From Waste to Hydrogen: Utilizing Waste as Feedstock or Catalysts for Hydrogen Generation
Abstract
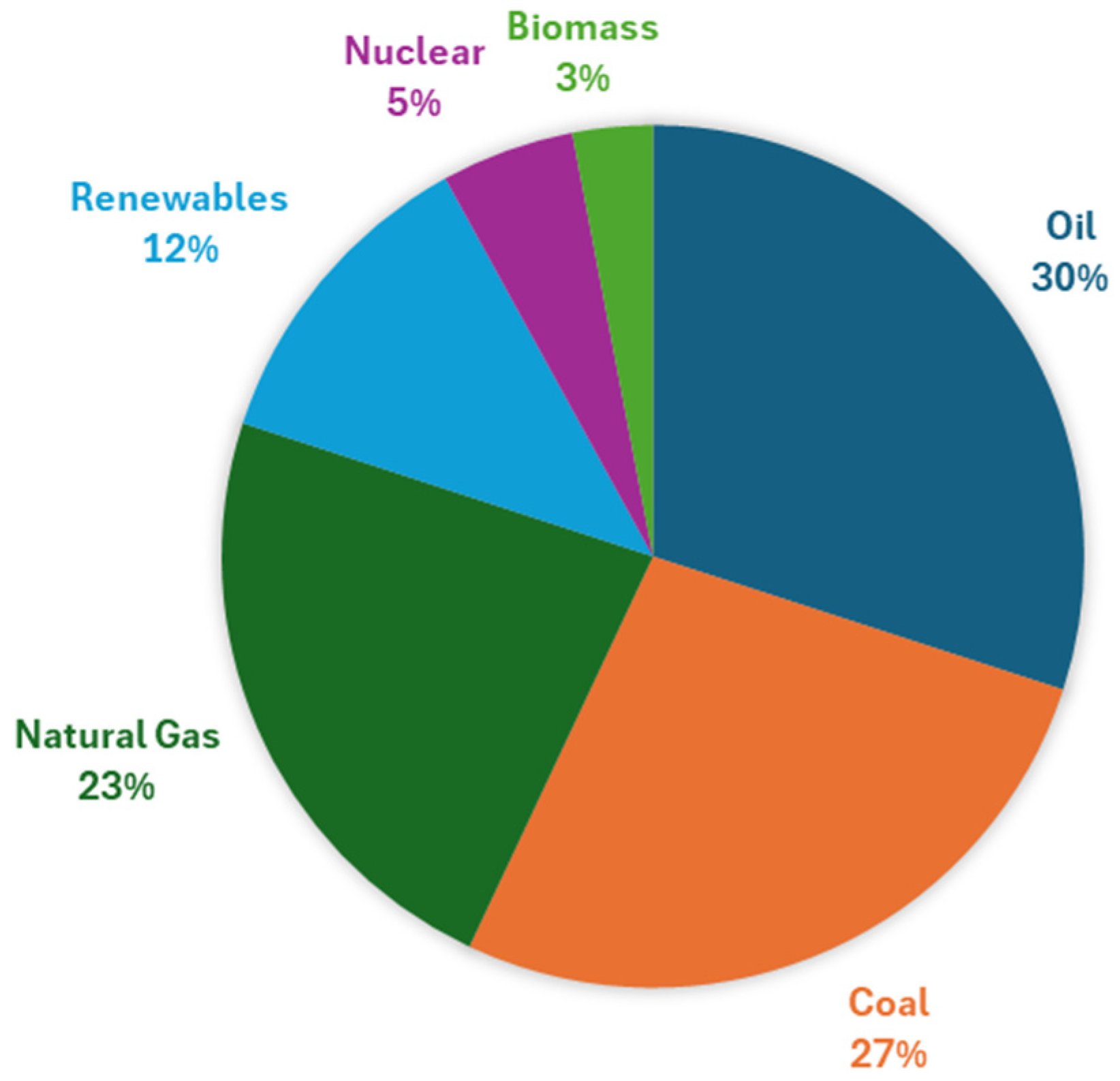
