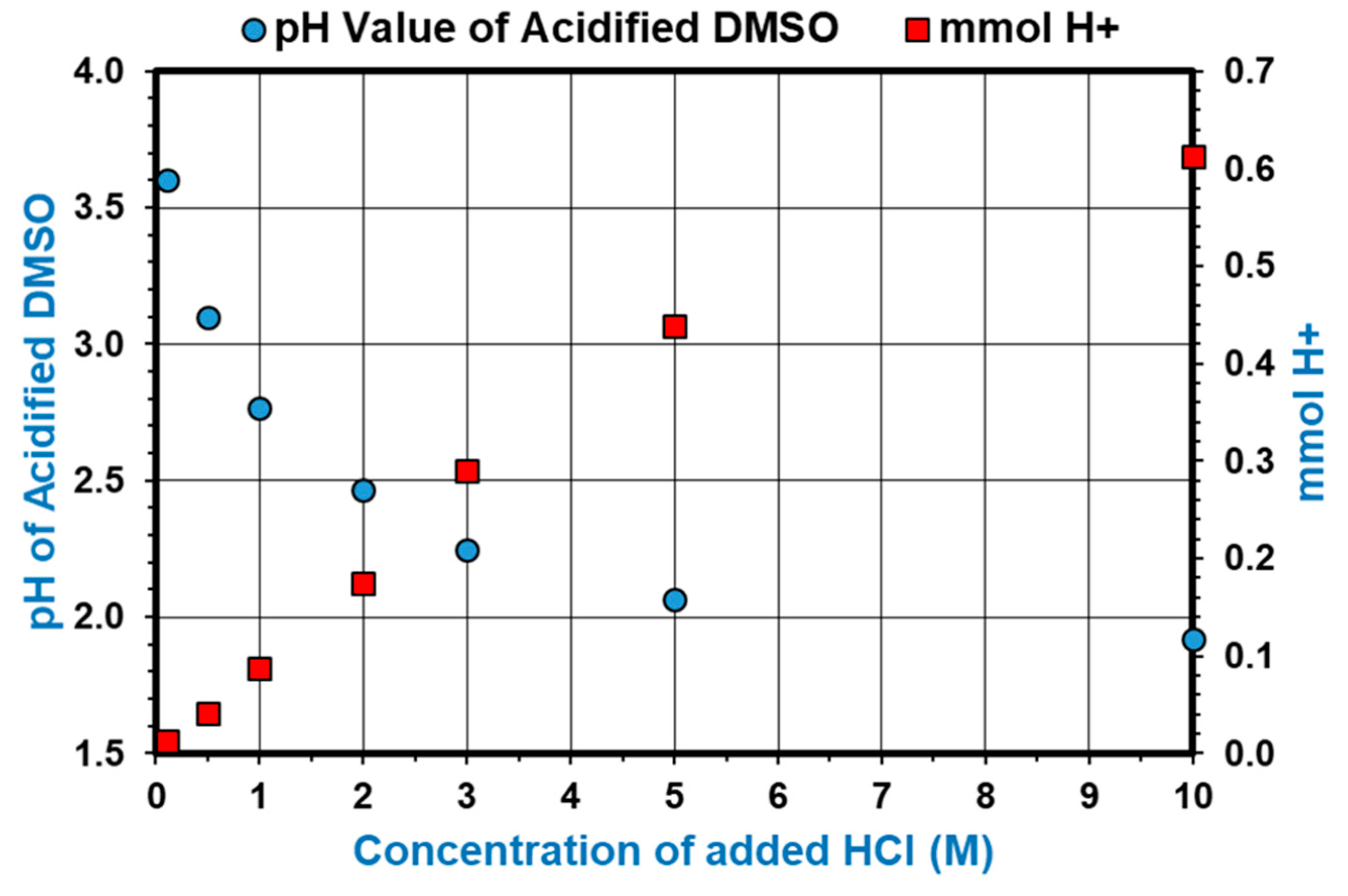
2.2. Optimization of Acidified DMSO as Hydrolysis Medium
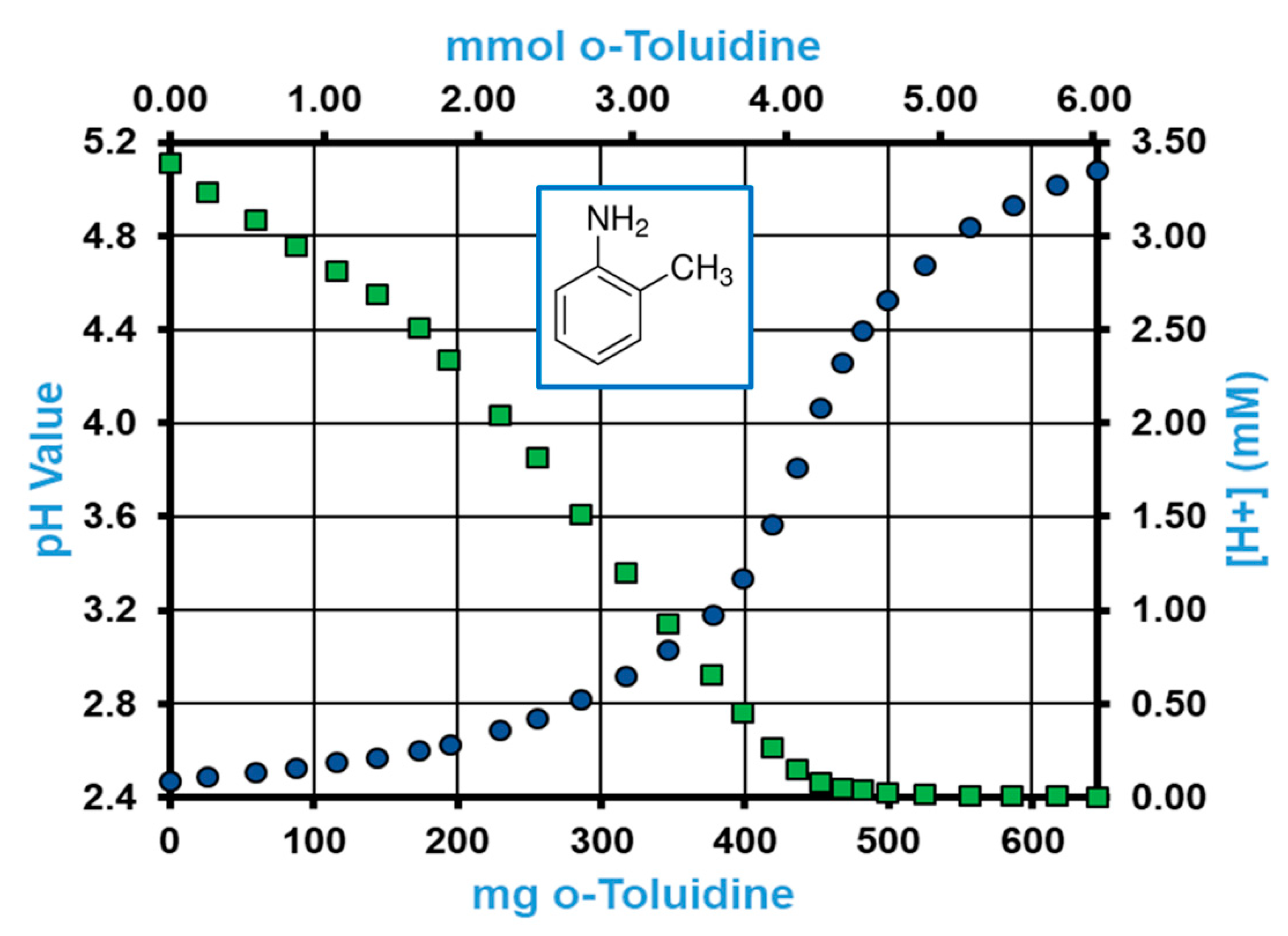
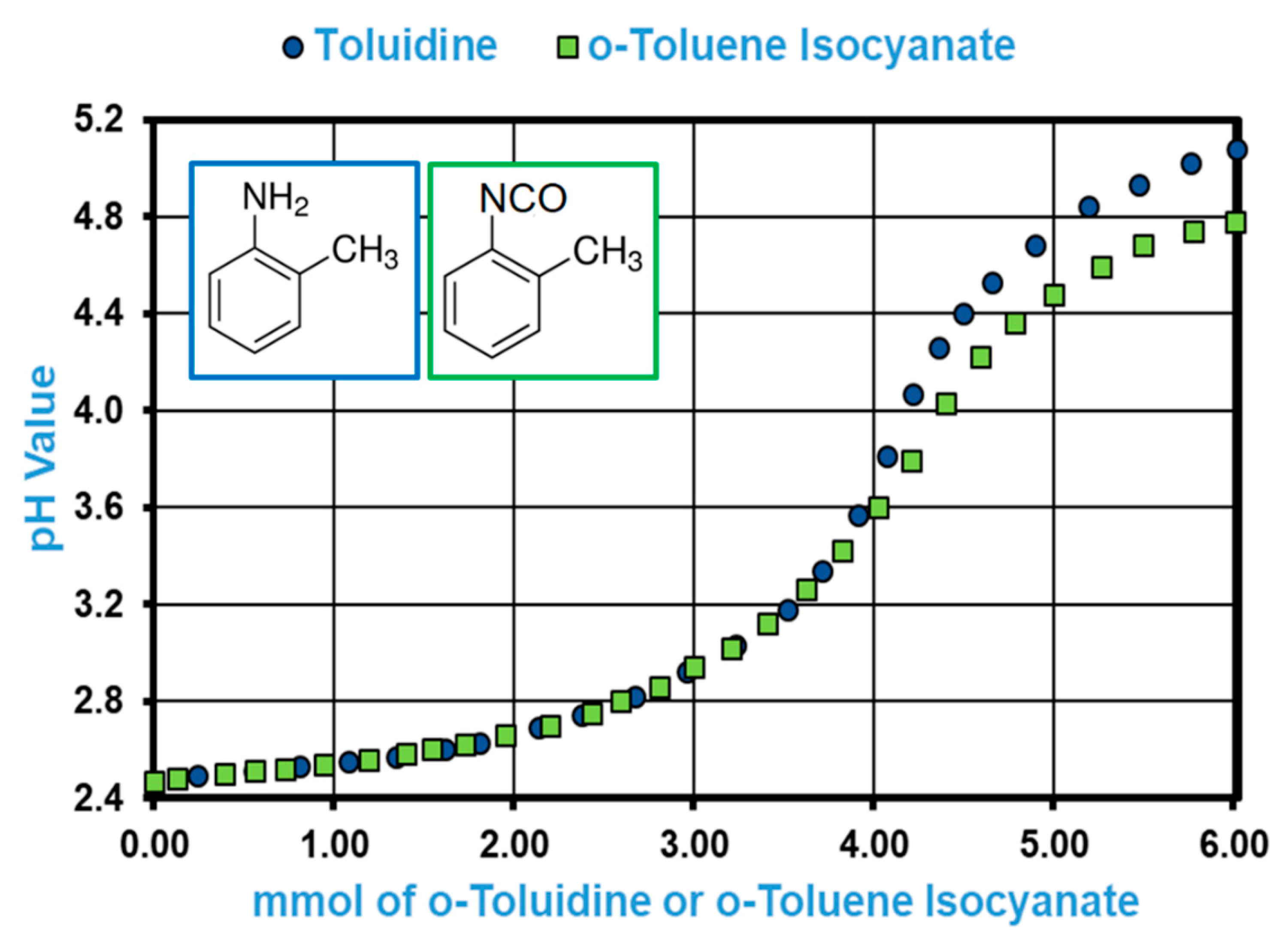
2.3. pH Profile of o-Toluidine and o-Toluene Isocyanate in Acidified DMSO
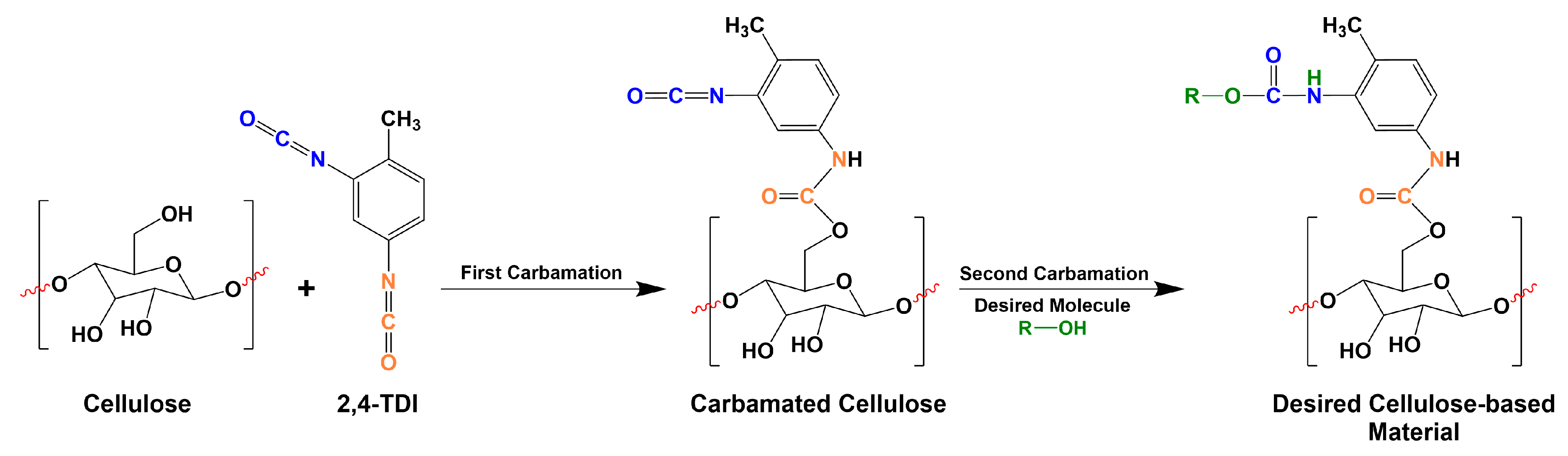
2.4. Carbamation of Cellulose Nanocrystals using TDI
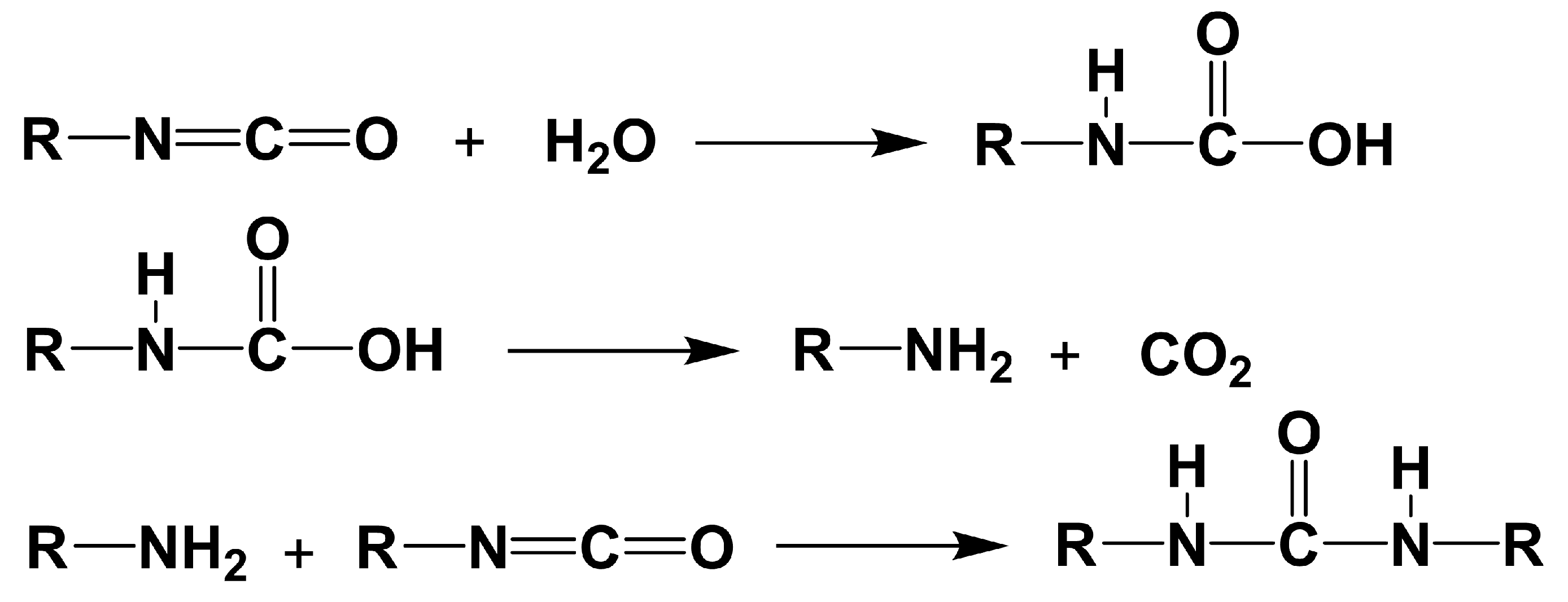
2.5. Hydrolysis of the Carbamated CNCs for the Determination of Free Isocyanates Degree of Substitution (DSNCO)
2.6. Determination of the Degree of Carbamation (DSTDI) using SEM-EDX
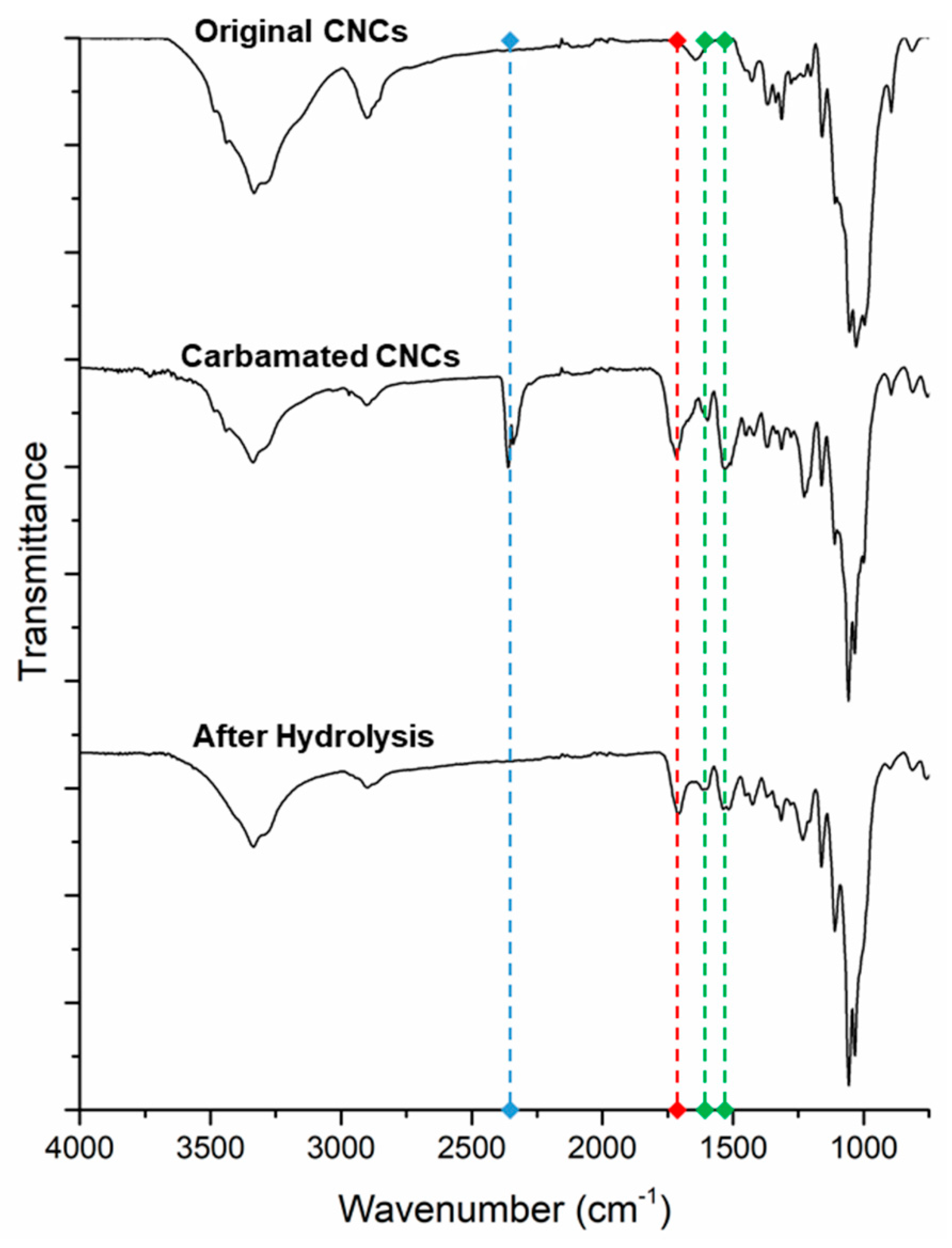
2.7. Characterization of the Carbamated and Hydrolyzed CNCs using FT-IR
2.8. Determination of CNC Thickness using Atomic Force Microscopy
3. Results and Discussion
3.1. Method Development
3.1.1. Optimization of the Hydrolysis Medium
3.1.2. pH Profiles of TDI Model Compounds in the Proposed Hydrolysis Medium
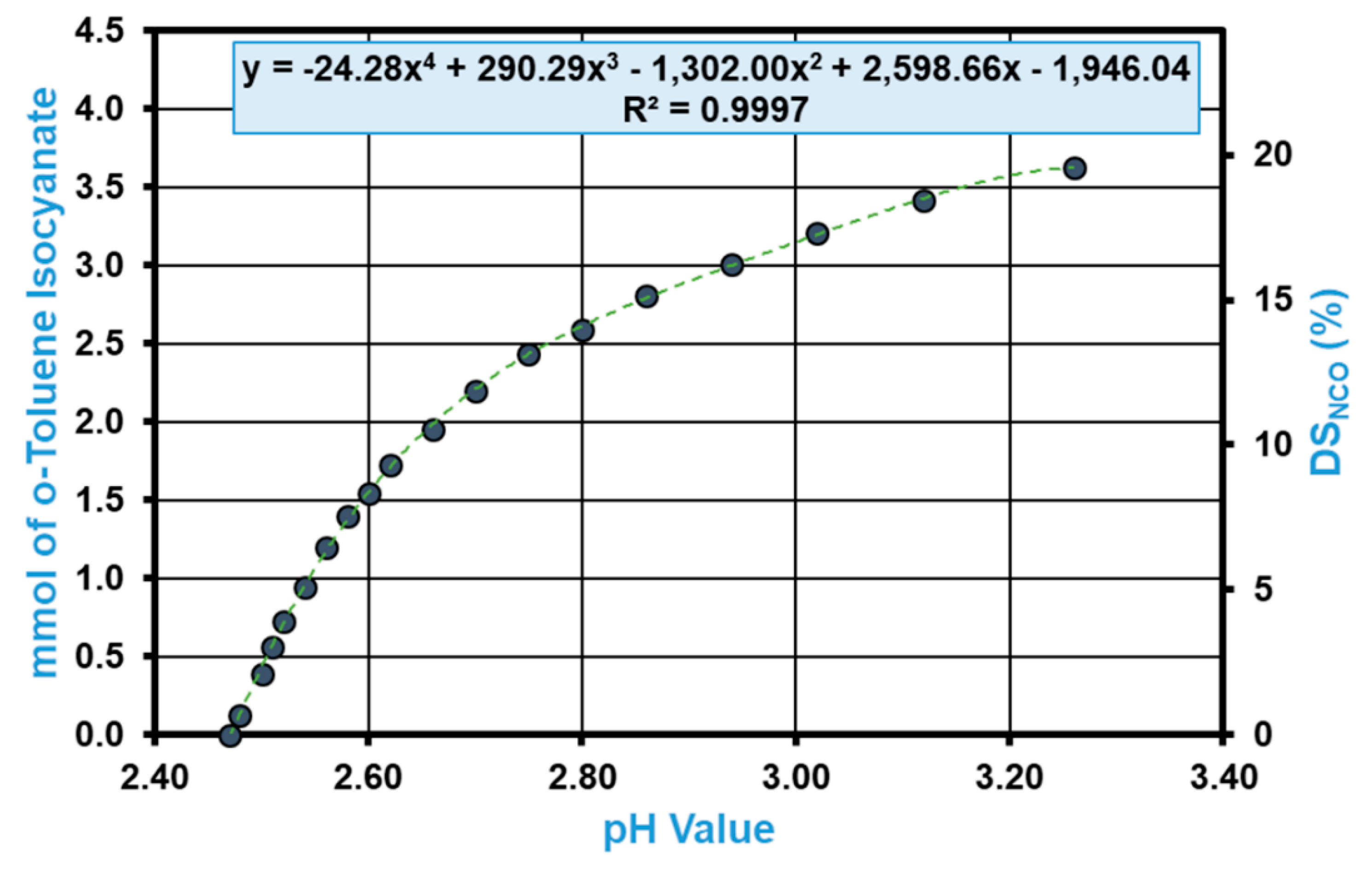
3.2. Quantification of the Free Isocyanates of TDI-Carbamated CNCs (DSNCO)
