Abstract
The former Artistic and Industrial Recreation Pavilion, which was designed by Antonio Palacios (1874–1945) and built for the Galician Regional Exhibition held in 1909 in Santiago de Compostela (Galicia, north-western Spain), and which currently houses a nursery school, was completely restored in 2018. The main purpose of the restoration was to recover the original exterior colour of the building. For this purpose, a study was undertaken to identify the original colour of the paintwork by first consulting historical archives and then conducting a micromorphological analysis of stratigraphic paint samples by stereomicroscopic examination and scanning electron microscopy (SEM) coupled with electron dispersive spectroscopy (EDS). Three reformations of the building are documented: one carried out in 1926, when the metal roof was replaced with a tile roof; another conducted between 1967 (when the old pavilion was described as a "destroyed building") and the mid-1970s (when it began to be used as a nursery); and finally, another in 1981, when the building was repainted. The analytical results revealed layers of white or yellow ochre (vanilla) paint corresponding to different periods. The presence of titanium (Ti) in the paint was used as a marker of its age, as titanium white was first formulated in 1921. The original layers include Zn in their composition, indicating that zinc oxide (ZnO) was the pigment used in the “snow” white paint probably used on the building in its first years of existence. In all cases, the pigment base is lime mixed with silicates, kaolin and other clays.
1. Introduction
The former Artistic and Industrial Recreation Pavilion (Figure 1), designed by the architect Antonio Palacios Ramilo (O Porriño, Pontevedra 1874 - El Plantío, Madrid 1945) and constructed by master builder Manuel Pereiro Caeiro, was one of the eleven pavilions constructed for the Galician Regional Exhibition held in 1909. After the exhibition, the building was used for other purposes, i.e., as a venue for exhibitions and other artistic events, as a laboratory, as a cinema, as headquarters of the Falange, and, since the mid-1970s, as a nursery school.

Figure 1.
Images of the Artistic and Industrial Recreation Pavilion. From left to right and top to bottom: original design of the Pavilion by Antonio Palacios in 1908 [2]; photograph of the building in 1915, by Manuel Chicharro Bissi (Source: La Voz de Galicia, newspaper); and two recent (2018) photographs of the building, housing the Santa Susana nursery.
Antonio Palacios was born in the town of O Porriño (south-west Galicia, north-west Spain, location of the most important granite quarries in Galicia), twenty-four years after Antoni Gaudí, a Catalan architect and greatest exponent of Modernism in Spain, and thirteen years after Le Corbusier, the father of modern architecture. Palacios took many architectural references from both of these designers. He was a prolific architect with an eclectic style including many historical influences. The work of Palacios was thus influenced by Plateresque, as seen in some of his Galician work, as well as by the Vienna Secession movement led by Otto Wagner, as indicated by his use of functional structures and clean materials, and even by Romanesque art [1]. At the early stage of his career, Palacios developed an architectural style that approached international trends, but he later distanced himself from these and directed his work towards regionalism, the result of his approach to Galician culture. His numerous works, built primarily of granite rock [1], include some monuments in Madrid, such as the Madrid City Council, the Fine Arts Circle and the Communications Palace, and also others in Galicia, such as the García Barbón Theatre in the city of Vigo and the O Porriño City Council Building.
The building under study, constructed between 1908 and 1909, is the only remaining work by Palacios in Santiago de Compostela (capital of Galicia and UNESCO World Heritage City since 1985). According to Barral Martínez [3], in the late 19th century, the city of Santiago de Compostela consisted of the old town around the Cathedral (the most important building in the city) and was defined by contrasts, with an important network of dark alleys and steep slopes next to wide squares and narrow streets. Likewise, the religious buildings, temples and other manors were close to farmhouses with buildings belonging to the poorest inhabitants. Thus, from an urbanistic point of view, the Galician Regional Exhibition held in 1909 in Santiago de Compostela, coinciding with the celebration of the first Jacobean Holy Year in the 20th century, consolidated the opening to the south of the modern expansion of the old site where the University of Santiago’s Campus Vida is now located. Furthermore, both events favoured internationalization of the city, with the number of visitors reaching around 140,000, a figure which was not matched by any Jacobean Holy Year until 1943.
The creation of the Jacobean Holy Year in Santiago dates back to the 15th century (1428 or 1434). Since then, the city has become a famous site of pilgrimage, one of the most important of Christianity. The Camino de Santiago (Way of St. James) leads to the Cathedral, where tradition has it that the remains of the apostle Saint James the Greater are buried. The route is currently one of the most important Christian pilgrimage routes, representing a hugely important international tourist attraction and very valuable economic resource [4].
