Antihypertensives’ Rock around the Clock
Abstract
1. Introduction
2. Methods
3. Types of Rhythmic Cycles
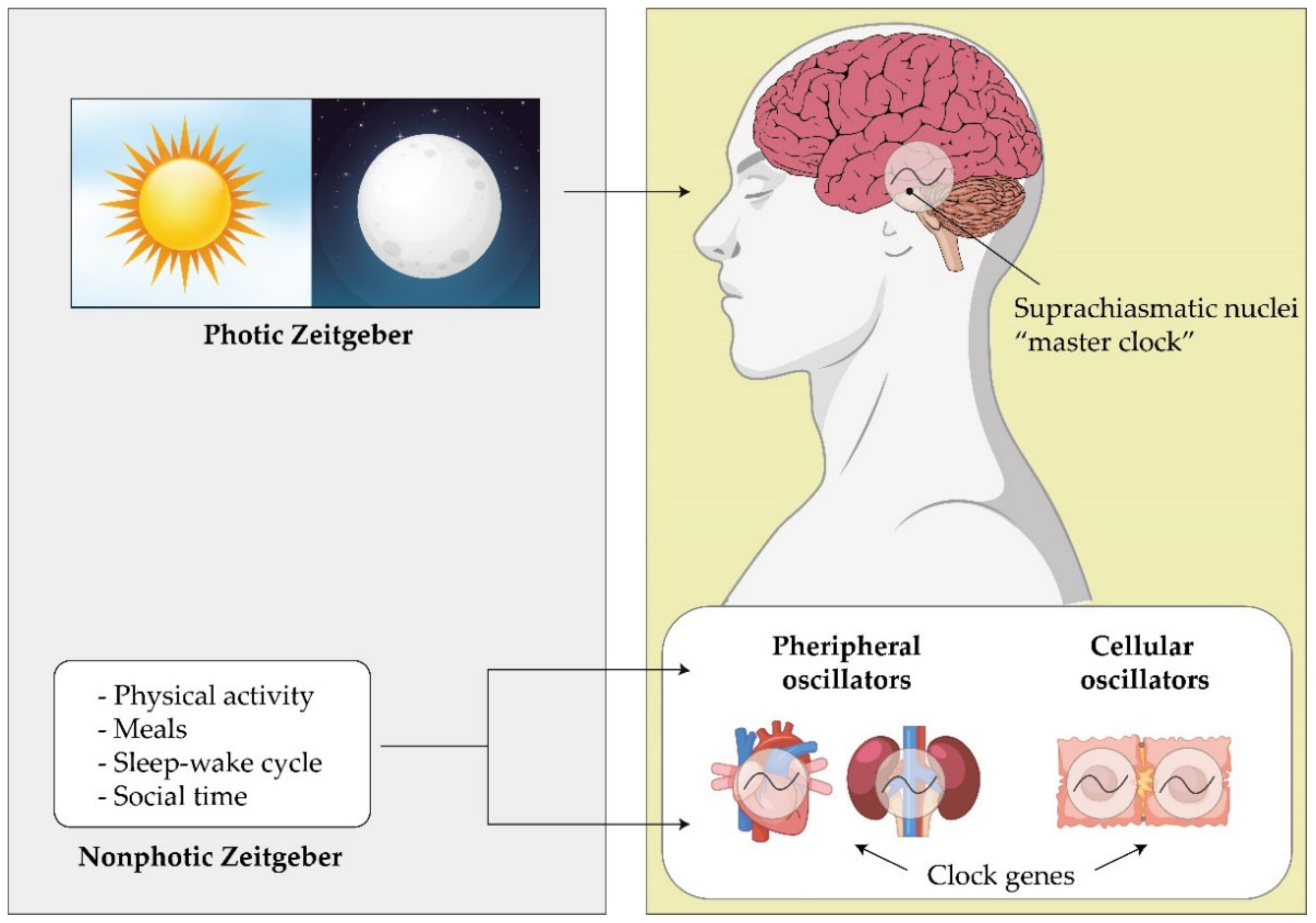
Circadian Rhythm
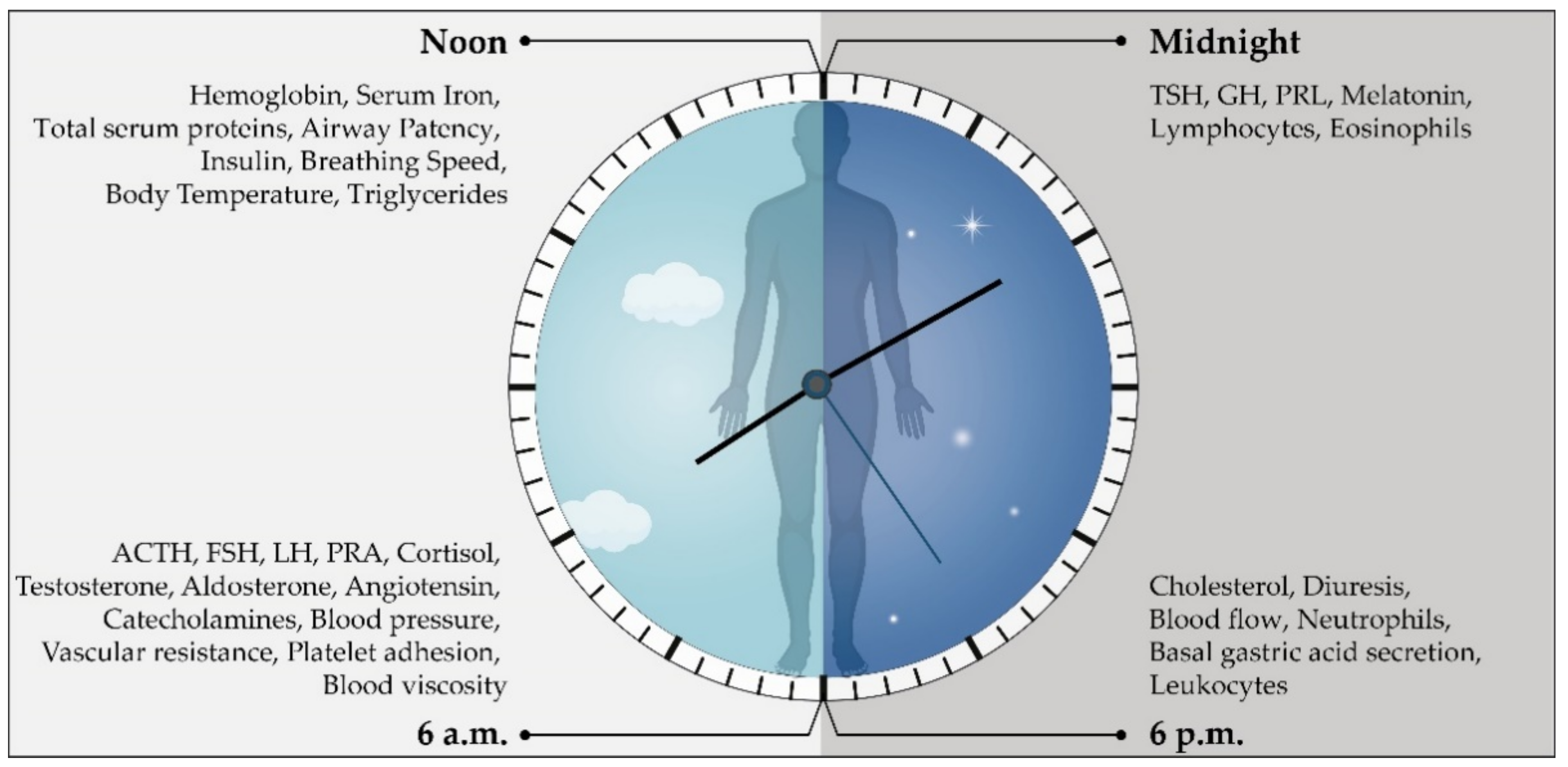
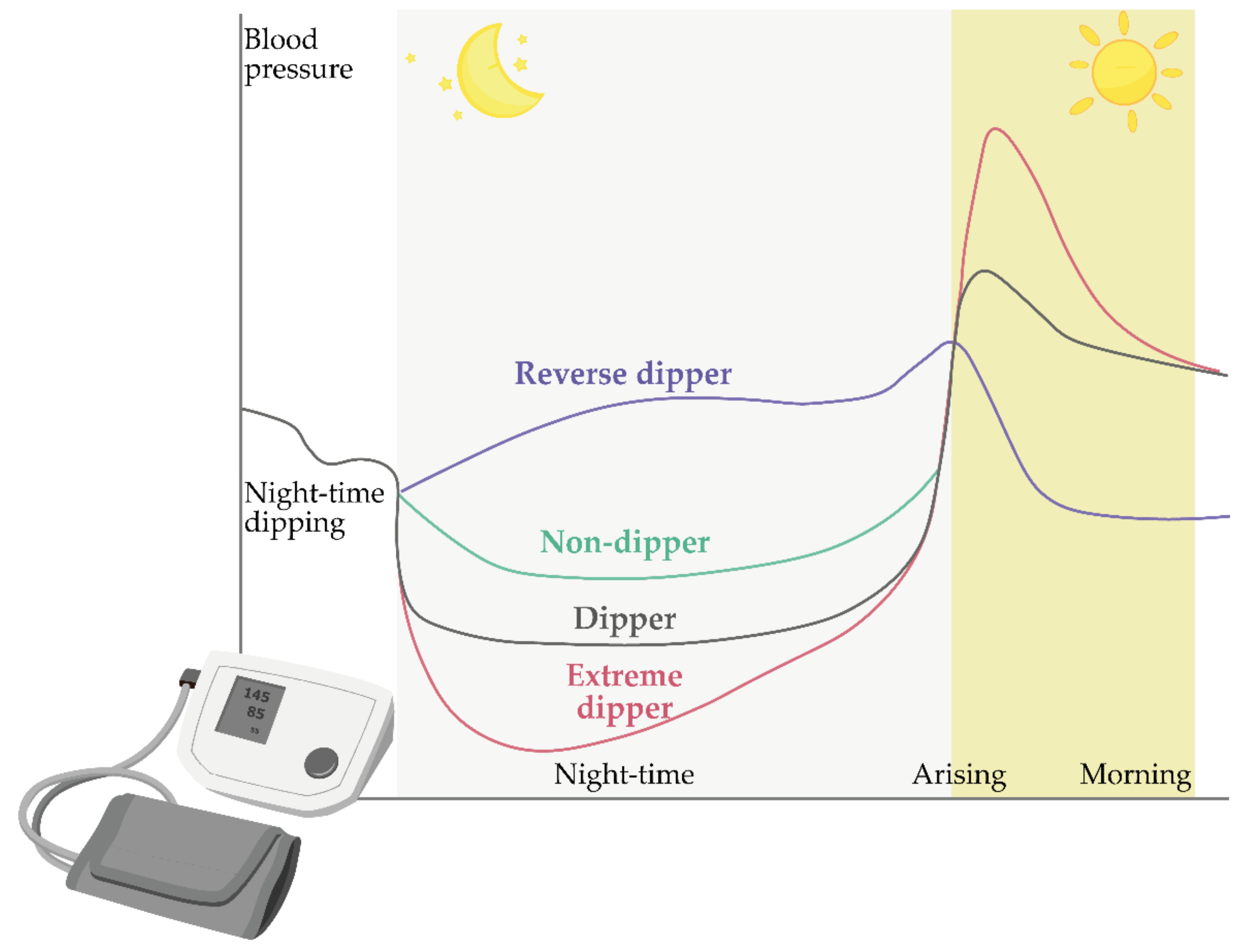
4. Circadian Blood Pressure Fluctuations
Models of Circadian Blood Pressure Fluctuations
5. Chronotherapy and Chronopharmaceutics
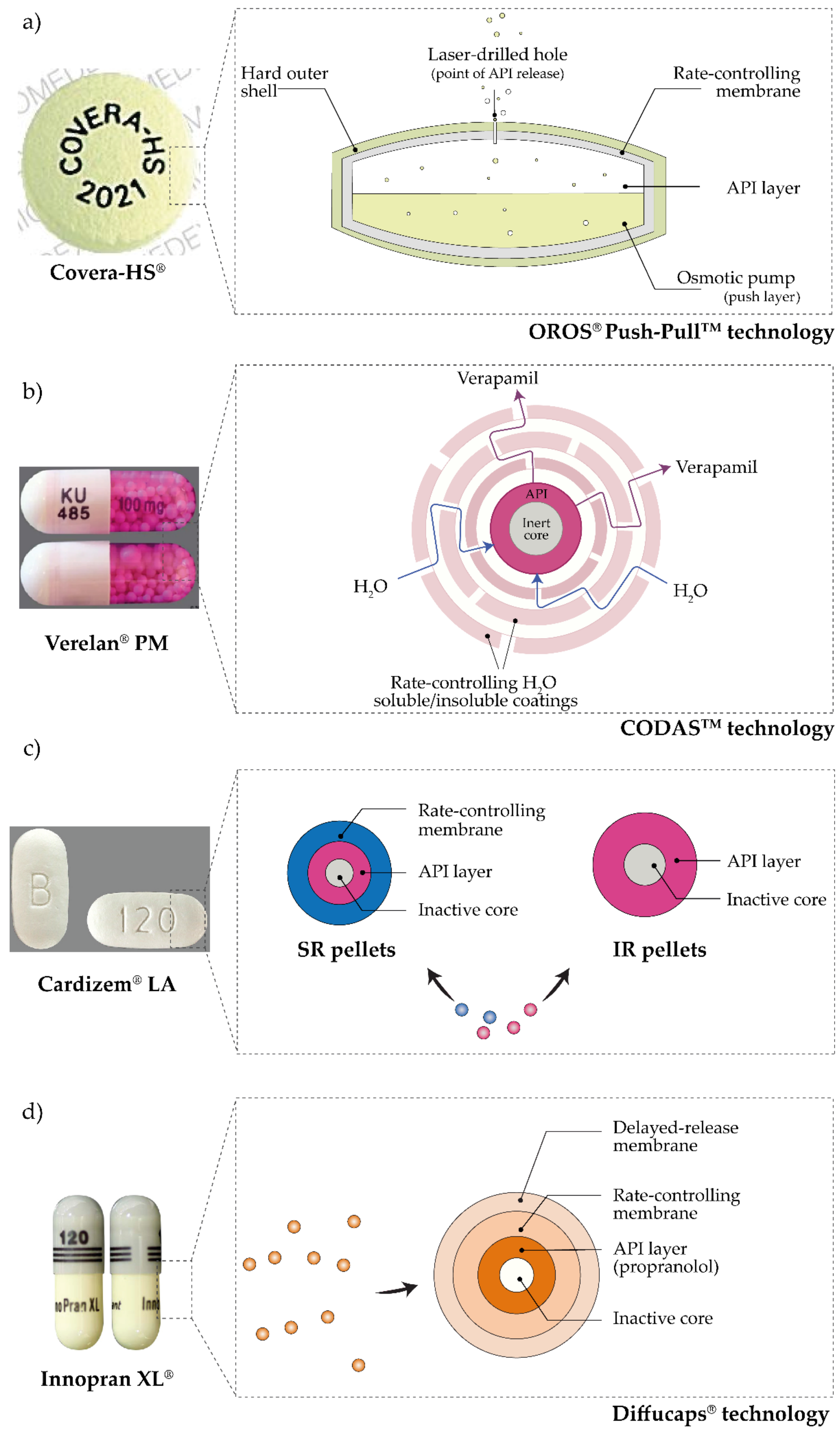
6. Pulsatile Antihypertensives Delivery Systems Approved for Use
7. Future Directions
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Duguay, D.; Cermakian, N. The crosstalk between physiology and circadian clock proteins. Chronobiol. Int. 2009, 26, 1479–1513. [Google Scholar] [CrossRef] [PubMed]
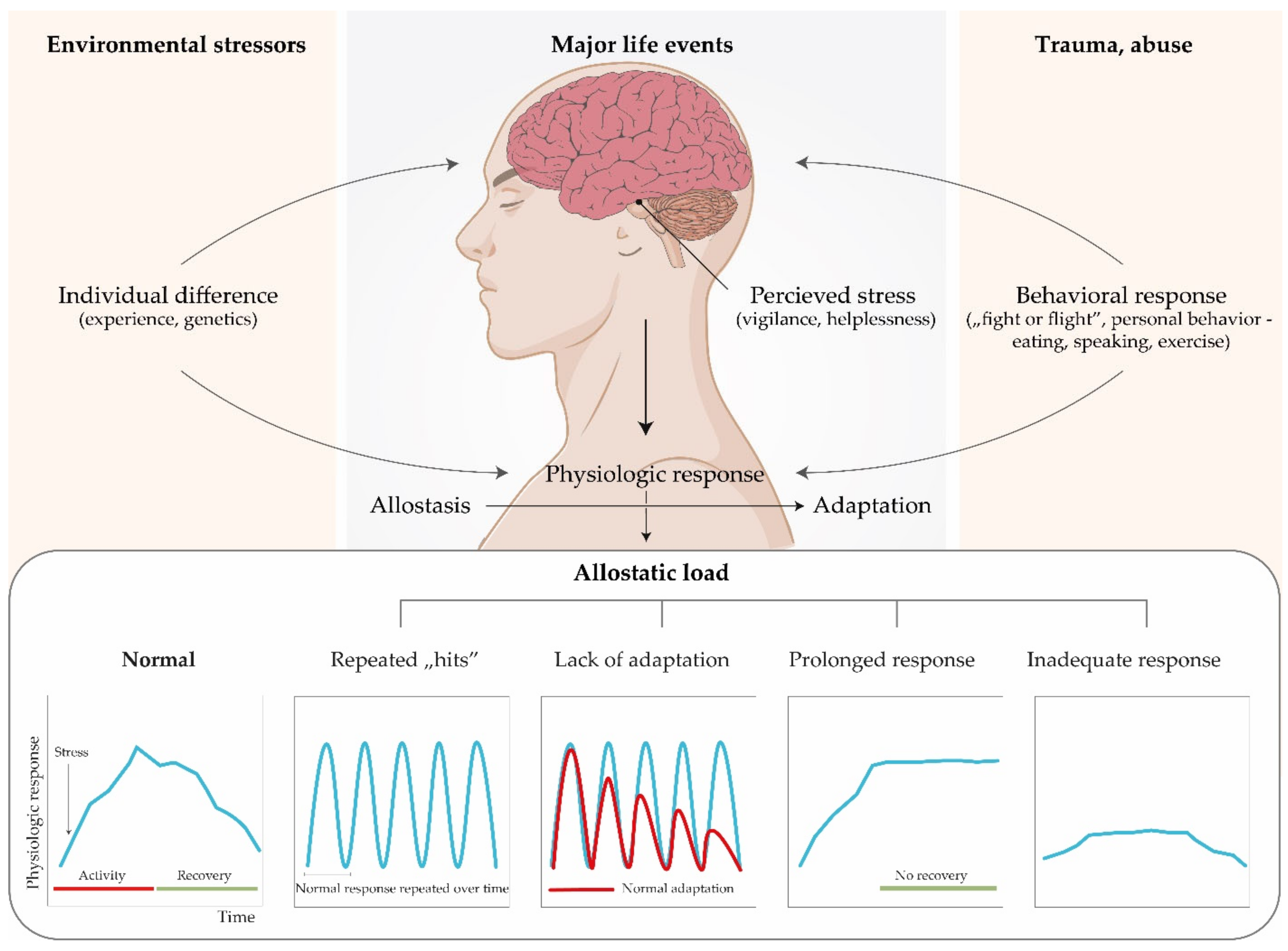