4. Conclusions
Supplementary Materials
Funding
Acknowledgments
Conflicts of Interest
References
- Badanova, A.K.; Taussarova, B.R.; Kutzhanova, A.Z. Hydrophobic finishing of cellulosic textile material. World Appl. Sci. J. 2014, 30, 1409–1416. [Google Scholar]
- Gwon, J.-G.; Cho, H.-J.; Chun, S.-J.; Lee, S.; Wu, Q.; Lee, S.-Y. Physiochemical, optical and mechanical properties of poly (lactic acid) nanocomposites filled with toluene diisocyanate grafted cellulose nanocrystals. RSC Adv. 2016, 6, 9438–9445. [Google Scholar] [CrossRef]
- Chen, L.F.; Tsao, G.T. Chemical procedures for enzyme immobilization on porous cellulose beads. Biotechnol. Bioeng. 1977, 19, 1463–1473. [Google Scholar] [CrossRef] [PubMed]
- Eyley, S.; Thielemans, W. Surface modification of cellulose nanocrystals. Nanoscale 2014, 6, 7764–7779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pang, L.; Gao, Z.; Zhang, S.; Li, Y.; Hu, S.; Ren, X. Preparation and anti-UV property of modified cellulose membranes for biopesticides controlled release. Ind. Crops Prod. 2016, 89, 176–181. [Google Scholar] [CrossRef]
