A Hydroalcoholic Gel-Based Disinfection System for Deteriogenic Fungi on the Contemporary Mixed Media Artwork Poesia by Alessandro Kokocinski
Abstract
1. Introduction
2. Materials and Methods
2.1. Diagnostic Study
2.2. Microbiological Analyses
2.3. Hydrogel Preparation
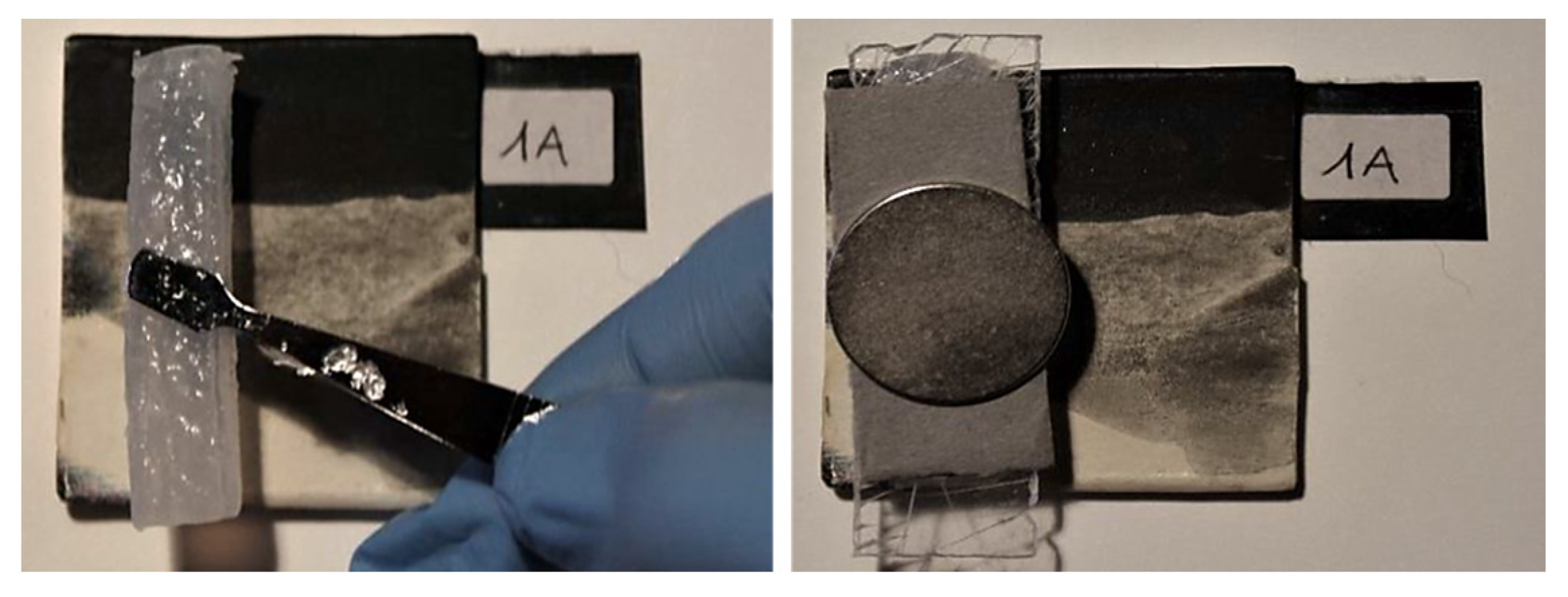
2.4. Mockups and Treatments
3. Results
3.1. Diagnostic Analysis
3.2. pH and Color Measurements
3.3. Microbiological Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hirsenberger, H.; Ranogajec, J.; Vucetic, S.; Lalic, B.; Gracanin, D. Collaborative projects in cultural heritage conservation–management challenges and risks. J. Cult. Herit. 2019, 37, 215–224. [Google Scholar] [CrossRef]
- Ambroselli, F. Poesia di Alessandro Kokocinski. Un approccio Multidisciplinare al Restauro di un Collage Polimaterico Affetto da Degrado Biologico. Master’s Thesis, University of Tuscia, Viterbo, Italy, 2021. [Google Scholar]
- Baglioni, M.; Poggi, G.; Chelazzi, D.; Baglioni, P. Advanced Materials in Cultural Heritage Conservation. Molecules 2021, 26, 3967. [Google Scholar] [CrossRef]
- Mazzuca, C.; Poggi, G.; Bonelli, N.; Micheli, L.; Baglioni, P.; Palleschi, A. Innovative Chemical Gels Meet Enzymes: A Smart Combination for Cleaning Paper Artworks. J. Colloid Interface Sci. 2017, 502, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Mazzuca, C.; Carbone, M.; Cancelliere, R.; Prati, S.; Sciutto, G.; Mazzeo, R.; Tositti, L.; Regazzi, R.; Mostacci, D.; Micheli, L. A new analytical approach to characterize the effect of γ-ray sterilization on wood. Microchem. J. 2018, 143, 493–502. [Google Scholar] [CrossRef]
- Mazzuca, C.; Severini, L.; Missori, M.; Tumiati, M.; Domenici, F.; Micheli, L.; Titubante, M.; Bragaglia, M.; Nanni, F.; Paradossi, G.; et al. Evaluating the Influence of Paper Characteristics on the Efficacy of New Poly(Vinyl Alcohol) Based Hydrogels for Cleaning Modern and Ancient Paper. Microchem. J. 2020, 155, 104716. [Google Scholar] [CrossRef]
- Mazzuca, C.; Micheli, L.; Carbone, M.; Basoli, F.; Cervelli, E.; Iannuccelli, S.; Sotgiu, S.; Palleschi, A. Gellan Hydrogel as a Powerful Tool in Paper Cleaning Process: A Detailed Study. J. Colloid Interface Sci. 2014, 416, 205–211. [Google Scholar] [CrossRef]
- Mazzuca, C.; Micheli, L.; Lettieri, R.; Cervelli, E.; Coviello, T.; Cencetti, C.; Sotgiu, S.; Iannuccelli, S.; Palleschi, G.; Palleschi, A. How to tune a paper cleaning process by means of modified gellan hydrogels. Microchem. J. 2016, 126, 359–367. [Google Scholar] [CrossRef]
- Petrella, G.; Mazzuca, C.; Micheli, L.; Cervelli, E.; De Fazio, D.; Iannuccelli, S.; Sotgiu, S.; Palleschi, G.; Palleschi, A. A new sustainable and innovative work for paper artworks cleaning process: Gellan hydrogel combined with hydrolytic enzymes. Int. J. Conserv. Sci. 2016, 7, 273–280. [Google Scholar]
- Mastrangelo, R.; Chelazzi, D.; Poggi, G.; Fratini, E.; Buemi, L.P.; Petruzzellis, M.L.; Baglioni, P. Twin-chain polymer hydrogels based on poly (vinyl alcohol) as new advanced tool for the cleaning of modern and contemporary art. Proc. Natl. Acad. Sci. USA 2020, 117, 7011–7020. [Google Scholar] [CrossRef]
