Redefining Quantum Dot Synthesis with Additive-Manufactured Microfluidics—A Review
Abstract
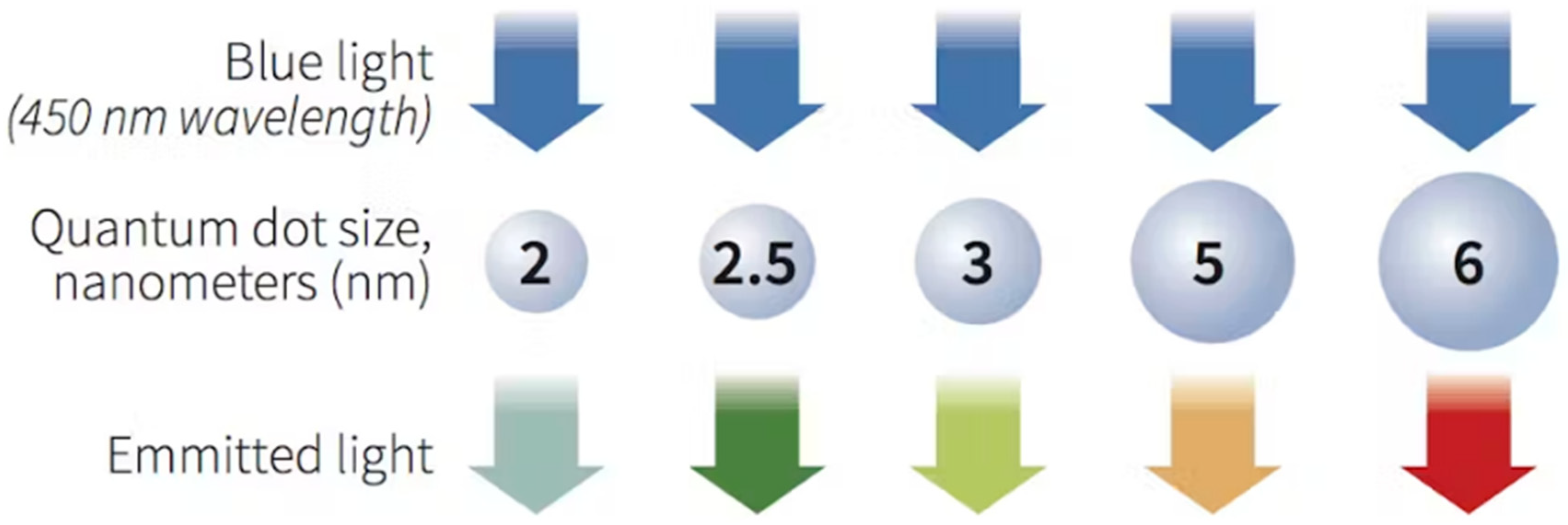
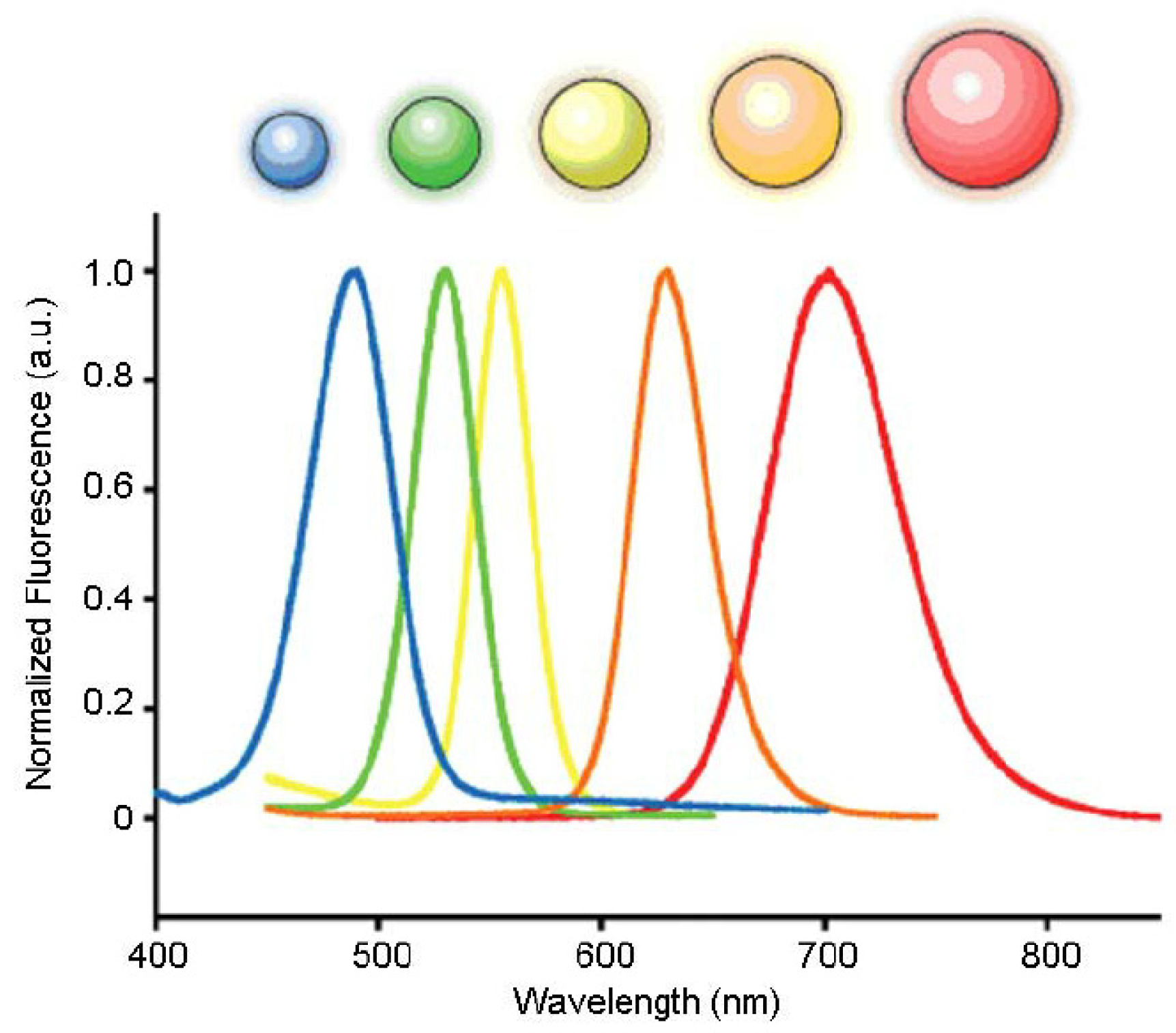
1. Introduction
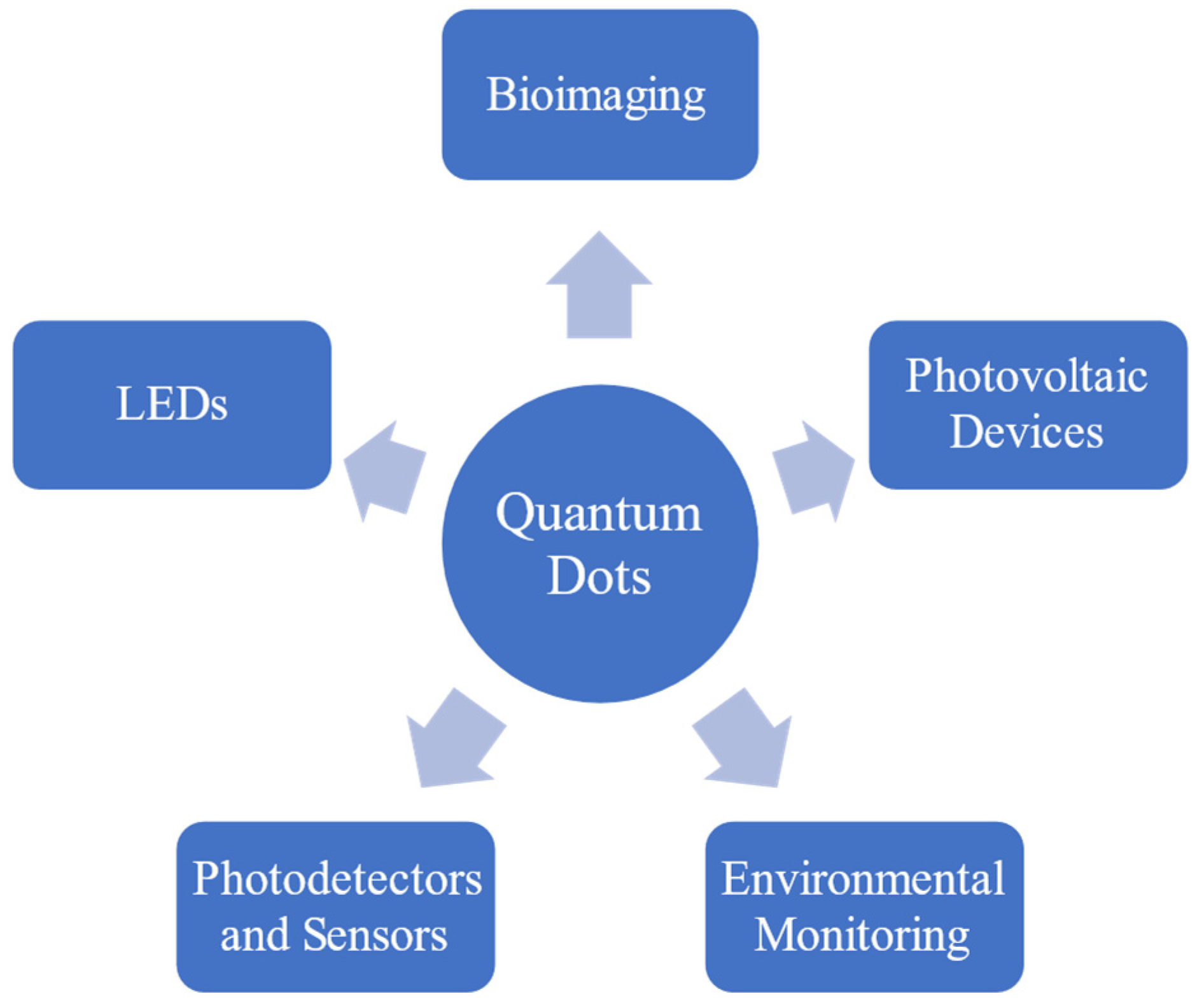
2. Applications
3. Synthesis Approaches
4. Hot Injection (HI)

5. Ultrasonication-Assisted Method
6. Wet and Dry Milling
7. Continuous Flow Synthesis
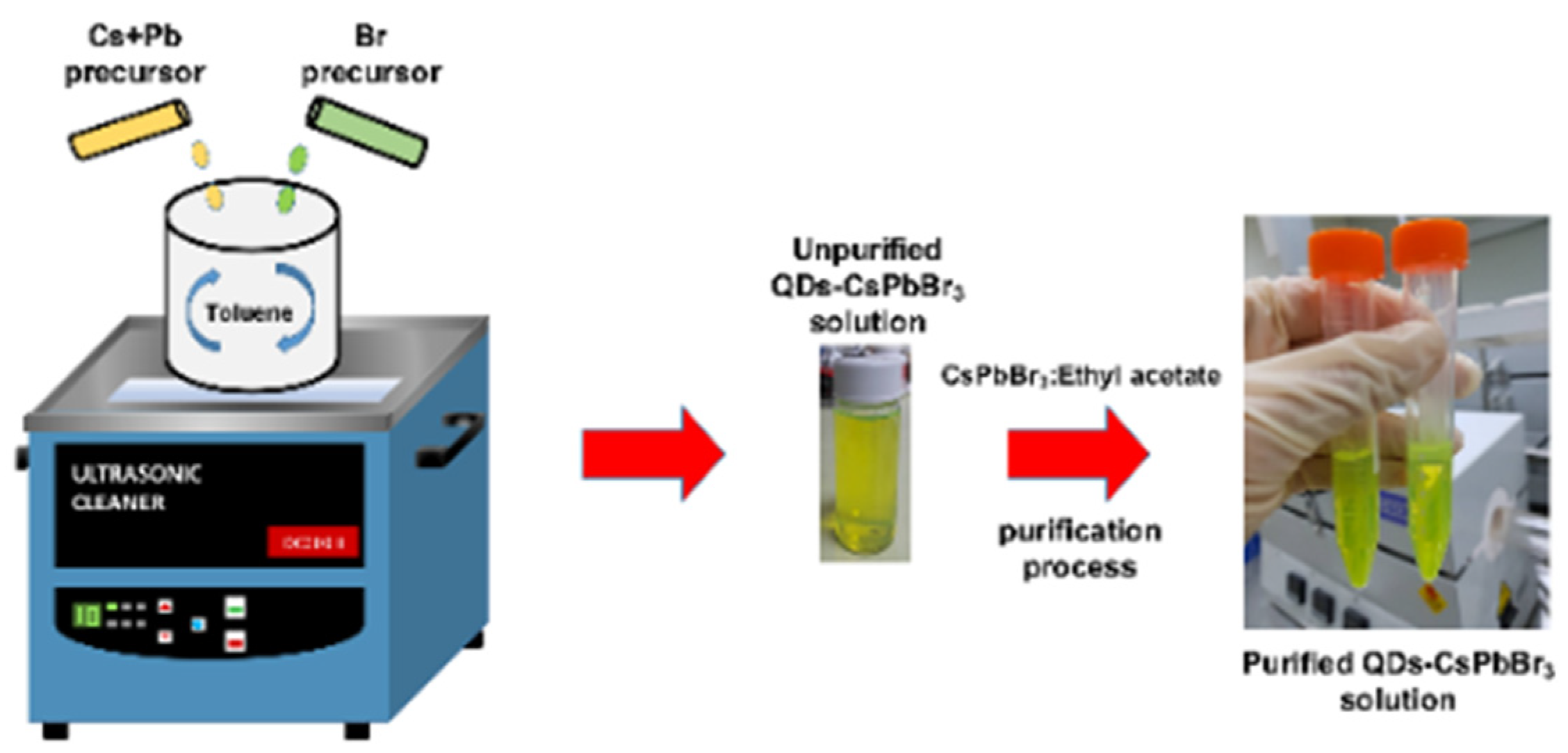
8. Advancing Quantum Dot Synthesis with Additive-Manufactured Microfluidics
8.1. Introduction to Microfluidics in QD Synthesis
8.2. Key Achievements of Microfluidics in QD Synthesis
8.3. Challenges and Limitations of Current PDMS-Based Microfluidic Systems
8.4. Additive-Manufactured Microfluidics
8.5. Current Challenges and Opportunities in Additive-Manufactured Microfluidics and Quantum Dot Synthesis
9. Discussion and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ashoori, R.C. Electrons in Artificial Atoms. Nature 1996, 379, 413–419. [Google Scholar] [CrossRef]
- Nguyen, H.; Hammel, B.; Sharp, D.; Kline, J.; Schwartz, G.; Harvey, S.; Nishiwaki, E.; Sandeno, S.; Ginger, D.; Majumdar, A.; et al. Colossal Core/Shell CdSe/CdS Quantum Dot Emitters. ACS Nano 2024, 18, 20726–20739. [Google Scholar] [CrossRef] [PubMed]
- Ornes, S. Quantum Dots. Proc. Natl. Acad. Sci. USA 2016, 113, 2796–2797. [Google Scholar] [CrossRef]
- The Royal Swedish Academy of Sciences. Scientific Background to the Nobel Prize in Chemistry 2023: Quantum Dots—Seeds of Nanoscience; The Royal Swedish Academy of Sciences: Stockholm, Sweden, 2023. [Google Scholar]
- Gao, X.; Cui, Y.; Levenson, R.M.; Chung, L.W.K.; Nie, S. In Vivo Cancer Targeting and Imaging with Semiconductor Quantum Dots. Nat. Biotechnol. 2004, 22, 969–976. [Google Scholar] [CrossRef] [PubMed]
- Ntziachristos, V.; Tung, C.H.; Bremer, C.; Weissleder, R. Fluorescence Molecular Tomography Resolves Protease Activity in Vivo. Nat. Med. 2002, 8, 757–761. [Google Scholar] [CrossRef]
- Agarwal, K.; Rai, H.; Mondal, S. Quantum Dots: An Overview of Synthesis, Properties, and Applications. Mater. Res. Express 2023, 10, 062001. [Google Scholar]
- Gidwani, B.; Sahu, V.; Shukla, S.S.; Pandey, R.; Joshi, V.; Jain, V.K.; Vyas, A. Quantum Dots: Prospectives, Toxicity, Advances and Applications. J. Drug Deliv. Sci. Technol. 2021, 61, 102308. [Google Scholar] [CrossRef]
- Ahuja, V.; Bhatt, A.K.; Varjani, S.; Choi, K.Y.; Kim, S.H.; Yang, Y.H.; Bhatia, S.K. Quantum Dot Synthesis from Waste Biomass and Its Applications in Energy and Bioremediation. Chemosphere 2022, 293, 133564. [Google Scholar] [CrossRef]
- Pandey, S.; Bodas, D. High-Quality Quantum Dots for Multiplexed Bioimaging: A Critical Review. Adv. Colloid Interface Sci. 2020, 278, 102137. [Google Scholar]
- Yang, B.; Valdiviezo, J. Introductory Information about Quantum Dots and Their Applications. ChemRxiv 2024. [Google Scholar] [CrossRef]
- Divsar, F. Introductory Chapter: Quantum Dots. In Quantum Dots: Fundamental and Applications; IntechOpen: Rijeka, Croatia, 2020. [Google Scholar]
- Pisanic, T.R.; Zhang, Y.; Wang, T.H. Quantum Dots in Diagnostics and Detection: Principles and Paradigms. Analyst 2014, 139, 2968–2981. [Google Scholar] [PubMed]
- Perrault, S.D. Gold Nanoparticles for Efficient Tumour Targeting: Materials, Biology & Application. Ph.D. Thesis, University of Toronto, Toronto, ON, Canada, 2009. [Google Scholar]
- Jang, E.; Jun, S.; Pu, L. High Quality CdSeS Nanocrystals Synthesized by Facile Single Injection Process and Their Electroluminescence. Chem. Commun. 2003, 3, 2964–2965. [Google Scholar] [CrossRef]
- Steckel, J.S.; Snee, P.; Coe-Sullivan, S.; Zimmer, J.P.; Halpert, J.E.; Anikeeva, P.; Kim, L.A.; Bulovic, V.; Bawendi, M.G. Color-Saturated Green-Emitting QD-LEDs. Angew. Chem. Int. Ed. 2006, 45, 5796–5799. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Zhu, Y.; Yang, X.; Li, C. Graphene Quantum Dots: Emergent Nanolights for Bioimaging, Sensors, Catalysis and Photovoltaic Devices. Chem. Commun. 2012, 48, 3686–3699. [Google Scholar] [CrossRef]
- Pleskova, S.; Mikheeva, E.; Gornostaeva, E. Using of Quantum Dots in Biology and Medicine. Adv. Exp. Med. Biol. 2018, 1048, 323–334. [Google Scholar]
- Dey, R.; Mazumder, S.; Mitra, M.K.; Mukherjee, S.; Das, G.C. Review: Biofunctionalized Quantum Dots in Biology and Medicine. J. Nanomater. 2009, 2009, 815734. [Google Scholar]
- Li, Y.; Peng, C.W. Application of Quantum Dots-Based Biotechnology in Cancer Diagnosis: Current Status and Future Perspectives. J. Nanomater. 2010, 2010, 676839. [Google Scholar]
- Yuan, T.; Meng, T.; He, P.; Shi, Y.; Li, Y.; Li, X.; Fan, L.; Yang, S. Carbon Quantum Dots: An Emerging Material for Optoelectronic Applications. J. Mater. Chem. C 2019, 7, 6820–6835. [Google Scholar]
- Moreels, I.; Lambert, K.; Smeets, D.; De Muynck, D.; Nollet, T.; Martins, J.C.; Vanhaecke, F.; Vantomme, A.; Delerue, C.; Allan, G.; et al. Size-Dependent Optical Properties of Colloidal PbS Quantum Dots. ACS Nano 2009, 3, 3023–3030. [Google Scholar] [CrossRef]
- Walling, M.A.; Novak, J.A.; Shepard, J.R.E. Quantum Dots for Live Cell and in Vivo Imaging. Int. J. Mol. Sci. 2009, 10, 441–491. [Google Scholar]
- Wen, G.W.; Lin, J.Y.; Jiang, H.X.; Chen, Z. Stark EfFects in Semiconductor Quantum Dots. Phys. Rev. B 1995, 52, 5913. [Google Scholar] [CrossRef]
- Jun, H.K.; Careem, M.A.; Arof, A.K. Quantum Dot-Sensitized Solar Cells-Perspective and Recent Developments: A Review of Cd Chalcogenide Quantum Dots as Sensitizers. Renew. Sustain. Energy Rev. 2013, 22, 148–167. [Google Scholar] [CrossRef]
- Kim, J.; Song, S.; Kim, Y.H.; Park, S.K. Recent Progress of Quantum Dot-Based Photonic Devices and Systems: A Comprehensive Review of Materials, Devices, and Applications. Small Struct 2021, 2, 2000024. [Google Scholar] [CrossRef]