- Gök, Ö.; Alkan, C.; Konuklu, Y. Developing a poly (ethylene glycol)/cellulose phase change reactive composite for cooling application. Sol. Energy Mater. Sol. Cells 2019, 191, 345–349. [Google Scholar] [CrossRef]
- Anghel, N.; Marius, N.; Spiridon, I. Heavy metal adsorption ability of a new composite material based on starch strengthened with chemically modified cellulose. Polym. Adv. Technol. 2019, 30, 1453–1460. [Google Scholar] [CrossRef]
- Bagheriasl, D.; Carreau, P.J.; Riedl, B.; Dubois, C. Enhanced properties of polylactide by incorporating cellulose nanocrystals. Polym. Compos. 2018, 39, 2685–2694. [Google Scholar] [CrossRef]
- Zoppe, J.O.; Peresin, M.S.; Habibi, Y.; Venditti, R.A.; Rojas, O.J. Reinforcing poly (ε-caprolactone) nanofibers with cellulose nanocrystals. ACS Appl. Mater. Interfaces 2009, 1, 1996–2004. [Google Scholar] [CrossRef] [PubMed]
- Dufresne, A.; Belgacem, M.N. Cellulose-reinforced composites: From micro-to nanoscale. Polímeros 2013, 23, 277–286. [Google Scholar] [CrossRef]