- Ramsay, D.S.; Woods, S.C. Clarifying the roles of homeostasis and allostasis in physiological regulation. Psychol. Rev. 2014, 121, 225–247. [Google Scholar] [CrossRef] [PubMed]
- Sterling, P.; Eyer, J. Allostasis: A new paradigm to explain arousal pathology. In Handbook of Life Stress, Cognition and Health; Fisher, S., Reason, J., Eds.; John Wiley & Sons: New York, NY, USA, 1988; pp. 629–649. [Google Scholar]
- Goldstein, D.S.; Mcewen, B. Allostasis, homeostats, and the nature of stress. Stress 2002, 5, 55–58. [Google Scholar] [CrossRef]
- McEwen, B.S. Protective and Damaging Effects of Stress Mediators. N. Engl. J. Med. 1998, 338, 171–179. [Google Scholar] [CrossRef]
- McEwen, B.S.; Gianaros, P.J. Stress- and Allostasis-Induced Brain Plasticity. Annu. Rev. Med. 2011, 62, 431–445. [Google Scholar] [CrossRef] [PubMed]
- Karatsoreos, I.N.; McEwen, B.S. Psychobiological allostasis: Resistance, resilience and vulnerability. Trends Cogn. Sci. 2011, 15, 576–584. [Google Scholar] [CrossRef]
- Marón, F.J.M.; Ferder, L.; Saraví, F.D.; Manucha, W. Hypertension linked to allostatic load: From psychosocial stress to inflammation and mitochondrial dysfunction. Stress 2019, 22, 169–181. [Google Scholar] [CrossRef]
- McEwen, B.S. Physiology and neurobiology of stress and adaptation: Central role of the brain. Physiol. Rev. 2007, 87, 873–904. [Google Scholar] [CrossRef]
- Adobe Inc. Adobe Illustrator. Available online: https://www.adobe.com/products/illustrator.html (accessed on 11 March 2021).
