Tagging Fluorescent Reporter to Epinecidin-1 Antimicrobial Peptide
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains, Vectors, and Reagents
2.2. Preparation of Competent Cells and Transformation
2.3. Plasmid Isolation
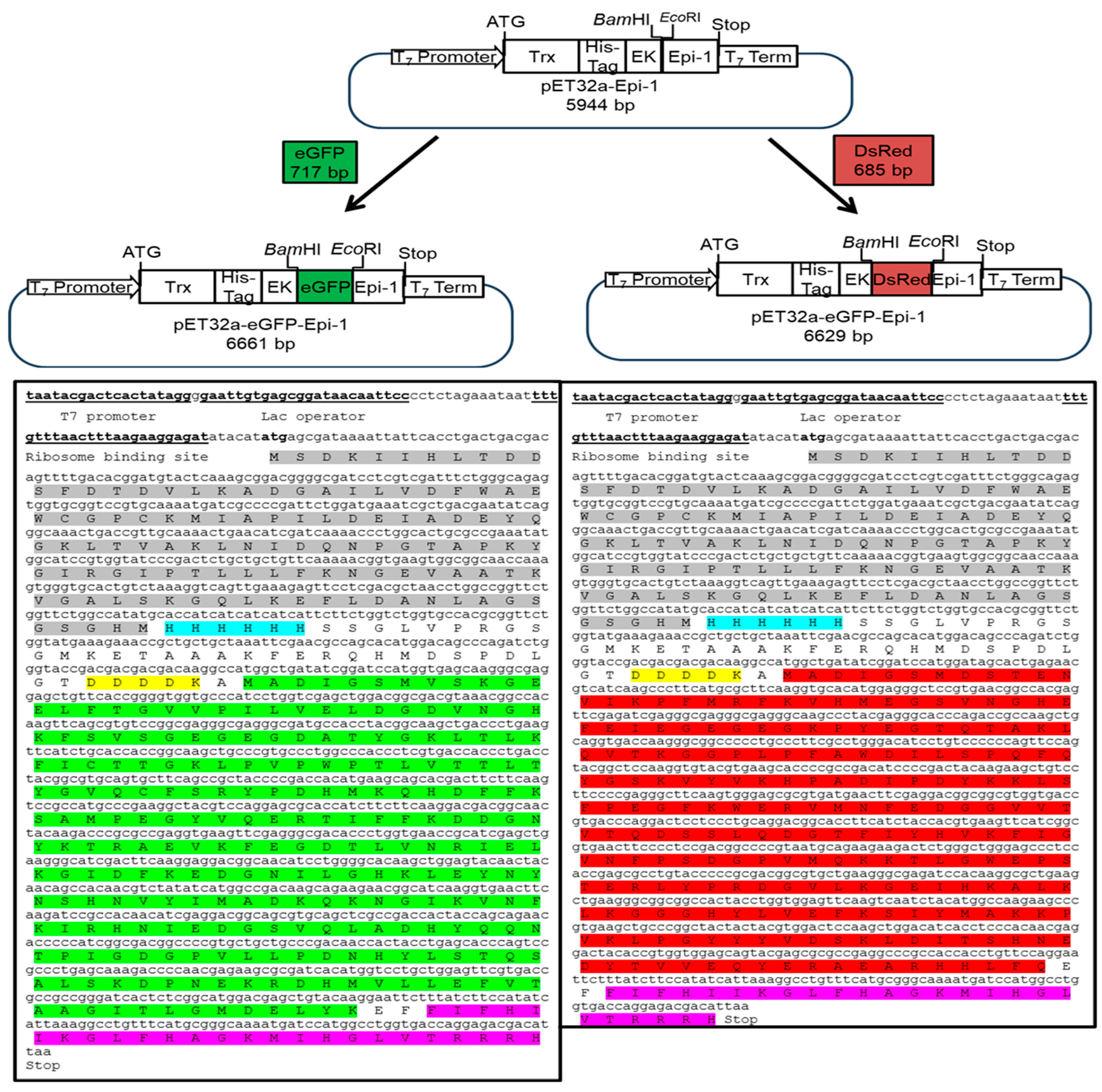
2.4. PCR Amplification of eGFP and DsRed
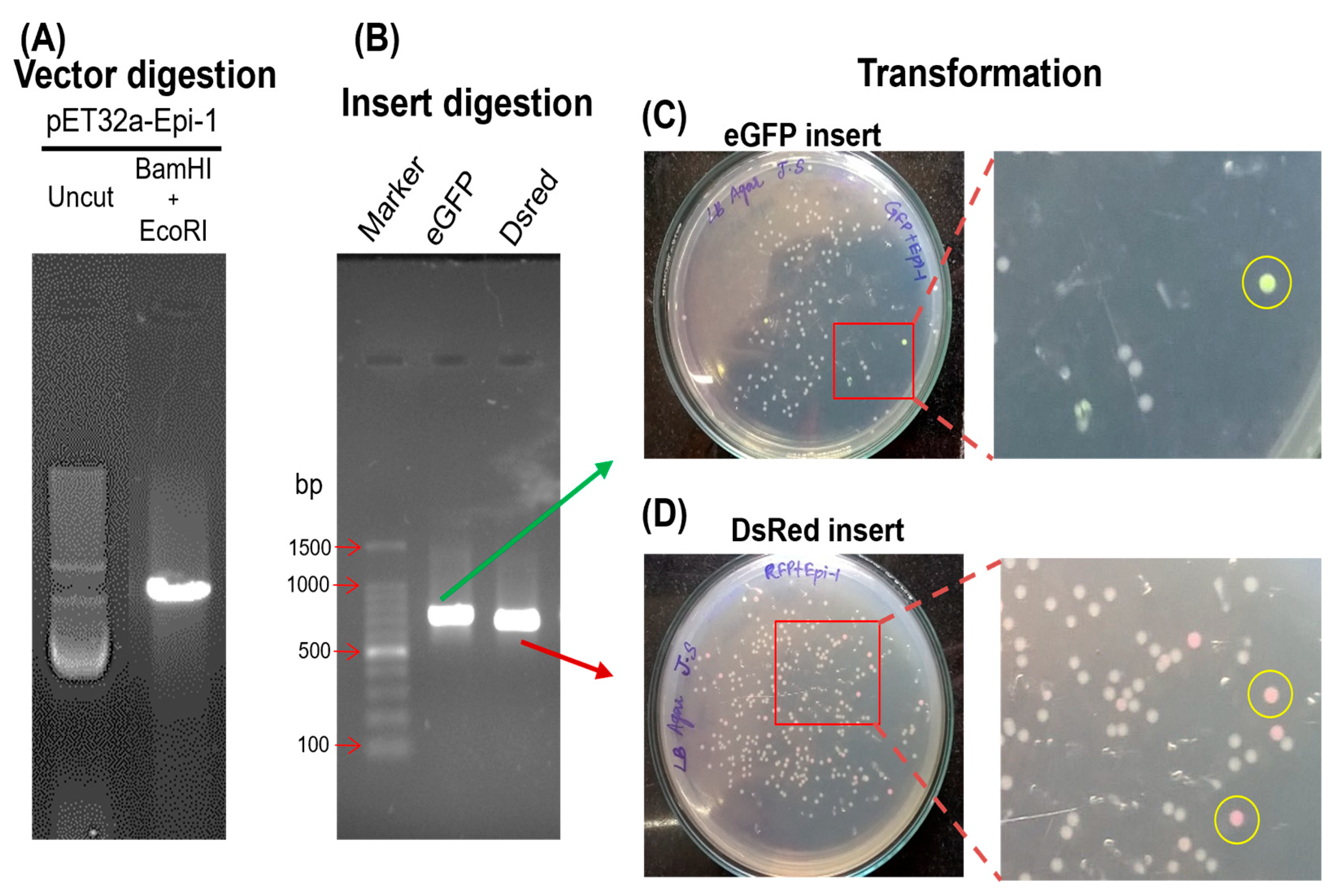
2.5. Restriction Digestion of Vector and Insert
2.6. Ligation and Transformation
2.7. Colony Patching, Screening and Validation
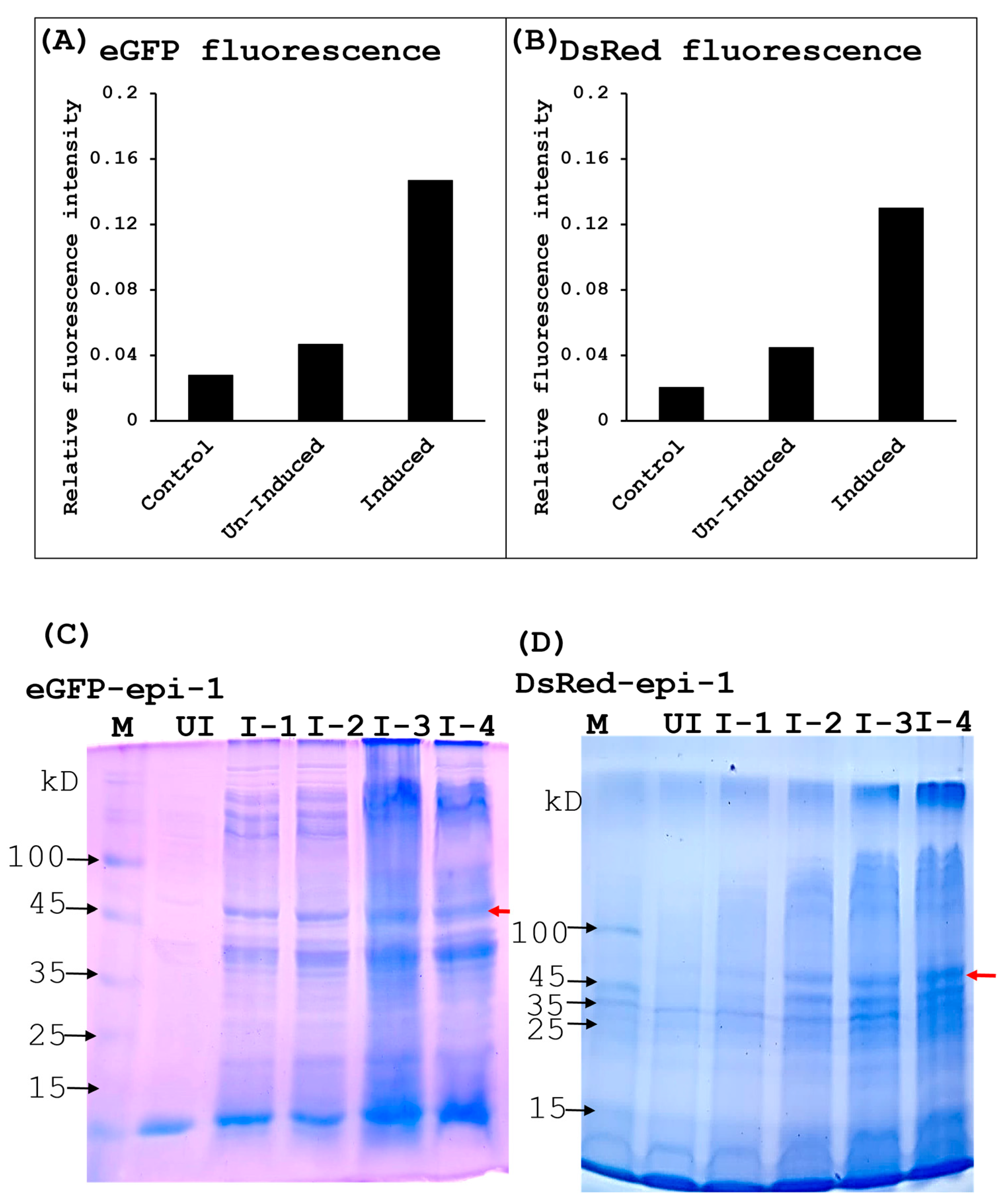
2.8. Overexpression, Fluorescent MEASUREMENT, and Purification of eGFP-epi-1 and Ds-Red-epi-1
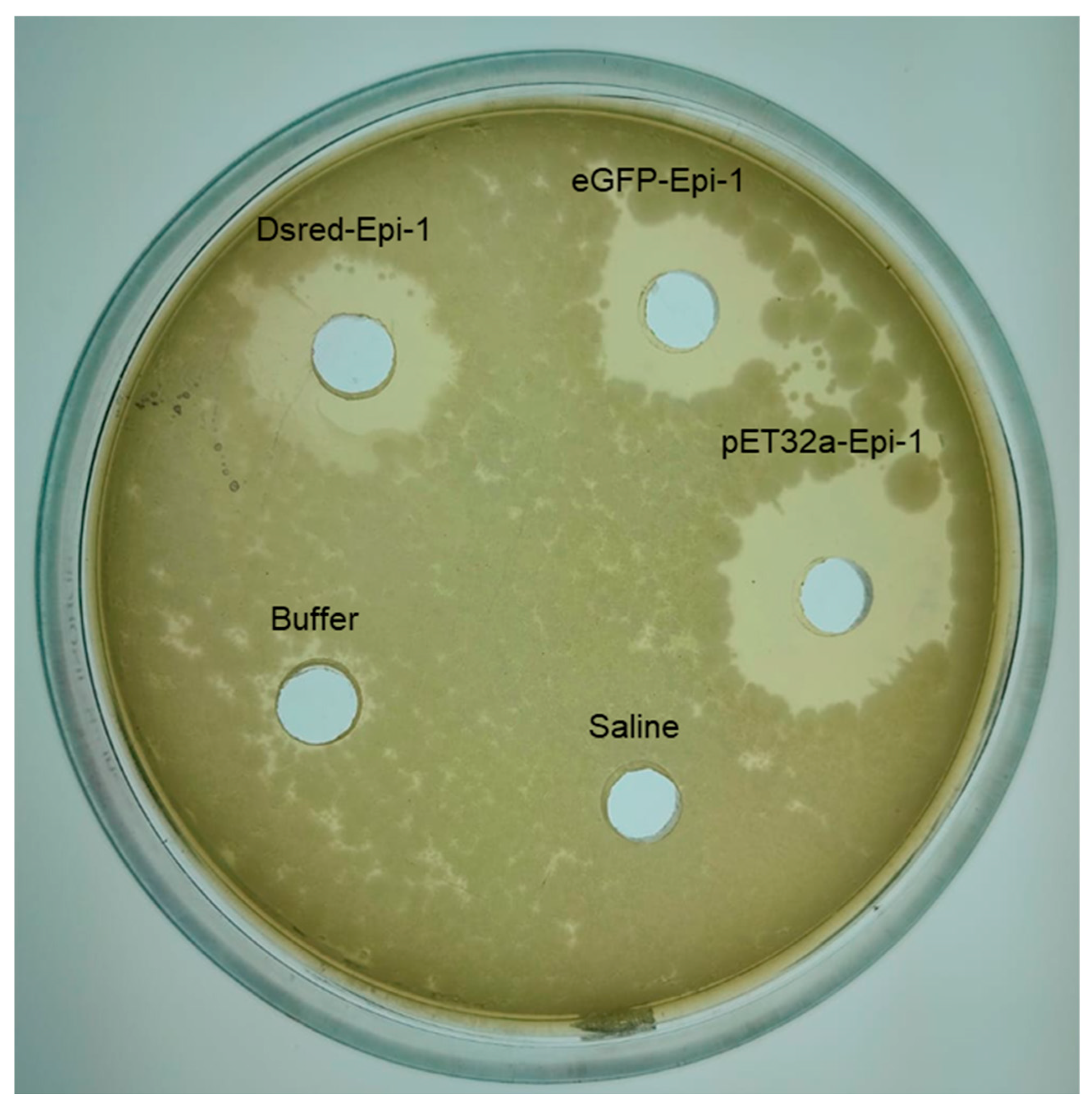
2.9. Antimicrobial Activity of eGFP-epi-1 and DsRed-epi-1
3. Results
3.1. Construction of Fluorescent Epi-1 Vector
3.2. Visual Screen of Fluorescent Reporter Epi-1 Clones
3.3. Validation of Visually Fluorescent Colonies by Molecular Techniques
3.4. Fluorescence Quantitation of Overexpressed eGFP-epi-1 and DsRed-epi-1