- Dounis, D.V.; Wilkes, G.L. Structure-property relationships of flexible polyurethane foams. Polymer 1997, 38, 2819–2828. [Google Scholar] [CrossRef]
- Belgacem, M.N.; Quillerou, J.; Gandini, A. Urethanes and polyurethanes bearing furan moieties—3. Synthesis, characterization and comparative kinetics of the formation of diurethanes. Eur. Polym. J. 1993, 29, 1217–1224. [Google Scholar] [CrossRef]
- Semsarzadeh, M.; Navarchian, A. Kinetic Study of the Bulk Reaction Between TDI and PPG in Prescence of DBTDL and FEAA Catalysts Using Quantitative FTIR Spectroscopy. J. Polym. Eng. 2003, 23, 225–240. [Google Scholar] [CrossRef]
- Gemeiner, P.; Augustin, J.; Drobnica, L. Reactions of cellulose isothiocyanates with thiol and amino compounds. Carbohydr. Res. 1977, 53, 217–222. [Google Scholar] [CrossRef]
- Habibi, Y.; Dufresne, A. Highly filled bionanocomposites from functionalized polysaccharide nanocrystals. Biomacromolecules 2008, 9, 1974–1980. [Google Scholar] [CrossRef] [PubMed]
- Thielemans, W.; Belgacem, M.N.; Dufresne, A. Starch nanocrystals with large chain surface modifications. Langmuir 2006, 22, 4804–4810. [Google Scholar] [CrossRef] [PubMed]
- Labet, M.; Thielemans, W.; Dufresne, A. Polymer grafting onto starch nanocrystals. Biomacromolecules 2007, 8, 2916–2927. [Google Scholar] [CrossRef] [PubMed]
- Shang, W.; Huang, J.; Luo, H.; Chang, P.R.; Feng, J.; Xie, G. Hydrophobic modification of cellulose nanocrystal via covalently grafting of castor oil. Cellulose 2013, 20, 179–190. [Google Scholar] [CrossRef]
- Morandi, G.; Thielemans, W. Synthesis of cellulose nanocrystals bearing photocleavable grafts by ATRP. Polym. Chem. 2012, 3, 1402–1407. [Google Scholar] [CrossRef]
- Yu, H.-Y.; Qin, Z.-Y. Surface grafting of cellulose nanocrystals with poly (3-hydroxybutyrate-co-3-hydroxyvalerate). Carbohydr. Polym. 2014, 101, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Mano, V.; Chimenti, S.; Ruggeri, G.; Pereira, F.V.; de Paula, E.L. P (CL-b-LLA) diblock copolymers grafting onto cellulosic nanocrystals. Polym. Bull. 2017, 74, 3673–3688. [Google Scholar] [CrossRef]