In 2018, the City Council decided to restore the Artistic and Industrial Recreation Pavilion, currently used as a nursery. The council contracted building restorers to repair the roof of the building, waterproof the existing terraces, repair the drains in the courtyards, build pathways in the interior yards, paint the entire building and to install a diesel tank in the inner courtyard. The contract was awarded under the condition that the work would not affect the internal or external structures of the building. However, the original appearance of the building had been modified by its numerous uses over the years, and a study was commissioned to identify the colour of the original paintwork.
This article presents the findings of the micromorphological analysis of stratigraphic paint samples taken from the exterior of the former Artistic and Industrial Recreation Pavilion (currently housing the Santa Susana nursery) in the city of Santiago de Compostela (north-west Spain). The findings were crosschecked with data from historical archives. The study findings generated the information required to identify the original colour so that the exterior of the building could be painted as originally conceived, guiding the restorers in their choice of the materials during the restoration of the building, which was carried out in late 2018.
2. Materials and Methods
2.1. History of the Building and Its Importance in the Work of Antonio Palacios
On 8 December, 1908, Juan Harguindey Pérez, president of the Artistic and Industrial Recreation of Santiago de Compostela, requested a building licence for constructing the Pavilion.
The licence was granted in February 1909, linking the building to the Regional Exhibition to be held the same year. Manuel Pereiro Caeiro was appointed master builder, and Antonio Flórez, who collaborated with Antonio Palacios in some projects in Madrid, was appointed architect for the project.
The Artistic and Industrial Recreation Pavilion is formally one of the most “European” works of Palacios, with only decorative features such as the female angels that crown the entrance and the bay leaves in horizontal and vertical bas-reliefs included as modernist elements. The building is essentially eclectic with some modernist touches. The Pavilion was built at a time when Palacios had not yet definitively established his own personal style, and therefore, the material loses all meaning and only the forms are perceived. Considered today from the perspective of the subsequent evolution of its author, the building represents a repertoire of tried and rejected elements, an ephemeral trial of a path that Palacios abandoned immediately and forever [5].
In 1916, due to the difficulty in meeting maintenance costs and the scant use of the building, its ownership was transferred to the City Council, which used it for different purposes (as a cinema, among other uses) before finally installing a municipal laboratory during a period of ten years. The original roof had become very fragile and new beams and braces were required, and the metal roof was finally replaced by tile roofing in 1926. This reformation gave rise to a building of very similar appearance to the present building, as the large vases that finished off the corners disappeared, as did the glass canopies on the sides. The Pavilion was later used as a cafeteria and restaurant, and during the Spanish Civil War (1936-1939) it was requisitioned by the Falange (led by the Spanish dictator Francisco Franco), an organisation of authoritarian, conservative ideology connected with Francoist Spain.
A nursery school was temporarily transferred to the Pavilion between 1955 and 1961. The building was then closed after being reported to be in very poor condition and in serious danger of collapse. Further reformation was carried out at some unspecified time between 1967 and 1976 and then again between 1981 and 1984, with the second of these works involving (as stated in writing) painting the entire building.
No information was found in the archives consulted about the building materials used in constructing the building or in the subsequent reformations.
2.2. Sampling and Description of the Analytical Techniques
As the purpose of the work reported here was to identify the original colour of the paintwork, the building was inspected for areas where the paint was chipped and the inner layer was exposed. Ten samples of about 70-100 mm of diameter were obtained from different areas. The sampling points were recorded, and the samples were examined by microphotography under a stereomicroscope (Nikon, SMZ-2T) and light microscope (Nikon Eclipse 50i). After the initial analysis, two representative samples in each set were chosen for further analysis (Figure 2): a building pillar (Figure 2A) and an ornamental part (Figure 2B). In order to observe the morphology of the pictorial preparations and determine the elementary composition of pigments and fillers, the samples were embedded in epoxy resin and, once this had set, cut transversally to the paint layers and polished. The cross-sections thus obtained enabled detailed study of the different paint layers. The cross- sections were observed under an OLYMPUS SZX7 (Germany) zoom stereomicroscope, and the general characteristics of the sample (number, colour, thickness, degree of adhesion and state of conservation of the different paint layers) were determined. The cross-sections were then studied under a Scanning Electronic Microscope EVO-LS15 (ZEISS, Germany) in variable pressure mode (10–400 Pa), which minimises the charge of the sample without the need for a metallic coating. The backscattered-electron images (BSE) were obtained at 15 kV. Analysis of the elemental composition was performed with a coupled energy-dispersive X-ray spectroscopy (EDS) micro-probe, with an X-Ray detector (Oxford Inca X-act, of resolution 129 eV). Measurements were made on spots and areas representative of the materials investigated and, in some cases, the distribution of elements was also mapped on the sample. The working conditions were as follows: 20 kV accelerating voltage, 2 3 1029 A; beam current, 100 s; and working distance, 8.5 mm.