- De Santis, P.C. Some observations on the use of enzymes in paper conservation. J. Am. Inst. Conserv. 1983, 23, 7–27. [Google Scholar] [CrossRef]
- Palla, F. Bioactive molecules: Innovative contributions of biotechnology to the restoration of cultural heritage. Conserv. Sci. Cult. Herit. 2013, 13, 369–373. [Google Scholar]
- Fidanza, M.R.; Caneva, G. Natural biocides for the conservation of stone cultural heritage: A review. J. Cult. Herit. 2019, 38, 271–286. [Google Scholar] [CrossRef]
- Proto, M.R.; Biondi, E.; Baldo, D.; Levoni, M.; Filippini, G.; Modesto, M.; Di Vito, M.; Bugli, F.; Ratti, C.; Minardi, P.; et al. Essential Oils and Hydrolates: Potential Tools for Defense against Bacterial Plant Pathogens. Microorganisms 2022, 10, 702. [Google Scholar] [CrossRef] [PubMed]
- Melis, M.; Miccoli, M.; Quarta, D. Multispectral hypercolorimetry and automatic guided pigment identification: Some master-pieces case studies. In Proceedings of the SPIE 8790, Optics for Arts, Architecture, and Archaeology IV; Munich, Germany, 13–16 May 2013, Pezzati, L., Targowski, P., Eds.; SPIE: Washington, WA, USA, 2013; Volume 8790. [Google Scholar]
- Colantonio, C.; Pelosi, C.; D’Alessandro, L.; Sottile, S.; Calabrò, G.; Melis, M. Hypercolorimetric Multispectral Imaging (HMI) system for cultural heritage diagnostics: An innovative study for copper painting examination. Eur. Phys. J. Plus 2018, 133, 526. [Google Scholar] [CrossRef]
- Lanteri, L.; Pelosi, C. 2D and 3D ultraviolet fluorescence applications on cultural heritage paintings and objects through a low-cost approach for diagnostics and documentation. In Proceedings of the SPIE 11784, Optics for Arts, Architecture, and Archaeology VIII; Munich, Germany, 21–25 June 2021, Liang, H., Groves, R., Eds.; SPIE: Washington, WA, USA, 2021; Volume 11784, p. 1178417. [Google Scholar]
- Learner, T. The Analysis of Synthetic Paints by Pyrolysis-Gas Chromatography-Mass Spectrometry (PyGCMS). Stud. Conserv. 2001, 46, 225–242. [Google Scholar]
- UNI EN 15886; Conservation of Cultural Property–Test Methods–Colour Measurement of Surfaces. CEN: Brussels, Belgium, 2010.
- Pelosi, C.; Capobianco, G.; Agresti, G.; Bonifazi, G.; Morresi, F.; Rossi, S.; Santamaria, S.; Serranti, S. A methodological approach to study the stability of selected watercolours for painting reintegration, through reflectance spectrophotometry, Fourier transform infrared spectroscopy and hyperspectral imaging. Spectrochim. Acta A 2018, 198, 92–106. [Google Scholar] [CrossRef]
- Pitt, J.I. A Laboratory Guide to Common Penicillium Species; Commonwealth Scientific and Industrial Research Organization; Division of Food Processing: North Ryde, Australia, 1991. [Google Scholar]
- Domsch, K.H.; Gams, W.; Anderson, T.H. Compendium of Soil Fungi, 2nd ed.; IHW-Verlag: Eching, Germany, 2007. [Google Scholar]
- Isola, D.; Zucconi, L.; Onofri, S.; Caneva, G.; De Hoog, G.S.; Selbmann, L. Extremotolerant rock inhabiting black fungi from Italian monumental sites. Fungal Divers. 2016, 76, 75–96. [Google Scholar] [CrossRef]
- ISO 5630-3; Paper and Board–Accelerated Ageing–Part 3: Moist Heat Treatment at 80 °C and 65% Relative Humidity. ISO: Genève, Switzerland, 1996.