3.5. eGFP-epi-1 and Ds-Red-epi-1 Functional Bioactivity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shimomura, O.; Johnson, F.H.; Saiga, Y. Extraction, Purification and Properties of Aequorin, a Bioluminescent Protein from the Luminous Hydromedusan, Aequorea. J. Cell Comp. Physiol. 1962, 59, 223–239. [Google Scholar] [CrossRef]
- Kahana, J.A.; Silver, P.A. Use of the A. Victoria Green Fluorescent Protein to Study Protein Dynamics in Vivo. Curr. Protoc. Pharmacol. 2001, 14, 5–15. [Google Scholar] [CrossRef]
- Shaner, N.C.; Lambert, G.G.; Chammas, A.; Ni, Y.; Cranfill, P.J.; Baird, M.A.; Sell, B.R.; Allen, J.R.; Day, R.N.; Israelsson, M.; et al. A Bright Monomeric Green Fluorescent Protein Derived from Branchiostoma Lanceolatum. Nat. Methods 2013, 10, 407–409. [Google Scholar] [CrossRef]
- Kremers, G.-J.; Gilbert, S.G.; Cranfill, P.J.; Davidson, M.W.; Piston, D.W. Fluorescent Proteins at a Glance. J. Cell Sci. 2011, 124, 157–160. [Google Scholar] [CrossRef]
- Stepanenko, O.V.; Verkhusha, V.V.; Kuznetsova, I.M.; Uversky, V.N.; Turoverov, K.K. Fluorescent Proteins as Biomarkers and Biosensors: Throwing Color Lights on Molecular and Cellular Processes. Curr. Protein Pept. Sci. 2008, 9, 338–369. [Google Scholar] [CrossRef]