- Pillai, K.V.; Gray, P.J.; Tien, C.C.; Bleher, R.; Sung, L.P.; Duncan, T.V. Environmental Release of Core-Shell Semiconductor Nanocrystals from Free-Standing Polymer Nanocomposite Films. Environ. Sci. Nano 2016, 3, 657–669. [Google Scholar] [CrossRef]
- Pu, Y.; Lin, L.; Wang, D.; Wang, J.X.; Qian, J.; Chen, J.F. Green Synthesis of Highly Dispersed Ytterbium and Thulium Co-Doped Sodium Yttrium Fluoride Microphosphors for in Situ Light Upconversion from near-Infrared to Blue in Animals. J. Colloid. Interface Sci. 2018, 511, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Deng, Y.; Peng, X.; Jin, Y. Quantum-Dot Light-Emitting Diodes for Large-Area Displays: Towards the Dawn of Commercialization. Adv. Mater. 2017, 29, 1607022. [Google Scholar] [CrossRef]
- Resch-Genger, U.; Grabolle, M.; Cavaliere-Jaricot, S.; Nitschke, R.; Nann, T. Quantum Dots versus Organic Dyes as Fluorescent Labels. Nat. Methods 2008, 5, 763–775. [Google Scholar] [CrossRef] [PubMed]
- Sargent, E.H. Colloidal Quantum Dot Solar Cells. Nat. Photonics 2012, 6, 12732–12763. [Google Scholar] [CrossRef]
- Cotta, M.A. Quantum Dots and Their Applications: What Lies Ahead? ACS Appl. Nano Mater. 2020, 3, 4920–4924. [Google Scholar] [CrossRef]
- Tongbram, B.; Sengupta, S.; Chakrabarti, S. Impact of an InxGa1−xAs Capping Layer in Impeding Indium Desorption from Vertically Coupled InAs/GaAs Quantum Dot Interfaces. ACS Appl. Nano Mater. 2018, 1, 4317–4331. [Google Scholar] [CrossRef]
- Rosa, B.L.T.; Parra-Murillo, C.A.; Chagas, T.; Garcia Junior, A.J.; Guimarães, P.S.S.; Deneke, C.; Magalhães-Paniago, R.; Malachias, A. Scanning Tunneling Measurements in Membrane-Based Nanostructures: Spatially-Resolved Quantum State Analysis in Postprocessed Epitaxial Systems for Optoelectronic Applications. ACS Appl. Nano Mater. 2019, 2, 4655–4664. [Google Scholar] [CrossRef]
- Ramasamy, P.; Kim, N.; Kang, Y.S.; Ramirez, O.; Lee, J.S. Tunable, Bright, and Narrow-Band Luminescence from Colloidal Indium Phosphide Quantum Dots. Chem. Mater. 2017, 29, 6893–6899. [Google Scholar] [CrossRef]
- Kim, Y.; Ham, S.; Jang, H.; Min, J.H.; Chung, H.; Lee, J.; Kim, D.; Jang, E. Bright and Uniform Green Light Emitting InP/ZnSe/ZnS Quantum Dots for Wide Color Gamut Displays. ACS Appl. Nano Mater. 2019, 2, 1496–1504. [Google Scholar] [CrossRef]
- Cho, E.; Kim, T.; Choi, S.M.; Jang, H.; Min, K.; Jang, E. Optical Characteristics of the Surface Defects in InP Colloidal Quantum Dots for Highly Efficient Light-Emitting Applications. ACS Appl. Nano Mater. 2018, 1, 7106–7114. [Google Scholar] [CrossRef]
- Xu, X.; Zhou, L.; Ding, D.; Wang, Y.; Huang, J.; He, H.; Ye, Z. Silicene Quantum Dots Confined in Few-Layer Siloxene Nanosheets for Blue-Light-Emitting Diodes. ACS Appl. Nano Mater. 2020, 3, 538–546. [Google Scholar] [CrossRef]
- Begum, R.; Chin, X.Y.; Damodaran, B.; Hooper, T.J.N.; Mhaisalkar, S.; Mathews, N. Cesium Lead Halide Perovskite Nanocrystals Prepared by Anion Exchange for Light-Emitting Diodes. ACS Appl. Nano Mater. 2020, 3, 1766–1774. [Google Scholar] [CrossRef]
- Suh, Y.H.; Kim, T.; Choi, J.W.; Lee, C.L.; Park, J. High-Performance CsPbX3 Perovskite Quantum-Dot Light-Emitting Devices via Solid-State Ligand Exchange. ACS Appl. Nano Mater. 2018, 1, 488–496. [Google Scholar] [CrossRef]
- Choi, J.; Choi, W.; Jeon, D.Y. Ligand-Exchange-Ready CuInS2/ZnS Quantum Dots via Surface-Ligand Composition Control for Film-Type Display Devices. ACS Appl. Nano Mater. 2019, 2, 5504–5511. [Google Scholar] [CrossRef]
- Shiman, D.I.; Sayevich, V.; Meerbach, C.; Nikishau, P.A.; Vasilenko, I.V.; Gaponik, N.; Kostjuk, S.V.; Lesnyak, V. Robust Polymer Matrix Based on Isobutylene (Co)Polymers for Efficient Encapsulation of Colloidal Semiconductor Nanocrystals. ACS Appl. Nano Mater. 2019, 2, 956–963. [Google Scholar] [CrossRef]
- Pachidis, P.; Cote, B.M.; Ferry, V.E. Tuning the Polarization and Directionality of Photoluminescence of Achiral Quantum Dot Films with Chiral Nanorod Dimer Arrays: Implications for Luminescent Applications. ACS Appl. Nano Mater. 2019, 2, 5681–5687. [Google Scholar] [CrossRef]
- Bose, R.; Dangerfield, A.; Rupich, S.M.; Guo, T.; Zheng, Y.; Kwon, S.; Kim, M.J.; Gartstein, Y.N.; Esteve, A.; Chabal, Y.J.; et al. Engineering Multilayered Nanocrystal Solids with Enhanced Optical Properties Using Metal Oxides for Photonic Applications. ACS Appl. Nano Mater. 2018, 1, 6782–6789. [Google Scholar] [CrossRef]
- Bederak, D.; Balazs, D.M.; Sukharevska, N.V.; Shulga, A.G.; Abdu-Aguye, M.; Dirin, D.N.; Kovalenko, M.V.; Loi, M.A. Comparing Halide Ligands in PbS Colloidal Quantum Dots for Field-Effect Transistors and Solar Cells. ACS Appl. Nano Mater. 2018, 1, 6882–6889. [Google Scholar] [CrossRef] [PubMed]
- Gudjonsdottir, S.; Van Der Stam, W.; Koopman, C.; Kwakkenbos, B.; Evers, W.H.; Houtepen, A.J. On the Stability of Permanent Electrochemical Doping of Quantum Dot, Fullerene, and Conductive Polymer Films in Frozen Electrolytes for Use in Semiconductor Devices. ACS Appl. Nano Mater. 2019, 2, 4900–4909. [Google Scholar] [CrossRef] [PubMed]
- Kokal, R.K.; Bredar, A.R.C.; Farnum, B.H.; Deepa, M. Solid-State Succinonitrile/Sulfide Hole Transport Layer and Carbon Fabric Counter Electrode for a Quantum Dot Solar Cell. ACS Appl. Nano Mater. 2019, 2, 7880–7887. [Google Scholar] [CrossRef]
- Liu, L.; Bisri, S.Z.; Ishida, Y.; Hashizume, D.; Aida, T.; Iwasa, Y. Ligand and Solvent Effects on Hole Transport in Colloidal Quantum Dot Assemblies for Electronic Devices. ACS Appl. Nano Mater. 2018, 1, 5217–5225. [Google Scholar] [CrossRef]