- Laureti, S.; Colantonio, C.; Burrascano, P.; Melis, M.; Calabrò, G.; Malekmohammadi, H.; Sfarra, S.; Ricci, M.; Pelosi, C. Development of integrated innovative techniques for paintings examination: The case studies of The Resurrection of Christ attributed to Andrea Mantegna and the Crucifixion of Viterbo attributed to Michelangelo’s workshop. J. Cult. Herit. 2019, 40, 1–16. [Google Scholar] [CrossRef]
- Ricci, M.; Laureti, S.; Malekmohammadi, H.; Sfarra, S.; Lanteri, L.; Colantonio, C.; Calabrò, G.; Pelosi, C. Surface and Interface Investigation of a 15th Century Wall Painting Using Multispectral Imaging and Pulse-Compression Infrared Thermography. Coatings 2021, 11, 546. [Google Scholar] [CrossRef]
- Cosentino, A.; Cosentino, A. Identification of pigments by multispectral imaging; a flowchart method. Herit. Sci. 2014, 2, 8. [Google Scholar] [CrossRef]
- Boust, C.; Wohlgelmuth, A. DATABASE: Pigment image database under UV and IR radiations. In Scientific Imaging for Cultural Heritage; Hypotheses: Ivry-sur-Seine, France, 2017; Available online: https://copa.hypotheses.org/552 (accessed on 13 February 2023).
- Wu, C.H.; Warren, H.L. Natural autofluorescence in fungi, and its correlation with viability. Mycologia 1984, 76, 1049–1058. [Google Scholar] [CrossRef]
- Lin, S.J.; Tan, H.Y.; Kuo, C.J.; Wu, R.J.; Wang, S.H.; Chen, W.L.; Jee, S.H.; Dong, C.Y. Multiphoton autofluorescence spectral analysis for fungus imaging and identification. Appl. Phys. Lett. 2009, 95, 043703. [Google Scholar]
- Bown, R. Particle size, shape and structure of paper fillers and their effect on paper properties. Pap. Technol. 1998, 39, 44–48. [Google Scholar]
- Schabel, S.; Putz, H.-J.; Hamm, U.; Kersten, A.; Bobek, B.; Hirsch, G.; Voss, D. Calcium carbonate in the paper industry-Blessing for coated papermaking and curse for recycling processes. Tappi J. 2014, 13, 47–54. [Google Scholar] [CrossRef]
- Bailão, A.; Šustic, S. Retouching with mica pigments. E-Conserv. J. 2013, 1, 45–60. [Google Scholar] [CrossRef]
- Pfaff, G. Special Effect Pigments: Technical Basics and Applications, 2nd ed.; Vincentz Network GmbH & Co KG: Hannover, Germany, 2008. [Google Scholar]
- Pfaff, G. The world of inorganic pigments. ChemTexts 2022, 8, 15. [Google Scholar] [CrossRef]
- Gadhave, R.V.I.; Gadhave, C.R. Adhesives for the Paper Packaging Industry: An Overview. Open J. Polym. Chem. 2022, 12, 55–79. [Google Scholar] [CrossRef]
- Doménech-Carbó, M.T.; Doménech-Carbó, A.; Gimeno-Adelantado, J.V.; Bosch-Reig, F. Identification of Syntethic Resins Used in Works of Art by Fourier Transform Infrared Spectroscopy. Appl. Spectrosc. 2001, 55, 1590–1602. [Google Scholar] [CrossRef]
- Mitchell, G.; France, F.; Nordon, A.; Leung Tang, P.; Gibson, L.T. Assessment of historical polymers using attenuated total reflectance-Fourier transform infrared spectroscopy with principal component analysis. Herit. Sci. 2013, 1, 28. [Google Scholar] [CrossRef]
- IRUG DATABASE. Available online: http://www.irug.org/search-spectral-database (accessed on 13 February 2023).