- Lippincott-Schwartz, J.; Snapp, E.; Kenworthy, A. Studying Protein Dynamics in Living Cells. Nat. Rev. Mol. Cell Biol. 2001, 2, 444–456. [Google Scholar] [CrossRef] [PubMed]
- Kjer-Nielsen, L.; van Vliet, C.; Erlich, R.; Toh, B.H.; Gleeson, P.A. The Golgi-Targeting Sequence of the Peripheral Membrane Protein P230. J. Cell Sci. 1999, 112 Pt 11, 1645–1654. [Google Scholar] [CrossRef] [PubMed]
- Saint-Jore, C.M.; Evins, J.; Batoko, H.; Brandizzi, F.; Moore, I.; Hawes, C. Redistribution of Membrane Proteins between the Golgi Apparatus and Endoplasmic Reticulum in Plants Is Reversible and Not Dependent on Cytoskeletal Networks. Plant J. 2002, 29, 661–678. [Google Scholar] [CrossRef] [PubMed]
- Atkins, D.; Izant, J.G. Expression and Analysis of the Green Fluorescent Protein Gene in the Fission Yeast Schizosaccharomyces Pombe. Curr. Genet. 1995, 28, 585–588. [Google Scholar] [CrossRef]
- Rizzuto, R.; Brini, M.; Pizzo, P.; Murgia, M.; Pozzan, T. Chimeric Green Fluorescent Protein as a Tool for Visualizing Subcellular Organelles in Living Cells. Curr. Biol. 1995, 5, 635–642. [Google Scholar] [CrossRef]
