Relationship between Plasma Lipopolysaccharide Concentration and Health Status in Healthy Subjects and Patients with Abnormal Glucose Metabolism in Japan: A Preliminary Cross-Sectional Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Approval and Consent to Participate
2.2. Study Design
2.3. Study 1
2.3.1. Subjects
2.3.2. Schedule
2.3.3. Data Collection
- Medical interview
- 2.
- Blood samples
- 3.
- Analysis of plasma LPS concentrations
2.4. Study 2
2.4.1. Subjects
2.4.2. Schedule
2.4.3. Data Collection
- Medical interview
- 2.
- Blood samples and analysis of plasma LPS concentration
- 3.
- Physiological and biochemical analyses
2.5. Statistical Methods
3. Results
3.1. Study 1
3.1.1. Subjects’ Characteristics
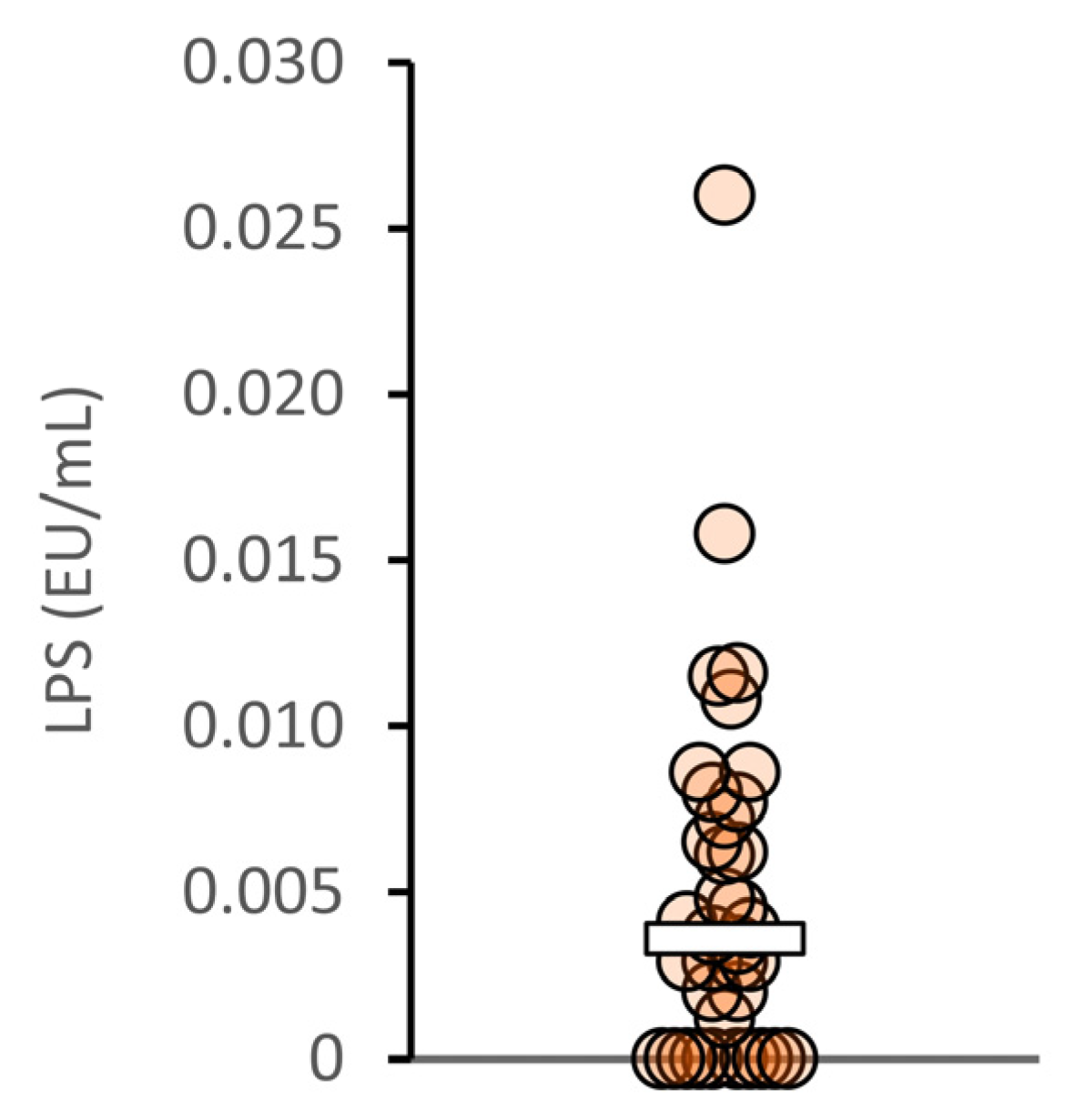
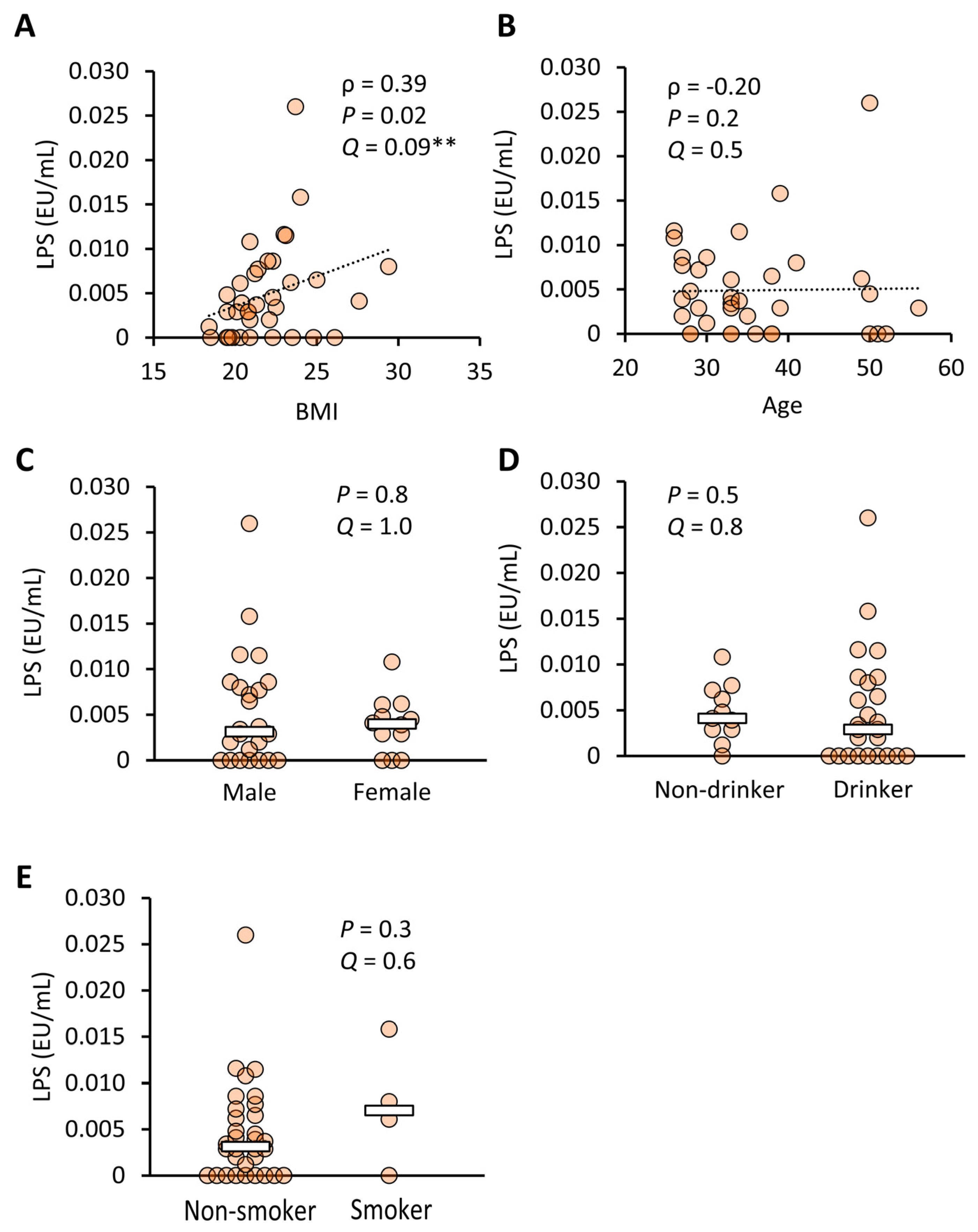
3.1.2. Plasma LPS Concentrations in Healthy Japanese Subjects
3.2. Study 2
3.2.1. Subjects’ Characteristics
3.2.2. Plasma LPS Concentrations in Japanese Patients with AGM
4. Discussion
4.1. Characteristics of the Study Subjects
4.2. Blood LPSs and Obesity
4.3. Blood LPSs and Glucose Metabolism
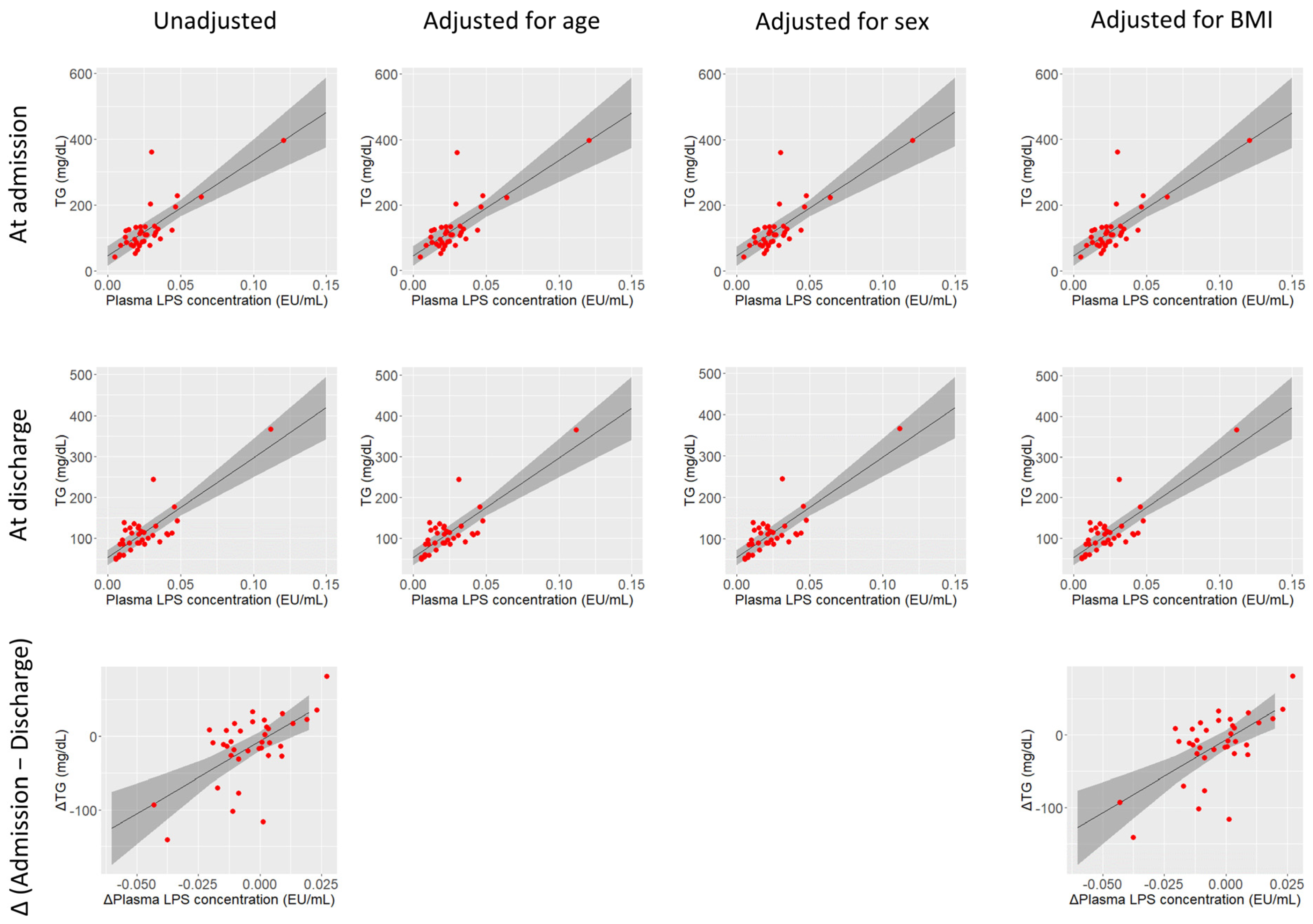
4.4. Blood LPSs and Lipid Metabolism
4.5. Blood LPSs and Renal Functions
4.6. Estimation of the Main Targets of Blood LPSs in AGM Patients
4.7. Implications for Clinical Applications
4.8. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cani, P.D.; Amar, J.; Iglesias, M.A.; Poggi, M.; Knauf, C.; Bastelica, D.; Neyrinck, A.M.; Fava, F.; Tuohy, K.M.; Chabo, C.; et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007, 56, 1761–1772. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Bibiloni, R.; Knauf, C.; Waget, A.; Neyrinck, A.M.; Delzenne, N.M.; Burcelin, R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 2008, 57, 1470–1481. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Hussey, S.E.; Sanchez-Avila, A.; Tantiwong, P.; Musi, N. Effect of lipopolysaccharide on inflammation and insulin action in human muscle. PLoS ONE 2013, 8, e63983. [Google Scholar] [CrossRef]
- Pussinen, P.J.; Havulinna, A.S.; Lehto, M.; Sundvall, J.; Salomaa, V. Endotoxemia is associated with an increased risk of incident diabetes. Diabetes Care 2011, 34, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, T.; Takahashi, N.; Sawaragi, Y.; Naknukool, S.; Yu, R.; Goto, T.; Kawada, T. Inflammation induced by RAW macrophages suppresses UCP1 mRNA induction via ERK activation in 10T1/2 adipocytes. Am. J. Physiol. Cell Physiol. 2013, 304, C729–C738. [Google Scholar] [CrossRef] [PubMed]
- Jeremic, N.; Chaturvedi, P.; Tyagi, S.C. Browning of White Fat: Novel Insight into Factors, Mechanisms, and Therapeutics. J. Cell. Physiol. 2017, 232, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Pilon, G.; Charbonneau, A.; White, P.J.; Dallaire, P.; Perreault, M.; Kapur, S.; Marette, A. Endotoxin mediated-iNOS induction causes insulin resistance via ONOO− induced tyrosine nitration of IRS-1 in skeletal muscle. PLoS ONE 2010, 5, e15912. [Google Scholar] [CrossRef]