- Poliszuk, A.; Ybarra, G. Analysis of Cultural Heritage Materials by Infrared Spectroscopy. In Infrared Spectroscopy: Theory, Developments and Applications; Cozzolino, D., Ed.; Nova Science Publishers: Hauppauge, NY, USA, 2013; pp. 1–18. [Google Scholar]
- Ramírez-Hernández, A.; Aguilar-Flores, C.; Aparicio-Saguilán, A. Fingerprint analysis of FTIR spectra of polymers containing vinyl acetate. DYNA 2019, 86, 198–205. [Google Scholar] [CrossRef]
- Crook, J.; Learner, T.J.S. The Impact of Modern Paints; Watson-Guptill publications: New York, NY, USA, 2000. [Google Scholar]
- Learner, T.J.S. Analysis of Modern Paints; The Getty Conservation Institute: Los Angeles, CA, USA, 2005. [Google Scholar]
- Jablonski, E.; Learner, T.; Hayes, J.; Golden, M. Conservation concerns for acrylic emulsion paints. Stud. Conserv. 2003, 48, 3–12. [Google Scholar] [CrossRef]
- López, F.A.; Martín, M.I.; Alguacil, F.J.; Rincón, J.M.; Centeno, T.A.; Romero, M. Thermolysis of fibreglass polyester composite and reutilisation of the glass fibre residue to obtain a glass–ceramic material. J. Anal. Appl. Pyrol. 2012, 93, 104–112. [Google Scholar] [CrossRef]
- Mansour, M.M.A.; Hamed, S.A.E.-K.M.; Salem, M.Z.M.; Ali, H.M. Illustration of the Effects of Five Fungi on Acacia saligna Wood Organic Acids and Ultrastructure Alterations in Wood Cell Walls by HPLC and TEM Examinations. Appl. Sci. 2020, 10, 2886. [Google Scholar] [CrossRef]