- Dong, Q.; Liu, H.; Hara, Y.; Starr, H.E.; Dempsey, J.L.; Lopez, R. Impact of Background Oxygen Pressure on the Pulsed-Laser Deposition of ZnO Nanolayers and on Their Corresponding Performance as Electron Acceptors in PbS Quantum-Dot Solar Cells. ACS Appl. Nano Mater. 2019, 2, 767–777. [Google Scholar] [CrossRef]
- Mumin, M.A.; Akhter, K.F.; Oyeneye, O.O.; Xu, W.Z.; Charpentier, P.A. Supercritical Fluid Assisted Dispersion of Nano-Silica Encapsulated CdS/ZnS Quantum Dots in Poly(Ethylene-Co-Vinyl Acetate) for Solar Harvesting Films. ACS Appl. Nano Mater. 2018, 1, 3186–3195. [Google Scholar] [CrossRef]
- Cui, P.; Tamukong, P.K.; Kilina, S. Effect of Binding Geometry on Charge Transfer in CdSe Nanocrystals Functionalized by N719 Dyes to Tune Energy Conversion Efficiency. ACS Appl. Nano Mater. 2018, 1, 3174–3185. [Google Scholar] [CrossRef]
- Tang, H.; Zhong, J.; Chen, W.; Shi, K.; Mei, G.; Zhang, Y.; Wen, Z.; Müller-Buschbaum, P.; Wu, D.; Wang, K.; et al. Lead Sulfide Quantum Dot Photodetector with Enhanced Responsivity through a Two-Step Ligand-Exchange Method. ACS Appl. Nano Mater. 2019, 2, 6135–6143. [Google Scholar] [CrossRef]
- Paliwal, A.; Singh, S.V.; Sharma, A.; Sugathan, A.; Liu, S.W.; Biring, S.; Pal, B.N. Microwave-Polyol Synthesis of Sub-10-Nm PbS Nanocrystals for Metal Oxide/Nanocrystal Heterojunction Photodetectors. ACS Appl. Nano Mater. 2018, 1, 6063–6072. [Google Scholar] [CrossRef]
- Hafiz, S.B.; Scimeca, M.R.; Zhao, P.; Paredes, I.J.; Sahu, A.; Ko, D.K. Silver Selenide Colloidal Quantum Dots for Mid-Wavelength Infrared Photodetection. ACS Appl. Nano Mater. 2019, 2, 1631–1636. [Google Scholar] [CrossRef]
- Cook, B.; Gong, M.; Ewing, D.; Casper, M.; Stramel, A.; Elliot, A.; Wu, J. Inkjet Printing Multicolor Pixelated Quantum Dots on Graphene for Broadband Photodetection. ACS Appl. Nano Mater. 2019, 2, 3246–3252. [Google Scholar] [CrossRef]
- Mahmoud, N.; Walravens, W.; Kuhs, J.; Detavernier, C.; Hens, Z.; Roelkens, G. Micro-Transfer-Printing of Al2O3-Capped Short-Wave-Infrared PbS Quantum Dot Photoconductors. ACS Appl. Nano Mater. 2019, 2, 299–306. [Google Scholar] [CrossRef]
- Singh, V.K.; Yadav, S.M.; Mishra, H.; Kumar, R.; Tiwari, R.S.; Pandey, A.; Srivastava, A. WS2 Quantum Dot Graphene Nanocomposite Film for UV Photodetection. ACS Appl. Nano Mater. 2019, 2, 3934–3942. [Google Scholar] [CrossRef]
- Lin, R.; Li, X.; Zheng, W.; Huang, F. Balanced Photodetection in Mixed-Dimensional Phototransistors Consisting of CsPbBr3 Quantum Dots and Few-Layer MoS2. ACS Appl. Nano Mater. 2019, 2, 2599–2605. [Google Scholar] [CrossRef]
- Amaral, P.E.M.; Hall, D.C.; Pai, R.; Król, J.E.; Kalra, V.; Ehrlich, G.D.; Ji, H.F. Fibrous Phosphorus Quantum Dots for Cell Imaging. ACS Appl. Nano Mater. 2020, 3, 752–759. [Google Scholar] [CrossRef]
- Mallick, S.; Kumar, P.; Koner, A.L. Freeze-Resistant Cadmium-Free Quantum Dots for Live-Cell Imaging. ACS Appl. Nano Mater. 2019, 2, 661–666. [Google Scholar] [CrossRef]
- Marqus, S.; Ahmed, H.; Ahmed, M.; Xu, C.; Rezk, A.R.; Yeo, L.Y. Increasing Exfoliation Yield in the Synthesis of MoS2 Quantum Dots for Optoelectronic and Other Applications through a Continuous Multicycle Acoustomicrofluidic Approach. ACS Appl. Nano Mater. 2018, 1, 2503–2508. [Google Scholar] [CrossRef]
- Gu, S.; Hsieh, C.T.; Tsai, Y.Y.; Ashraf Gandomi, Y.; Yeom, S.; Kihm, K.D.; Fu, C.C.; Juang, R.S. Sulfur and Nitrogen Co-Doped Graphene Quantum Dots as a Fluorescent Quenching Probe for Highly Sensitive Detection toward Mercury Ions. ACS Appl. Nano Mater. 2019, 2, 790–798. [Google Scholar] [CrossRef]
- Anh, N.T.N.; Doong, R.A. One-Step Synthesis of Size-Tunable Gold@Sulfur-Doped Graphene Quantum Dot Nanocomposites for Highly Selective and Sensitive Detection of Nanomolar 4-Nitrophenol in Aqueous Solutions with Complex Matrix. ACS Appl. Nano Mater. 2018, 1, 2153–2163. [Google Scholar] [CrossRef]
- Hu, H.; Quan, H.; Zhong, B.; Li, Z.; Huang, Y.; Wang, X.; Zhang, M.; Chen, D. A Reduced Graphene Oxide Quantum Dot-Based Adsorbent for Efficiently Binding with Organic Pollutants. ACS Appl. Nano Mater. 2018, 1, 6502–6513. [Google Scholar] [CrossRef]
- Li, L.; Zhu, X. Enhanced Photocatalytic Hydrogen Evolution of Carbon Quantum Dot Modified 1D Protonated Nanorods of Graphitic Carbon Nitride. ACS Appl. Nano Mater. 2018, 1, 5337–5344. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, S.; Fan, D.; Reilly, J.; Zhang, H.; Yao, W.; Huang, J. Carbon Quantum Dot/TiO2 Nanohybrids: Efficient Photocatalysts for Hydrogen Generation via Intimate Contact and Efficient Charge Separation. ACS Appl. Nano Mater. 2019, 2, 1027–1032. [Google Scholar] [CrossRef]
- Calabro, R.L.; Yang, D.S.; Kim, D.Y. Controlled Nitrogen Doping of Graphene Quantum Dots Through Laser Ablation in Aqueous Solutions for Photoluminescence and Electrocatalytic Applications. ACS Appl. Nano Mater. 2019, 2, 6948–6959. [Google Scholar] [CrossRef]
- Liu, H.; Wang, H.; Qian, Y.; Zhuang, J.; Hu, L.; Chen, Q.; Zhou, S. Nitrogen-Doped Graphene Quantum Dots as Metal-Free Photocatalysts for Near-Infrared Enhanced Reduction of 4-Nitrophenol. ACS Appl. Nano Mater. 2019, 2, 7043–7050. [Google Scholar] [CrossRef]
- De Melo, F.M.; Grasseschi, D.; Brandao, B.B.N.S.; Fu, Y.; Toma, H.E. Superparamagnetic Maghemite-Based Cdte Quantum Dots as Efficient Hybrid Nanoprobes for Water-Bath Magnetic Particle Inspection. ACS Appl. Nano Mater. 2018, 1, 2858–2868. [Google Scholar] [CrossRef]
- Keshavarz, A.; Riahinasab, S.T.; Hirst, L.S.; Stokes, B.J. New Promesogenic Ligands for Host Medium Microencapsulation by Quantum Dots via Liquid Crystal Phase Transition Templating. ACS Appl. Nano Mater. 2019, 2, 2542–2547. [Google Scholar] [CrossRef]