- Dabrowski, S.; Brillowska-Dabrowska, A.; Kur, J. Fluorescent Protein Vector for Directional Selection of PCR Clones. Biotechniques 2000, 29, 800–808. [Google Scholar] [CrossRef]
- Okabe, M.; Ikawa, M.; Kominami, K.; Nakanishi, T.; Nishimune, Y. “Green Mice” as a Source of Ubiquitous Green Cells. FEBS Lett. 1997, 407, 313–319. [Google Scholar] [CrossRef]
- Bahar, A.A.; Ren, D. Antimicrobial Peptides. Pharmaceuticals 2013, 6, 1543–1575. [Google Scholar] [CrossRef] [PubMed]
- Thankappan, B.; Jeyarajan, S.; Hiroaki, S.; Anbarasu, K.; Natarajaseenivasan, K.; Fujii, N. Antimicrobial and Antibiofilm Activity of Designed and Synthesized Antimicrobial Peptide, KABT-AMP. Appl. Biochem. Biotechnol. 2013, 170, 1184–1193. [Google Scholar] [CrossRef] [PubMed]
- Thankappan, B.; Sivakumar, J.; Asokan, S.; Ramasamy, M.; Pillai, M.M.; Selvakumar, R.; Angayarkanni, J. Dual Antimicrobial and Anticancer Activity of a Novel Synthetic α-Helical Antimicrobial Peptide. Eur. J. Pharm. Sci. 2021, 161, 105784. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Zong, X.; Jin, M.; Min, J.; Wang, F.; Wang, Y. Mechanisms and Regulation of Defensins in Host Defense. Signal Transduct. Target. Ther. 2023, 8, 300. [Google Scholar] [CrossRef]