- Chen, S.-N.; Tan, Y.; Xiao, X.-C.; Li, Q.; Wu, Q.; Peng, Y.-Y.; Ren, J.; Dong, M.-L. Deletion of TLR4 attenuates lipopolysaccharide-induced acute liver injury by inhibiting inflammation and apoptosis. Acta Pharmacol. Sin. 2021, 42, 1610–1619. [Google Scholar] [CrossRef]
- Fuke, N.; Nagata, N.; Suganuma, H.; Ota, T. Regulation of gut microbiota and metabolic endotoxemia with dietary factors. Nutrients 2019, 11, 2277. [Google Scholar] [CrossRef] [PubMed]
- Gnauck, A.; Lentle, R.G.; Kruger, M.C. Chasing a ghost?—Issues with the determination of circulating levels of endotoxin in human blood. Crit. Rev. Clin. Lab. Sci. 2016, 53, 197–215. [Google Scholar] [CrossRef]
- Kadowaki, S.; Tamura, Y.; Sugimoto, D.; Kaga, H.; Suzuki, R.; Someya, Y.; Yamasaki, N.; Sato, M.; Kakehi, S.; Kanazawa, A.; et al. A Short-Term High-Fat Diet Worsens Insulin Sensitivity with Changes in Metabolic Parameters in Non-Obese Japanese Men. J. Clin. Med. 2023, 12, 4084. [Google Scholar] [CrossRef]
- Tomooka, S.; Oishi, E.; Asada, M.; Sakata, S.; Hata, J.; Chen, S.; Honda, T.; Suzuki, K.; Watanabe, H.; Murayama, N.; et al. Serum Lipopolysaccharide-binding Protein Levels and the Incidence of Metabolic Syndrome in a General Japanese Population: The Hisayama Study. J. Epidemiol. 2022, JE20220232. [Google Scholar] [CrossRef]
- Gonai, M.; Shigehisa, A.; Kigawa, I.; Kurasaki, K.; Chonan, O.; Matsuki, T.; Yoshida, Y.; Aida, M.; Hamano, K.; Terauchi, Y. Galacto-oligosaccharides ameliorate dysbiotic Bifidobacteriaceae decline in Japanese patients with type 2 diabetes. Benef. Microbes 2017, 8, 705–716. [Google Scholar] [CrossRef] [PubMed]
- Tamaki, S.; Kanazawa, A.; Sato, J.; Tamura, Y.; Asahara, T.; Takahashi, T.; Matsumoto, S.; Yamashiro, Y.; Watada, H. Clinical factors associated with bacterial translocation in Japanese patients with type 2 diabetes: A retrospective study. PLoS ONE 2019, 14, e0222598. [Google Scholar] [CrossRef]
- Watanabe, H.; Katsura, T.; Takahara, M.; Miyashita, K.; Katakami, N.; Matsuoka, T.; Kawamori, D.; Shimomura, I. Plasma lipopolysaccharide binding protein level statistically mediates between body mass index and chronic microinflammation in Japanese patients with type 1 diabetes. Diabetol. Int. 2020, 11, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Geller, D.A.; Kispert, P.H.; Su, G.L.; Wang, S.C.; Di Silvio, M.; Tweardy, D.J.; Billiar, T.R.; Simmons, R.L. Induction of hepatocyte lipopolysaccharide binding protein in models of sepsis and the acute-phase response. Arch. Surg. 1993, 128, 22–27; discussion 27–28. [Google Scholar] [CrossRef]
- Liu, X.; Lu, L.; Yao, P.; Ma, Y.; Wang, F.; Jin, Q.; Ye, X.; Li, H.; Hu, F.B.; Sun, L.; et al. Lipopolysaccharide binding protein, obesity status and incidence of metabolic syndrome: A prospective study among middle-aged and older Chinese. Diabetologia 2014, 57, 1834–1841. [Google Scholar] [CrossRef]