- Aranguren, M.I.; Williams, R.J. Kinetic and statistical aspects of the formation of polyurethanes from toluene diisocyanate. Polymer 1986, 27, 425–430. [Google Scholar] [CrossRef]
- Gilbert, D. Fate of TDI and MDI in air, soil, and water. J. Cell. Plast. 1988, 24, 178–192. [Google Scholar] [CrossRef]
- Castro, E.A.; Moodie, R.B.; Sansom, P.J. The kinetics of hydrolysis of methyl and phenyl lsocyanates. J. Chem. Soc. Perkin Trans. 2 1985, 5, 737–742. [Google Scholar] [CrossRef]
- Brown, W.E.; Green, A.H.; Cedel, T.E.; Cairns, J. Biochemistry of protein-isocyanate interactions: A comparison of the effects of aryl vs. alkyl isocyanates. Environ. Health Perspect. 1987, 72, 5. [Google Scholar] [CrossRef]
- Li, Z.; Xu, X.; Peng, Y.; Jiang, Z.; Ding, C.; Qian, X. An Unusual Synthesis of Tröger’s Bases Using DMSO/HCl as Formaldehyde Equivalent. Synthesis 2005, 2005, 1228–1230. [Google Scholar] [CrossRef]
- Tanaka, S.-Y.; Yasuda, M.; Baba, A. Practical and simple synthesis of substituted quinolines by an HCl−DMSO system on a large scale: Remarkable effect of the chloride ion. J. Organ. Chem. 2006, 71, 800–803. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, Y.; Langan, P.; Chanzy, H. Crystal structure and hydrogen-bonding system in cellulose Iβ from synchrotron X-ray and neutron fiber diffraction. J. Am. Chem. Soc. 2002, 124, 9074–9082. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zhang, X.; Zhang, X.; Huang, H.; Chang, J.; Chen, H. Structural investigations of toluene diisocyanate (TDI) and trimethylolpropane (TMP)-based polyurethane prepolymer. J. Ind. Eng. Chem. 2012, 18, 1620–1627. [Google Scholar] [CrossRef]
- Špírková, M.; Kubin, M.; Dušek, K. Side reactions in the formation of polyurethanes: Model reactions between phenylisocyanate and 1-butanol. J. Macromol. Sci. Chem. 1987, 24, 1151–1166. [Google Scholar] [CrossRef]
| T (°C) | Final pH | mmol NCO | DSNCO (%) | N/C Ratio | DSTDI (%) | DSNCO/DSTDI (%) |
|---|---|---|---|---|---|---|