Figure 2.
General image (left) and detail (right) of the sampled areas of the building: (A) pillar and (B) ornamental part. Photographs: Marta Díaz.
3. Results and Discussion
The front view of the samples taken from the pillars (Figure 2A) revealed vanilla (yellow ochre) coloured remains of a superficial and more recent layer of a largely disintegrated layer of paint (Figure 3).
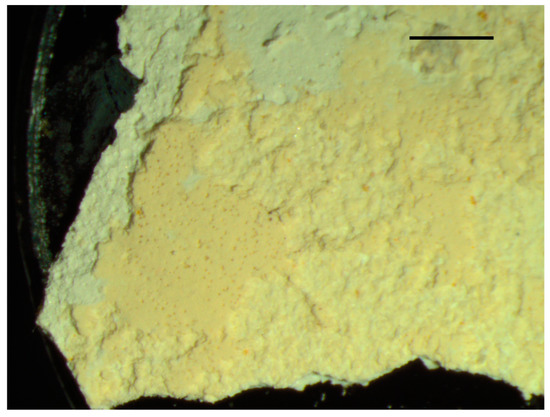
Figure 3.
Front view of the surface of a sample taken from the pillars. Scale bar is 1 mm.
The cross-sectional samples of the pillars (henceforth, sample A) consisted of seven different layers, of different thicknesses, on a cement substrate (Figure 4). The layers, ordered from the most superficial (i.e., the most recent) to that adhered to the cement substrate (i.e., the oldest or original layer), were characterised as follows: (1) remains of a highly disintegrated vanilla-coloured layer, (2) a white layer of approximately 110 microns thick, (3) a thin, pinkish layer of about 40 microns thick, (4) a yellow ochre layer of between 100 and 130 microns thick, (5) a thick porous, whitish layer of between 200 and 600 microns thick, (6) a yellowish-whitish layer ranging from 80 to 300 microns thick, followed by (7) another very similar layer of 100 microns thick, but of a more solid colour (Figure 4).

Figure 4.
Stereomicroscope images of a cross-section of samples taken from the pillar (sample A), embedded in acrylic resin (upper image) and prepared for micromorphological study by scanning electron microscopy and elemental analysis (lower image). In both images, six segments (indicated in red) corresponding to the layers of different thicknesses were observed. The most recent and the oldest two levels (upper and lower) were observed and analysed by SEM/EDS.
As seen in Figure 5, the microanalysis by SEM/EDS of the most superficial layer (layer 1), vanilla-coloured and highly disintegrated (Figure 3), indicates that the major elements are Ca and O, both indicative of the presence of lime, and also Ba, which indicates the presence of barite (barium sulphate). Si also appears along with other elements, such as Mg, Al, S, K, Fe and Zn, typical of clays, such as kaolin (Al, Si, O).
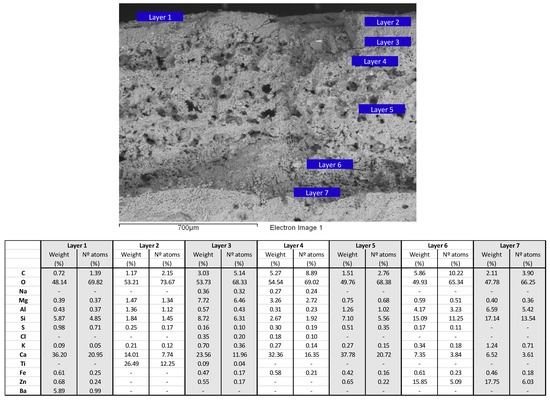
Figure 5.
Results of the micromorphological analysis by SEM/EDS of the seven layers of sample A, taken from the pillars of the building.