- Durmusoglu, E.G.; Turker, Y.; Yagci Acar, H. Luminescent PbS and PbS/CdS Quantum Dots with Hybrid Coatings as Nanotags for Authentication of Petroleum Products. ACS Appl. Nano Mater. 2019, 2, 7737–7746. [Google Scholar] [CrossRef]
- Giroux, M.S.; Zahra, Z.; Salawu, O.A.; Burgess, R.M.; Ho, K.T.; Adeleye, A.S. Assessing the Environmental Effects Related to Quantum Dot Structure, Function, Synthesis and Exposure. Environ. Sci. Nano 2021, 9, 867–910. [Google Scholar] [CrossRef]
- Sun, H.; Ji, H.; Ju, E.; Guan, Y.; Ren, J.; Qu, X. Synthesis of Fluorinated and Nonfluorinated Graphene Quantum Dots through a New Top-down Strategy for Long-Time Cellular Imaging. Chem.—A Eur. J. 2015, 21, 3791–3797. [Google Scholar] [CrossRef]
- Sharma, A.; Das, J. Small Molecules Derived Carbon Dots: Synthesis and Applications in Sensing, Catalysis, Imaging, and Biomedicine. J. Nanobiotechnol. 2019, 17, 92. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xia, Y. Bottom-up and Top-down Approaches to the Synthesis of Monodispersed Spherical Colloids of Low Melting-Point Metals. Nano Lett. 2004, 4, 2047–2050. [Google Scholar] [CrossRef]
- Murray, C.; Norris, D.; Bawendi, M. Synthesis and Characterization of Nearly Monodisperse CdE (E = Sulfur, Selenium, Tellurium) Semiconductor Nanocrystallites. J. Am. Chem. Soc. 1993, 115, 8706–8715. [Google Scholar] [CrossRef]
- Protesescu, L.; Yakunin, S.; Bodnarchuk, M.I.; Krieg, F.; Caputo, R.; Hendon, C.H.; Yang, R.X.; Walsh, A.; Kovalenko, M.V. Nanocrystals of Cesium Lead Halide Perovskites (CsPbX3, X = Cl, Br, and I): Novel Optoelectronic Materials Showing Bright Emission with Wide Color Gamut. Nano Lett. 2015, 15, 3692–3696. [Google Scholar] [CrossRef]
- Yuan, G.; Ritchie, C.; Ritter, M.; Murphy, S.; Gómez, D.E.; Mulvaney, P. The Degradation and Blinking of Single CsPbI3 Perovskite Quantum Dots. J. Phys. Chem. C 2018, 122, 13407–13415. [Google Scholar] [CrossRef]
- Park, Y.S.; Guo, S.; Makarov, N.S.; Klimov, V.I. Room Temperature Single-Photon Emission from Individual Perovskite Quantum Dots. ACS Nano 2015, 9, 10386–10393. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Li, J.; Li, X.; Xu, L.; Dong, Y.; Zeng, H. Quantum Dot Light-Emitting Diodes Based on Inorganic Perovskite Cesium Lead Halides (CsPbX3). Adv. Mater. 2015, 27, 7162–7167. [Google Scholar] [CrossRef]
- Yu, H.; Tian, G.; Xu, W.; Wang, S.; Zhang, H.; Niu, J.; Chen, X. Green Light-Emitting Devices Based on Perovskite CsPbBr3 Quantum Dots. Front. Chem. 2018, 6, 381. [Google Scholar] [CrossRef]
- Yan, D.; Zhao, S.; Zhang, Y.; Wang, H.; Zang, Z. Highly Efficient Emission and High-CRI Warm White Light-Emitting Diodes from Ligand-Modified CsPbBr3 Quantum Dots. Opto-Electron. Adv. 2022, 5, 200075. [Google Scholar] [CrossRef]
- Hassanabadi, E.; Latifi, M.; Gualdrón-Reyes, A.F.; Masi, S.; Yoon, S.J.; Poyatos, M.; Julián-López, B.; Mora-Seró, I. Ligand & Band Gap Engineering: Tailoring the Protocol Synthesis for Achieving High-Quality CsPbI3 quantum Dots. Nanoscale 2020, 12, 14194–14203. [Google Scholar] [CrossRef]
- Thapa, S.; Bhardwaj, K.; Basel, S.; Pradhan, S.; Eling, C.J.; Adawi, A.M.; Bouillard, J.S.G.; Stasiuk, G.J.; Reiss, P.; Pariyar, A.; et al. Long-Term Ambient Air-Stable Cubic CsPbBr3 Perovskite Quantum Dots Using Molecular Bromine. Nanoscale Adv. 2019, 1, 3388–3391. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Guo, J.; Sun, S.; Lu, P.; Zhang, X.; Shi, Z.; Yu, W.W.; Zhang, Y. Surface Ligand Engineering-Assisted CsPbI3 Quantum Dots Enable Bright and Efficient Red Light-Emitting Diodes with a Top-Emitting Structure. Chem. Eng. J. 2021, 404, 126563. [Google Scholar] [CrossRef]
- Chaudhary, B.; Kshetri, Y.K.; Kim, H.S.; Lee, S.W.; Kim, T.H. Current Status on Synthesis, Properties and Applications of CsPbX3(X = Cl, Br, I) Perovskite Quantum Dots/Nanocrystals. Nanotechnology 2021, 32, 502007. [Google Scholar] [CrossRef]
- Vighnesh, K.; Wang, S.; Liu, H.; Rogach, A.L. Hot-Injection Synthesis Protocol for Green-Emitting Cesium Lead Bromide Perovskite Nanocrystals. ACS Nano 2022, 16, 19618–19625. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; He, H.; Fang, Z.; Lou, H.; Lin, C.; Chen, L.; Ye, Z. Ultrasonication-Assisted Ambient-Air Synthesis of Monodispersed Blue-Emitting CsPbBr3 Quantum Dots for White Light Emission. ACS Appl. Nano Mater. 2019, 2, 6874–6879. [Google Scholar] [CrossRef]
- Tien, C.H.; Chen, L.C.; Lee, K.Y.; Tseng, Z.L.; Dong, Y.S.; Lin, Z.J. High-Quality All-Inorganic Perovskite CsPbBr3 Quantum Dots Emitter Prepared by a Simple Purified Method and Applications of Light-Emitting Diodes. Energies 2019, 12, 3507. [Google Scholar] [CrossRef]
- Palazon, F.; El Ajjouri, Y.; Sebastia-Luna, P.; Lauciello, S.; Manna, L.; Bolink, H.J. Mechanochemical Synthesis of Inorganic Halide Perovskites: Evolution of Phase-Purity, Morphology, and Photoluminescence. J. Mater. Chem. C Mater. 2019, 7, 11406–11410. [Google Scholar] [CrossRef]
- Jana, A.; Mittal, M.; Singla, A.; Sapra, S. Solvent-Free, Mechanochemical Syntheses of Bulk Trihalide Perovskites and Their Nanoparticles. Chem. Commun. 2017, 53, 3046–3049. [Google Scholar] [CrossRef]
- Zhu, Z.Y.; Yang, Q.Q.; Gao, L.F.; Zhang, L.; Shi, A.Y.; Sun, C.L.; Wang, Q.; Zhang, H.L. Solvent-Free Mechanosynthesis of Composition-Tunable Cesium Lead Halide Perovskite Quantum Dots. J. Phys. Chem. Lett. 2017, 8, 1610–1614. [Google Scholar] [CrossRef]
- Protesescu, L.; Yakunin, S.; Nazarenko, O.; Dirin, D.N.; Kovalenko, M.V. Low-Cost Synthesis of Highly Luminescent Colloidal Lead Halide Perovskite Nanocrystals by Wet Ball Milling. ACS Appl. Nano Mater. 2018, 1, 1300–1308. [Google Scholar] [CrossRef]
