Prediction of Autonomy Loss in Alzheimer’s Disease
Abstract
:1. Introduction and Objective
2. Method
2.1. Study Design—Data
2.2. Construction and Validation—Bayesian Network
2.3. Comparison with Logistic Regression
2.4. Statistical Analysis
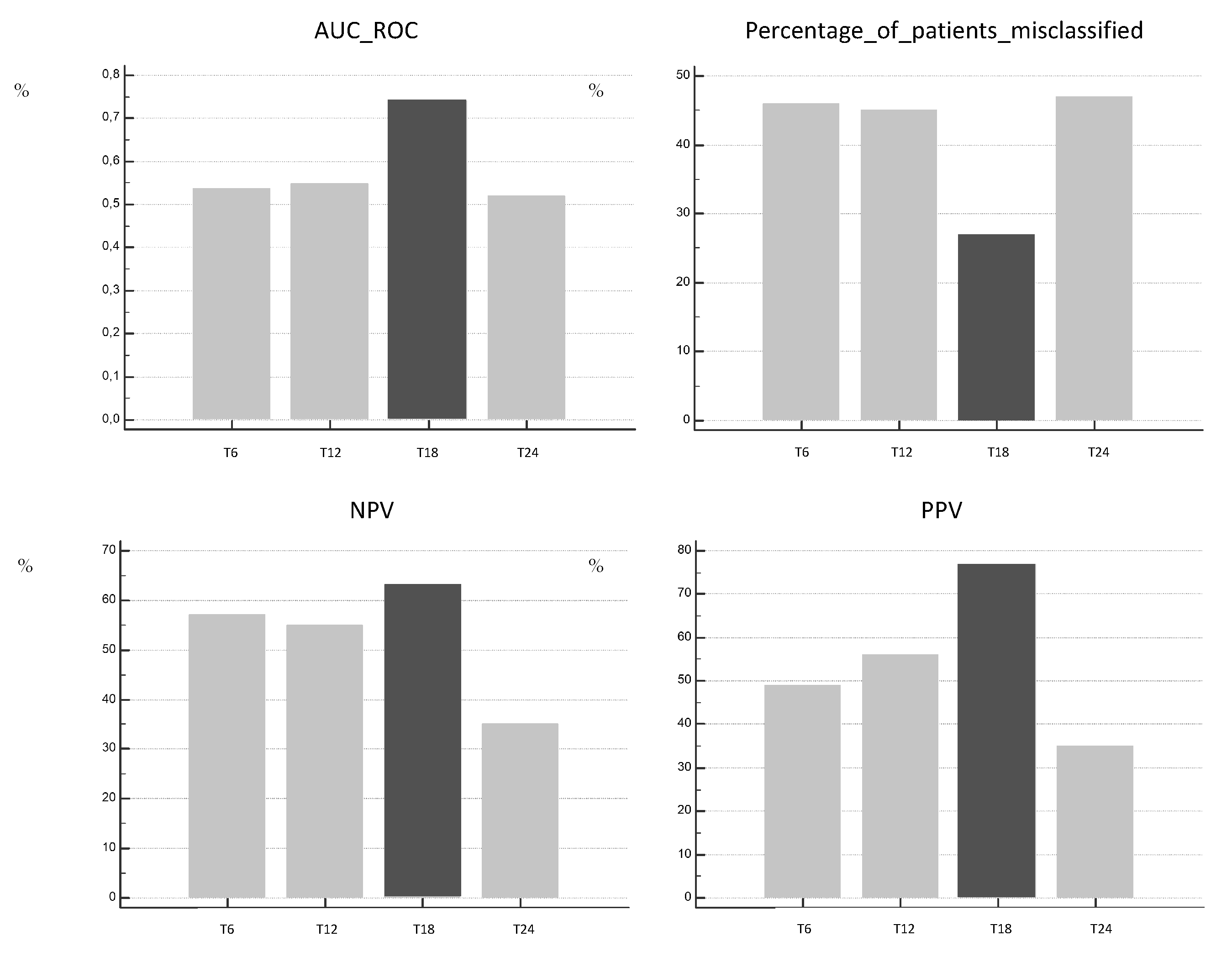
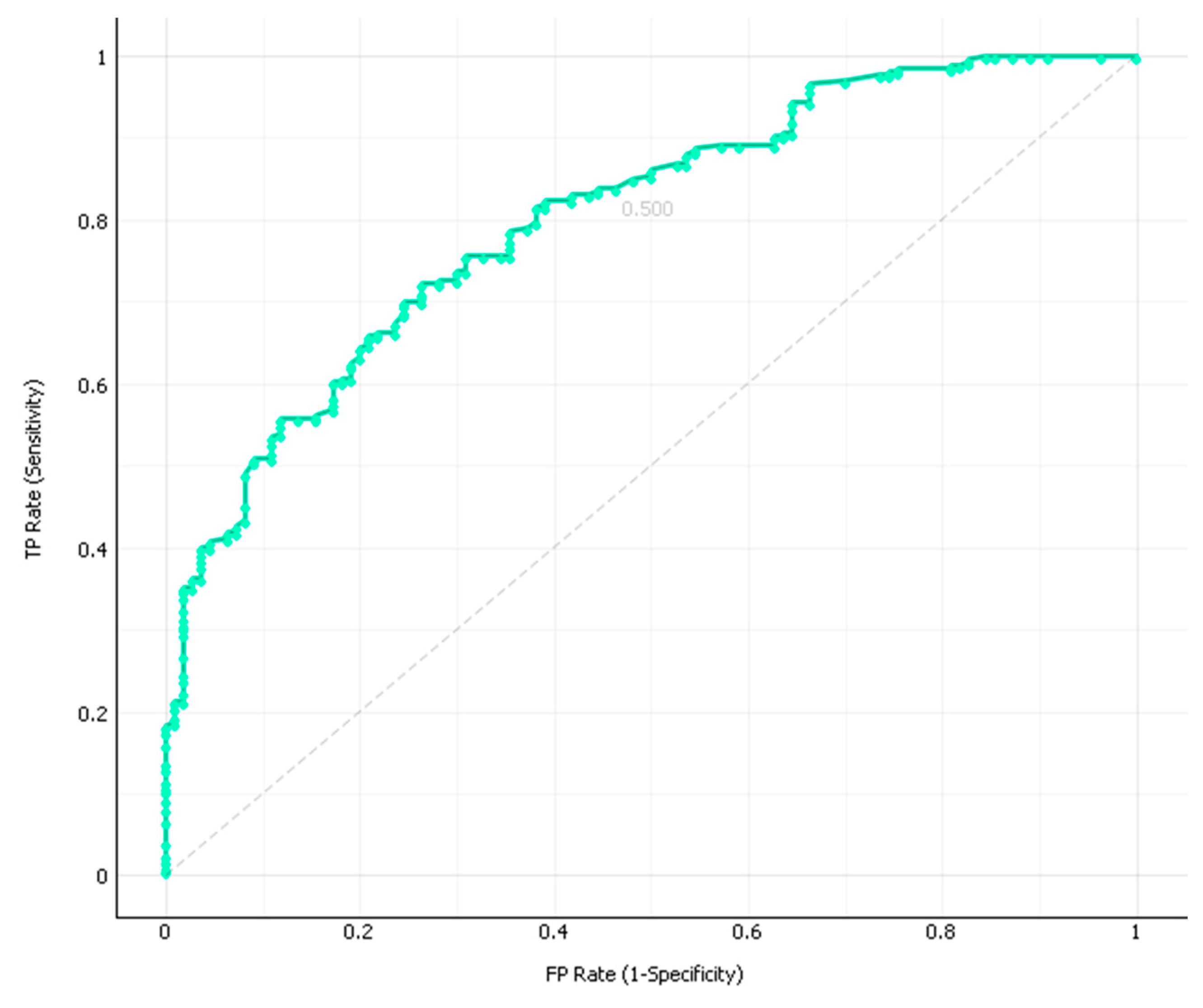
3. Results
4. Discussion
5. Conclusions/Relevance
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alzheimer’s Association 2018 Alzheimer’s disease facts and figures. Alzheimers Dement 2018, 14, 367–429. [CrossRef]
- Alzheimer’s Disease International, Dementia Statistics. Available online: https://www.alz.co.uk/research/statistics (accessed on 14 April 2018).