- Schulkin, J.; McEwen, B.S.; Gold, P.W. Allostasis, amygdala, and anticipatory angst. Neurosci. Biobehav. Rev. 1994, 18, 385–396. [Google Scholar] [CrossRef]
- Youan, B.B.C. Chronopharmaceutics; Youan, B.B.C., Ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2009; ISBN 9780470498392. [Google Scholar]
- Moszczynski, A.; Murray, B.J. Neurobiological Aspects of Sleep Physiology. Neurol. Clin. 2012, 30, 963–985. [Google Scholar] [CrossRef]
- Dijk, D.-J.; Czeisler, C.A. Contribution of the Circadian Pacemaker and the Sleep Homeostat to Sleep Propensity, Sleep Structure, Electroencephalographic Slow Waves, and Sleep Spindle Activity in Humans. J. Neurosci. 1995, 15, 3526–3528. [Google Scholar] [CrossRef] [PubMed]
- Lahav, G. The strength of indecisiveness: Oscillatory behavior for better cell fate determination. Sci. STKE 2004, 2004, pe55. [Google Scholar] [CrossRef] [PubMed]
- Spiga, F.; Pooley, J.; Russell, G.; Lightman, S.L. Ultradian Rhythms. In Stress: Neuroendocrinology and Neurobiology; Elsevier Inc.: Amsterdam, The Netherlands, 2017; Volume 2, pp. 429–437. ISBN 9780128024232. [Google Scholar]
- Lamont, E.W.; Amir, S. Circadian and Ultradian Clocks/Rhythms. In Reference Module in Neuroscience and Biobehavioral Psychology; Koob, G., Thompson, R.F., Le Moal, M., Eds.; Elsevier Ltd.: Oxford, UK, 2016; pp. 257–261. ISBN 9780128093245. [Google Scholar]
- Lemmer, B. The importance of biological rhythms in drug treatment of hypertension and sex-dependent modifications. ChronoPhysiology Ther. 2012, 2, 9. [Google Scholar] [CrossRef]
- McEwen, B.S.; Karatsoreos, I.N. Sleep deprivation and circadian disruption: Stress, allostasis, and allostatic load. Sleep Med. Clin. 2015, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Rao, R.; Androulakis, I.P. The physiological significance of the circadian dynamics of the HPA axis: Interplay between circadian rhythms, allostasis and stress resilience. Horm. Behav. 2019, 110, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Koch, C.E.; Leinweber, B.; Drengberg, B.C.; Blaum, C.; Oster, H. Interaction between circadian rhythms and stress. Neurobiol. Stress 2017, 6, 57–67. [Google Scholar] [CrossRef]
- Cuninkova, L.; Brown, S.A. Peripheral Circadian Oscillators. Ann. N. Y. Acad. Sci. 2008, 1129, 358–370. [Google Scholar] [CrossRef]
- Stokkan, K.A.; Yamazaki, S.; Tei, H.; Sakaki, Y.; Menaker, M. Entrainment of the circadian clock in the liver by feeding. Science 2001, 291, 490–493. [Google Scholar] [CrossRef]
- Balsalobre, A.; Brown, S.A.; Marcacci, L.; Tronche, F.; Kellendonk, C.; Reichardt, H.M.; Schutz, G.; Schibler, U. Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science 2000, 289, 2344–2347. [Google Scholar] [CrossRef] [PubMed]
- Morf, J.; Rey, G.; Schneider, K.; Stratmann, M.; Fujita, J.; Naef, F.; Schibler, U. Cold-inducible RNA-binding protein modulates circadian gene expression posttranscriptionally. Science 2012, 338, 379–383. [Google Scholar] [CrossRef]
- Ramsey, K.M.; Yoshino, J.; Brace, C.S.; Abrassart, D.; Kobayashi, Y.; Marcheva, B.; Hong, H.K.; Chong, J.L.; Buhr, E.D.; Lee, C.; et al. Circadian clock feedback cycle through NAMPT-Mediated NAD+ biosynthesis. Science 2009, 324, 651–654. [Google Scholar] [CrossRef] [PubMed]
- Yamakawa, G.R.; Basu, P.; Cortese, F.; Macdonnell, J.; Whalley, D.; Smith, V.M.; Antle, M.C. The cholinergic forebrain arousal system acts directly on the circadian pacemaker. Proc. Natl. Acad. Sci. USA 2016, 113, 13498–13503. [Google Scholar] [CrossRef] [PubMed]
- Davies, S.K.; Ang, J.E.; Revell, V.L.; Holmes, B.; Mann, A.; Robertson, F.P.; Cui, N.; Middleton, B.; Ackermann, K.; Kayser, M.; et al. Effect of sleep deprivation on the human metabolome. Proc. Natl. Acad. Sci. USA 2014, 111, 10761–10766. [Google Scholar] [CrossRef] [PubMed]
- Mazzoccoli, G.; Francavilla, M.; Pazienza, V.; Benegiamo, G.; Piepoli, A.; Vinciguerra, M.; Giuliani, F.; Yamamoto, T.; Takumi, T. Differential patterns in the periodicity and dynamics of clock gene expression in mouse liver and stomach. Chronobiol. Int. 2012, 29, 1300–1311. [Google Scholar] [CrossRef]
- Wirz-Justice, A. How to measure circadian rhythms in humans. Medicographia 2007, 29, 84–90. [Google Scholar]
- Provencio, I.; Rodriguez, I.R.; Jiang, G.; Hayes, W.P.; Moreira, E.F.; Rollag, M.D. A novel human opsin in the inner retina. J. Neurosci. 2000, 20, 600–605. [Google Scholar] [CrossRef]
- Johnson, R.F.; Moore, R.Y.; Morin, L.P. Loss of entrainment and anatomical plasticity after lesions of the hamster retinohypothalamic tract. Brain Res. 1988, 460, 297–313. [Google Scholar] [CrossRef]
- Oren, D.A.; Koziorowski, M.; Desan, P.H. SAD and the not-so-single photoreceptors. Am. J. Psychiatry 2013, 170, 1403–1412. [Google Scholar] [CrossRef]
- Aschoff, J.; Pohl, H. Phase relations between a circadian rhythm and its zeitgeber within the range of entrainment. Naturwissenschaften 1978, 65, 80–84. [Google Scholar] [CrossRef]
- Moore, R.Y. Circadian Rhythms: Basic Neurobiology and Clinical Applications. Annu. Rev. Med. 1997, 48, 253–266. [Google Scholar] [CrossRef]