- Tilves, C.M.; Zmuda, J.M.; Kuipers, A.L.; Nestlerode, C.S.; Evans, R.W.; Bunker, C.H.; Patrick, A.L.; Miljkovic, I. Association of Lipopolysaccharide-Binding Protein with Aging-Related Adiposity Change and Prediabetes among African Ancestry Men. Diabetes Care 2016, 39, 385–391. [Google Scholar] [CrossRef]
- Asada, M.; Oishi, E.; Sakata, S.; Hata, J.; Yoshida, D.; Honda, T.; Furuta, Y.; Shibata, M.; Suzuki, K.; Watanabe, H.; et al. Serum Lipopolysaccharide-Binding Protein Levels and the Incidence of Cardiovascular Disease in a General Japanese Population: The Hisayama Study. J. Am. Heart Assoc. 2019, 8, e013628. [Google Scholar] [CrossRef]
- Fang, H.; Liu, A.; Dirsch, O.; Sun, J.; Jin, H.; Lu, M.; Yang, D.; Dahmen, U. Serum LBP levels reflect the impaired synthetic capacity of the remnant liver after partial hepatectomy in rats. J. Immunol. Methods 2012, 382, 68–75. [Google Scholar] [CrossRef]
- Su, G.L.; Fontana, R.J.; Jinjuvadia, K.; Bayliss, J.; Wang, S.C. Lipopolysaccharide Binding Protein Is Down-Regulated During Acute Liver Failure. Dig. Dis. Sci. 2012, 57, 918–924. [Google Scholar] [CrossRef]
- Moreno-Navarrete, J.M.; Escoté, X.; Ortega, F.; Serino, M.; Campbell, M.; Michalski, M.-C.; Laville, M.; Xifra, G.; Luche, E.; Domingo, P.; et al. A role for adipocyte-derived lipopolysaccharide-binding protein in inflammation- and obesity-associated adipose tissue dysfunction. Diabetologia 2013, 56, 2524–2537. [Google Scholar] [CrossRef]
- Vors, C.; Pineau, G.; Drai, J.; Meugnier, E.; Pesenti, S.; Laville, M.; Laugerette, F.; Malpuech-Brugère, C.; Vidal, H.; Michalski, M.-C. Postprandial Endotoxemia Linked with Chylomicrons and Lipopolysaccharides Handling in Obese Versus Lean Men: A Lipid Dose-Effect Trial. J. Clin. Endocrinol. Metab. 2015, 100, 3427–3435. [Google Scholar] [CrossRef] [PubMed]
- Dheda, S.; Min, H.; Vesey, D.; Hawley, C.; Johnson, D.W.; Fahim, M. Establishing a stable platform for the measurement of blood endotoxin levels in the dialysis population. Diagnosis 2021, 8, 249–256. [Google Scholar] [CrossRef]
- Fuke, N.; Ushida, Y.; Sato, I.; Suganuma, H. Inter-Day Variation in the Fasting Plasma Lipopolysaccharide Concentration in the Morning Is Associated with Inter-Day Variation in Appetite in Japanese Males: A Short-Term Cohort Study. Metabolites 2023, 13, 395. [Google Scholar] [CrossRef]
- Yuan, X.; Wang, R.; Han, B.; Sun, C.; Chen, R.; Wei, H.; Chen, L.; Du, H.; Li, G.; Yang, Y.; et al. Functional and metabolic alterations of gut microbiota in children with new-onset type 1 diabetes. Nat. Commun. 2022, 13, 6356. [Google Scholar] [CrossRef]
- Roth-Schulze, A.J.; Penno, M.A.S.; Ngui, K.M.; Oakey, H.; Bandala-Sanchez, E.; Smith, A.D.; Allnutt, T.R.; Thomson, R.L.; Vuillermin, P.J.; Craig, M.E.; et al. Type 1 diabetes in pregnancy is associated with distinct changes in the composition and function of the gut microbiome. Microbiome 2021, 9, 167. [Google Scholar] [CrossRef]
