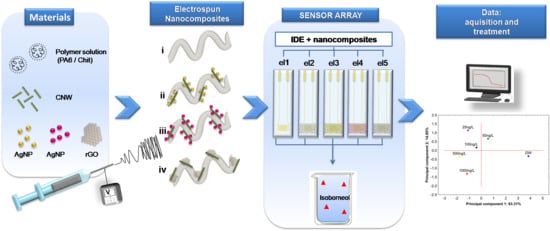
Tuning the Electrical Properties of Electrospun Nanofibers with Hybrid Nanomaterials for Detecting Isoborneol in Water Using an Electronic Tongue
Abstract
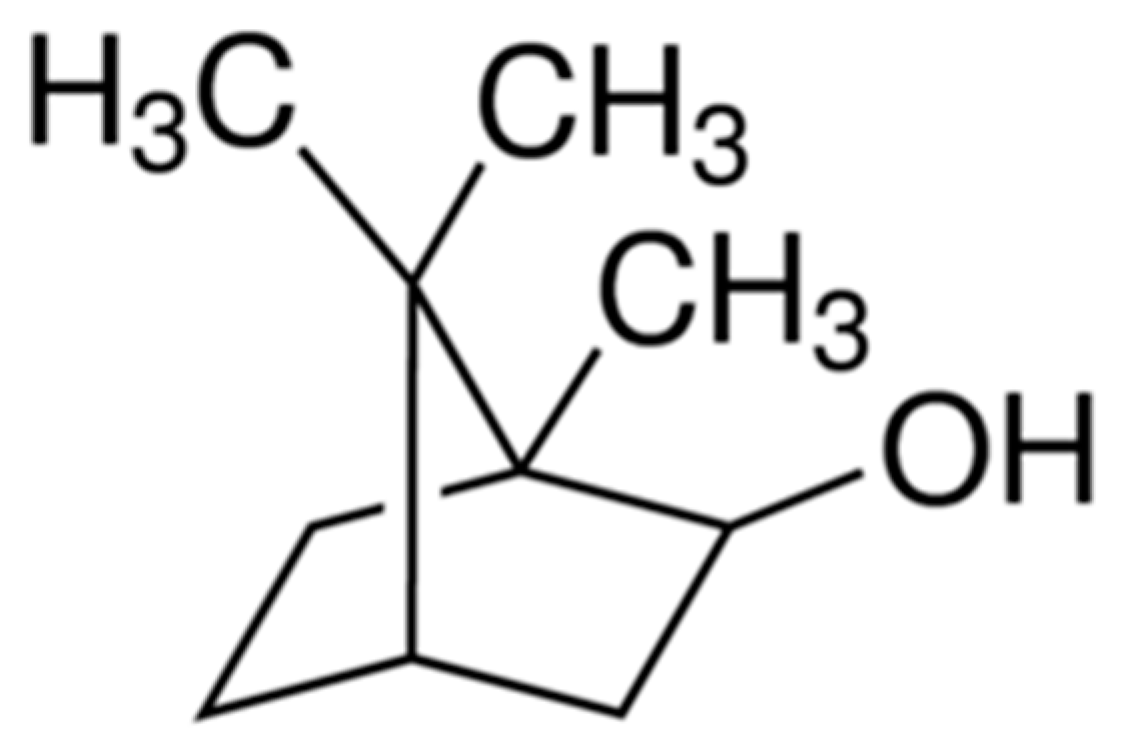
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Synthesis of Cellulose Nanowhiskers (CNW)
2.3. Synthesis of CNW:Ag, CNW:Au, and CNW:rGO
2.4. Production of Nanocomposites
2.5. CNW:Ag, CNW:Au, and CNW:rGO Characterization
2.6. Design of Sensing Units of the e-Tongue
2.7. Detection Experiments
3. Results and Discussion
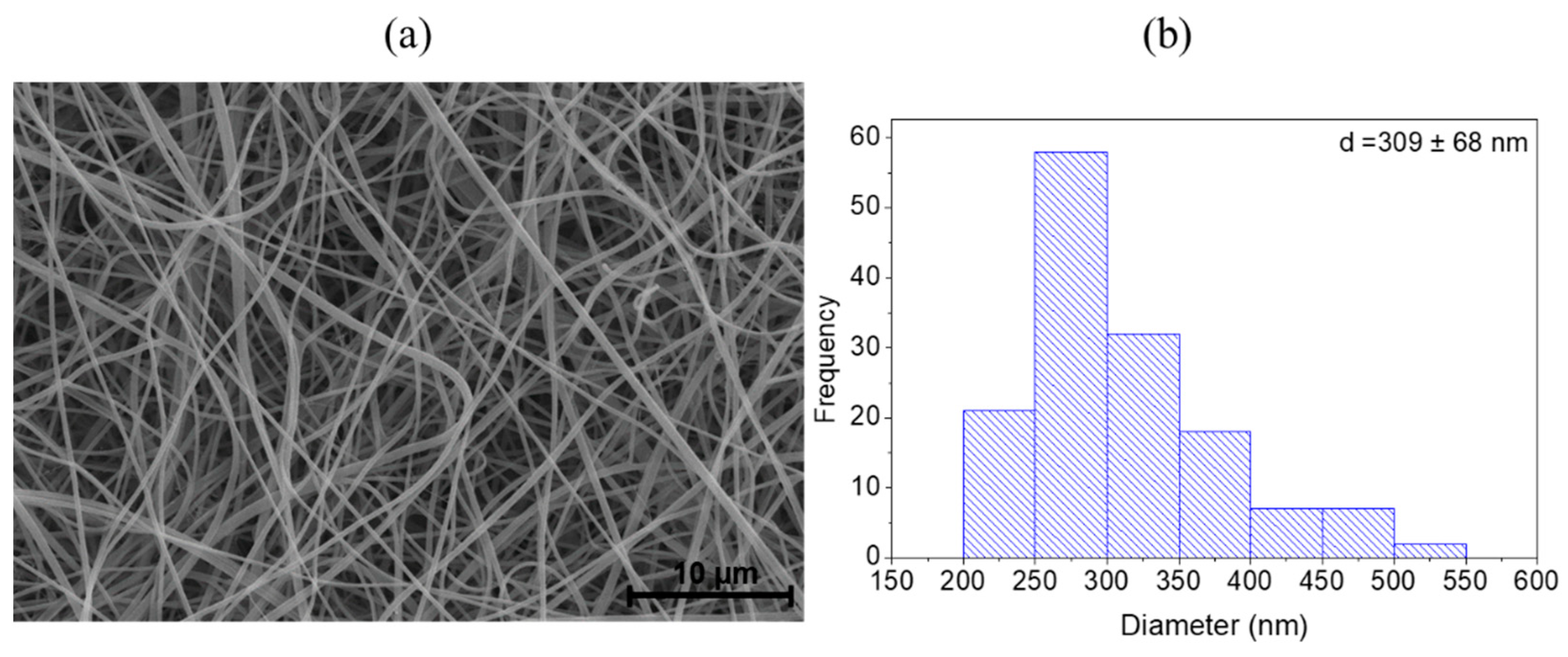
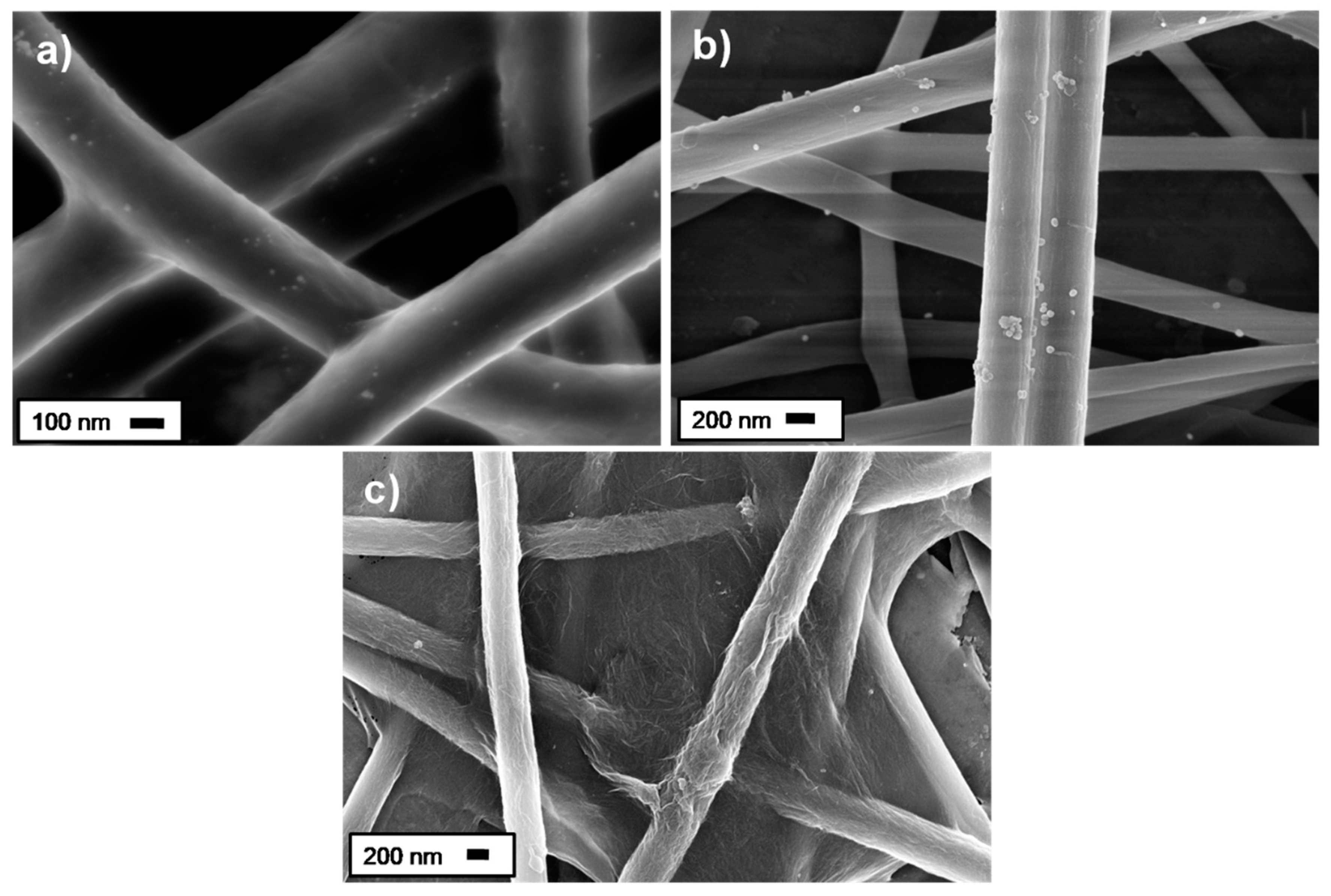
3.1. Nanocomposites Characterization
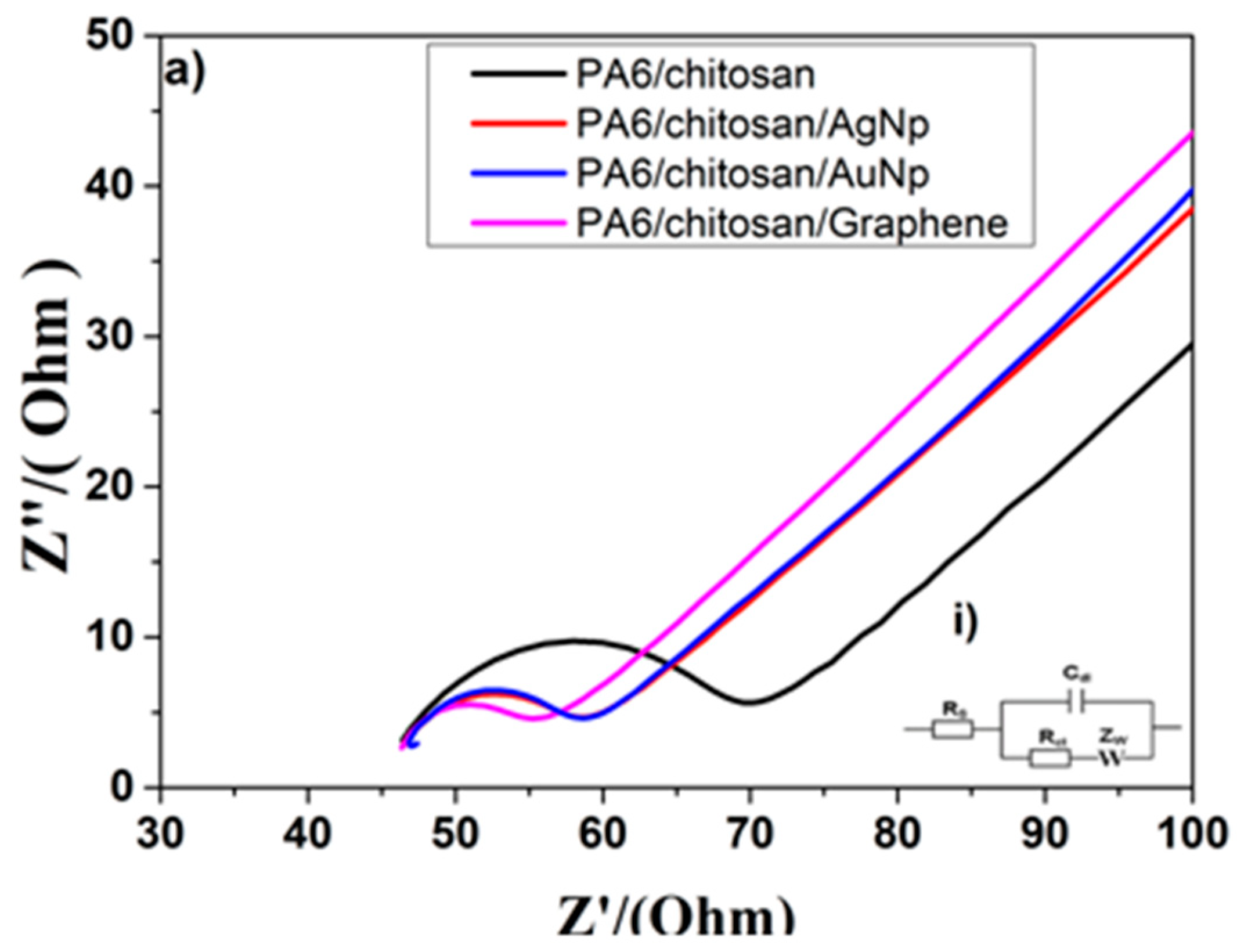
3.2. Electrochemical Characterization of Electrodes
3.3. E-Tongue Experiments
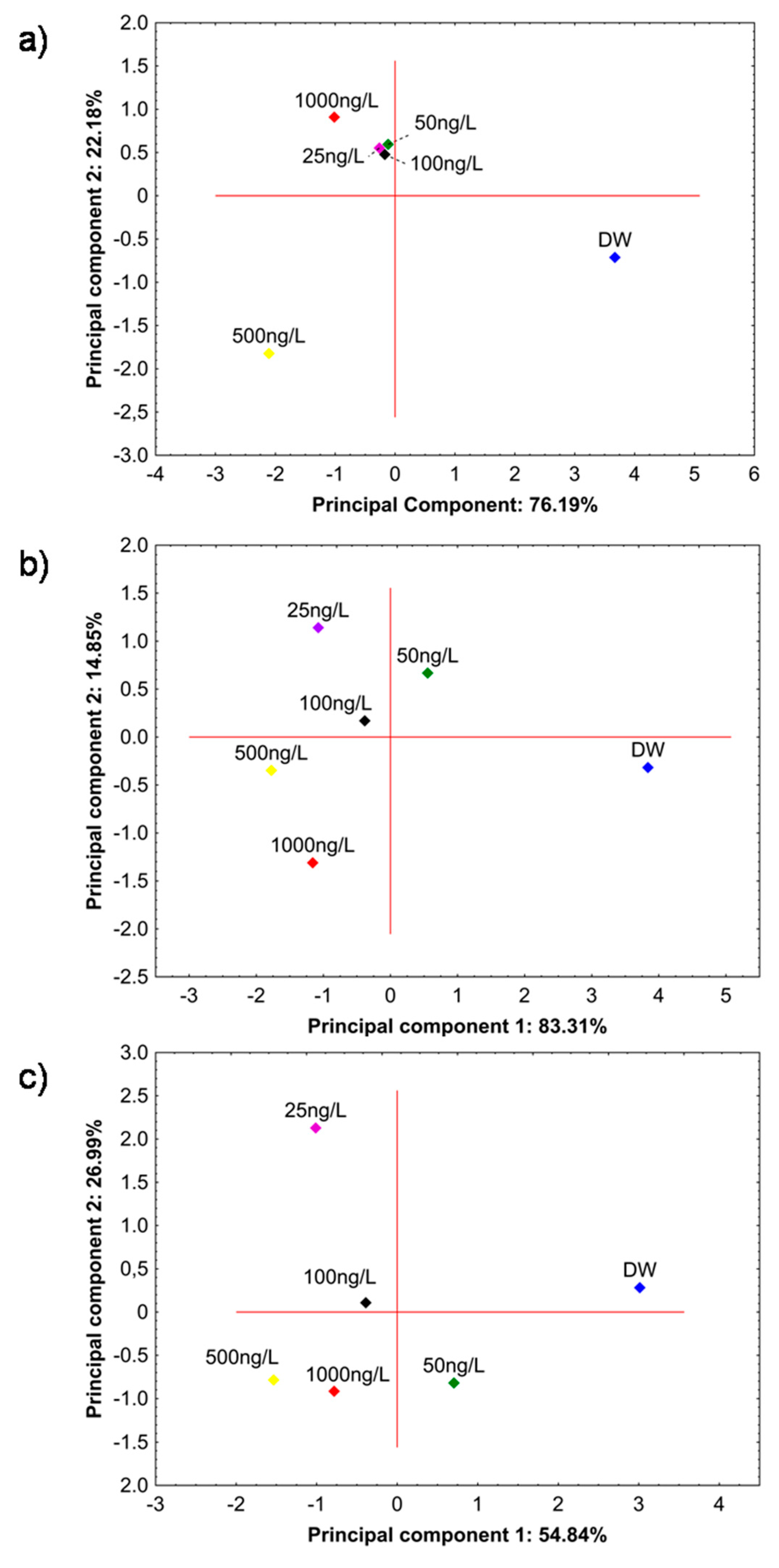
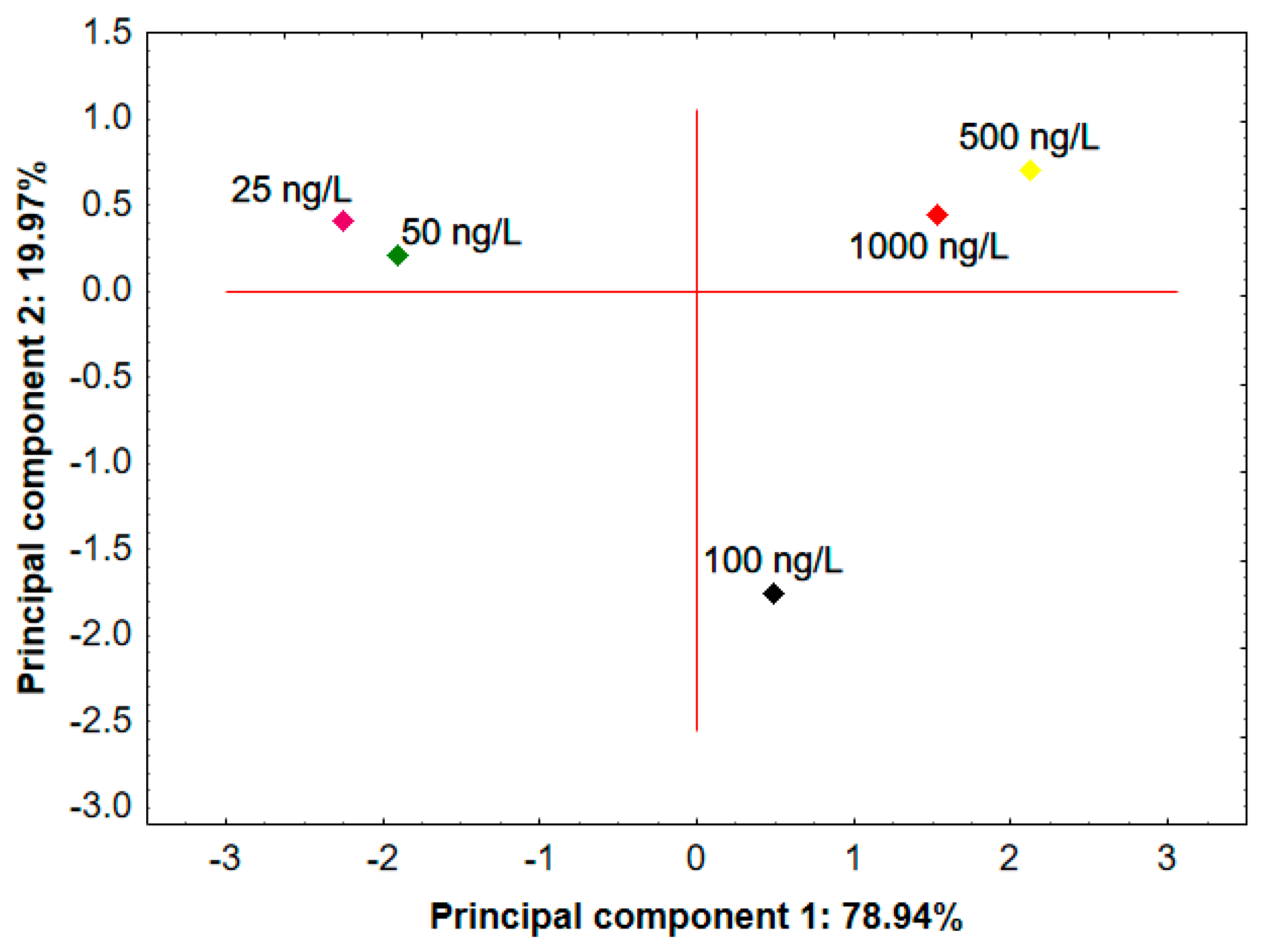
3.3.1. Water Classification—e-Tongue Measurements
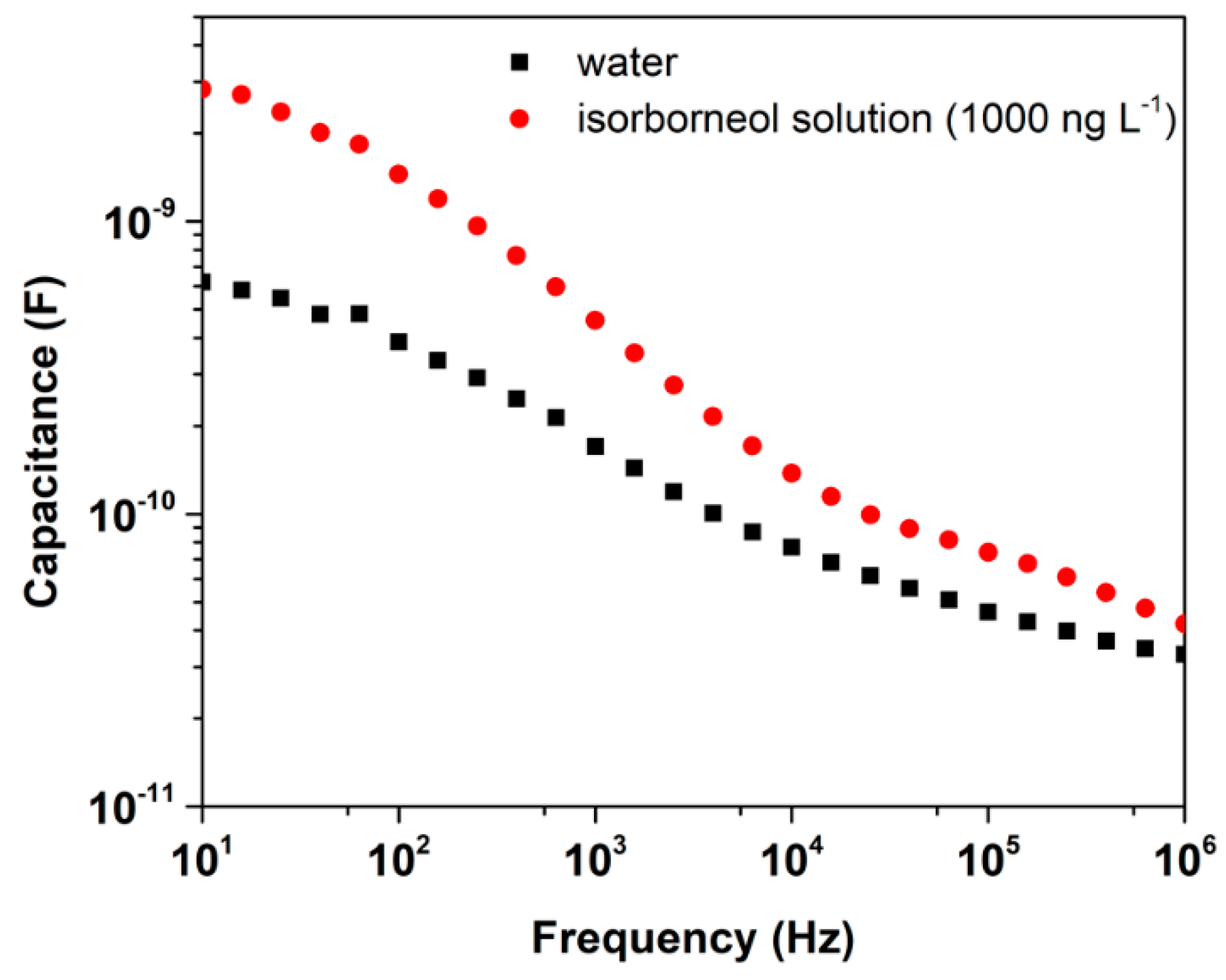
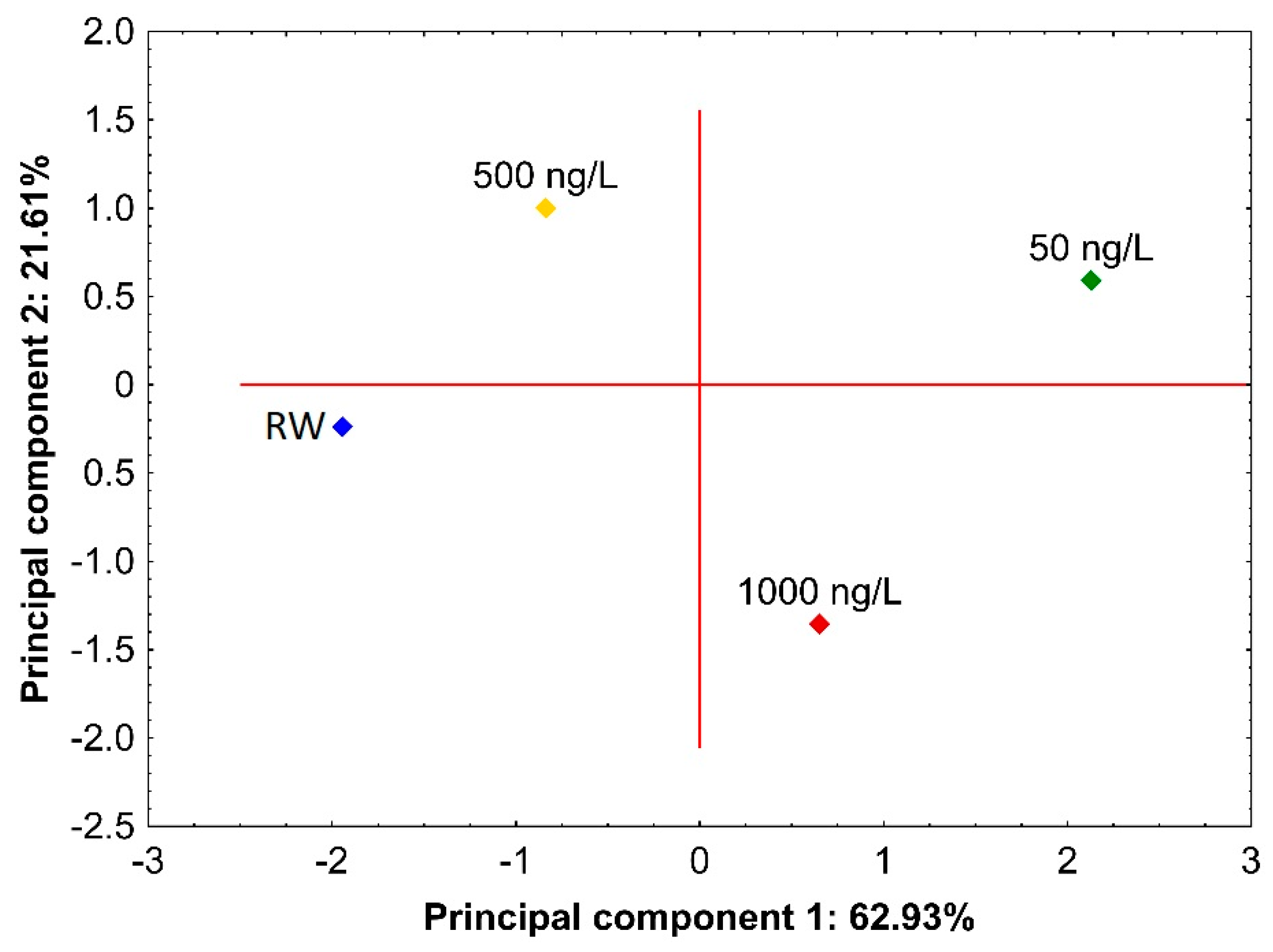
3.3.2. Real Samples
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Zhang, W.; Webb, D.J.; Lao, L.; Hammond, D.; Carpenter, M.; Williams, C. Water content detection in aviation fuel by using PMMA based optical fiber grating. Sens. Actuators B Chem. 2019, 282, 774–779. [Google Scholar] [CrossRef]
- Khan, S.; Ali, F.; Akhtar, K. Chitosan nanocomposite fibers supported copper nanoparticles based perceptive sensor and active catalyst for nitrophenol in real water. Carbohydr. Polym. 2019, 207, 650–662. [Google Scholar] [CrossRef] [PubMed]