- Bjarnason, G.A.; Jordan, R.C.K.; Wood, P.A.; Li, Q.; Lincoln, D.W.; Sothern, R.B.; Hrushesky, W.J.M.; Ben-David, Y. Circadian expression of clock genes in human oral mucosa and skin: Association with specific cell-cycle phases. Am. J. Pathol. 2001, 158, 1793–1801. [Google Scholar] [CrossRef]
- Selfridge, J.M.; Gotoh, T.; Schiffhauer, S.; Liu, J.J.; Stauffer, P.E.; Li, A.; Capelluto, D.G.S.; Finkielstein, C.V. Chronotherapy: Intuitive, Sound, Founded…But Not Broadly Applied. Drugs 2016, 76, 1507–1521. [Google Scholar] [CrossRef] [PubMed]
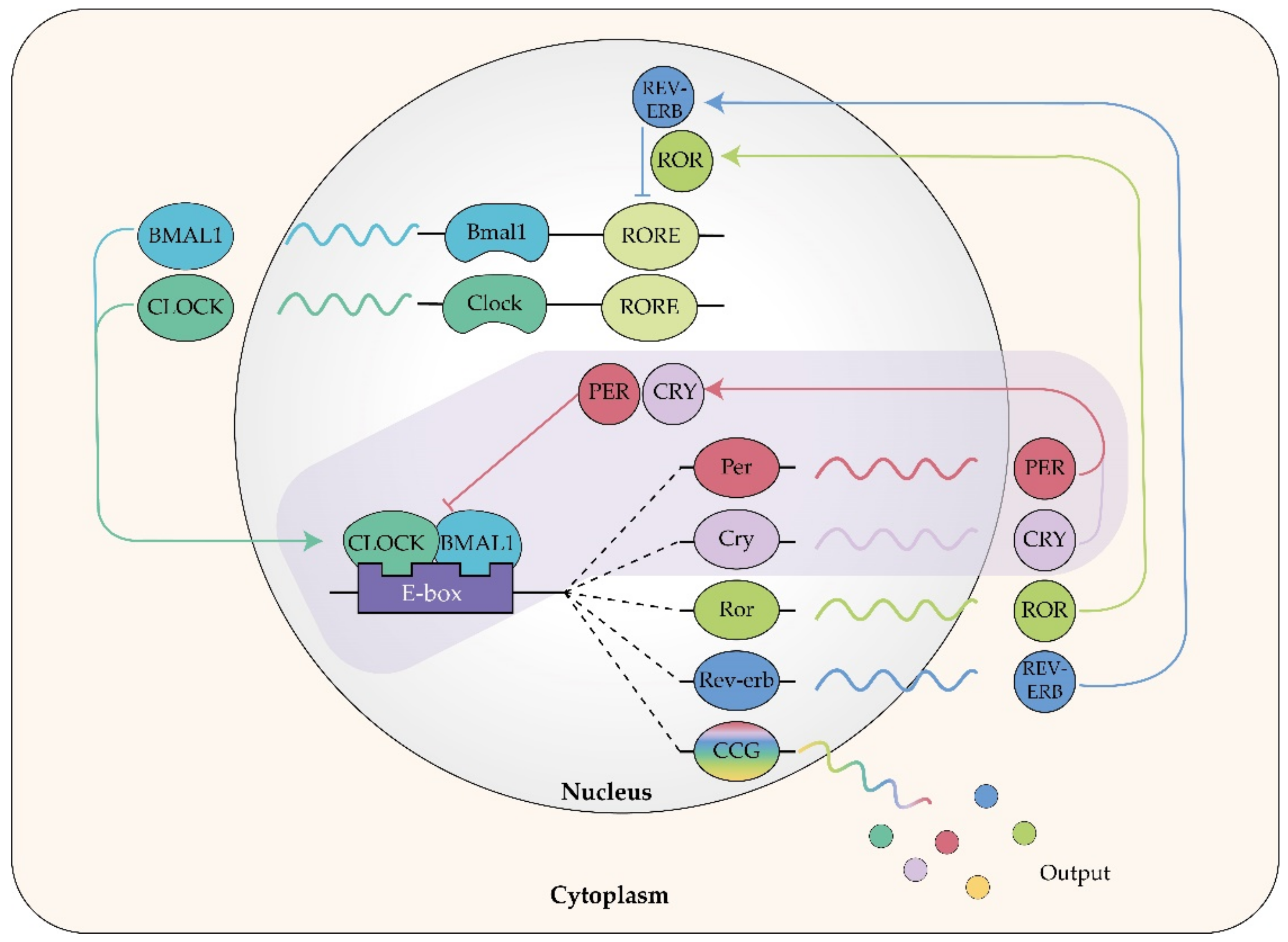
- Shearman, L.P.; Sriram, S.; Weaver, D.R.; Maywood, E.S.; Chaves, I.; Zheng, B.; Kume, K.; Lee, C.C.; Van Der Horst, G.T.J.; Hastings, M.H.; et al. Interacting molecular loops in the mammalian circadian clock. Science 2000, 288, 1013–1019. [Google Scholar] [CrossRef]
- Preitner, N.; Damiola, F.; Lopez-Molina, L.; Zakany, J.; Duboule, D.; Albrecht, U.; Schibler, U. The orphan nuclear receptor REV-ERBα controls circadian transcription within the positive limb of the mammalian circadian oscillator. Cell 2002, 110, 251–260. [Google Scholar] [CrossRef]
- Crnko, S.; Du Pré, B.C.; Sluijter, J.P.G.; Van Laake, L.W. Circadian rhythms and the molecular clock in cardiovascular biology and disease. Nat. Rev. Cardiol. 2019, 16, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Kelleher, F.C.; Rao, A.; Maguire, A. Circadian molecular clocks and cancer. Cancer Lett. 2014, 342, 9–18. [Google Scholar] [CrossRef]
- Antypa, N.; Mandelli, L.; Nearchou, F.A.; Vaiopoulos, C.; Stefanis, C.N.; Serretti, A.; Stefanis, N.C. The 3111TC polymorphism interacts with stressful life events to influence patterns of sleep in females. Chronobiol. Int. 2012, 29, 891–897. [Google Scholar] [CrossRef]
- Škrlec, I. Circadian rhythm and myocardial infarction. Med. Flum. 2019, 55, 32–42. [Google Scholar] [CrossRef][Green Version]
- Takeda, N.; Maemura, K. Cardiovascular disease, chronopharmacotherapy, and the molecular clock. Adv. Drug Deliv. Rev. 2010, 62, 956–966. [Google Scholar] [CrossRef]
- Langmesser, S.; Tallone, T.; Bordon, A.; Rusconi, S.; Albrecht, U. Interaction of circadian clock proteins PER2 and CRY with BMAL1 and CLOCK. BMC Mol. Biol. 2008, 9. [Google Scholar] [CrossRef]
- Akashi, M.; Takumi, T. The orphan nuclear receptor RORα regulates circadian transcription of the mammalian core-clock Bmal1. Nat. Struct. Mol. Biol. 2005, 12, 441–448. [Google Scholar] [CrossRef]
- Solt, L.A.; Kojetin, D.J.; Burris, T.P. The REV-ERBs and RORs: Molecular links between circadian rhythms and lipid homeostasis. Future Med. Chem. 2011, 3, 623–638. [Google Scholar] [CrossRef]
- Ikeda, R.; Tsuchiya, Y.; Koike, N.; Umemura, Y.; Inokawa, H.; Ono, R.; Inoue, M.; Sasawaki, Y.; Grieten, T.; Okubo, N.; et al. REV-ERBα and REV-ERBβ function as key factors regulating Mammalian Circadian Output. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef]
- Beytebiere, J.R.; Greenwell, B.J.; Sahasrabudhe, A.; Menet, J.S. Clock-controlled rhythmic transcription: Is the clock enough and how does it work? Transcription 2019, 10, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Pawar, V.K.; Awasthi, R. Chronotherapy: An Approach to Synchronize Drug Delivery with Circadian Rhythm. J. Chronother. Drug Deliv. 2010, 1, 1–8. [Google Scholar]
- Drake, C.L.; Roehrs, T.; Richardson, G.; Walsh, J.K.; Roth, T. Shift Work Sleep Disorder: Prevalence and Consequences Beyond that of Symptomatic Day Workers. Sleep 2004, 27, 1453–1462. [Google Scholar] [CrossRef]
- Czeisler, C.A.; Walsh, J.K.; Roth, T.; Hughes, R.J.; Wright, K.P.; Kingsbury, L.; Arora, S.; Schwartz, J.R.L.; Niebler, G.E.; Dinges, D.F. Modafinil for Excessive Sleepiness Associated with Shift-Work Sleep Disorder. N. Engl. J. Med. 2005, 353, 476–486. [Google Scholar] [CrossRef]
- Neil-Sztramko, S.E.; Pahwa, M.; Demers, P.A.; Gotay, C.C. Health-related interventions among night shift workers: A critical review of the literature. Scand. J. Work. Environ. Health 2014, 40, 543–556. [Google Scholar] [CrossRef]
- Crowther, M.E.; Ferguson, S.A.; Vincent, G.E.; Reynolds, A.C. Non-Pharmacological Interventions to Improve Chronic Disease Risk Factors and Sleep in Shift Workers: A Systematic Review and Meta-Analysis. Clocks Sleep 2021, 3, 9. [Google Scholar] [CrossRef]
- Postolache, T.T.; Raheja, U.K. Body Rhythms/Biological Clocks. In Encyclopedia of Mental Health; Friedman, H.S., Ed.; Academic Press: Waltham, MA, USA, 2016; Volume 1, pp. 193–203. ISBN 9780123970459. [Google Scholar]
- Hermida, R.C.; Ayala, D.E.; Portaluppi, F. Circadian variation of blood pressure: The basis for the chronotherapy of hypertension. Adv. Drug Deliv. Rev. 2007, 59, 904–922. [Google Scholar] [CrossRef]
- Casagrande, M.; Favieri, F.; Langher, V.; Guarino, A.; Di Pace, E.; Germanò, G.; Forte, G. The night side of blood pressure: Nocturnal blood pressure dipping and emotional (dys)regulation. Int. J. Environ. Res. Public Health 2020, 17, 1–11. [Google Scholar] [CrossRef]
- Pena-Hernandez, C.; Nugent, K.; Tuncel, M. Twenty-Four-Hour Ambulatory Blood Pressure Monitoring. J. Prim. Care Community Health 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Rhoads, M.K.; Balagee, V.; Thomas, S.J. Circadian Regulation of Blood Pressure: Of Mice and Men. Curr. Hypertens. Rep. 2020, 22. [Google Scholar] [CrossRef]