- Hassan, R.R.A.; Mohamed, W.S. Effect of methyl methacrylate/hydroxyethyl methacrylate copolymer on optical and mechanical properties and long-term durability of paper under accelerated ageing. Int. J. Conserv. Sci. 2017, 8, 237–250. [Google Scholar]
- Szczepanowska, H.; Lovett, C.M. A study of the removal and prevention of fungal stains on paper. J. Am. Inst. Conserv. 1992, 31, 147–160. [Google Scholar] [CrossRef]
- Sterflinger, K.; Pinzari, F. The revenge of time: Fungal deterioration of cultural heritage with particular reference to books, paper and parchment. Environ. Microbiol. 2012, 14, 559–566. [Google Scholar] [CrossRef]
- Cremonesi, P. L’uso Degli Enzimi Nella Pulitura di Opera Policrome; Il Prato, Arti Grafiche Padovane: Padova, Italy, 1999. [Google Scholar]
- Palla, F. Biotecnologie per i Beni Cultuali. Innovazioni scientifiche Bio-cleaning, Bio-reinforcing. CRPR/InForma Riv. Semest. Del Cent. Reg. Progett. E Restauro 2006, 1, 22–26. [Google Scholar]
- Paulus, W. Directory of Microbiocides for the Protection of Materials. A Handbook; Springer Dordrecht: Berlin, Germany, 2005. [Google Scholar]
- Sequeira, S.O.; Phillips, A.J.L.; Cabrita, E.J.; Macedo, M.F. Ethanol as an antifungal treatment for paper: Short-term and long-term effects. Stud. Conserv. 2017, 62, 33–42. [Google Scholar] [CrossRef]
- Cassanelli, M.; Norton, I.; Mills, T. Effect of alcohols on gellan gum gel structure: Bridging the molecular level and the three-dimensional network. Food Struct. 2017, 14, 112–120. [Google Scholar] [CrossRef]