- Braga, G.S.; Paterno, L.G.; Fonseca, F.J. Performance of an electronic tongue during monitoring 2-methylisoborneol and geosmin in water samples. Sens. Actuators B Chem. 2012, 171–172, 181–189. [Google Scholar] [CrossRef]
- Braga, G.S.; Lieberzeit, P.A.; Fonseca, F.J. Molecularly imprinted polymer based sensor to detect isoborneol in aqueous samples. Procedia Eng. 2016, 168, 448–451. [Google Scholar] [CrossRef]
- Son, M.; Cho, D.G.; Lim, J.H.; Park, J.; Hong, S.; Ko, H.J.; Park, T.H. Real-time monitoring of geosmin and 2-methylisoborneol, representative odor compounds in water pollution using bioelectronic nose with human-like performance. Biosens. Bioelectron. 2015, 74, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.L.; Boyer, G.L.; Zimba, P.V. A review of cyanobacterial odorous and bioactive metabolites: Impacts and management alternatives in aquaculture. Aquaculture 2008, 280, 5–20. [Google Scholar] [CrossRef]
- Morales-Valle, H.; Silva, L.C.; Paterson, R.R.M.; Oliveira, J.M.; Venâncio, A.; Lima, N. Microextraction and gas chromatography/mass spectrometry for improved analysis of geosmin and other fungal “off” volatiles in grape juice. J. Microbiol. Methods 2010, 83, 48–52. [Google Scholar] [CrossRef]
- Howgate, P. Tainting of farmed fish by geosmin and 2-methyl-iso-borneol: A review of sensory aspects and of uptake/depuration. Aquaculture 2004, 234, 155–181. [Google Scholar] [CrossRef]
- Armaka, M.; Papanikolaou, E.; Sivropoulou, A.; Arsenakis, M. Antiviral properties of isoborneol, a potent inhibitor of herpes simplex virus type 1. Antivir. Res. 1999, 43, 79–92. [Google Scholar] [CrossRef]