- Smolensky, M.H.; Hermida, R.C.; Portaluppi, F. Circadian mechanisms of 24-hour blood pressure regulation and patterning. Sleep Med. Rev. 2017, 33, 4–16. [Google Scholar] [CrossRef]
- James, G.D. Ambulatory blood pressure variation: Allostasis and adaptation. Auton. Neurosci. Basic Clin. 2013, 177, 87–94. [Google Scholar] [CrossRef]
- James, G.D. Continuous Blood Pressure Variation: Hidden Adaptability. In Biological Measures of Human Experience across the Lifespan: Making Visible the Invisible; Sievert, L.L., Brown, D.E., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 143–169. ISBN 9783319441030. [Google Scholar]
- Modesti, P.A.; Morabito, M.; Bertolozzi, I.; Massetti, L.; Panci, G.; Lumachi, C.; Giglio, A.; Bilo, G.; Caldara, G.; Lonati, L.; et al. Weather-related changes in 24-hour blood pressure profile: Effects of age and implications for hypertension management. Hypertension 2006, 47, 155–161. [Google Scholar] [CrossRef]
- James, G.D.; Gates, E.M.; Pickering, T.G.; Laragh, J.H. Parity and Perceived Job Stress Elevate Blood Pressure in Young Normotensive Working Women. Am. J. Hypertens. 1989, 2, 637–639. [Google Scholar] [CrossRef]
- Gerin, W.; James, G.D. Psychosocial determinants of hypertension: Laboratory and field models. Blood Press. Monit. 2010, 15, 93–99. [Google Scholar] [CrossRef]
- Van Berge-Landry, H.; James, G.D. Serum electrolyte, serum protein, serum fat and renal responses to a dietary sodium challenge: Allostasis and allostatic load. Ann. Hum. Biol. 2004, 31, 477–487. [Google Scholar] [CrossRef]
- James, G.D.; Brown, D.E. The Biological Stress Response and Lifestyle: Catecholamines and Blood Pressure. Annu. Rev. Anthropol. 1997, 26, 313–335. [Google Scholar] [CrossRef]
- Hermida, R.C.; Ayala, D.E.; Fernández, J.R.; Mojón, A.; Smolensky, M.H.; Fabbian, F.; Portaluppi, F. Administration-Time Differences in Effects of Hypertension Medications on Ambulatory Blood Pressure Regulation. Chronobiol. Int. 2013, 30, 280–314. [Google Scholar] [CrossRef] [PubMed]
- Chugh, A.R.; Loughran, J.H.; Slaughter, M.S. Circadian variations in blood pressure in health and disease: Implications for patient management. ChronoPhysiology Ther. 2011, 1, 17–31. [Google Scholar] [CrossRef][Green Version]
- Smolensky, M.H.; Siegel, R.A.; Haus, E.; Hermida, R.; Portaluppi, F. Biological rhythms, drug delivery, and chronotherapeutics. In Fundamentals and Applications of Controlled Release Drug Delivery; Springer: New York, NY, USA, 2012; pp. 359–443. ISBN 9781461408819. [Google Scholar]
- Gowthami, B.; Krishna, S.V.G.; Rao, D.S. Application of coating technology to chronotherapeutic drug delivery systems: Recent publications and patents. Curr. Res. Pharmacol. Drug Discov. 2021, 2, 100015. [Google Scholar] [CrossRef]
- Hermida, R.C.; Ayala, D.E.; Smolensky, M.H.; Portaluppi, F. Chronotherapy in hypertensive patients: Administration-time dependent effects of treatment on blood pressure regulation. Expert Rev. Cardiovasc. Ther. 2007, 5, 463–475. [Google Scholar] [CrossRef]
- Myburgh, D.P.; Verho, M.; Botes, J.H.; Erasmus, T.P.; Luus, H.G. 24-hour blood pressure control with ramipril: Comparison of once-daily morning and evening administration. Curr. Ther. Res. 1995, 56, 1298–1306. [Google Scholar] [CrossRef]
- Palatini, P.; Racioppa, A.; Raule, G.; Zaninotto, M.; Penzo, M.; Pessina, A.C. Effect of timing of administration on the plasma ACE inhibitory activity and the antihypertensive effect of quinapril. Clin. Pharmacol. Ther. 1992, 52, 378–383. [Google Scholar] [CrossRef]
- Witte, K.; Weisser, K.; Neubeck, M.; Mutschler, E.; Lehmann, K.; Hopf, R.; Lemmer, B. Cardiovascular effects, pharmacokinetics, and converting enzyme inhibition of enalapril after morning versus evening administration. Clin. Pharmacol. Ther. 1993, 54, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Hermida, R.C.; Ayala, D.E.; Calvo, C.; Portaluppi, F.; Smolensky, M.H. Chronotherapy of hypertension: Administration-time-dependent effects of treatment on the circadian pattern of blood pressure. Adv. Drug Deliv. Rev. 2007, 59, 923–939. [Google Scholar] [CrossRef]
- Hermida, R.C.; Calvo, C.; Ayala, D.E.; Fernández, J.R.; Covelo, M.; Mojón, A.; López, J.E. Treatment of non-dipper hypertension with bedtime administration of valsartan. J. Hypertens. 2005, 23, 1913–1922. [Google Scholar] [CrossRef]
- Smolensky, M.H.; Hermida, R.C.; Portaluppi, F. Comparison of the efficacy of morning versus evening administration of olmesartan in uncomplicated essential hypertension. Chronobiol. Int. 2007, 24, 171–181. [Google Scholar] [CrossRef]
- Fukuda, M.; Yamanaka, T.; Mizuno, M.; Motokawa, M.; Shirasawa, Y.; Miyagi, S.; Nishio, T.; Yoshida, A.; Kimura, G. Angiotensin II type 1 receptor blocker, olmesartan, restores nocturnal blood pressure decline by enhancing daytime natriuresis. J. Hypertens. 2008, 26, 583–588. [Google Scholar] [CrossRef]
- Hermida, R.C.; Calvo, C.; Ayala, D.E.; Domínguez, M.J.; Covelo, M.; Fernández, J.R.; Fontao, M.J.; López, J.E. Administration-time-dependent effects of doxazosin GITS on ambulatory blood pressure of hypertensive subjects. Chronobiol. Int. 2004, 21, 277–296. [Google Scholar] [CrossRef]