- Jeyarajan, S.; Peter, A.S.; Ranjith, S.; Sathyan, A.; Duraisamy, S.; Kandasamy, I.; Chidambaram, P.; Kumarasamy, A. Glycine-Replaced Epinecidin-1 Variant Bestows Better Stability and Stronger Antimicrobial Activity against a Range of Nosocomial Pathogenic Bacteria. Biotechnol. Appl. Biochem. 2024, 71, 1384–1404. [Google Scholar] [CrossRef]
- Jeyarajan, S.; Peter, A.S.; Sathyan, A.; Ranjith, S.; Kandasamy, I.; Duraisamy, S.; Chidambaram, P.; Kumarasamy, A. Expression and Purification of Epinecidin-1 Variant (Ac-Var-1) by Acid Cleavage. Appl. Microbiol. Biotechnol. 2024, 108, 176. [Google Scholar] [CrossRef]
- Jeyarajan, S.; Sathyan, A.; Peter, A.S.; Ranjith, S.; Duraisamy, S.; Natarajaseenivasan, S.M.; Chidambaram, P.; Kumarasamy, A. Bioproduction and Characterization of Epinecidin-1 and Its Variants Against Multi Drug Resistant Bacteria Through In Silico and In Vitro Studies. Int. J. Pept. Res. Ther. 2023, 29, 66. [Google Scholar] [CrossRef]