- Wu, X.; Li, Y.; Xu, D.; Zhou, H.; Wang, J.; Guo, X.; Zhang, Y. Gas chromatography-mass spectrometry and high-performance liquid chromotagraphy analysis of the drug absorption characteristics in the buccal mucosa via a circulating device. Biomed. Rep. 2014, 3, 51–54. [Google Scholar] [CrossRef] [Green Version]
- Sun, H.; Zhang, F.; Chen, S.; Guan, Z.; Jiang, J.; Fang, W.; Chen, F. Effects of aphid herbivory on volatile organic compounds of Artemisia annua and Chrysanthemum morifolium. Biochem. Syst. Ecol. 2015, 60, 225–233. [Google Scholar] [CrossRef]
- Di Natale, C.; Macagnano, A.; Davide, F.; D’Amico, A.; Legin, A.; Vlasov, Y.; Rudnitskaya, A.; Selezenev, B. Multicomponent analysis on polluted waters by means of an electronic tongue. Sens. Actuators B Chem. 1997, 44, 423–428. [Google Scholar] [CrossRef]
- Karkra, R.; Kumar, P.; Bansod, B.K.S.; Bagchi, S.; Sharma, P.; Krishna, C.R. Classification of heavy metal ions present in multi-frequency multi-electrode potable water data using evolutionary algorithm. Appl. Water Sci. 2017, 7, 3679–3689. [Google Scholar] [CrossRef]
- Riul, A., Jr.; Dantas, C.A.R.; Miyazaki, C.M.; Oliveira, O.N., Jr. Recents advances in electronic tongues. Analyst 2010, 135, 2481–2495. [Google Scholar] [CrossRef] [PubMed]
- Facure, M.H.M.; Mercante, L.A.; Mattoso, L.H.C.; Correa, D.S. Detection of trace levels of organophosphate pesticides using an electronic tongue based on graphene hybrid nanocomposites. Talanta 2017, 167, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H. Chemical preparation of graphene-based nanomaterials and their applications in chemical and biological sensors. Small 2011, 7, 2413–2427. [Google Scholar] [CrossRef]