- Leiva-Gea, I.; Sánchez-Alcoholado, L.; Martín-Tejedor, B.; Castellano-Castillo, D.; Moreno-Indias, I.; Urda-Cardona, A.; Tinahones, F.J.; Fernández-García, J.C.; Queipo-Ortuño, M.I. Gut Microbiota Differs in Composition and Functionality Between Children with Type 1 Diabetes and MODY2 and Healthy Control Subjects: A Case-Control Study. Diabetes Care 2018, 41, 2385–2395. [Google Scholar] [CrossRef]
- Simonsen, J.R.; Järvinen, A.; Hietala, K.; Harjutsalo, V.; Forsblom, C.; Groop, P.-H.; Lehto, M. Bacterial infections as novel risk factors of severe diabetic retinopathy in individuals with type 1 diabetes. Br. J. Ophthalmol. 2021, 105, 1104–1110. [Google Scholar] [CrossRef]
- Simonsen, J.R.; Järvinen, A.; Harjutsalo, V.; Forsblom, C.; Groop, P.-H.; Lehto, M. The association between bacterial infections and the risk of coronary heart disease in type 1 diabetes. J. Intern. Med. 2020, 288, 711–724. [Google Scholar] [CrossRef]
- Huang, X.; Yan, D.; Xu, M.; Li, F.; Ren, M.; Zhang, J.; Wu, M. Interactive association of lipopolysaccharide and free fatty acid with the prevalence of type 2 diabetes: A community-based cross-sectional study. J. Diabetes Investig. 2019, 10, 1438–1446. [Google Scholar] [CrossRef]
- Amar, J.; Burcelin, R.; Ruidavets, J.B.; Cani, P.D.; Fauvel, J.; Alessi, M.C.; Chamontin, B.; Ferriéres, J. Energy intake is associated with endotoxemia in apparently healthy men. Am. J. Clin. Nutr. 2008, 87, 1219–1223. [Google Scholar] [CrossRef]
- American Diabetes Association 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2021. Diabetes Care 2021, 44, S15–S33. [Google Scholar] [CrossRef] [PubMed]
- Wada, T.; Haneda, M.; Furuichi, K.; Babazono, T.; Yokoyama, H.; Iseki, K.; Araki, S.; Ninomiya, T.; Hara, S.; Suzuki, Y.; et al. Clinical impact of albuminuria and glomerular filtration rate on renal and cardiovascular events, and all-cause mortality in Japanese patients with type 2 diabetes. Clin. Exp. Nephrol. 2014, 18, 613–620. [Google Scholar] [CrossRef]
- Ohkura, T.; Shiochi, H.; Fujioka, Y.; Sumi, K.; Yamamoto, N.; Matsuzawa, K.; Izawa, S.; Kinoshita, H.; Ohkura, H.; Kato, M.; et al. 20/(fasting C-peptide × fasting plasma glucose) is a simple and effective index of insulin resistance in patients with type 2 diabetes mellitus: A preliminary report. Cardiovasc. Diabetol. 2013, 12, 21. [Google Scholar] [CrossRef] [PubMed]
- Varma, M.C.; Kusminski, C.M.; Azharian, S.; Gilardini, L.; Kumar, S.; Invitti, C.; McTernan, P.G. Metabolic endotoxaemia in childhood obesity. BMC Obes. 2016, 3, 3. [Google Scholar] [CrossRef]
- Mokkala, K.; Pellonperä, O.; Röytiö, H.; Pussinen, P.; Rönnemaa, T.; Laitinen, K. Increased intestinal permeability, measured by serum zonulin, is associated with metabolic risk markers in overweight pregnant women. Metabolism 2017, 69, 43–50. [Google Scholar] [CrossRef]