- Jeyarajan, S.; Kumarasamy, A. (PDF) Anti-Candida and Antibiofilm Activity of Epinecidin-1 and Its Variants. Available online: https://www.researchgate.net/publication/383685655_Anti-Candida_and_antibiofilm_activity_of_epinecidin-1_and_its_variants (accessed on 29 October 2025).
- Jeyarajan, S.; Kumarasamy, A. (PDF) Antifungal Activity of Protamine. Available online: https://www.researchgate.net/publication/386541174_Antifungal_activity_of_protamine (accessed on 29 October 2025).
- Jeyarajan, S.; Ranjith, S.; Veerapandian, R.; Natarajaseenivasan, K.; Chidambaram, P.; Kumarasamy, A. Antibiofilm Activity of Epinecidin-1 and Its Variants Against Drug-Resistant Candida Krusei and Candida Tropicalis Isolates from Vaginal Candidiasis Patients. Infect. Dis. Rep. 2024, 16, 1214–1229. [Google Scholar] [CrossRef]
- Bauer, A.W.; Kirby, W.M.; Sherris, J.C.; Turck, M. Antibiotic Susceptibility Testing by a Standardized Single Disk Method. Am. J. Clin. Pathol. 1966, 45, 493–496. [Google Scholar] [CrossRef] [PubMed]
- Novagen. pET System Manual. In: Novagen (Ed .11). 2005. Available online: https://kirschner.med.harvard.edu/files/protocols/Novagen_petsystem.pdf (accessed on 15 October 2025).