- Mercante, L.A.; Pavinatto, A.; Iwaki, L.E.O.; Scagion, V.P.; Zucolotto, V.; Oliveira, O.N.; Mattoso, L.H.C.; Correa, D.S. Electrospun polyamide 6/poly(allylamine hydrochloride) nanofibers functionalized with carbon nanotubes for electrochemical detection of dopamine. ACS Appl. Mater. Interfaces 2015, 7, 4784–4790. [Google Scholar] [CrossRef] [PubMed]
- Migliorini, F.L.; Sanfelice, R.C.; Pavinatto, A.; Steffens, J.; Steffens, C.; Correa, D.S. Voltammetric cadmium(II) sensor based on a fluorine doped tin oxide electrode modified with polyamide 6/chitosan electrospun nanofibers and gold nanoparticles. Microchim. Acta 2017, 184, 1077–1084. [Google Scholar] [CrossRef]
- Oliveira, J.E.; Grassi, V.; Scagion, V.P.; Mattoso, L.H.C.; Glenn, G.M.; Medeiros, E.S. Sensor array for water analysis based on interdigitated electrodes modified with fiber films of poly(lactic acid)/multiwalled carbon nanotubes. IEEE Sens. J. 2013, 13, 759–766. [Google Scholar] [CrossRef]
- Lvova, L.; Pudi, R.; Galloni, P.; Lippolis, V.; Di Natale, C.; Lundström, I.; Paolesse, R. Multi-transduction sensing films for electronic tongue applications. Sens. Actuators B Chem. 2015, 207, 1076–1086. [Google Scholar] [CrossRef]
- Cetó, X.; González-Calabuig, A.; Crespo, N.; Pérez, S.; Capdevila, J.; Puig-Pujol, A.; Del Valle, M. Electronic tongues to assess wine sensory descriptors. Talanta 2017, 162, 218–224. [Google Scholar] [CrossRef] [PubMed]