- Dartigues, J.-F.; Helmer, C.; Letenneur, L.; Péres, K.; Amieva, H.; Auriacombe, S.; Orgogozo, J.-M.; Commenges, D.; Jacqmin-Gadda, H.; Richard-Harston, S.; et al. Paquid 2012: Illustration and overview. Geriatr. Psychol. Neuropsychiatr. Vieil. 2012, 10, 325–331. [Google Scholar] [CrossRef]
- Feldman, H.H.; Woodward, M. The staging and assessment of moderate to severe Alzheimer disease. Neurology 2005, 65, S10. [Google Scholar] [CrossRef]
- Le Duff, F.; Develay, A.E.; Quetel, J.; Lafay, P.; Schück, S.; Pradier, C.; Robert, P. French National Alzheimer dataBank (BNA) The 2008–2012 French Alzheimer plan: Description of the national Alzheimer information system. J. Alzheimers Dis. 2012, 29, 891–902. [Google Scholar] [CrossRef] [Green Version]
- Dauphinot, V.; Delphin-Combe, F.; Mouchoux, C.; Dorey, A.; Bathsavanis, A.; Makaroff, Z.; Rouch, I.; Krolak-Salmon, P. Risk factors of caregiver burden among patients with Alzheimer’s disease or related disorders: A cross-sectional study. J. Alzheimers Dis. 2015, 44, 907–916. [Google Scholar] [CrossRef] [Green Version]
- Crespo, M.; Hornillos, C.; de Quirós, M.B. Factors associated with quality of life in dementia patients in long-term care. Int. Psychogeriatr. 2013, 25, 577–585. [Google Scholar] [CrossRef]
- Balardy, L.; Voisin, T.; Cantet, C.; Vellas, B.; REAL. FR Group. Predictive factors of emergency hospitalisation in Alzheimer’s patients: Results of one-year follow-up in the REAL.FR Cohort. J. Nutr. Health Aging 2005, 9, 112–116. [Google Scholar]
- Wattmo, C.; Wallin, A.K.; Londos, E.; Minthon, L. Risk factors for nursing home placement in Alzheimer’s disease: A longitudinal study of cognition, ADL, service utilization, and cholinesterase inhibitor treatment. Gerontologist 2011, 51, 17–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bérard, A.; Gervès, C.; Fontaine, R.; Aquino, J.-P.; Plisson, M. Combien coûte la maladie d’Alzheimer. Fond. Médéric Alzheimer 2015, 98, 9. [Google Scholar]
- Lalande, L.; Bourguignon, L.; Carlier, C.; Ducher, M. Bayesian networks: A new method for the modeling of bibliographic knowledge: Application to fall risk assessment in geriatric patients. Med. Biol. Eng. Comput. 2013, 51, 657–664. [Google Scholar] [CrossRef]
- Ducher, M.; Mounier-Vehier, C.; Lantelme, P.; Vaisse, B.; Baguet, J.-P.; Fauvel, J.-P. Reliability of a Bayesian network to predict an elevated aldosterone-to-renin ratio. Arch. Cardiovasc. Dis. 2015, 108, 293–299. [Google Scholar] [CrossRef]
- Kaewprag, P.; Newton, C.; Vermillion, B.; Hyun, S.; Huang, K.; Machiraju, R. Predictive models for pressure ulcers from intensive care unit electronic health records using Bayesian networks. BMC Med. Inform. Decis. Mak. 2017, 17, 65. [Google Scholar] [CrossRef] [PubMed]
- Sakai, S.; Kobayashi, K.; Nakamura, J.; Toyabe, S.; Akazawa, K. Accuracy in the diagnostic prediction of acute appendicitis based on the Bayesian network model. Methods Inf. Med. 2007, 46, 723–726. [Google Scholar] [CrossRef] [PubMed]