The next, white-coloured layer (layer 2, Figure 5) mainly contained O, Ti and Ca, and also Si, Mg, Al, S and K. The base of the paint therefore appears to be lime and the white pigment, Ti oxide or titanium white (TiO2), which was not manufactured regularly or marketed until the 1920s [6,7], after which it became the most abundantly used pigment in the 20th century. Si, Mg, Al, S and K, which appear in a proportion of less than 2% (percentage weight), form part of the clays that make up the base of the paint. S may also be associated with the presence of gypsum or other sulphates, soluble salts that are very common in plasters.
The underlying pink-coloured layer (layer 3, Figure 5) was found to contain large amounts of Ca and O, and also Si and Mg, in non-negligible proportions, of percentage weight of around 8% and 9 %, respectively. Other elements such as K, Al, S, Na, Fe, Ti, and Zn were detected in smaller quantities, and the amount of the latter two (Ti and Zn) was almost negligible. It is therefore a plaster or lime preparation (Ca, O) with silicates as kaolin minerals (Al, Si, O) and other clays (Mg, Si, O) in its composition. The clays contain iron, giving rise to the pink colour. Cl, an uncommon component of paint, was also detected, and assumed to be associated with the presence of sodium (Figure 5), indicating the possible origin of marine aerosols usually found in the air, even in inland environments [8].
The next yellow ochre layer (layer 4, Figure 5) also contains elements of lime and silicates that make up the mortar or plaster, mainly Ca and O, and also C (a lighter element, the content of which is difficult to measure by EDS, especially in samples embedded in epoxy resin), Mg and Si. Other elements, already present in low proportions, include Na, Al, S, K, Fe and also Cl, as in the previous layer.
The next thick, porous white layer (layer 5, Figure 5) also mainly contained Ca and O, as well as a non-negligible amount of Si, around 7% by weight. Al, Mg, S, K and Fe appear in proportions of around 1%, indicating that lime was again mixed with some silicates to form the plaster. Some Zn was also detected, but may represent contamination from the underlying layers 6 and 7.
The last two layers (6 and 7, Figure 5) are of very similar composition, mainly containing Ca, O, C and Si, typical of the paint bases already mentioned, and also Al, which in these two oldest layers appear in non-negligible proportions of around 4% and 7% respectively (percentage weight). Both layers contain important amounts of Zn, an element that appeared in almost residual amounts in the previous layers. The amounts of Zn and Si in these layers are very similar, with percentage weights of around 15% and 17% respectively. The composition indicates that the base support of pigments or load is lime mixed with silicates rich in Al, and the pigment material is zinc white (ZnO). This pigment provides a “snow” white colour, of a cooler shade than lead and titanium white. Formulation of this pigment dates back to the 18th century and is therefore consistent with a paint formulation used in 1909.
Zinc oxide as a white pigment was widely diffused across Europe for about a century, between 1834 [9] and the late 1960s, when it was gradually replaced by titanium white [10]. Compared to the highly toxic white lead, another formerly widely used white pigment, its practical benefits are its fineness, a lesser tendency to yellowing and its resistance to darkening when mixed with hydrogen sulphide pigments [10,11]. The well-known greater resistance and anti-microbial action (mainly against fungal attack) of zinc oxide also favour use of this component over lead white and titanium oxide [12].
According to Downs [13], white zinc paint began to attract serious interest in America in 1848-1849. Its snowy white colour, hardness and relatively low price, compared with that of white lead, as well as its high resistance to weathering, led to the first American patent for its manufacture being obtained in 1850.
White zinc paint was commonly used on buildings in the second half of the 19th century and the beginning of the 20th century. For example, it was used on the Said Halim palace, Cairo (Egypt), which was designed and constructed by the Italian architect Antonio Lasciac between 1899 and 1901 and which belonged to Mohamed Ali’s family during the period 1805-1952 [14]. Zinc oxide was also the white pigment used in the ceiling paintings in the historic Dauis Church and Loay Church, in Bohol (Philippines), construction of which began in the late 19th century [15]. In the USA, the 1854 specifications for the remodelling of the Salem Custom House specified the use of zinc oxide for painting the woodwork [16].
The samples from the ornamental part of the building (Figure 2B, henceforth, sample B) contained an upper white coating and a lower yellow ochre coating (Figure 6), each consisting of several layers. SEM-EDS analysis revealed that both coatings contain large amounts of Ti (Figure 7), indicating that the entire sample contained coats of paint applied after the 1920s and, therefore, that this sample did not contain the original exterior paint of the building.