- Radilla-Vázquez, R.B.; Parra-Rojas, I.; Martínez-Hernández, N.E.; Márquez-Sandoval, Y.F.; Illades-Aguiar, B.; Castro-Alarcón, N. Gut Microbiota and Metabolic Endotoxemia in Young Obese Mexican Subjects. Obes. Facts 2016, 9, 1–11. [Google Scholar] [CrossRef]
- Jayashree, B.; Bibin, Y.S.; Prabhu, D.; Shanthirani, C.S.; Gokulakrishnan, K.; Lakshmi, B.S.; Mohan, V.; Balasubramanyam, M. Increased circulatory levels of lipopolysaccharide (LPS) and zonulin signify novel biomarkers of proinflammation in patients with type 2 diabetes. Mol. Cell. Biochem. 2014, 388, 203–210. [Google Scholar] [CrossRef]
- Lassenius, M.I.; Ahola, A.J.; Harjutsalo, V.; Forsblom, C.; Groop, P.-H.; Lehto, M. Endotoxins are associated with visceral fat mass in type 1 diabetes. Sci. Rep. 2016, 6, 38887. [Google Scholar] [CrossRef]
- Kiddle, S.J.; Sattlecker, M.; Proitsi, P.; Simmons, A.; Westman, E.; Bazenet, C.; Nelson, S.K.; Williams, S.; Hodges, A.; Johnston, C.; et al. Candidate blood proteome markers of Alzheimer’s disease onset and progression: A systematic review and replication study. J. Alzheimer’s Dis. 2014, 38, 515–531. [Google Scholar] [CrossRef]
- Smith, S.B.; Mir, E.; Bair, E.; Slade, G.D.; Dubner, R.; Fillingim, R.B.; Greenspan, J.D.; Ohrbach, R.; Knott, C.; Weir, B.; et al. Genetic variants associated with development of TMD and its intermediate phenotypes: The genetic architecture of TMD in the OPPERA prospective cohort study. J. Pain 2013, 14, T91–T101.E3. [Google Scholar] [CrossRef]
- Kanda, Y. Investigation of the freely available easy-to-use software “EZR” for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Waldhäusl, W.K.; Gasić, S.; Bratusch-Marrain, P.; Korn, A.; Nowotny, P. Feedback inhibition by biosynthetic human insulin of insulin release in healthy human subjects. Am. J. Physiol. 1982, 243, E476–E482. [Google Scholar] [CrossRef] [PubMed]
- Garvey, W.T.; Revers, R.R.; Kolterman, O.G.; Rubenstein, A.H.; Olefsky, J.M. Modulation of insulin secretion by insulin and glucose in type II diabetes mellitus. J. Clin. Endocrinol. Metab. 1985, 60, 559–568. [Google Scholar] [CrossRef]
- The Statistics Bureau, Ministry of Internal Affairs and Communications. Population Survey, Japan. 2016. Available online: https://www.e-stat.go.jp/stat-search/files?page=1&layout=datalist&toukei=00200524&tstat=000000090001&cycle=0&tclass1=000001011678&tclass2=000001091515&stat_infid=000031504515&tclass3val=0 (accessed on 1 October 2023).
- J. Ministry of Health, Labour and Welfare. The National Health and Nutrition Survey, Japan. 2017. Available online: https://www.e-stat.go.jp/stat-search/files?page=1&layout=datalist&toukei=00450171&kikan=00450&tstat=000001041744&cycle=7&tclass1=000001123258&survey=健康&result_page=1&cycle_facet=cycle&tclass2val=0 (accessed on 1 October 2023).