- Langarizadeh, M.; Moghbeli, F. Applying Naive Bayesian Networks to Disease Prediction: A Systematic Review. Acta Inf. Med. 2016, 24, 364–369. [Google Scholar] [CrossRef]
- Seixas, F.L.; Zadrozny, B.; Laks, J.; Conci, A.; Muchaluat Saade, D.C. A Bayesian network decision model for supporting the diagnosis of dementia, Alzheimer’s disease and mild cognitive impairment. Comput. Biol. Med. 2014, 51, 140–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Y.; Lv, S.; Tang, Y. Construction and Application of Bayesian Network in Early Diagnosis of Alzheimer Disease’s System. In Proceedings of the 2007 IEEE/ICME International Conference on Complex Medical Engineering, Gold Coast, QLD, Australia, 13–15 July 2007; pp. 924–929. [Google Scholar]
- Lechowski, L.; De Stampa, M.; Tortrat, D.; Teillet, L.; Benoit, M.; Robert, P.H.; Vellas, B.; REAL. FR Group. Predictive factors of rate of loss of autonomy in Alzheimer’s disease patients. A prospective study of the REAL.FR Cohort. J. Nutr. Health Aging 2005, 9, 100–104. [Google Scholar]
- Lechowski, L.; Dieudonne, B.; Tortrat, D.; Teillet, L.; Robert, P.H.; Benoit, M.; Forette, B.; Vellas, B. Role of behavioural disturbance in the loss of autonomy for activities of daily living in Alzheimer patients. Int. J. Geriatr. Psychiatry 2003, 18, 977–982. [Google Scholar] [CrossRef]
- Melis, R.J.F.; Marengoni, A.; Rizzuto, D.; Kivipelto, M.; Angleman, S.; Fratiglioni, L. The influence of multimorbidity on clinical progression of dementia in a population-based cohort. Alzheimers Dement 2013, 11, P261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lawton, M.P.; Brody, E.M. Assessment of older people: Self-maintaining and instrumental activities of daily living. Gerontologist 1969, 9, 179–186. [Google Scholar] [CrossRef]
- Delva, F.; Auriacombe, S.; Letenneur, L.; Foubert-Samier, A.; Bredin, A.; Clementy, A.; Latxague, C.; Puymirat, E.; Ballan, G.; Delabrousse-Mayoux, J.-P.; et al. Natural history of functional decline in Alzheimer’s disease: A systematic review. J. Alzheimers Dis. 2014, 40, 57–67. [Google Scholar] [CrossRef]
- Mok, W.Y.W.; Chu, L.W.; Chung, C.P.; Chan, N.Y.; Hui, S.L. The relationship between non-cognitive symptoms and functional impairment in Alzheimer’s disease. Int. J. Geriatr. Psychiatry 2004, 19, 1040–1046. [Google Scholar] [CrossRef]
- Tekin, S.; Fairbanks, L.A.; O’Connor, S.; Rosenberg, S.; Cummings, J.L. Activities of Daily Living in Alzheimer’s Disease: Neuropsychiatric, Cognitive, and Medical Illness Influences. Am. J. Geriatr. Psychiatry 2001, 9, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Frisoni, G.B.; Fox, N.C.; Jack, C.R.J.; Scheltens, P.; Thompson, P.M. The clinical use of structural MRI in Alzheimer disease. Nat. Rev. Neurol. 