- López, C.A.; Abia, C.; Alvarez-Galván, M.C.; Hong, B.K.; Martínez-Huerta, M.V.; Serrano-Sánchez, F.; Carrascoso, F.; Castellanos-Gómez, A.; Fernández-Dĺaz, M.T.; Alonso, J.A. Crystal Structure Features of CsPbBr3 Perovskite Prepared by Mechanochemical Synthesis. ACS Omega 2020, 5, 5931–5938. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Xie, H.; Guo, X.; Wang, K.; Yang, C.; Wang, N.; Ge, C. Luminescence, Stability, and Applications of CsPbBr3 Quantum Dot/Polymethyl Methacrylate Composites Prepared by a Solvent- and Ligand-Free Ball Milling Method. Opt. Mater. 2023, 136, 113398. [Google Scholar] [CrossRef]
- Kim, J.; Manh, N.T.; Thai, H.T.; Jeong, S.K.; Lee, Y.W.; Cho, Y.; Ahn, W.; Choi, Y.; Cho, N. Improving the Stability of Ball-Milled Lead Halide Perovskites via Ethanol/Water-Induced Phase Transition. Nanomaterials 2022, 12, 920. [Google Scholar] [CrossRef]
- Zhang, F.; Zhong, H.; Chen, C.; Wu, X.G.; Hu, X.; Huang, H.; Han, J.; Zou, B.; Dong, Y. Brightly Luminescent and Color-Tunable Colloidal CH3NH3PbX3 (X = Br, I, Cl) Quantum Dots: Potential Alternatives for Display Technology. ACS Nano 2015, 9, 4533–4542. [Google Scholar] [CrossRef]
- Vybornyi, O.; Yakunin, S.; Kovalenko, M.V. Polar-Solvent-Free Colloidal Synthesis of Highly Luminescent Alkylammonium Lead Halide Perovskite Nanocrystals. Nanoscale 2016, 8, 6278–6283. [Google Scholar] [CrossRef] [PubMed]
- Lignos, I.; Stavrakis, S.; Nedelcu, G.; Protesescu, L.; Demello, A.J.; Kovalenko, M.V. Synthesis of Cesium Lead Halide Perovskite Nanocrystals in a Droplet-Based Microfluidic Platform: Fast Parametric Space Mapping. Nano Lett. 2016, 16, 1869–1877. [Google Scholar] [CrossRef]
- MacEiczyk, R.M.; Dümbgen, K.; Lignos, I.; Protesescu, L.; Kovalenko, M.V.; Demello, A.J. Microfluidic Reactors Provide Preparative and Mechanistic Insights into the Synthesis of Formamidinium Lead Halide Perovskite Nanocrystals. Chem. Mater. 2017, 29, 8433–8439. [Google Scholar] [CrossRef]
- Lignos, I.; Protesescu, L.; Emiroglu, D.B.; MacEiczyk, R.; Schneider, S.; Kovalenko, M.V.; DeMello, A.J. Unveiling the Shape Evolution and Halide-Ion-Segregation in Blue-Emitting Formamidinium Lead Halide Perovskite Nanocrystals Using an Automated Microfluidic Platform. Nano Lett. 2018, 18, 1246–1252. [Google Scholar] [CrossRef]
- Krishna, K.S.; Li, Y.; Li, S.; Kumar, C.S.S.R. Lab-on-a-Chip Synthesis of Inorganic Nanomaterials and Quantum Dots for Biomedical Applications. Adv. Drug Deliv. Rev. 2013, 65, 1470–1495. [Google Scholar] [CrossRef]
- Hartman, R.L.; McMullen, J.P.; Jensen, K.F. Deciding Whether to Go with the Flow: Evaluating the Merits of Flow Reactors for Synthesis. Angew. Chem. Int. Ed. 2011, 50, 7502–7519. [Google Scholar] [CrossRef]
- Song, Y.; Hormes, J.; Kumar, C.S.S.R. Microfluidic Synthesis of Nanomaterials. Small 2008, 4, 698–711. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.J.; De Varine Bohan, G.M.; Torrente-Murciano, L. Synthesis of Narrow Sized Silver Nanoparticles in the Absence of Capping Ligands in Helical Microreactors. React. Chem. Eng. 2017, 2, 116–128. [Google Scholar] [CrossRef]
- Erdem, E.Y.; Cheng, J.C.; Doyle, F.M.; Pisano, A.P. Multi-Temperature Zone, Droplet-Based Microreactor for Increased Temperature Control in Nanoparticle Synthesis. Small 2014, 10, 1076–1080. [Google Scholar] [CrossRef]
- Yen, B.K.H.; Günther, A.; Schmidt, M.A.; Jensen, K.F.; Bawendi, M.G. A Microfabricated Gas-Liquid Segmented Flow Reactor for High-Temperature Synthesis: The Case of CdSe Quantum Dots. Angew. Chem. 2005, 44, 5583–5587. [Google Scholar] [CrossRef]
- Marre, S.; Park, J.; Rempel, J.; Guan, J.; Bawendi, M.G.; Jensen, K.F. Supercritical Continuous-Microflow Synthesis of Narrow Size Distribution Quantum Dots. Adv. Mater. 2008, 20, 4830–4834. [Google Scholar] [CrossRef]
- Liang, X.; Baker, R.W.; Wu, K.; Deng, W.; Ferdani, D.; Kubiak, P.S.; Marken, F.; Torrente-Murciano, L.; Cameron, P.J. Continuous Low Temperature Synthesis of MAPbX3 Perovskite Nanocrystals in a Flow Reactor. React. Chem. Eng. 2018, 3, 640–644. [Google Scholar] [CrossRef]
- Palaniyandi, T.; Ravi, M.; Sivaji, A.; Baskar, G.; Viswanathan, S.; Wahab, M.R.A.; Surendran, H.; Nedunchezhian, S.; Ahmad, I.; Veettil, V.N. Recent Advances in Microfluidic Chip Technologies for Applications as Preclinical Testing Devices for the Diagnosis and Treatment of Triple-Negative Breast Cancers. Pathol. Res. Pract. 2024, 264, 155711. [Google Scholar] [CrossRef]
- Jurinjak Tušek, A.; Šalić, A.; Valinger, D.; Jurina, T.; Benković, M.; Kljusurić, J.G.; Zelić, B. The Power of Microsystem Technology in the Food Industry—Going Small Makes It Better. Innov. Food Sci. Emerg. Technol. 2021, 68, 102613. [Google Scholar] [CrossRef]
- Kumar, P.S.; Madapusi, S.; Goel, S. Sub-Second Synthesis of Silver Nanoparticles in 3D Printed Monolithic Multilayered Microfluidic Chip: Enhanced Chemiluminescence Sensing Predictions via Machine Learning Algorithms. Int. J. Biol. Macromol. 2023, 245, 125502. [Google Scholar] [CrossRef]
- Kobayashi, M.; Akitsu, T.; Furuya, M.; Sekiguchi, T.; Shoji, S.; Tanii, T.; Tanaka, D. Efficient Synthesis of a Schiff Base Copper(II) Complex Using a Microfluidic Device. Micromachines 2023, 14, 890. [Google Scholar] [CrossRef]
- Dai, J.; Yang, X.; Hamon, M.; Kong, L. Particle Size Controlled Synthesis of CdS Nanoparticles on a Microfluidic Chip. Chem. Eng. J. 2015, 280, 385–390. [Google Scholar] [CrossRef]
- Li, G.; Liu, C.; Zhang, X.; Zhai, P.; Lai, X.; Jiang, W. Low Temperature Synthesis of Carbon Dots in Microfluidic Chip and Their Application for Sensing Cefquinome Residues in Milk. Biosens. Bioelectron. 2023, 228, 115187. [Google Scholar] [CrossRef]