- González-Calabuig, A.; Del Valle, M. Voltammetric electronic tongue to identify Brett character in wines. On-site quantification of its ethylphenol metabolites. Talanta 2018, 179, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Andre, R.S.; Pavinatto, A.; Mercante, L.A.; Paris, E.C.; Mattoso, L.H.C.; Correa, D.S. Improving the electrochemical properties of polyamide 6/polyaniline electrospun nanofibers by surface modification with ZnO nanoparticles. RSC Adv. 2015, 5, 73875–73881. [Google Scholar] [CrossRef]
- Scagion, V.P.; Mercante, L.A.; Sakamoto, K.Y.; Oliveira, J.E.; Fonseca, F.J.; Mattoso, L.H.C.; Ferreira, M.D.; Correa, D.S. An electronic tongue based on conducting electrospun nanofibers for detecting tetracycline in milk samples. RSC Adv. 2016, 6, 103740–103746. [Google Scholar] [CrossRef]
- Khan, A.A.; Akhtar, T. Synthesis, characterization and ion-exchange properties of a fibrous type ‘polymeric-inorganic’ composite cation-exchanger Nylon-6,6 Sn(IV) phosphate: Its application in making Hg(II) selective membrane electrode. Electrochim. Acta 2009, 54, 3320–3329. [Google Scholar] [CrossRef]
- Choi, J.; Park, E.J.; Park, D.W.; Shim, S.E. MWCNT–OH adsorbed electrospun nylon 6,6 nanofibers chemiresistor and their application in low molecular weight alcohol vapours sensing. Synth. Met. 2010, 160, 2664–2669. [Google Scholar] [CrossRef]
- Shrestha, B.K.; Mousa, H.M.; Tiwari, A.P.; Ko, S.W.; Park, C.H.; Kim, C.S. Development of polyamide-6,6/chitosan electrospun hybrid nanofibrous scaffolds for tissue engineering application. Carbohydr. Polym. 2016, 148, 107–114. [Google Scholar] [CrossRef]