2010, 6, 67–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berrut, G.; Decker, L. Comprehensive assessment of comorbidity in the elderly. Geriatr. Psychol. Neuropsychiatr. Viei. 2015, 13, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.D.; Paradis, C.F.; Houck, P.R.; Mazumdar, S.; Stack, J.A.; Rifai, A.H.; Mulsant, B.; Reynolds, C.F., 3rd. Rating chronic medical illness burden in geropsychiatric practice and research: Application of the Cumulative Illness Rating Scale. Psychiatry Res. 1992, 41, 237–248. [Google Scholar] [CrossRef]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic. Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]


| Variables | All AD Patients (n = 668) | AD Patients with ≥2 Visits (n = 485) | p |
|---|---|---|---|
| Age | 82.1 ± 6.13 | 82.1 ± 6.2 | n.s |
| Sex (Female) | 61.4 | 64.3 | n.s |
| Geographic location | |||
| CMRR city | 81.4 | 82.1 | n.s |
| <50 km not in CMRR city | 16.5 | 15.7 | n.s |
| >50 km | 1.8 | 1.9 | n.s |
| Out of region | 0.3 | 0.4 | n.s |
| Family situation | |||
| Lived with someone | 63.0 | 63.9 | n.s |
| Lived alone | 33.4 | 32.8 | n.s |
| Lived out of home | 3.0 | 2.9 | n.s |
| Lived at home without other information | 0.4 | 0.4 | n.s |
| Other | 0.1 | 0.0 | n.s |
| Comorbidities: number | 1.3 ± 1.7 | 1.3 ± 1.7 | n.s |
| IADL score | 3.3 ± 2.1 | 3.4 ± 2.1 | n.s |
| MMSE score | 18.1 ± 5.8 | 18.2 ± 5.6 | n.s |
| Antidepressant drug prescription (No) | 62.1 | 62.3 | n.s |
| Antipsychotic drug prescription (No) | 94.9 | 95.3 | n.s |
| Anxiolytic drug prescription (No) | 84.1 | 84.9 | n.s |
| Hypnotic drug prescription (No) | 97.3 | 96.9 | n.s |
| Alzheimer’s specific-drug prescription | |||
| No | 41.5 | 37.1 | n.s |
| ChEI | 38.0 | 40.8 | n.s |
| ChEI and memantine (EBIXA) | 3.6 | 4.7 | n.s |
| Two ChEIs | 0.7 | 0.6 | n.s |
| Memantine (EBIXA) | 16.2 | 16.7 | n.s |
| Group | Number of Visits Initially | Visits Excluded | Number of Visits Included |
|---|---|---|---|
| T6 | 307 | 68 | 239 (153 patients) |
| T12 | 261 | 50 | 211 (159 patients) |
| T18 | 109 | 18 | 91 (74 patients) |
| T24 | 64 | 13 | 51 (43 patients) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nicolas, A.-S.; Ducher, M.; Bourguignon, L.; Dauphinot, V.; Krolak-Salmon, P. Prediction of Autonomy Loss in Alzheimer’s Disease. Forecasting 2022, 4, 26-35. https://doi.org/10.3390/forecast4010002
Nicolas A-S, Ducher M, Bourguignon L, Dauphinot V, Krolak-Salmon P. Prediction of Autonomy Loss in Alzheimer’s Disease. Forecasting. 2022; 4(1):26-35. https://doi.org/10.3390/forecast4010002
Chicago/Turabian StyleNicolas, Anne-Sophie, Michel Ducher, Laurent Bourguignon, Virginie Dauphinot, and Pierre Krolak-Salmon. 2022. "Prediction of Autonomy Loss in Alzheimer’s Disease" Forecasting 4, no. 1: 26-35. https://doi.org/10.3390/forecast4010002
APA StyleNicolas, A.-S., Ducher, M., Bourguignon, L., Dauphinot, V., & Krolak-Salmon, P. (2022). Prediction of Autonomy Loss in Alzheimer’s Disease. Forecasting, 4(1), 26-35. https://doi.org/10.3390/forecast4010002