- Sequeira, S.O.; Cabrita, E.J.; Macedoequeira, M.F. Fungal biodeterioration of paper: How are paper and book conservators dealing with it? An international survey. Restaurator. Int. J. Preserv. Libr. Arch. Mater. 2014, 35, 181–199. [Google Scholar] [CrossRef]
- Florian, M.L.; Purinton, N. Determination of location of stains in fungal spots and enzymatic removal of pigmented hyphae in paper. In Biodeterioration of Cultural Property 3, Proceedings of the 3rd International Conference on Biodeterioration of Cultural Property, Bangkok, Thailand, 4–7 July 1995; The Conservation Science Division, The Fine Arts Department: Bangkok, Thailand, 1995; pp. 414–425. [Google Scholar]
- Di Vito, M.; Vergari, L.; Mariotti, M.; Proto, M.R.; Barbanti, L.; Garzoli, S.; Sanguinetti, M.; Sabatini, L.; Peduzzi, A.; Bellardi, M.G.; et al. Anti-Mold Effectiveness of a Green Emulsion Based on Citrus aurantium Hydrolate and Cinnamomum zeylanicum Essential Oil for the Modern Paintings Restoration. Microorganisms 2022, 10, 205. [Google Scholar] [CrossRef] [PubMed]
- Zmeu, N.; Bosch-Roig, P. Risk analysis of biodeterioration in contemporary art collections: The poly-material challenge. J. Cult. Herit. 2022, 58, 33–48. [Google Scholar] [CrossRef]



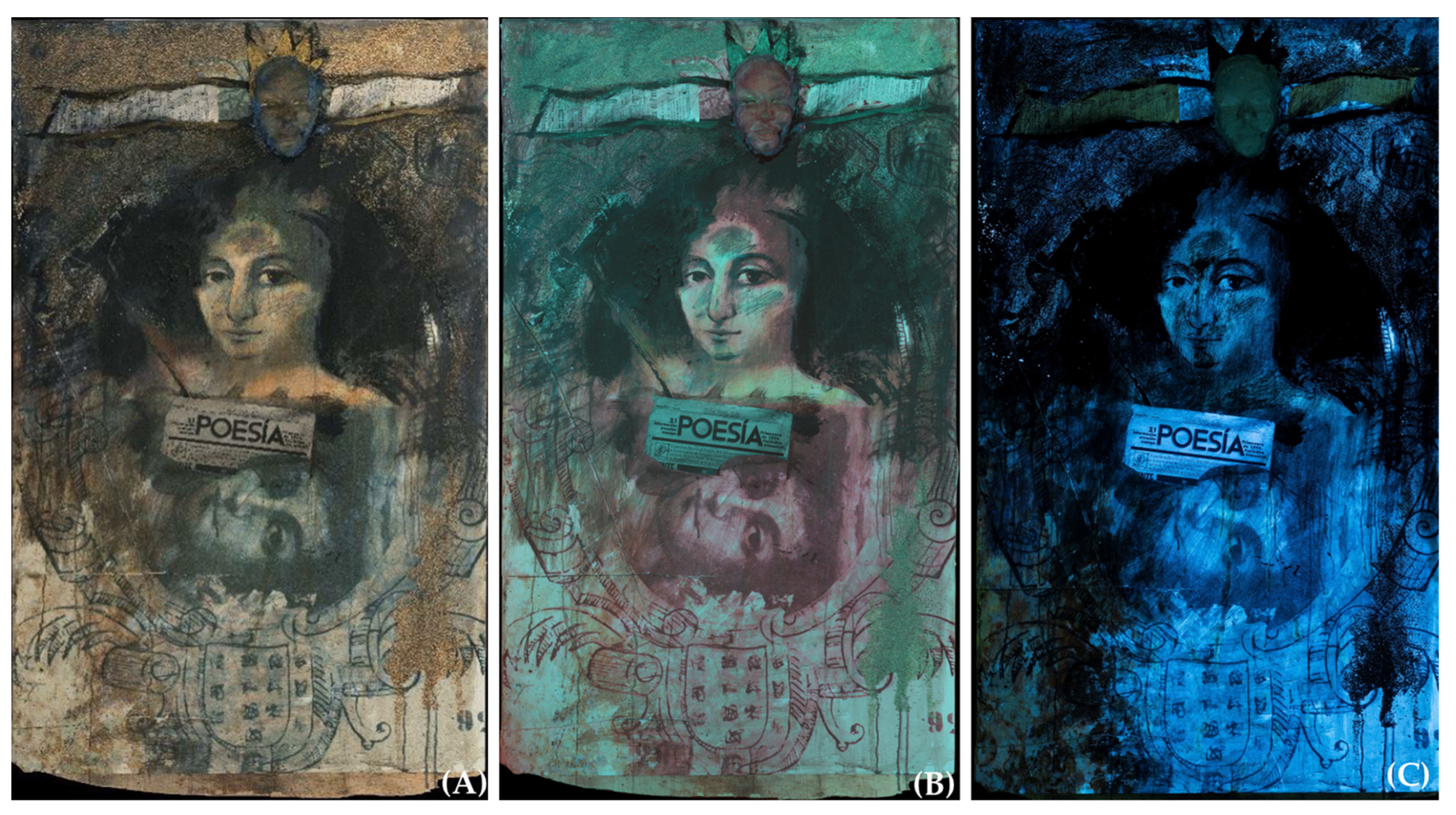

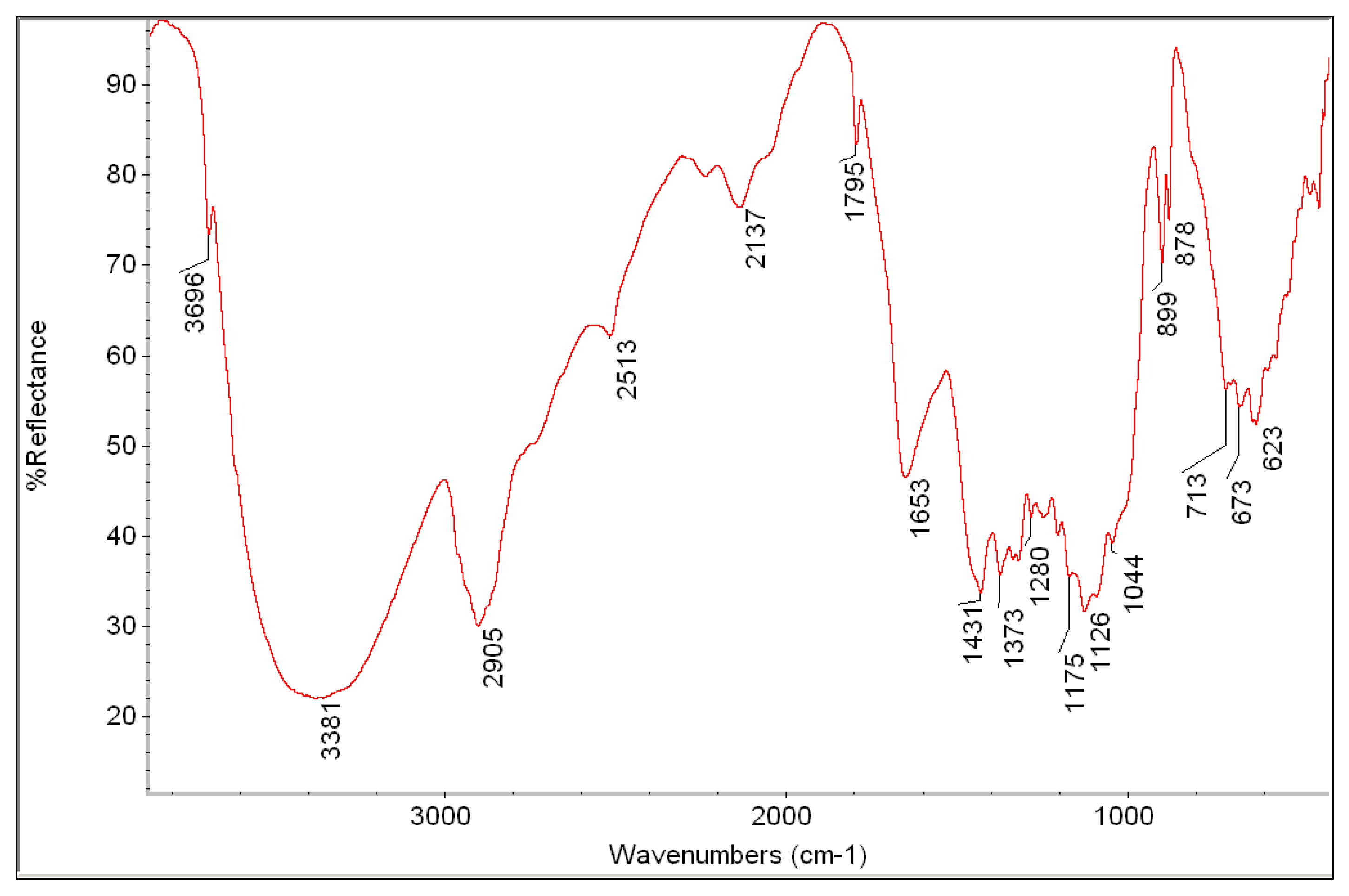
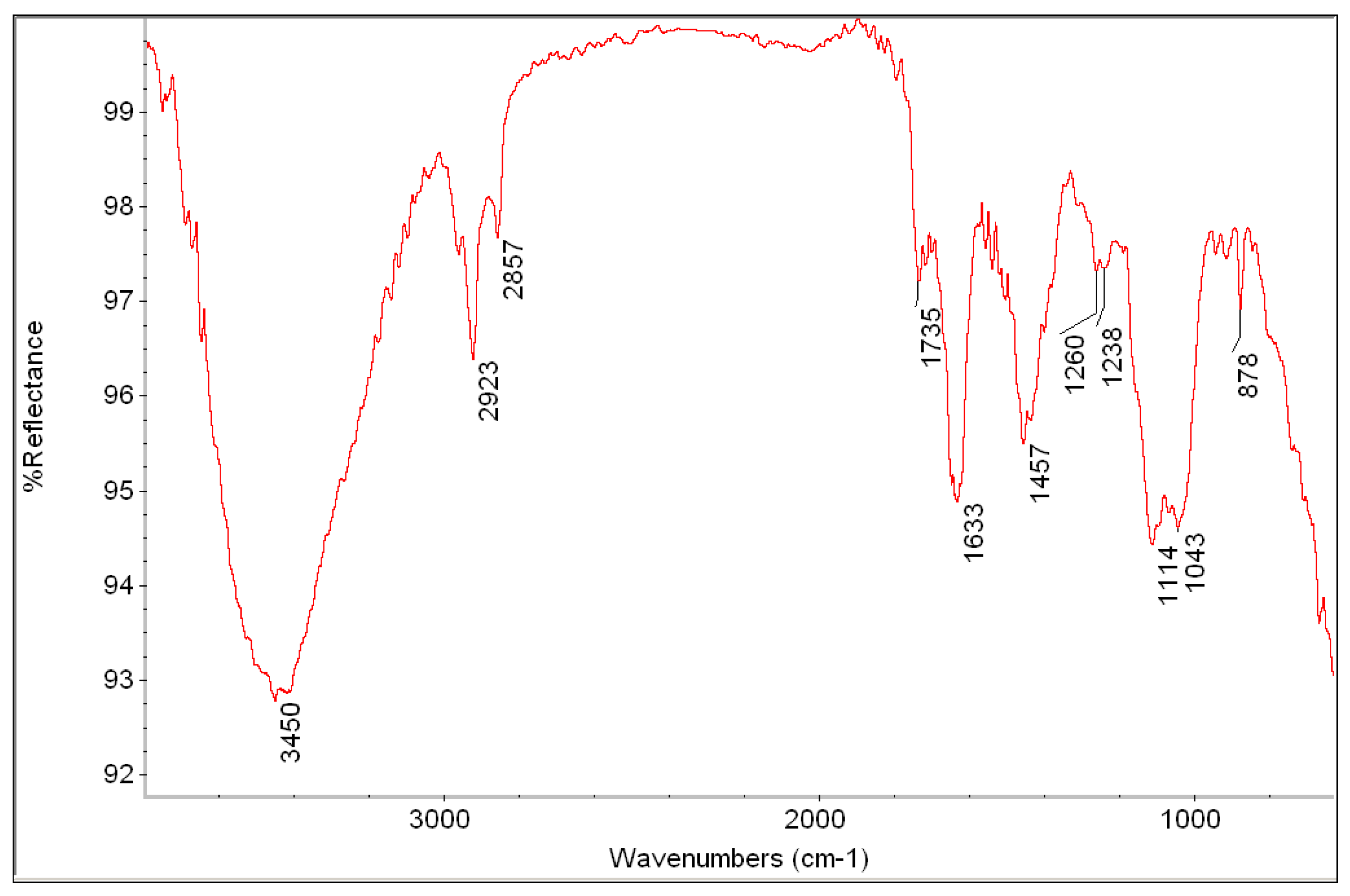
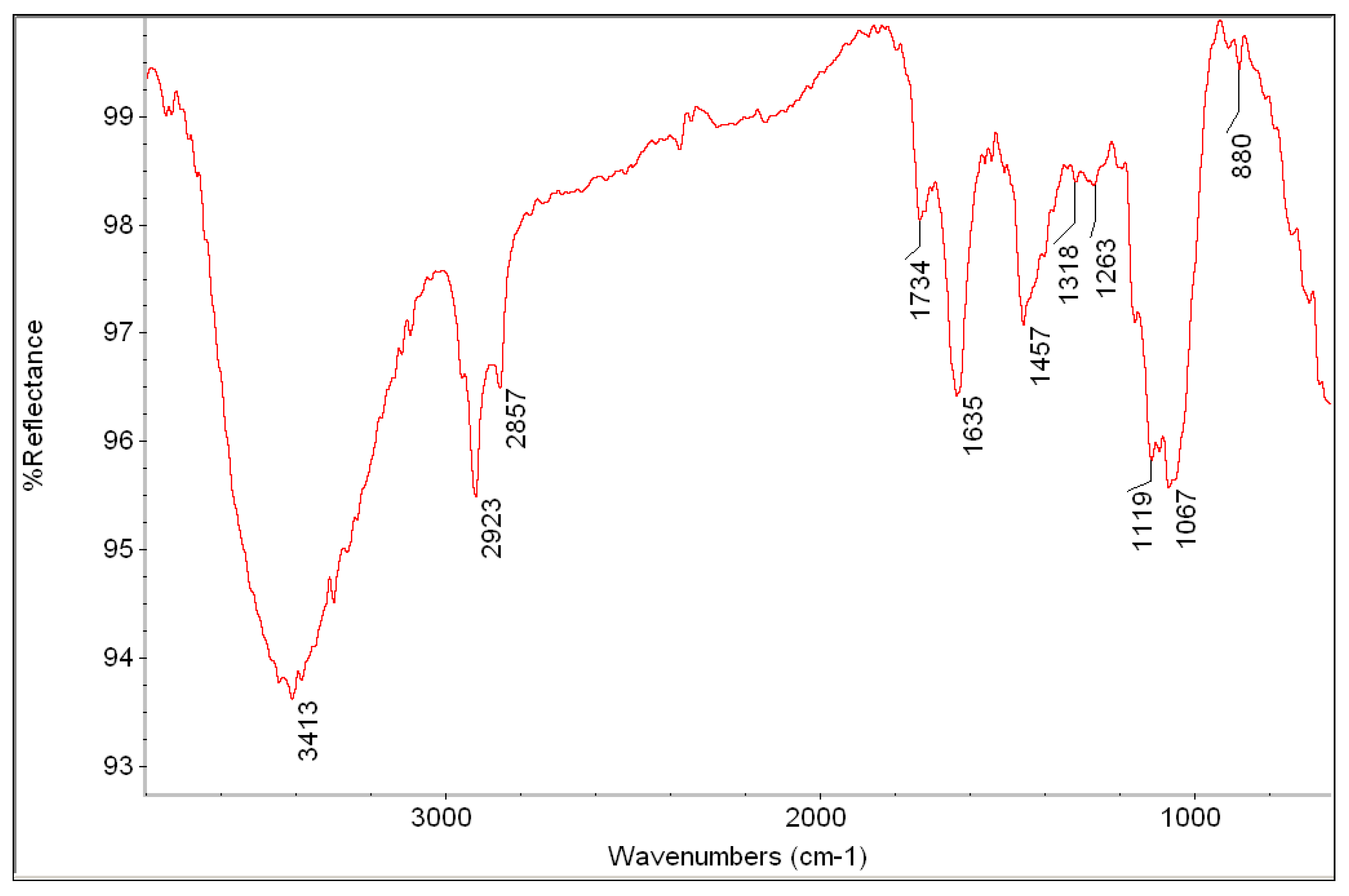

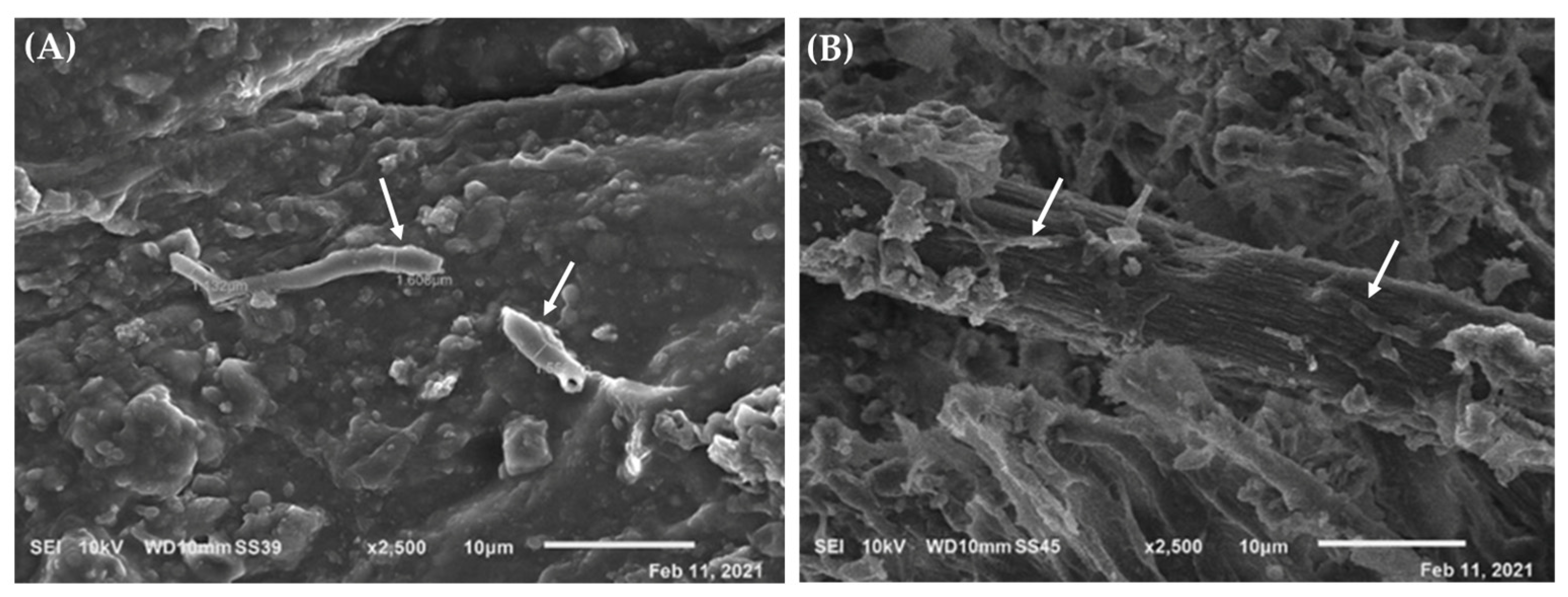
| Measured Point | Detected Elements | Hypothesis about Pigments and Materials |