- Koga, H.; Hayashi, J.; Yamamoto, M.; Kitamoto, K. Prevention of morning surge of hypertension by the evening administration of carvedilol. Jpn. Med. Assoc. J. 2005, 48, 398–403. [Google Scholar]
- Hermida, R.C.; Calvo, C.; Ayala, D.; Rodríguez, M.; Chayán, L.; López, J. Administration time-dependent effects of nebivolol on the diurnal/nocturnal blood pressure ratio in hypertensive patients. J. Hypertens. 2006, 24, S89. [Google Scholar]
- Hermida, R.C.; Calvo, C.; Ayala, D.E.; Lopez, J.; Rodriguez, M.; Covelo, M. Administration time-dependent effects of amlodipine on ambulatory blood pressure in patients with essential hypertension. Am. J. Hypertens. 2005, 18, A61. [Google Scholar] [CrossRef][Green Version]
- Kitahara, Y.; Saito, F.; Akao, M.; Fujita, H.; Takahashi, A.; Taguchi, H.; Hino, T.; Otsuka, Y.; Kushiro, T.; Kanmatsuse, K. Effect of Morning and Bedtime Dosing with Cilnidipine on Blood Pressure, Heart Rate, and Sympathetic Nervous Activity in Essential Hypertensive Patients. J. Cardiovasc. Pharmacol. 2004, 43, 68–73. [Google Scholar] [CrossRef]
- Kohno, I.; Iwasaki, H.; Okutani, M.; Mochizuki, Y.; Sano, S.; Satoh, Y.; Ishihara, T.; Ishii, H.; Mukaiyama, S.; Ijiri, H.; et al. Administration-time-dependent effects of diltiazem on the 24-hour blood pressure profile of essential hypertension patients. Chronobiol. Int. 1997, 14, 71–84. [Google Scholar] [CrossRef]
- Portaluppi, F.; Vergnani, L.; Manfredini, R.; Degli Uberti, E.C.; Fersini, C. Time-dependent effect of isradipine on the nocturnal hypertension in chronic renal failure. Am. J. Hypertens. 1995, 8, 719–726. [Google Scholar] [CrossRef]
- Hermida, R.C.; Ayala, D.E.; Mojón, A.; Alonso, I.; Fernández, J.R. Reduction of morning blood pressure surge after treatment with nifedipine GITS at bedtime, but not upon awakening, in essential hypertension. Blood Press. Monit. 2009, 14, 152–159. [Google Scholar] [CrossRef]
- White, W.B.; Mansoor, G.A.; Pickering, T.G.; Vidt, D.G.; Hutchinson, H.G.; Johnson, R.B.; Noveck, R. Differential effects of morning and evening dosing of nisoldipine ER on circadian blood pressure and heart rate. Am. J. Hypertens. 1999, 12, 806–814. [Google Scholar] [CrossRef]
- Meilhac, B.; Mallion, J.M.; Carre, A.; Chanudet, X.; Poggi, L.; Gosse, P.; Dallocchio, M. Study of the influence of the time of administration on the antihypertensive effect and nitrendipine tolerance in mild to moderate essential hypertensive patients. Value of ambulatory recording of blood pressure on 24 hours. Therapie 1992, 47, 205–210. [Google Scholar] [PubMed]
- Cutler, N.R.; Anders, R.J.; Jhee, S.S.; Sramek, J.J.; Awan, N.A.; Bultas, J.; Lahiri, A.; Woroszylska, M. Placebo-controlled evaluation of three doses of a controlled-onset, extended-release formulation of verapamil in the treatment of stable angina pectoris. Am. J. Cardiol. 1995, 75, 1102–1106. [Google Scholar] [CrossRef]
- Frishman, W.H.; Glasser, S.; Stone, P.; Deedwania, P.C.; Johnson, M.; Fakouhi, T.D. Comparison of controlled-onset, extended-release verapamil with amlodipine and amlodipine plus atenolol on exercise performance and ambulatory ischemia in patients with chronic stable angina pectoris. Am. J. Cardiol. 1999, 83, 507–514. [Google Scholar] [CrossRef]
- FDA; CDER. COVERA-HS ® (Verapamil Hydrochloride) Extended-Release Tablets Controlled-Onset; FDA: Silver Spring, MA, USA, 2011. [Google Scholar]
- Maroni, A.; Foppoli, A.; Palugan, L.; Gazzaniga, A. Drug Delivery: Pulsatile Systems. In Encyclopedia of Pharmaceutical Science and Technology; Swarbrick, J., Ed.; CRC Press: Boca Raton, FL, USA, 2013; pp. 1173–1182. [Google Scholar]
- Davar, N.; Ghosh, S. Oral Controlled Release-Based Products for Life Cycle Management. In Oral Controlled Release Formulation Design and Drug Delivery; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2010; pp. 305–320. [Google Scholar]
- Montaigne, D.; Marechal, X.; Modine, T.; Coisne, A.; Mouton, S.; Fayad, G.; Ninni, S.; Klein, C.; Ortmans, S.; Seunes, C.; et al. Daytime variation of perioperative myocardial injury in cardiac surgery and its prevention by Rev-Erbα antagonism: A single-centre propensity-matched cohort study and a randomised study. Lancet 2018, 391, 59–69. [Google Scholar] [CrossRef]
- Woldt, E.; Sebti, Y.; Solt, L.A.; Duhem, C.; Lancel, S.; Eeckhoute, J.; Hesselink, M.K.C.; Paquet, C.; Delhaye, S.; Shin, Y.; et al. Rev-erb-α modulates skeletal muscle oxidative capacity by regulating mitochondrial biogenesis and autophagy. Nat. Med. 2013, 19, 1039–1046. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Yoo, S.H.; Takahashi, J.S. Small molecule modifiers of circadian clocks. Cell. Mol. Life Sci. 2013, 70, 2985–2998. [Google Scholar] [CrossRef] [PubMed]
- Manzano, M.; Colilla, M.; Vallet-Reg, M. Drug delivery from ordered mesoporous matrices. Expert Opin. Drug Deliv. 2009, 6, 1383–1400. [Google Scholar] [CrossRef]
- Qu, F.; Zhu, G.; Huang, S.; Li, S.; Sun, J.; Zhang, D.; Qiu, S. Controlled release of Captopril by regulating the pore size and morphology of ordered mesoporous silica. Microporous Mesoporous Mater. 2006, 92, 1–9. [Google Scholar] [CrossRef]