- Murai, J.; Nishizawa, H.; Otsuka, A.; Fukuda, S.; Tanaka, Y.; Nagao, H.; Sakai, Y.; Suzuki, M.; Yokota, S.; Tada, H.; et al. Low muscle quality in Japanese type 2 diabetic patients with visceral fat accumulation. Cardiovasc. Diabetol. 2018, 17, 112. [Google Scholar] [CrossRef] [PubMed]
- Sato, J.; Kanazawa, A.; Ikeda, F.; Yoshihara, T.; Goto, H.; Abe, H.; Komiya, K.; Kawaguchi, M.; Shimizu, T.; Ogihara, T.; et al. Gut dysbiosis and detection of “live gut bacteria” in blood of Japanese patients with type 2 diabetes. Diabetes Care 2014, 37, 2343–2350. [Google Scholar] [CrossRef]
- Omran, F.; Murphy, A.M.; Younis, A.Z.; Kyrou, I.; Vrbikova, J.; Hainer, V.; Sramkova, P.; Fried, M.; Ball, G.; Tripathi, G.; et al. The impact of metabolic endotoxaemia on the browning process in human adipocytes. BMC Med. 2023, 21, 154. [Google Scholar] [CrossRef]
- Yeager, M.P.; Rassias, A.J.; Pioli, P.A.; Beach, M.L.; Wardwell, K.; Collins, J.E.; Lee, H.-K.; Guyre, P.M. Pretreatment with stress cortisol enhances the human systemic inflammatory response to bacterial endotoxin. Crit. Care Med. 2009, 37, 2727–2732. [Google Scholar] [CrossRef] [PubMed]
- Kox, M.; van Eijk, L.T.; Zwaag, J.; van den Wildenberg, J.; Sweep, F.C.G.J.; van der Hoeven, J.G.; Pickkers, P. Voluntary activation of the sympathetic nervous system and attenuation of the innate immune response in humans. Proc. Natl. Acad. Sci. USA 2014, 111, 7379–7384. [Google Scholar] [CrossRef]
- Vergès, B.; Duvillard, L.; Lagrost, L.; Vachoux, C.; Garret, C.; Bouyer, K.; Courtney, M.; Pomié, C.; Burcelin, R. Changes in lipoprotein kinetics associated with type 2 diabetes affect the distribution of lipopolysaccharides among lipoproteins. J. Clin. Endocrinol. Metab. 2014, 99, E1245–E1253. [Google Scholar] [CrossRef]
- Emancipator, K.; Csako, G.; Elin, R.J. In vitro inactivation of bacterial endotoxin by human lipoproteins and apolipoproteins. Infect. Immun. 1992, 60, 596–601. [Google Scholar] [CrossRef] [PubMed]
- Harm, S.; Schildböck, C.; Strobl, K.; Hartmann, J. An in vitro study on factors affecting endotoxin neutralization in human plasma using the Limulus amebocyte lysate test. Sci. Rep. 2021, 11, 4192. [Google Scholar] [CrossRef] [PubMed]
- Van Deventer, S.J.H.; Buller, H.R.; Ten Cate, J.W.; Aarden, L.A.; Hack, C.E.; Sturk, A. Experimental endotoxemia in humans: Analysis of cytokine release and coagulation, fibrinolytic, and complement pathways. Blood 1990, 76, 2520–2526. [Google Scholar] [CrossRef] [PubMed]
- Hudgins, L.C.; Parker, T.S.; Levine, D.M.; Gordon, B.R.; Saal, S.D.; Jiang, X.; Seidman, C.E.; Tremaroli, J.D.; Lai, J.; Rubin, A.L. A single intravenous dose of endotoxin rapidly alters serum lipoproteins and lipid transfer proteins in normal volunteers. J. Lipid Res. 2003, 44, 1489–1498. [Google Scholar] [CrossRef] [PubMed]
- Victorov, A.V.; Gladkaya, E.M.; Novikov, D.K.; Kosykh, V.A.; Yurkiv, V.A. Lipopolysaccharide toxin can directly stimulate the intracellular accumulation of lipids and their secretion into medium in the primary culture of rabbit hepatocytes. FEBS Lett. 1989, 256, 155–158. [Google Scholar] [CrossRef]
- Harris, H.W.; Grunfeld, C.; Feingold, K.R.; Rapp, J.H. Human very low density lipoproteins and chylomicrons can protect against endotoxin-induced death in mice. J. Clin. Investig. 1990, 86, 696–702. [Google Scholar] [CrossRef]
- Rood, J.; Smith, S.R. Triglyceride concentrations and endotoxemia. Am. J. Clin. Nutr. 2008, 88, 248–249. [Google Scholar] [CrossRef]
- Harte, A.L.; Varma, M.C.; Tripathi, G.; McGee, K.C.; Al-Daghri, N.M.; Al-Attas, O.S.; Sabico, S.; O’Hare, J.P.; Ceriello, A.; Saravanan, P.; et al. High fat intake leads to acute postprandial exposure to circulating endotoxin in type 2 diabetic subjects. Diabetes Care 2012, 35, 375–382. [Google Scholar] [CrossRef]
- Lassenius, M.I.; Pietiläinen, K.H.; Kaartinen, K.; Pussinen, P.J.; Syrjänen, J.; Forsblom, C.; Pörsti, I.; Rissanen, A.; Kaprio, J.; Mustonen, J.; et al. Bacterial endotoxin activity in human serum is associated with dyslipidemia, insulin resistance, obesity, and chronic inflammation. Diabetes Care 2011, 34, 1809–1815. [Google Scholar] [CrossRef] [PubMed]
- Kajiwara, K.; Takata, S.; To, T.T.; Takara, K.; Hatakeyama, Y.; Tamaoki, S.; Darveau, R.P.; Ishikawa, H.; Sawa, Y. The promotion of nephropathy by Porphyromonas gingivalis lipopolysaccharide via toll-like receptors. Diabetol. Metab. Syndr. 2017, 9, 73. [Google Scholar] [CrossRef]