|---|---|---|
| X1, gold color of the crown | Ti (main), Fe, Ca | Mica pigment based on Ti-oxide and Fe-oxide |
| X2, gold color of the crown | Ti (main), Fe, Ca | Mica pigment based on Ti-oxide and Fe-oxide |
| X3, blue color | Ca (main), Cu, Zn, Fe | Cu-based pigment |
| X4, black color | Ca (main), Fe | Fe-based ink |
| X5, brown glazing | Ca (main), Fe, Zn, Ti | Fe-based pigment |
| X6, sand | Ca, Fe | |
| X7, white of the paper | Ca (main), Fe | Material used in paper treatment |
| X8, brown on the shoulder | Ca (main), Fe | Iron-based pigment |
| Sample Nr. | Layer | Original | After Aging | After Treatment |
|---|---|---|---|---|
| 1 | Paint layer | 6.64 | 5.45 | 5.98 |
| Paper + velatura | 7.44 | 6.67 | 6.58 | |
| Paper | 7.64 | 6.89 | 8.09 | |
| Plywood | 5.75 | 5.48 | 5.67 | |
| 2 | Paint layer | 7.03 | 6.43 | 6.60 |
| Paper + velatura | 7.44 | 6.44 | 7.12 | |
| Paper | 7.70 | 7.63 | 7.39 | |
| Plywood | 6.68 | 6.27 | 6.59 | |
| 3 | Paint layer | 7.07 | 6.61 | 6.62 |
| Paper + velatura | 7.50 | 7.01 | 8.75 | |
| Paper | 7.65 | 6.52 | 7.97 | |
| Plywood | 5.68 | 6.16 | 5.80 | |
| 4 | Paint layer | 6.93 | 6.70 | 6.79 |
| Paper + velatura | 7.52 | 7.47 | 8.30 | |
| Paper | 7.68 | 7.68 | 6.71 | |
| Plywood | 6.33 | 6.28 | 6.22 |
| Measured Point | ΔL* | Δa* | Δb* | ΔE* |
|---|---|---|---|---|
| 1 | −5.00 | 0.82 | 1.57 | 5.30 |
| 2 | 2.32 | −0.94 | −0.083 | 2.50 |
| 3 | 2.78 | −0.91 | −0.30 | 2.94 |
| 4 | −0.91 | 0.01 | 0.72 | 1.17 |