- Cheng, Y.; Ling, S.D.; Geng, Y.; Wang, Y.; Xu, J. Microfluidic Synthesis of Quantum Dots and Their Applications in Bio-Sensing and Bio-Imaging. Nanoscale Adv. 2021, 3, 2180–2195. [Google Scholar] [CrossRef] [PubMed]
- Li, G.X.; Li, Q.; Cheng, R.; Chen, S. Synthesis of Quantum Dots Based on Microfluidic Technology. Curr. Opin. Chem. Eng. 2020, 29, 34–41. [Google Scholar] [CrossRef]
- Hou, C.; Gu, Y.; Yuan, W.; Zhang, W.; Xiu, X.; Lin, J.; Gao, Y.; Liu, P.; Chen, X.; Song, L. Application of Microfluidic Chips in the Simulation of the Urinary System Microenvironment. Mater. Today Bio 2023, 19, 100553. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Yang, J. Biomimetic Microfluidic Chips for Toxicity Assessment of Environmental Pollutants. Sci. Total Environ. 2024, 919, 170745. [Google Scholar] [CrossRef]
- Chandnani, K.; Rajput, N.; Jadav, T.; Pillai, M.; Dhakne, P.; Tekade, R.K.; Sengupta, P. Technological Advancement and Current Standing of Microfluidic Chip Based Devices for Targeted Analysis of Biomarkers. Microchem. J. 2023, 195, 109532. [Google Scholar] [CrossRef]
- Xiao, B.; Zhao, R.; Wang, N.; Zhang, J.; Sun, X.; Chen, A. Recent Advances in Centrifugal Microfluidic Chip-Based Loop-Mediated Isothermal Amplification. TrAC Trends Anal. Chem. 2023, 158, 116836. [Google Scholar] [CrossRef]
- Zheng, Y.; Luo, C.; Chai, Z.; Liu, R.; Yang, Z.; Liao, X.; Li, X.; Wang, N.; Li, D.; Ji, X.; et al. High-Throughput Preparation of Monodisperse Biocompatible Core-Shell Capsules by 3D-Printed Microfluidics. Chem. Eng. Sci. 2025, 304, 121104. [Google Scholar] [CrossRef]
- Becker, H. Hype, Hope and Hubris: The Quest for the Killer Application in Microfluidics. Lab Chip 2009, 9, 2119–2122. [Google Scholar] [CrossRef]
- Fadlelmula, M.M.; Mazinani, B.; Subramanian, V. Towards Personalized Microfluidics: 3D Printing of High-Performance Micropumps by Control and Optimization of Fabrication-Induced Surface Roughness. Addit. Manuf. 2024, 94, 104468. [Google Scholar] [CrossRef]
- Krause, M.; Marshall, A.; Catterlin, J.K.; Kartalov, E. Self-Assembled Electrically Conductive Biocompatible CNF Wiring Enables 3D-Printed Microfluidics Applications. Sens. Actuators A Phys. 2025, 381, 116070. [Google Scholar] [CrossRef]
- Chin, C.D.; Linder, V.; Sia, S.K. Commercialization of Microfluidic Point-of-Care Diagnostic Devices. Lab Chip 2012, 12, 2118–2134. [Google Scholar] [CrossRef]
- IDTechEx. 3D Printing 2015–2025: Technologies, Markets, Players; IDTechEx: Cambridge, UK, 2015. [Google Scholar]
- 3D Printing Market to Grow to US$16.2 Billion in 2018. Met. Powder Rep. 2014, 69, 42. [CrossRef]
- Singh, M.; Haverinen, H.M.; Dhagat, P.; Jabbour, G.E. Inkjet Printing-Process and Its Applications. Adv. Mater. 2010, 22, 673–685. [Google Scholar] [CrossRef] [PubMed]
- Bonyár, A.; Sántha, H.; Ring, B.; Varga, M.; Kovács, J.G.; Harsányi, G. 3D Rapid Prototyping Technology (RPT) as a Powerful Tool in Microfluidic Development. Procedia Eng. 2010, 5, 291–294. [Google Scholar] [CrossRef]
- De Gans, B.J.; Duineveld, P.C.; Schubert, U.S. Inkjet Printing of Polymers: State of the Art and Future Developments. Adv. Mater. 2004, 16, 203–213. [Google Scholar] [CrossRef]
- Bonyár, A.; Sántha, H.; Varga, M.; Ring, B.; Vitéz, A.; Harsányi, G. Characterization of Rapid PDMS Casting Technique Utilizing Molding Forms Fabricated by 3D Rapid Prototyping Technology (RPT). Int. J. Mater. Form. 2014, 7, 189–196. [Google Scholar] [CrossRef]
- Walczak, R.; Adamski, K. Inkjet 3D Printing of Microfluidic Structures—On the Selection of the Printer towards Printing Your Own Microfluidic Chips. J. Micromech. Microeng. 2015, 25, 085013. [Google Scholar] [CrossRef]
- Bártolo, P.J. Stereolithography—Materials, Processes and Applications; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Melchels, F.P.W.; Feijen, J.; Grijpma, D.W. A Review on Stereolithography and Its Applications in Biomedical Engineering. Biomaterials 2010, 31, 6121–6130. [Google Scholar] [CrossRef]
- Comina, G.; Suska, A.; Filippini, D. PDMS Lab-on-a-Chip Fabrication Using 3D Printed Templates. Lab Chip 2014, 14, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Shallan, A.I.; Smejkal, P.; Corban, M.; Guijt, R.M.; Breadmore, M.C. Cost-Effective Three-Dimensional Printing of Visibly Transparent Microchips within Minutes. Anal. Chem. 2014, 86, 3124–3130. [Google Scholar] [CrossRef] [PubMed]
- Au, A.K.; Lee, W.; Folch, A. Mail-Order Microfluidics: Evaluation of Stereolithography for the Production of Microfluidic Devices. Lab Chip 2014, 14, 1294–1301. [Google Scholar] [CrossRef]
- Xing, J.F.; Zheng, M.L.; Duan, X.M. Two-Photon Polymerization Microfabrication of Hydrogels: An Advanced 3D Printing Technology for Tissue Engineering and Drug Delivery. Chem. Soc. Rev. 2015, 44, 5031–5039. [Google Scholar] [CrossRef]
- Kumi, G.; Yanez, C.O.; Belfield, K.D.; Fourkas, J.T. High-Speed Multiphoton Absorption Polymerization: Fabrication of Microfluidic Channels with Arbitrary Cross-Sections and High Aspect Ratios. Lab Chip 2010, 10, 1057–1060. [Google Scholar] [CrossRef] [PubMed]
- Stoneman, M.; Fox, M.; Zeng, C.; Raicu, V. Real-Time Monitoring of Two-Photon Photopolymerization for Use in Fabrication of Microfluidic Devices. Lab Chip 2009, 9, 819–827. [Google Scholar] [CrossRef]
- Wu, D.; Chen, Q.D.; Niu, L.G.; Wang, J.N.; Wang, J.; Wang, R.; Xia, H.; Sun, H.B. Femtosecond Laser Rapid Prototyping of Nanoshells and Suspending Components towards Microfluidic Devices. Lab Chip 2009, 9, 2391–2394. [Google Scholar] [CrossRef]
- Crump, S. Apparatus and Method for Creating Three-Dimensional Objects. US Patent 5,121,329, 9 June 1992. Available online: https://patentimages.storage.googleapis.com/21/01/d3/69165ba25d15e0/US5121329.pdf (accessed on 15 May 2025).