- Suginta, W.; Khunkaewla, P.; Schulte, A. Electrochemical biosensor applications of polysaccharides chitin and chitosan. Chem. Rev. 2013, 113, 5458–5479. [Google Scholar] [CrossRef]
- Yang, D.; Li, L.; Chen, B.; Shi, S.; Nie, J.; Ma, G. Functionalized chitosan electrospun nanofiber membranes for heavy-metal removal. Polymer 2019, 163, 74–85. [Google Scholar] [CrossRef]
- Muxika, A.; Etxabide, A.; Uranga, J.; Guerrero, P.; de la Caba, K. Chitosan as a bioactive polymer: Processing, properties and applications. Int. J. Biol. Macromol. 2017, 105, 1358–1368. [Google Scholar] [CrossRef]
- Miretzky, P.; Cirelli, A.F. Hg(II) removal from water by chitosan and chitosan derivatives: A review. J. Hazard. Mater. 2009, 167, 10–23. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Han, T.; Wang, J.; Shao, L.; Qi, C.; Zhang, X.M.; Liu, C.; Liu, X. Removal of heavy metal chromium using cross-linked chitosan composite nanofiber mats. Int. J. Biol. Macromol. 2018, 120, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Fiamingo, A.; de Moura Delezuk, J.A.; Trombotto, S.; David, L.; Campana-Filho, S.P. Extensively deacetylated high molecular weight chitosan from the multistep ultrasound-assisted deacetylation of beta-chitin. Ultrason. Sonochem. 2016, 32, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, D.M.; de Lacerda Bukzem, A.; Campana-Filho, S.P. Response surface methodology applied to the study of the microwave-assisted synthesis of quaternized chitosan. Carbohydr. Polym. 