| 35 | 2.51 ± 0.00 | 0.59 ± 0.00 | 3.2 ± 0.0 | 0.045 ± 0.001 | 4.5 ± 0.1 | 72 ± 1.6 |
| 55 | 2.55 ± 0.01 | 1.07 ± 0.11 | 5.8 ± 0.6 | 0.077 ± 0.001 | 9.6 ± 0.2 | 61 ± 6.4 |
| 75 | 2.54 ± 0.00 | 0.91 ± 0.06 | 4.9 ± 0.3 | 0.096 ± 0.001 | 14.2 ± 0.3 | 34 ± 2.2 |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abushammala, H. A Simple Method for the Quantification of Free Isocyanates on the Surface of Cellulose Nanocrystals upon Carbamation using Toluene Diisocyanate. Surfaces 2019, 2, 444-454. https://doi.org/10.3390/surfaces2020032
Abushammala H. A Simple Method for the Quantification of Free Isocyanates on the Surface of Cellulose Nanocrystals upon Carbamation using Toluene Diisocyanate. Surfaces. 2019; 2(2):444-454. https://doi.org/10.3390/surfaces2020032
Chicago/Turabian StyleAbushammala, Hatem. 2019. "A Simple Method for the Quantification of Free Isocyanates on the Surface of Cellulose Nanocrystals upon Carbamation using Toluene Diisocyanate" Surfaces 2, no. 2: 444-454. https://doi.org/10.3390/surfaces2020032