- Sawa, Y.; Takata, S.; Hatakeyama, Y.; Ishikawa, H.; Tsuruga, E. Expression of toll-like receptor 2 in glomerular endothelial cells and promotion of diabetic nephropathy by Porphyromonas gingivalis lipopolysaccharide. PLoS ONE 2014, 9, e97165. [Google Scholar] [CrossRef]
- Samuel, V.T.; Shulman, G.I. The pathogenesis of insulin resistance: Integrating signaling pathways and substrate flux. J. Clin. Investig. 2016, 126, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Kelley, D.E.; Mandarino, L.J. Fuel selection in human skeletal muscle in insulin resistance: A reexamination. Diabetes 2000, 49, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Galicia-Garcia, U.; Benito-Vicente, A.; Jebari, S.; Larrea-Sebal, A.; Siddiqi, H.; Uribe, K.B.; Ostolaza, H.; Martín, C. Pathophysiology of Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2020, 21, 6275. [Google Scholar] [CrossRef] [PubMed]
- Rutledge, J.C.; Ng, K.F.; Aung, H.H.; Wilson, D.W. Role of triglyceride-rich lipoproteins in diabetic nephropathy. Nat. Rev. Nephrol. 2010, 6, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Araki, E.; Goto, A.; Kondo, T.; Noda, M.; Noto, H.; Origasa, H.; Osawa, H.; Taguchi, A.; Tanizawa, Y.; Tobe, K.; et al. Japanese Clinical Practice Guideline for Diabetes 2019. Diabetol. Int. 2020, 11, 165–223. [Google Scholar] [CrossRef] [PubMed]
- Malaguarnera, M.; Vacante, M.; Antic, T.; Giordano, M.; Chisari, G.; Acquaviva, R.; Mastrojeni, S.; Malaguarnera, G.; Mistretta, A.; Li Volti, G.; et al. Bifidobacterium longum with fructo-oligosaccharides in patients with non alcoholic steatohepatitis. Dig. Dis. Sci. 2012, 57, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Imai, K.; Kim, I.S.; Wang, E.H. Matching Methods for Causal Inference with Time-Series Cross-Sectional Data. Am. J. Pol. Sci. 2023, 67, 587–605. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Y.; Liu, J.; Li, Y.; Quan, J. Association of Lipopolysaccharide-Toll-Like Receptor 4 Signaling and Microalbuminuria in Patients with Type 2 Diabetes Mellitus. Diabetes Metab. Syndr. Obes. 2022, 15, 3143–3152. [Google Scholar] [CrossRef]

| Characteristic | Number or Median (IQR) | |
|---|---|---|
| n | 36 | |
| Age (years) | 33 | (29–39) |
| Sex (male:female) | 24:12 | |
| Body mass index (kg/m2) | 21 | (20–23) |
| Diabetes (yes/no) | 0/36 | |
| Metabolic syndrome (yes/no) | 0/36 | |
| Habitual drinking (yes/no) | 25/11 | |
| Current smoker (yes/no) | 4/32 | |
| Characteristic | At Admission | At Discharge | p | Q |
|---|---|---|---|---|
| Number or Median (IQR) | Number or Median (IQR) | |||
| n | 36 | - | - | - |
| Age (years) | 64 (52–73) | - | - | - |
| Sex (male:female) | 21:15 | - | - | - |
| Diabetes (n) | ||||
| Type 1 | 3 | - | - | - |
| Type 2 | 30 | - | - | - |
| IGT | 3 | - | - | - |
| Insulin use | 28 | - | - | - |
| CPR (ng/mL) | 2.1 (1.5–3.3) † | - | - | - |
| HbA1c (%) | 8.7 (6.8–10.0) | - | - | - |
| Stage of diabetic nephropathy (1:2:3:4:5:ND) | 22:3:8:0:1:2 | - | - | - |
| 20/(CPR × FPG) | 1.3 (0.9–1.8) † | - | - | - |
| FPG (mg/dL) | 130 (96–151) | 103 (88–119) | <0.01 | <0.05 *** |
| GA (%) | 22 (18–27) | 20 (15–22) | <0.01 | <0.05 *** |
| TGs (mg/dL) | 109 (85–128) | 109 (87–126) | 0.17 | 0.37 |
| TC (mg/dL) | 174 (162–199) | 156 (131–179) | <0.01 | <0.05 *** |
| HDL-C (mg/dL) | 40 (36–48) | 40 (37–47) | 0.15 | 0.36 |
| LDL-C (mg/dL) | 105 (87–121) | 90 (69–116) | <0.01 | <0.05 *** |
| AST (U/L) | 21 (18–28) | 22 (19–30) | 0.19 | 0.39 |
| ALT (U/L) | 22 (18–31) | 25 (16–31) | 0.59 | 0.83 |
| γ-GTP (U/L) | 29 (16–43) | 23 (14–45) | <0.01 | <0.05 *** |
| SBP (mmHg) | 124 (113–144) | 119 (111–125) | <0.01 | <0.05 *** |
| BMI (kg/m2) | 26 (23–31) | 25 (23–30) | <0.01 | <0.05 *** |
| hs-CRP (mg/dL) | 0.101 (0.037–0.193) | 0.076 (0.028–0.184) | 0.1 | 0.30 |
| LPSs (EU/mL) | 0.0232 (0.0187–0.0306) | 0.0211 (0.0113–0.0286) | 0.12 | 0.35 |
| Characteristic | Model 1 | Model 2 | Model 3 | Model 4 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | p | Q | β | p | Q | β | p | Q | β | p | Q | |
| CPR | 42 | <0.01 | <0.05 *** | 43 | <0.01 | <0.05 *** | 42 | <0.01 | <0.05 *** | 42 | <0.01 | <0.05 *** |
| HbA1c | 34 | 0.15 | 0.36 | 34 | 0.15 | 0.36 | 34 | 0.15 | 0.36 | 36 | 0.07 | 0.22 |
| Diabetic nephropathy | 20 | <0.05 | <0.1 ** | 21 | <0.05 | <0.1 ** | 21 | <0.05 | <0.1 ** | 22 | <0.01 | <0.1 ** |
| 20/(CPR × FPG) | −24 | <0.05 | <0.2 * | −25 | <0.05 | <0.1 ** | −24 | <0.05 | <0.2 * | −24 | <0.05 | <0.2 * |
| FPG | 801 | <0.05 | <0.2 * | 800 | <0.05 | <0.2 * | 790 | <0.05 | <0.2 * | 827 | <0.05 | <0.2 * |