2016, 138, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Rinaudo, M.; Milas, M.; Le Dung, P. Characterization of chitosan. Influence of ionic strength and degree of acetylation on chain expansion. Int. J. Biol. Macromol. 1993, 15, 281–285. [Google Scholar] [CrossRef]
- Teodoro, K.B.R.; Sanfelice, R.C.; Mattoso, L.H.C.; Correa, D.S. Cellulose whiskers influence the morphology and antibacterial properties of silver nanoparticles composites. J. Nanosci. Nanotechnol. 2018, 18, 4876–4883. [Google Scholar] [CrossRef]
- Babaei, M.; Ganjalikhani, M. A systematic review of gold nanoparticles as novel cancer therapeutics gold nanoparticles as novel cancer therapeutics. Nanomed. J. 2013, 1, 211–219. [Google Scholar]
- Teodoro, K.B.R.; Migliorini, F.L.; Facure, M.H.M.; Correa, D.S. Conductive electrospun nanofibers containing cellulose nanowhiskers and reduced graphene oxide for the electrochemical detection of mercury(II). Carbohydr. Polym. 2019, 207, 747–754. [Google Scholar] [CrossRef]
- Wilson, D.; Alegret, S.; del Valle, M. Simultaneous titration of ternary mixtures of Pb(II), Cd(II) and Cu(II) with potentiometric electronic tongue detection. Electroanalysis 2015, 27, 336–342. [Google Scholar] [CrossRef]
- Correa, A.C.; Teixeira, E.M.; Carmona, V.B.; Teodoro, K.B.; Ribeiro, C.; Mattoso, L.H.C.; Marconcini, J.M. Obtaining nanocomposites of polyamide 6 and cellulose whiskers via extrusion and injection molding. Cellulose 2014, 21, 311–322. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, B.; Cheng, Z.; Yang, X.; Dong, S.; Wang, E. A facile approach to immobilize protein for biosensor: Self-assembled supported bilayer lipid membranes on glassy carbon electrode. Biosens. Bioelectron. 