- Novakova-Marcincinova, L.; Novak-Marcincin, J. Testing of Materials for Rapid Prototyping Fused Deposition Modelling Technology. Int. J. Mech. Aerosp. Ind. Mechatron. Manuf. Eng. 2012, 6, 73. [Google Scholar]
- Symes, M.D.; Kitson, P.J.; Yan, J.; Richmond, C.J.; Cooper, G.J.T.; Bowman, R.W.; Vilbrandt, T.; Cronin, L. Integrated 3D-Printed Reactionware for Chemical Synthesis and Analysis. Nat. Chem. 2012, 4, 349–354. [Google Scholar] [CrossRef]
- Kitson, P.J.; Rosnes, M.H.; Sans, V.; Dragone, V.; Cronin, L. Configurable 3D-Printed Millifluidic and Microfluidic “lab on a Chip” Reactionware Devices. Lab Chip 2012, 12, 3267–3271. [Google Scholar] [CrossRef]
- Bishop, G.W.; Satterwhite, J.E.; Bhakta, S.; Kadimisetty, K.; Gillette, K.M.; Chen, E.; Rusling, J.F. 3D-Printed Fluidic Devices for Nanoparticle Preparation and Flow-Injection Amperometry Using Integrated Prussian Blue Nanoparticle-Modified Electrodes. Anal. Chem. 2015, 87, 5437–5443. [Google Scholar] [CrossRef]
- Waheed, S.; Cabot, J.M.; Macdonald, N.P.; Lewis, T.; Guijt, R.M.; Paull, B.; Breadmore, M.C. 3D Printed Microfluidic Devices: Enablers and Barriers. Lab Chip 2016, 16, 1993–2013. [Google Scholar] [CrossRef]
- O’Connor, J.; Punch, J.; Jeffers, N.; Stafford, J. A Dimensional Comparison between Embedded 3D-Printed and Silicon Microchannels. In Journal of Physics: Conference Series, Proceedings of the Eurotherm Seminar 102: Thermal Management of Electronic Systems, Limerick, Ireland, 18–20 June 2014; IOP Publishing: Bristol, UK, 2014; Volume 525. [Google Scholar]
- Sochol, R.D.; Sweet, E.; Glick, C.C.; Venkatesh, S.; Avetisyan, A.; Ekman, K.F.; Raulinaitis, A.; Tsai, A.; Wienkers, A.; Korner, K.; et al. 3D Printed Microfluidic Circuitry via Multijet-Based Additive Manufacturing. Lab Chip 2016, 16, 668–678. [Google Scholar] [CrossRef] [PubMed]
- Takenaga, S.; Schneider, B.; Erbay, E.; Biselli, M.; Schnitzler, T.; Schöning, M.J.; Wagner, T. Fabrication of Biocompatible Lab-on-Chip Devices for Biomedical Applications by Means of a 3D-Printing Process. Phys. Status Solidi (A) Appl. Mater. Sci. 2015, 212, 1347–1352. [Google Scholar] [CrossRef]
- Moore, J.L.; McCuiston, A.; Mittendorf, I.; Ottway, R.; Johnson, R.D. Behavior of Capillary Valves in Centrifugal Microfluidic Devices Prepared by Three-Dimensional Printing. Microfluid. Nanofluidics 2011, 10, 877–888. [Google Scholar] [CrossRef]
- da Costa, P.F.G.M.; Merízio, L.G.; Wolff, N.; Terraschke, H.; de Camargo, A.S.S. Real-Time Monitoring of CdTe Quantum Dots Growth in Aqueous Solution. Sci. Rep. 2024, 14, 7884. [Google Scholar] [CrossRef]
- Abdel-Latif, K.; Epps, R.W.; Kerr, C.B.; Papa, C.M.; Castellano, F.N.; Abolhasani, M. Facile Room-Temperature Anion Exchange Reactions of Inorganic Perovskite Quantum Dots Enabled by a Modular Microfluidic Platform. Adv. Funct. Mater. 2019, 29, 1900712. [Google Scholar] [CrossRef]
- Dittrich, P.S.; Manz, A. Lab-on-a-Chip: Microfluidics in Drug Discovery. Nat. Rev. Drug Discov. 2006, 5, 210–218. [Google Scholar] [CrossRef]
- Ho, C.M.B.; Ng, S.H.; Li, K.H.H.; Yoon, Y.J. 3D Printed Microfluidics for Biological Applications. Lab Chip 2015, 15, 3627–3637. [Google Scholar] [CrossRef]
- Sackmann, E.K.; Fulton, A.L.; Beebe, D.J. The Present and Future Role of Microfluidics in Biomedical Research. Nature 2014, 507, 181–189. [Google Scholar] [CrossRef]
- Fuard, D.; Tzvetkova-Chevolleau, T.; Decossas, S.; Tracqui, P.; Schiavone, P. Optimization of Poly-Di-Methyl-Siloxane (PDMS) Substrates for Studying Cellular Adhesion and Motility. Microelectron. Eng. 2008, 85, 1289–1293. [Google Scholar] [CrossRef]
- Moran, M.; Hobbs, C. From Communities of Interest to Communities Of Practice: The Role and Impact of Professional Development in Nuclear Security Education. Br. J. Educ. Stud. 2018, 66, 87–107. [Google Scholar] [CrossRef]
- Xia, Y.; Whitesides, G.M. Soft Lithography. Annu. Rev. Mater. Sci. 1998, 28, 550–575. [Google Scholar] [CrossRef]
- Ma, P.; Jia, X.; He, Y.; Tao, J.; Wang, Q.; Wei, C.I. Recent Progress of Quantum Dots for Food Safety Assessment: A Review. Trends Food Sci. Technol. 2024, 143, 104310. [Google Scholar] [CrossRef]

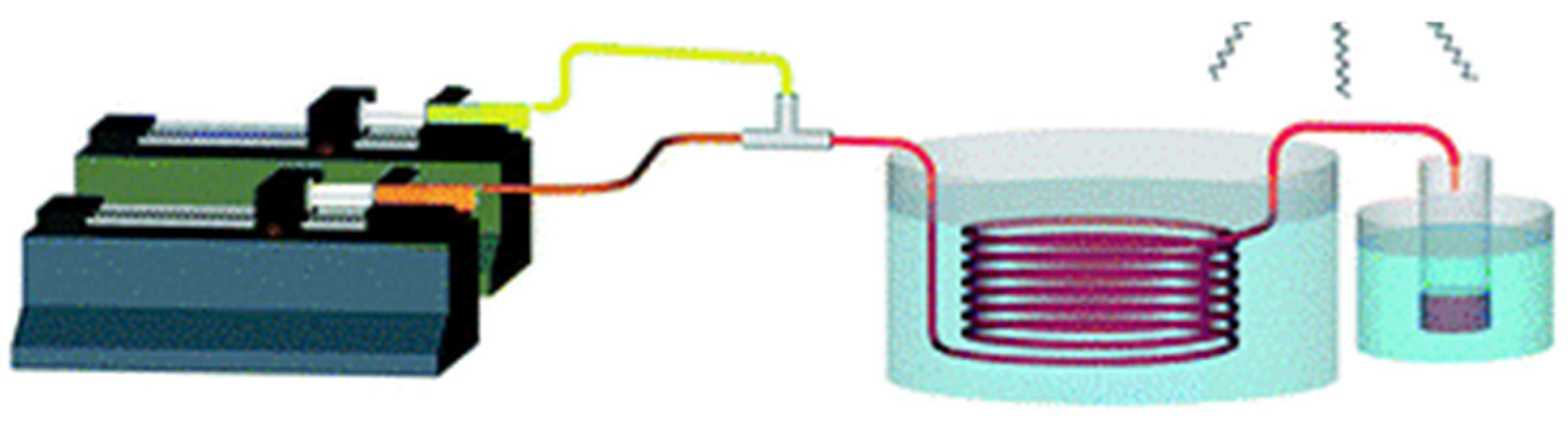
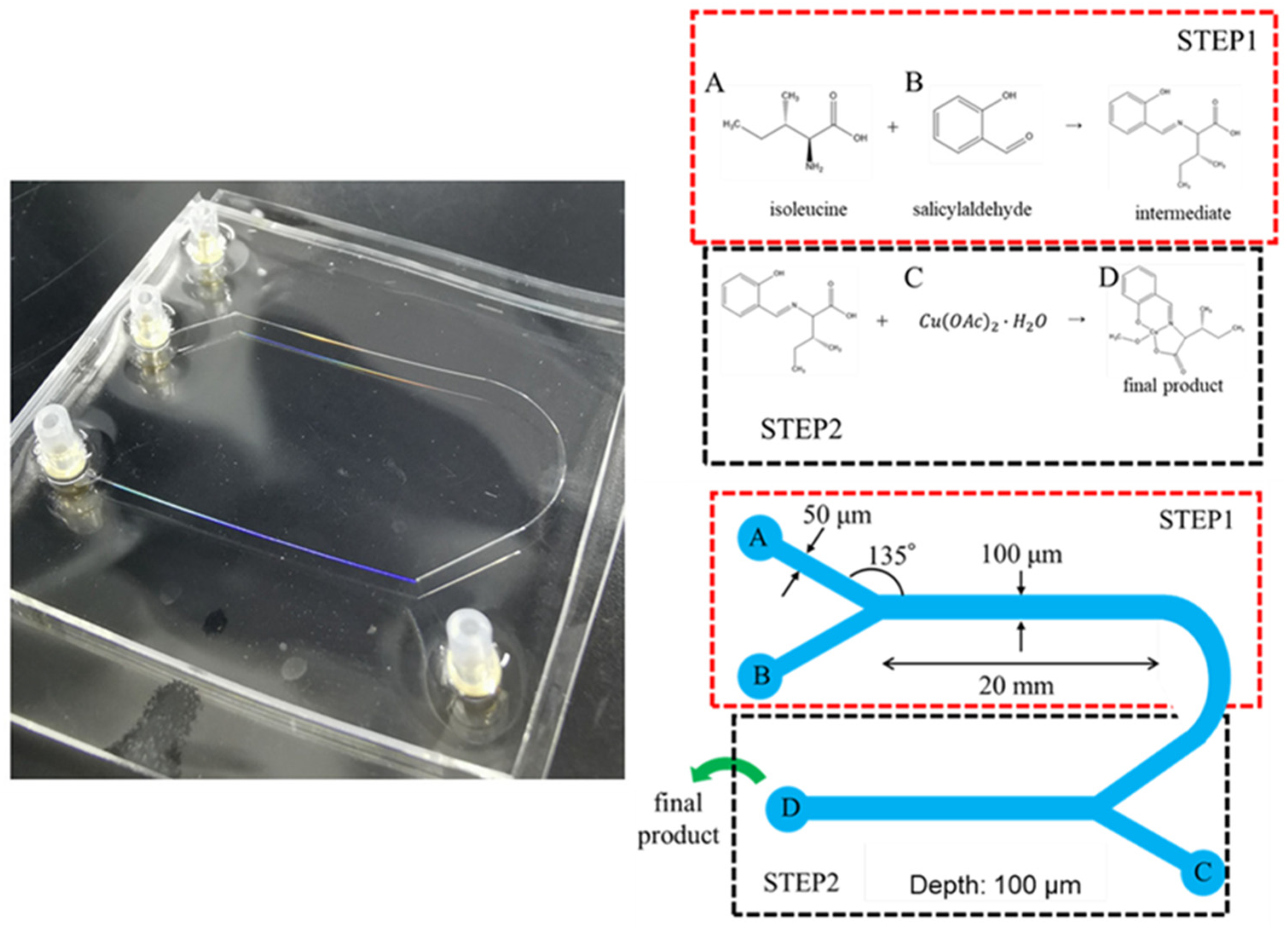
| Method | Process Description | Advantages | Disadvantages | Reaction Environment | Time Efficiency |
|---|---|---|---|---|---|
| Hot Injection (HI) | High-temperature injection of reactants in a controlled environment enables the production of quantum dots with precise size and shape control. | Allows for precise control over size and optical properties; high-quality quantum dots. | It requires high temperatures and rapid injection, which is challenging without an oxygen-free environment. | Requires an inert atmosphere, typically nitrogen gas, and high temperatures. | Rapid nucleation and growth occur within seconds, but require preparation time. |
| Ultrasonication-Assisted | A scalable method involving ultrasonication in ambient air enables the rapid formation of nanoparticles without the need for inert gas protection. | No need for an isolated environment; produces monodispersed quantum dots with high photoluminescence yield. | Results can vary depending on ultrasonication conditions and are less consistent than those of other methods. | Operates in ambient air; no exceptional environment required. | Swift process (within minutes) with immediate nucleation. |
| Wet and Dry Milling | Simple grinding or milling components to produce quantum dots can avoid solubility issues, but often results in lower-quality products. | A cost-effective and straightforward process with fewer risks, thereby avoiding solubility issues. | Lower photoluminescence quantum yield; surface defects can reduce final product quality. | No specific environmental requirements, simple grinding/milling. | Relatively slow, depending on milling time and desired particle size. |
| Continuous Flow | Reactants are mixed in a continuous flow reactor at controlled temperatures, providing consistent and reproducible quantum dot synthesis. | Enhanced reproducibility and yield; no need for high temperatures or specialized environments. | Complex setup; not as straightforward as batch processes. | Controlled environment within a flow reactor; usually does not require inert gases. | Efficient for continuous production; reaction times are short, but setup can be time-consuming. |
| Metric | Traditional Synthesis Methods | Microfluidic Methods | References |
|---|---|---|---|
| Size Control | Wide particle size distribution due to uncontrollable reaction conditions. | Produces nanoparticles with <10% standard deviation in size distribution through precise reaction control. | [114] |
| Cost Efficiency | High material and energy consumption; expensive fabrication processes. | Reduces material and energy usage; microreactors are inexpensive and rapidly fabricated (e.g., CO2-laser). | [104,112] |
| Scalability | Struggles to maintain consistent quality when scaled up; batch processes can be inefficient. | Designed for continuous flow production, allowing scalable and consistent QD synthesis. | [115,117] |
| Reproducibility | Variable conditions often lead to inconsistent quality between batches. | Ensures thermal and chemical homogeneity, resulting in consistent reaction conditions and reproducible QDs. | [116] |