- Jeyarajan, S.; Ranjith, S.; Thankappan, B.; Kumarasamy, A. A Step-by-Step Guide for Biosynthesis of Recombinant Fusion Antimicrobial Peptide and Release of the Active Peptide from Its Fusion Partner by Formic Acid Cleavage. Methods Mol. Biol. 2025, 2931, 155–175. [Google Scholar] [CrossRef] [PubMed]
- Metlitskaia, L.; Cabralda, J.E.; Suleman, D.; Kerry, C.; Brinkman, J.; Bartfeld, D.; Guarna, M.M. Recombinant Antimicrobial Peptides Efficiently Produced Using Novel Cloning and Purification Processes. Biotechnol. Appl. Biochem. 2004, 39, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Luan, C.; Xie, Y.G.; Pu, Y.T.; Zhang, H.W.; Han, F.F.; Feng, J.; Wang, Y.Z. Recombinant Expression of Antimicrobial Peptides Using a Novel Self-Cleaving Aggregation Tag in Escherichia Coli. Can. J. Microbiol. 2014, 60, 113–120. [Google Scholar] [CrossRef]
- Osusky, M.; Zhou, G.; Osuska, L.; Hancock, R.E.; Kay, W.W.; Misra, S. Transgenic Plants Expressing Cationic Peptide Chimeras Exhibit Broad-Spectrum Resistance to Phytopathogens. Nat. Biotechnol. 2000, 18, 1162–1166. [Google Scholar] [CrossRef]
- Morin, K.M.; Arcidiacono, S.; Beckwitt, R.; Mello, C.M. Recombinant Expression of Indolicidin Concatamers in Escherichia Coli. Appl. Microbiol. Biotechnol. 2006, 70, 698–704. [Google Scholar] [CrossRef]
- Butvilovskaya, V.I.; Tsybulskaya, M.V.; Tikhonov, A.A.; Talibov, V.O.; Belousov, P.V.; Sazykin, A.Y.; Schwartz, A.M.; Putlyaeva, L.V.; Surzhikov, S.A.; Stomakhin, A.A.; et al. Preparation of recombinant serpins B3 and B4 and investigation of their specific interactions with antibodies using hydrogel-based microarrays. Mol Biol 2015, 49, 790–799. [Google Scholar] [CrossRef]
- Chahardooli, M.; Niazi, A.; Aram, F.; Sohrabi, S.M. Expression of Recombinant Arabian Camel Lactoferricin-Related Peptide in Pichia Pastoris and Its Antimicrobial Identification. J. Sci. Food Agric. 2016, 96, 569–575. [Google Scholar] [CrossRef]
- Kumarasamy, A.; Jeyarajan, S.; Kimler, V.A.; Premceski, A.; Cheon, J.; Mishra, V.; Giblin, F.J. In Vitro Studies on the Interaction of Guinea Pig αA Crystallin and αA Crystallin (66-80) Peptide Using Fluorescence Polarization and Transmission Electron Microscopy. Investig. Ophthalmol. Vis. Sci. 2017, 58, 5301. [Google Scholar]
- Jeyarajan, S.; Kumarasamy, A.; Cheon, J.; Premceski, A.; Seidel, E.; Kimler, V.A.; Giblin, F.J. TEM Analysis of αA66-80 Peptide-Induced Protein Aggregates and Amyloid Fibrils in Human and Guinea Pig αA-Crystallins. Investig. Ophthalmol. Vis. Sci. 2018, 59, 3043. [Google Scholar]
- Yadav, P.; Su, M.; Sivakumar, J.; Giblin, F.; Ohi, M. Structural Organization of the Guinea Pig αA-Crystallin and αA 66−80 Peptide Complex. Microsc. Microanal. 2019, 25, 1318–1319. [Google Scholar] [CrossRef]
- Anbarasu, K.; Sivakumar, J. Multidimensional Significance of Crystallin Protein-Protein Interactions and Their Implications in Various Human Diseases. Biochim. Biophys. Acta 2016, 1860, 222–233. [Google Scholar] [CrossRef] [PubMed]
- Li, Y. Carrier Proteins for Fusion Expression of Antimicrobial Peptides in Escherichia Coli. Biotechnol. Appl. Biochem. 2009, 54, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Li, Y. Recombinant Production of Antimicrobial Peptides in Escherichia Coli: A Review. Protein Expr. Purif. 2011, 80, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Govindarajan, S.; Sivakumar, J.; Garimidi, P.; Rangaraj, N.; Kumar, J.M.; Rao, N.M.; Gopal, V. Targeting Human Epidermal Growth Factor Receptor 2 by a Cell-Penetrating Peptide-Affibody Bioconjugate. Biomaterials 2012, 33, 2570–2582. [Google Scholar] [CrossRef] [PubMed]