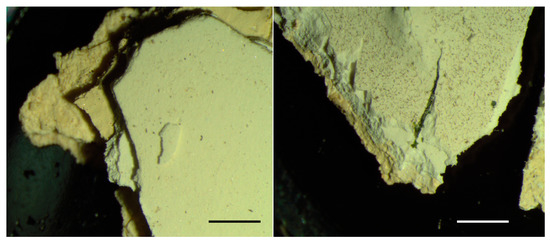
Figure 6.
Stereomicroscope images of the samples taken from the ornamental parts of the building (sample B). In both front views, the upper white coating and the lower yellow ochre coating can be observed: both are thick coatings consisting of several layers. Scale bar is 1 mm.
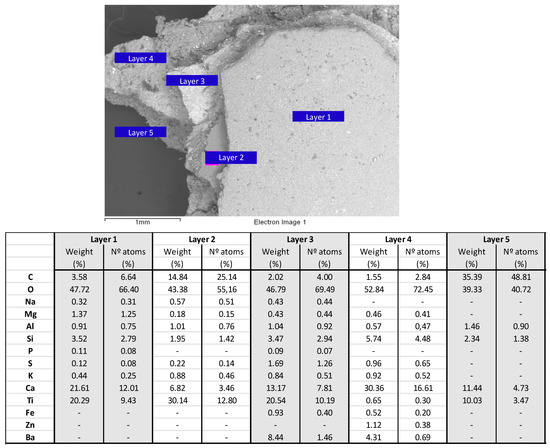
Figure 7.
Results of the micromorphological analysis by SEM/EDS of the five layers of sample B, taken from the ornamental part of the building.
The upper and more recent white coating consists of two distinguishable layers (layers 1 and 2, Figure 7), a lower one with a high content of C, O, Ca and Ti (layer 2) and an upper one with a high content of O, Ca and Ti (layer 1), apparently indicating that the paint contained lime and titanium oxide (titanium white). Na, Mg, Al, Si (in a higher proportion in the upper layer), S and K, silicate elements, also appear in both layers.
Three layers were distinguished in the yellow ochre coating, which corresponds to the innermost part of the sample (layers 3-5, Figure 7). The oldest layer mainly contains C and O and, to a lesser extent, Ca and Ti, and it therefore appears to be a lime preparation (Ca, C, O) with Ti oxide as pigment filler (layer 5). Al and Si, typical of the clays that also make up the pigment charge, also appear. The next layer, i.e., the layer above the previous one, also yellow ochre (layer 4), contains large amounts of Ca and O, as well as Si and Ba, with scarce presence of Ti. This finding seems to indicate that the layer represents a silicates/barite lime preparation that serves as a plaster or mortar for the upper layer (layer 3), of the same colour and containing significant amounts of Ca, O, Ti, Ba and Si, i.e., lime with silicates and containing titanium white as pigment (TiO2). In all three layers, the ochre colour is due to the presence of Fe.
4. Conclusions
The above reported findings indicate that the original pigment used in the Artistic and Industrial Recreation Pavilion designed by Antonio Palacios and built for the Galician Regional Exhibition held in 1909 in Santiago de Compostela (NW Spain) was zinc oxide (ZnO), which gave the building a “snow” white colouration. However, this pigment was not used in subsequent restorations. Other white pigments such as barium sulphate and titanium white were used, the latter (first formulated in 1921) in the 1926 restoration.
The ochre layers are all not original, as they contain titanium oxide, and the colour is due to the presence of Fe.
The basis of both white and ochre pigments, and hence original and non-original pigments, is the same: lime mixed with silicates, including kaolin and other clays.
Author Contributions
P.S. and B.P. devised the methodology; P.S. and B.P. validated the method; P.S. and B.P. conducted the formal analysis; P.S. and B.P. conducted the research; P.S. prepared and wrote the original draft of the manuscript, P.S. and B.P. wrote, revised and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
The Xunta de Galicia, Consellería de Servizos Sociais financially supported this work under a contract for the ’Rehabilitación Escuela infantil de Sta Susana (Santiago de Compostela)’ with Tragsa and Arrokabe architects, who commissioned the painting characterisation project to the GEMAP-USC research group, to which both authors of the manuscript belong.