2001, 16, 47–52. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Migliorini, F.L.; Teodoro, K.B.R.; Scagion, V.P.; dos Santos, D.M.; Fonseca, F.J.; Mattoso, L.H.C.; Correa, D.S. Tuning the Electrical Properties of Electrospun Nanofibers with Hybrid Nanomaterials for Detecting Isoborneol in Water Using an Electronic Tongue. Surfaces 2019, 2, 432-443. https://doi.org/10.3390/surfaces2020031
Migliorini FL, Teodoro KBR, Scagion VP, dos Santos DM, Fonseca FJ, Mattoso LHC, Correa DS. Tuning the Electrical Properties of Electrospun Nanofibers with Hybrid Nanomaterials for Detecting Isoborneol in Water Using an Electronic Tongue. Surfaces. 2019; 2(2):432-443. https://doi.org/10.3390/surfaces2020031
Chicago/Turabian StyleMigliorini, Fernanda L., Kelcilene B. R. Teodoro, Vanessa P. Scagion, Danilo M. dos Santos, Fernando J. Fonseca, Luiz H. C. Mattoso, and Daniel S. Correa. 2019. "Tuning the Electrical Properties of Electrospun Nanofibers with Hybrid Nanomaterials for Detecting Isoborneol in Water Using an Electronic Tongue" Surfaces 2, no. 2: 432-443. https://doi.org/10.3390/surfaces2020031
APA StyleMigliorini, F. L., Teodoro, K. B. R., Scagion, V. P., dos Santos, D. M., Fonseca, F. J., Mattoso, L. H. C., & Correa, D. S. (2019). Tuning the Electrical Properties of Electrospun Nanofibers with Hybrid Nanomaterials for Detecting Isoborneol in Water Using an Electronic Tongue. Surfaces, 2(2), 432-443. https://doi.org/10.3390/surfaces2020031