- Jeyarajan, S.; Xavier, J.; Rao, N.M.; Gopal, V. Plasmid DNA Delivery into MDA-MB-453 Cells Mediated by Recombinant Her-NLS Fusion Protein. Int. J. Nanomed. 2010, 5, 725–733. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.; Wang, G. On-Resin Cleavage of Bacterially Expressed Fusion Proteins for Purification of Active Recombinant Peptides SK-29, KR-20, LL-29, and LL-23 from Human Sweat or Skin. Protein Expr. Purif. 2007, 55, 395–405. [Google Scholar] [CrossRef]
- Ke, T.; Liang, S.; Huang, J.; Mao, H.; Chen, J.; Dong, C.; Huang, J.; Liu, S.; Kang, J.; Liu, D.; et al. A Novel PCR-Based Method for High Throughput Prokaryotic Expression of Antimicrobial Peptide Genes. BMC Biotechnol. 2012, 12, 10. [Google Scholar] [CrossRef]
- Skosyrev, V.S.; Rudenko, N.V.; Yakhnin, A.V.; Zagranichny, V.E.; Popova, L.I.; Zakharov, M.V.; Gorokhovatsky, A.Y.; Vinokurov, L.M. EGFP as a Fusion Partner for the Expression and Organic Extraction of Small Polypeptides. Protein Expr. Purif. 2003, 27, 55–62. [Google Scholar] [CrossRef]
- Chalfie, M.; Tu, Y.; Euskirchen, G.; Ward, W.W.; Prasher, D.C. Green Fluorescent Protein as a Marker for Gene Expression. Science 1994, 263, 802–805. [Google Scholar] [CrossRef]
- Liu, B.-F.; Anbarasu, K.; Liang, J.J.-N. Confocal Fluorescence Resonance Energy Transfer Microscopy Study of Protein-Protein Interactions of Lens Crystallins in Living Cells. Mol. Vis. 2007, 13, 854–861. [Google Scholar]
- Jeyarajan, S.; Zhang, I.X.; Arvan, P.; Lentz, S.I.; Satin, L.S. Simultaneous Measurement of Changes in Mitochondrial and Endoplasmic Reticulum Free Calcium in Pancreatic Beta Cells. Biosensors 2023, 13, 382. [Google Scholar] [CrossRef]
- Zhang, I.X.; Herrmann, A.; Leon, J.; Jeyarajan, S.; Arunagiri, A.; Arvan, P.; Gilon, P.; Satin, L.S. ER Stress Increases Expression of Intracellular Calcium Channel RyR1 to Modify Ca2+ Homeostasis in Pancreatic Beta Cells. J. Biol. Chem. 2023, 299, 105065. [Google Scholar] [CrossRef]
- Banerjee, S.; Kumar, J.; Apte-Deshpande, A.; Padmanabhan, S. A Novel Prokaryotic Vector for Identification and Selection of Recombinants: Direct Use of the Vector for Expression Studies in E. coli. Microb. Cell Factories 2010, 9, 30. [Google Scholar] [CrossRef]
- Schultz, T.; Martinez, L.; de Marco, A. The Evaluation of the Factors That Cause Aggregation during Recombinant Expression in E. coli Is Simplified by the Employment of an Aggregation-Sensitive Reporter. Microb. Cell Factories 2006, 5, 28. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeyarajan, S.; Ramesh, H.P.; Anbarasu, A.; Jayachandran, J.; Kumarasamy, A. Tagging Fluorescent Reporter to Epinecidin-1 Antimicrobial Peptide. J 2025, 8, 42. https://doi.org/10.3390/j8040042
Jeyarajan S, Ramesh HP, Anbarasu A, Jayachandran J, Kumarasamy A. Tagging Fluorescent Reporter to Epinecidin-1 Antimicrobial Peptide. J. 2025; 8(4):42. https://doi.org/10.3390/j8040042
Chicago/Turabian StyleJeyarajan, Sivakumar, Harini Priya Ramesh, Atchyasri Anbarasu, Jayasudha Jayachandran, and Anbarasu Kumarasamy. 2025. "Tagging Fluorescent Reporter to Epinecidin-1 Antimicrobial Peptide" J 8, no. 4: 42. https://doi.org/10.3390/j8040042
APA StyleJeyarajan, S., Ramesh, H. P., Anbarasu, A., Jayachandran, J., & Kumarasamy, A. (2025). Tagging Fluorescent Reporter to Epinecidin-1 Antimicrobial Peptide. J, 8(4), 42. https://doi.org/10.3390/j8040042