- Qu, F.; Zhu, G.; Huang, S.; Li, S.; Qiu, S. Effective Controlled Release of Captopril by Silylation of Mesoporous MCM-41. ChemPhysChem 2006, 7, 400–406. [Google Scholar] [CrossRef]
- Biswas, N. Modified mesoporous silica nanoparticles for enhancing oral bioavailability and antihypertensive activity of poorly water soluble valsartan. Eur. J. Pharm. Sci. 2017, 99, 152–160. [Google Scholar] [CrossRef]
- Biswas, N.; Kuotsu, K. Chronotherapeutically Modulated Pulsatile System of Valsartan Nanocrystals—an In Vitro and In Vivo Evaluation. AAPS PharmSciTech 2017, 18, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Pasparakis, G.; Bouropoulos, N. Swelling studies and in vitro release of verapamil from calcium alginate and calcium alginate-chitosan beads. Int. J. Pharm. 2006, 323, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Thampi, N.K. Formulation, Optimization and Evaluation of Floating Pulsatile Beads of Captopril. World J. Pharm. Pharm. Sci. 2017, 6, 1619–1637. [Google Scholar] [CrossRef][Green Version]
- Das, S.; Varma Vegesna, N.S.K.; Shivakumar, H.G. Design and development of a dual-drug loaded pulsatile capsule for treatment of hypertension—In vitro and ex vivo studies. RSC Adv. 2015, 5, 100424–100433. [Google Scholar] [CrossRef]
- Gadad, A.P.; Reddy, A.D.; Dandagi, P.M.; Masthiholimath, V.S. Design and characterization of hollow/porous floating beads of captopril for pulsatile drug delivery. Asian J. Pharm. 2012, 6, 137–143. [Google Scholar] [CrossRef]
- De Paula, W.X.; Denadai, Â.M.L.; Braga, A.N.G.; Shastri, V.P.; Pinheiro, S.V.B.; Frezard, F.; Santos, R.A.S.; Sinisterra, R.D. A long-lasting oral preformulation of the angiotensin II AT1 receptor antagonist losartan. Drug Dev. Ind. Pharm. 2018, 44, 1498–1505. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.K.; Das, P.; Mondal, A.; Mandal, A.; Kuotsu, K. Design, formulation and evaluation of multiparticulate time programmed system of ramipril for pulsed release: An approach in the management of early morning surge in blood pressure. J. Drug Deliv. Sci. Technol. 2021, 62, 102344. [Google Scholar] [CrossRef]
- Tucak, A.; Sirbubalo, M.; Hindija, L.; Rahić, O.; Hadžiabdić, J.; Muhamedagić, K.; Čekić, A.; Vranić, E. Microneedles: Characteristics, materials, production methods and commercial development. Micromachines 2020, 11, 961. [Google Scholar] [CrossRef]
- Kelchen, M.N.; Brogden, N.K. In Vitro Skin Retention and Drug Permeation through Intact and Microneedle Pretreated Skin after Application of Propranolol Loaded Microemulsions. Pharm. Res. 2018, 35, 1–12. [Google Scholar] [CrossRef]
- Ita, K.; Hatsakorzian, N.; Tolstikov, V. Microneedle-Mediated Delivery of Atenolol and Bisoprolol Hemifumarate. J. Nanopharm. Drug Deliv. 2013, 1, 38–44. [Google Scholar] [CrossRef]
- Kaur, M.; Ita, K.B.; Popova, I.E.; Parikh, S.J.; Bair, D.A. Microneedle-assisted delivery of verapamil hydrochloride and amlodipine besylate. Eur. J. Pharm. Biopharm. 2014, 86, 284–291. [Google Scholar] [CrossRef]
- Li, Y.; Liu, F.; Su, C.; Yu, B.; Liu, D.; Chen, H.J.; Lin, D.-A.; Yang, C.; Zhou, L.; Wu, Q.; et al. Biodegradable Therapeutic Microneedle Patch for Rapid Antihypertensive Treatment. ACS Appl. Mater. Interfaces 2019, 11, 30575–30584. [Google Scholar] [CrossRef]
- Luu, E.; Ita, K.B.; Morra, M.J.; Popova, I.E. The Influence of Microneedles on the Percutaneous Penetration of Selected Antihypertensive Agents: Diltiazem Hydrochloride and Perindopril Erbumine. Curr. Drug Deliv. 2018, 15, 1449–1458. [Google Scholar] [CrossRef] [PubMed]
- Alhnan, M.A.; Okwuosa, T.C.; Sadia, M.; Wan, K.W.; Ahmed, W.; Arafat, B. Emergence of 3D Printed Dosage Forms: Opportunities and Challenges. Pharm. Res. 2016, 33, 1817–1832. [Google Scholar] [CrossRef] [PubMed]
- Goyanes, A.; Buanz, A.B.M.; Basit, A.W.; Gaisford, S. Fused-filament 3D printing (3DP) for fabrication of tablets. Int. J. Pharm. 2014, 476, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Kadry, H.; Al-Hilal, T.A.; Keshavarz, A.; Alam, F.; Xu, C.; Joy, A.; Ahsan, F. Multi-purposable filaments of HPMC for 3D printing of medications with tailored drug release and timed-absorption. Int. J. Pharm. 2018, 544, 285–296. [Google Scholar] [CrossRef]
- Hermida, R.C.; Crespo, J.J.; Domínguez-Sardiña, M.; Otero, A.; Moyá, A.; Ríos, M.T.; Sineiro, E.; Castiñeira, M.C.; Callejas, P.A.; Pousa, L.; et al. Bedtime hypertension treatment improves cardiovascular risk reduction: The Hygia Chronotherapy Trial. Eur. Heart J. 2020, 41, 4565–4576. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahić, O.; Tucak, A.; Sirbubalo, M.; Hindija, L.; Hadžiabdić, J. Antihypertensives’ Rock around the Clock. J 2021, 4, 62-81. https://doi.org/10.3390/j4010005
Rahić O, Tucak A, Sirbubalo M, Hindija L, Hadžiabdić J. Antihypertensives’ Rock around the Clock. J. 2021; 4(1):62-81. https://doi.org/10.3390/j4010005
Chicago/Turabian StyleRahić, Ognjenka, Amina Tucak, Merima Sirbubalo, Lamija Hindija, and Jasmina Hadžiabdić. 2021. "Antihypertensives’ Rock around the Clock" J 4, no. 1: 62-81. https://doi.org/10.3390/j4010005
APA StyleRahić, O., Tucak, A., Sirbubalo, M., Hindija, L., & Hadžiabdić, J. (2021). Antihypertensives’ Rock around the Clock. J, 4(1), 62-81. https://doi.org/10.3390/j4010005