| Reaction Efficiency | Slower reaction rates and limited separation of nucleation and growth processes. | Facilitates rapid heat and mass transfer, enabling faster reactions and better control of growth processes. | [102] |
| Environmental Impact | High waste generation and energy demands. | Minimizes waste through precise control and efficient material use. | [111] |
| QD Type | Synthesis Parameters | Achieved Properties | Application |
|---|---|---|---|
| Ag2S | Liquid droplet micro-reactor using soybean oil and glycol as the medium | Water solubility is crucial for biological applications | Not specified |
| ZnO | Combined ultrasonic and microfluidic technology | Quantum yield of 64.7; increased with ultrasonic temperature | Not specified |
| Carbon QDs (CQDs) | Polytetrafluoroethylene microtubes with thermal decomposition | Not specified | Not specified |
| CQDs | Multilayer structure chip system with ascorbic acid and dimethyl sulfoxide | Diameter: 3.3 nm; quantum yield of 2.6 (moderate photoluminescence) | Precise control of the reaction process for consistent QD production |
| CsPbX3 Perovskite | Droplet-based microfluidic platform with online absorbance and fluorescence detection | Enabled real-time characterization, crucial for optimizing synthesis | Not specified |
| Challenge Type | Impact on QD Synthesis | Proposed Solutions | References |
|---|---|---|---|
| Material Limitations | Alteration of reaction conditions due to absorption, solvent incompatibility, and structural instability | Exploration of alternative materials (e.g., COC, PS) and surface modifications | [119,120,121] |
| Design Constraints for Scaling Up | Inconsistent QD quality, clogging, and difficulty in maintaining uniform reaction conditions | Use of trunk–branch structures, three-dimensional channel designs, and automated control systems | [116,122] |
| Cost Constraints | High costs of traditional methods and cleanroom facilities | Use of 3D printing to reduce costs and enable complex designs | [112,123] |
| Technique | Fundamentals | Capabilities | Strengths | Weaknesses | Source |
|---|---|---|---|---|---|
| Inkjet 3D Printing (i3DP) | i3DP uses inkjet technology to deposit material layer by layer, operating in continuous or drop-on-demand modes, with drop-on-demand preferred for better droplet control. | Capable of creating complex devices but faces challenges in removing support material from enclosed channels; decent resolution but limited by surface roughness and material availability. | Suitable for rapid prototyping; decent resolution. | Difficult to remove support material, higher costs, and surface roughness. | [129,130,131,132,133] |
| Stereolithography (SLA) | SLA involves the spatially controlled photopolymerization of liquid resin, using either a laser or DLP; objects are built layer by layer, with both free surface and constrained surface configurations. | Known for good resolution and ability to create transparent, biocompatible devices; limited by available resins and challenges in removing uncured resin from small channels. | Better resolution, material variety, and biocompatibility. | Limited by resin options, challenging post-processing for enclosed structures. | [134,135,136,137,138] |
| Two-Photon Polymerization (2PP) | 2PP uses a femtosecond laser for high-resolution polymerization, enabling the creation of intricate three-dimensional microstructures within devices and achieving sub-micron precision. | It provides the highest resolution, ideal for nanoscale features; however, it is prolonged and costly, limiting its practicality for routine or large-scale fabrication. | Superior resolution; ideal for nanoscale features. | Very slow; extremely costly; requires specialized equipment; not suitable for large-scale production. | [139,140,141,142] |
| Extrusion Printing (FDM) | FDM extrudes thermoplastic filament through a heated nozzle layer by layer, making it widely used due to its simplicity and material versatility. | Cost-effective and compatible with mass production, but lower resolution and rough surface finishes make it less suitable for fine microfluidic work. | Cost-effective, compatible with mass production, and versatile material options. | Lower resolution, rough surfaces, limited minimum channel size, and slower than other techniques. | [143,144,145,146,147] |
| Manufacturing Technology | Model/Manufacturer | Material | Resolution (x, y, z) μm | Advantages Reported | Disadvantages Reported | Application | Source |
|---|---|---|---|---|---|---|---|
| Inkjet 3D Printing (i3DP) | ProJet 3500 HD | Acrylonitrile | 39 × 39 × 29 | Vertically printed channels have dimensional stability and smooth surfaces. | Features along the Y-axis have rough surfaces and low dimensional accuracy. | Study of printing performance for microfluidic features. | [149] |
| Inkjet 3D Printing (i3DP) | ProJet 3000 HD | VisiJet M3 Polymer | 38 × 38 × 32 | Modular assembly and integrated microfluidic circuits. | Residual flow observed through closed interactions; limited optical properties, biocompatibility. | Fabrication of fluidic circuit components like capacitors, diodes, and transistors. | [150] |
| Stereolithography (SLA) | Miicraft (Taiwan) | Acrylate-based resin | 56 × 56 × 50 | Transparent, low-cost microfluidic chips. | It requires improvement in resin properties and hardware; coatings are needed for templates. | Gradient generation, droplet extraction, and glucose sensing. | [136,137] |
| Stereolithography (SLA) | Asiga PicoPlus 27 | PlasCLEAR | 27 × 27 × 0.25 | Biocompatible microfluidic chips with <100 μm channels can be printed. | Not reported. | Fabrication of microfluidic chips for cell culturing and sensor integration. | [151] |
| Two-Photon Polymerization (2PP) | Ti laser (Kapteyn-Murnane) | SU-8-negative photoresist | Not reported | High-resolution and small-scale structures were fabricated. | Time-consuming for complex structures. | Fabrication of microchannels and trapping yeast cells. | [141] |
| Fused Deposition Modeling (FDM) | Dimension SST 768 | ABS-P400 | 254 × 254 × 254 | Variable widths are achievable in single devices at low cost. | Surface roughness affects laminar flow; there is a limited choice of polymers. | Capillary valves in centrifugal microfluidic devices. | [152] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarbaland, F.b.N.; Kobayashi, M.; Tanaka, D.; Fujita, R.; Furuya, M. Redefining Quantum Dot Synthesis with Additive-Manufactured Microfluidics—A Review. J 2025, 8, 18. https://doi.org/10.3390/j8020018
Sarbaland FbN, Kobayashi M, Tanaka D, Fujita R, Furuya M. Redefining Quantum Dot Synthesis with Additive-Manufactured Microfluidics—A Review. J. 2025; 8(2):18. https://doi.org/10.3390/j8020018
Chicago/Turabian StyleSarbaland, Faisal bin Nasser, Masashi Kobayashi, Daiki Tanaka, Risa Fujita, and Masahiro Furuya. 2025. "Redefining Quantum Dot Synthesis with Additive-Manufactured Microfluidics—A Review" J 8, no. 2: 18. https://doi.org/10.3390/j8020018
APA StyleSarbaland, F. b. N., Kobayashi, M., Tanaka, D., Fujita, R., & Furuya, M. (2025). Redefining Quantum Dot Synthesis with Additive-Manufactured Microfluidics—A Review. J, 8(2), 18. https://doi.org/10.3390/j8020018