| 5 | −2.77 | 0.37 | 0.62 | 2.86 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ambroselli, F.; Canini, F.; Lanteri, L.; Marconi, M.; Mazzuca, C.; Pelosi, C.; Vinciguerra, V.; Wicks, E.; Zucconi, L. A Hydroalcoholic Gel-Based Disinfection System for Deteriogenic Fungi on the Contemporary Mixed Media Artwork Poesia by Alessandro Kokocinski. Heritage 2023, 6, 2716-2734. https://doi.org/10.3390/heritage6030144
Ambroselli F, Canini F, Lanteri L, Marconi M, Mazzuca C, Pelosi C, Vinciguerra V, Wicks E, Zucconi L. A Hydroalcoholic Gel-Based Disinfection System for Deteriogenic Fungi on the Contemporary Mixed Media Artwork Poesia by Alessandro Kokocinski. Heritage. 2023; 6(3):2716-2734. https://doi.org/10.3390/heritage6030144
Chicago/Turabian StyleAmbroselli, Francesca, Fabiana Canini, Luca Lanteri, Martina Marconi, Claudia Mazzuca, Claudia Pelosi, Vittorio Vinciguerra, Elizabeth Wicks, and Laura Zucconi. 2023. "A Hydroalcoholic Gel-Based Disinfection System for Deteriogenic Fungi on the Contemporary Mixed Media Artwork Poesia by Alessandro Kokocinski" Heritage 6, no. 3: 2716-2734. https://doi.org/10.3390/heritage6030144
APA StyleAmbroselli, F., Canini, F., Lanteri, L., Marconi, M., Mazzuca, C., Pelosi, C., Vinciguerra, V., Wicks, E., & Zucconi, L. (2023). A Hydroalcoholic Gel-Based Disinfection System for Deteriogenic Fungi on the Contemporary Mixed Media Artwork Poesia by Alessandro Kokocinski. Heritage, 6(3), 2716-2734. https://doi.org/10.3390/heritage6030144