| GA | −0.7 | 0.99 | 1.00 | 0 | 1.00 | 1.00 | 0 | 1.00 | 1.00 | 12 | 0.84 | 0.97 |
| TGs | 2910 | <0.01 | <0.05 *** | 2910 | <0.01 | <0.05 *** | 2934 | <0.01 | <0.05 *** | 2904 | <0.01 | <0.05 *** |
| TC | 185 | 0.53 | 0.79 | 185 | 0.54 | 0.79 | 226 | 0.35 | 0.59 | 180 | 0.55 | 0.79 |
| LDL-C | 332 | <0.05 | 0.83 | 185 | 0.54 | 0.79 | 226 | 0.35 | 0.59 | 180 | 0.55 | 0.79 |
| HDL-C | −171 | 0.10 | 0.31 | −170 | 0.11 | 0.31 | −163 | 0.11 | 0.31 | −165 | 0.11 | 0.32 |
| AST | −207 | 0.16 | 0.36 | −209 | 0.13 | 0.36 | −209 | 0.16 | 0.36 | −212 | 0.15 | 0.36 |
| ALT | −350 | 0.35 | 0.59 | −358 | 0.28 | 0.51 | −356 | 0.35 | 0.59 | −365 | 0.33 | 0.58 |
| γ-GTP | −485 | 0.23 | 0.46 | −486 | 0.24 | 0.46 | −507 | 0.21 | 0.43 | −486 | 0.24 | 0.46 |
| SBP | −1.7 | 0.99 | 1.00 | 0 | 1.00 | 1.00 | 10 | 0.96 | 1.00 | 0 | 1.00 | 1.00 |
| BMI | 16 | 0.83 | 0.97 | 14 | 0.82 | 0.97 | 15 | 0.84 | 0.97 | - | - | - |
| hs-CRP | −0.4 | 0.85 | 0.97 | 0 | 0.84 | 0.97 | 0 | 0.88 | 0.97 | −1 | 0.67 | 0.90 |
| Characteristic | Model 1 | Model 2 | Model 3 | Model 4 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | p | Q | β | p | Q | β | p | Q | β | p | Q | |
| FPG | 94 | 0.64 | 0.88 | 113 | 0.54 | 0.79 | 79 | 0.69 | 0.90 | 64 | 0.72 | 0.94 |
| GA | 3 | 0.97 | 1.00 | 8 | 0.89 | 0.98 | 2 | 0.97 | 1.00 | −11 | 0.80 | 0.97 |
| TGs | 2434 | <0.01 | <0.05 *** | 2431 | <0.01 | <0.05 *** | 2416 | <0.01 | <0.05 *** | 2450 | <0.01 | <0.05 *** |
| TC | −274 | 0.37 | 0.61 | −269 | 0.38 | 0.62 | −301 | 0.29 | 0.53 | −269 | 0.39 | 0.62 |
| LDL-C | −421 | 0.12 | 0.35 | −426 | 0.12 | 0.35 | −438 | 0.10 | 0.30 | −402 | 0.14 | 0.36 |
| HDL-C | −168 | <0.05 | <0.2 * | −162 | <0.05 | <0.2 * | −172 | <0.05 | <0.2 * | −180 | <0.05 | <0.1 ** |
| AST | −400 | 0.15 | 0.36 | −402 | 0.15 | 0.36 | −386 | 0.15 | 0.36 | −415 | 0.13 | 0.36 |
| ALT | −604 | 0.14 | 0.36 | −624 | 0.13 | 0.35 | −583 | 0.15 | 0.36 | −616 | 0.14 | 0.36 |
| γ-GTP | −449 | 0.22 | 0.44 | −451 | 0.22 | 0.45 | −430 | 0.23 | 0.46 | −455 | 0.22 | 0.44 |
| SBP | 20 | 0.85 | 0.97 | 22 | 0.84 | 0.97 | 18 | 0.87 | 0.97 | 21 | 0.84 | 0.97 |
| BMI | −20 | 0.76 | 0.97 | −27 | 0.65 | 0.88 | −20 | 0.77 | 0.97 | - | - | - |
| hs-CRP | −1 | 0.63 | 0.87 | −1 | 0.60 | 0.85 | −1 | 0.64 | 0.87 | 0 | 0.71 | 0.93 |
| Characteristic | Model 1 | Model 2 | ||||
|---|---|---|---|---|---|---|
| β | p | Q | β | p | Q | |
| FPG | 5 | 0.99 | 1.00 | 69 | 0.91 | 0.99 |
| GA | −27 | 0.53 | 0.79 | −1 | 0.98 | 1.00 |
| TGs | 2041 | <0.01 | <0.05 *** | 2076 | <0.01 | <0.05 *** |
| TC | −78 | 0.83 | 0.97 | −26 | 0.94 | 1.00 |
| LDL-C | −139 | 0.66 | 0.90 | −82 | 0.80 | 0.97 |
| HDL-C | −208 | <0.05 | <0.1 ** | −225 | <0.05 | <0.1 ** |
| AST | 21 | 0.95 | 1.00 | 13 | 0.97 | 1.00 |
| ALT | 104 | 0.81 | 0.97 | 87 | 0.85 | 0.97 |
| γ-GTP | 145 | 0.34 | 0.59 | 145 | 0.36 | 0.60 |
| SBP | 207 | 0.32 | 0.57 | 230 | 0.28 | 0.52 |
| BMI | 12 | 0.23 | 0.46 | - | - | - |
| hs-CRP | 2 | 0.41 | 0.65 | 2 | 0.42 | 0.67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fuke, N.; Sawada, S.; Ito-Sasaki, T.; Inoue, K.Y.; Ushida, Y.; Sato, I.; Matsue, T.; Katagiri, H.; Ueda, H.; Suganuma, H. Relationship between Plasma Lipopolysaccharide Concentration and Health Status in Healthy Subjects and Patients with Abnormal Glucose Metabolism in Japan: A Preliminary Cross-Sectional Study. J 2023, 6, 605-626. https://doi.org/10.3390/j6040040
Fuke N, Sawada S, Ito-Sasaki T, Inoue KY, Ushida Y, Sato I, Matsue T, Katagiri H, Ueda H, Suganuma H. Relationship between Plasma Lipopolysaccharide Concentration and Health Status in Healthy Subjects and Patients with Abnormal Glucose Metabolism in Japan: A Preliminary Cross-Sectional Study. J. 2023; 6(4):605-626. https://doi.org/10.3390/j6040040
Chicago/Turabian StyleFuke, Nobuo, Shojiro Sawada, Takahiro Ito-Sasaki, Kumi Y. Inoue, Yusuke Ushida, Ikuo Sato, Tomokazu Matsue, Hideki Katagiri, Hiroyuki Ueda, and Hiroyuki Suganuma. 2023. "Relationship between Plasma Lipopolysaccharide Concentration and Health Status in Healthy Subjects and Patients with Abnormal Glucose Metabolism in Japan: A Preliminary Cross-Sectional Study" J 6, no. 4: 605-626. https://doi.org/10.3390/j6040040
APA StyleFuke, N., Sawada, S., Ito-Sasaki, T., Inoue, K. Y., Ushida, Y., Sato, I., Matsue, T., Katagiri, H., Ueda, H., & Suganuma, H. (2023). Relationship between Plasma Lipopolysaccharide Concentration and Health Status in Healthy Subjects and Patients with Abnormal Glucose Metabolism in Japan: A Preliminary Cross-Sectional Study. J, 6(4), 605-626. https://doi.org/10.3390/j6040040