Acknowledgments
The authors thank Oscar Andrés, the chief architect of the construction Management, and Marta Díaz, of BIC materiales y conservación SL, for their valuable assistance in the study. The authors are also grateful for funding awarded by the Xunta de Galicia within the project “Consolidación y estructuración de unidades de investigación competitivas—Grupos de referencia competitiva (GRC)” (Ref. ED431C 2018/32).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Franco Taboada, J.A. Antonio Palacios, unique modern architecture seen through his drawings. Rev. Expresión Gráfica Arquit. 2017, 22, 268–287. [Google Scholar] [CrossRef][Green Version]
- University Historical Archive. Santiago. (A.H.U.S.) Section: License for works. Book, 1 of 1920. (23.2.5) File Concession of Work License to the Artistic and Industrial Recreation of This City to Build a Pavilion on the Bóveda Esplanade.
- Barral Martínez, M. La exposición regional gallega de 1909: Objetivos y logros en clave moderna. In La Historia: Lost in translation? Actas del XIII Congreso de la Asociación de Historia Contemporánea; González Madrid, D.A., Ortiz Heras, M., Pérez Garzón, J.S., Eds.; Servicio de Publicaciones de la Universidad de Castilla-La Mancha: Cuenca, Spain, 2017; pp. 3283–3296. (In Spanish) [Google Scholar]
- Gonzalez, R.; Medina, J.; Medina, X.S. Cultural tourism and urban management in northwestern Spain: The pilgrimage to Santiago de Compostela. Tour. Geogr. 2003, 5, 446–460. [Google Scholar] [CrossRef]
- Beramendi, X. Pabellón de Recreo en Santiago, de Antonio Palacios. Revista Arquitectura Colexio oficial Arquitectos Galicia 1984, 52–57. (In Spanish) [Google Scholar]
- Gettens, R.J.; Stout, G.L. Painting Materials. A Short Encyclopaedia; Dover Publications, Inc.: New York, NY, USA, 1966. [Google Scholar]
- Prieto, B.; Sanmartín, P.; Pereira-Pardo, L.; Silva, B. Recovery of the traditional colours of painted woodwork in the Historical Centre of Lugo (NW Spain). J. Cult. Herit. 2011, 12, 279–286. [Google Scholar] [CrossRef]
- Silva, B.; Rivas, T.; García- Rodeja, E.; Prieto, B. Distribution of ions of marine origin in Galicia (NW Spain) as a function of distance from the sea. Atmos. Environ. 2007, 41, 4396–4407. [Google Scholar] [CrossRef]
- Eastaugh, N.; Walsh, V.; Chaplin, T.; Siddall, R. The Pigment Compendium: A Dictionary of Historical Pigments; Elsevier–Butterworth Heinemen: Oxford, UK, 2004; p. 499. [Google Scholar]
- Pratali, E. Zinc oxide grounds in the 19th and 20th century oil paintings and their role in picture degradation processes. CeROArt 2013, 3. [Google Scholar] [CrossRef]
- Kühn, H.; dans Feller, R.L. (Eds.) Artist’s Pigments: A Handbook of Their History and Characteristics; Cambridge University Press: Cambridge, UK, 1986; pp. 169–186. [Google Scholar]
- Gaylarde, C.C.; Morton, L.H.G.; Loh, K.; Shirakawa, M.A. Biodeterioration of external architectural paint films—A review. Int. Biodeterior. Biodegrad. 2011, 65, 1189–1198. [Google Scholar] [CrossRef]
- Downs, A.C., Jr. Zinc for Paint and Architectural Use in the 19th Century. Bull. Assoc. Preserv. Technol. 1976, 8, 80. [Google Scholar] [CrossRef]
- Aly, M.F.; Bakr, A.M. Characterization of artificial marble “Scagliola” used in Historical Building in Cairo, Egypt. In Proceedings of the 4th International Congress on “Science and Technology for the Safeguard of Cultural Heritage in the Mediterranean Basin, Cairo, Egypt, 6–8 December 2009; pp. 408–414. [Google Scholar]
- Roxas, G.C.; Han, M.S.; Moon, D.H. Scientific Analysis of Pigments in 20th Century Paintings for Selected Historical Churches of the Bohol, Philippines. J. Conserv. Sci. 2017, 33, 507–518. [Google Scholar] [CrossRef]
- Perrault, C.L. Techniques Employed at the North Atlantic Historic Preservation Center for the Sampling and Analysis of Historic Architectural Paints and Finishes. Bull. Assoc. Preserv. Technol. 1978, 10, 6. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).