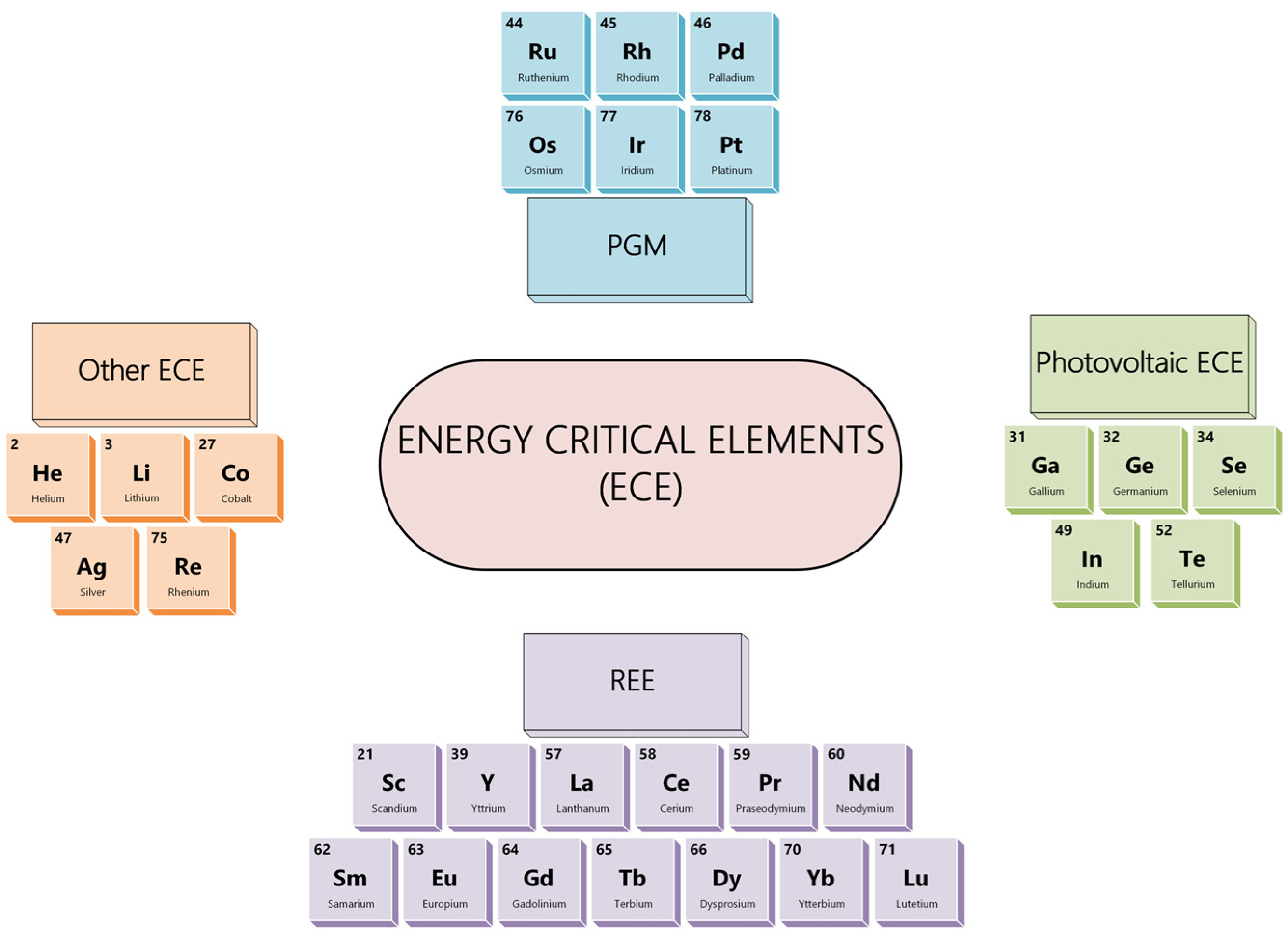
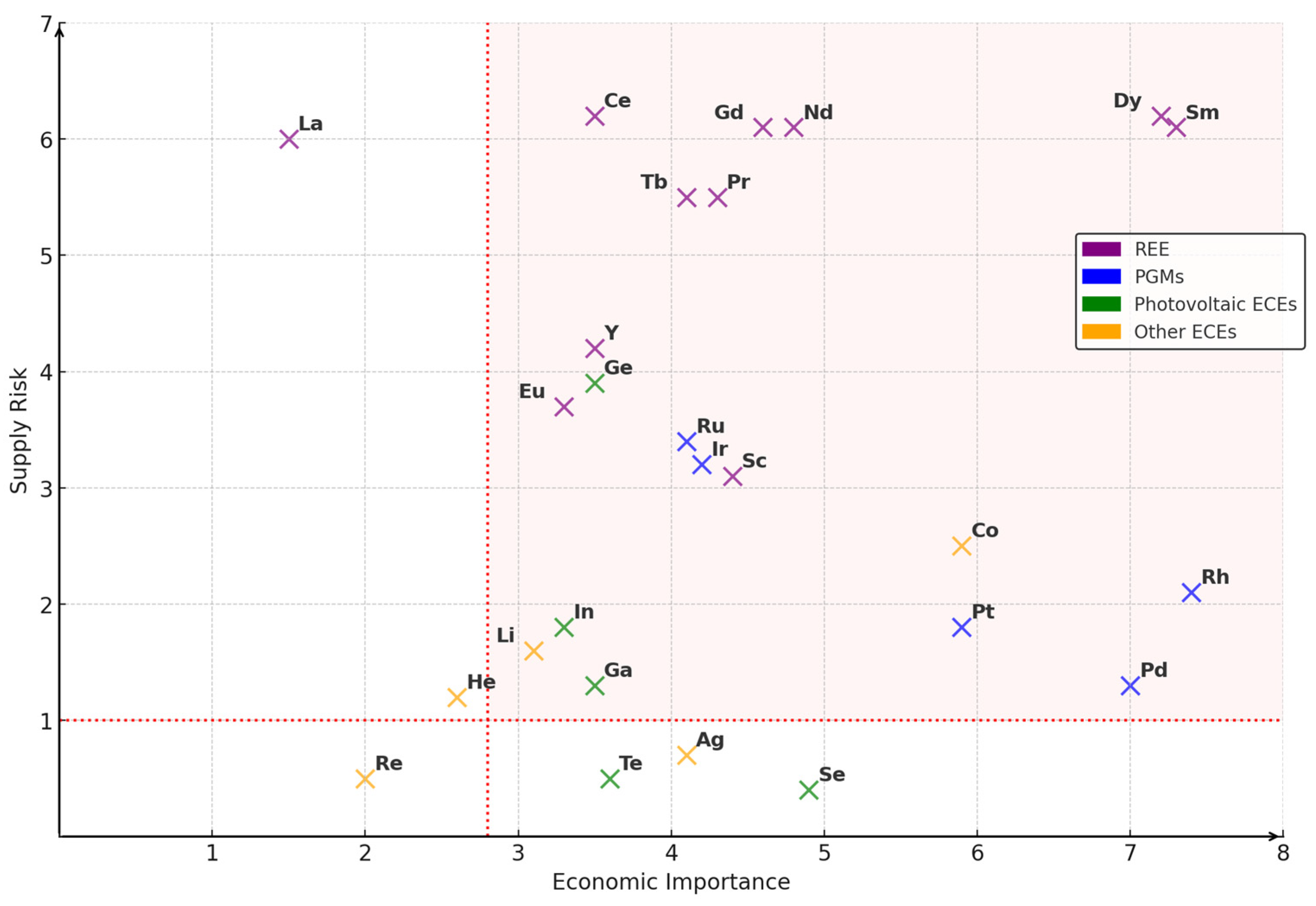
1. Introduction
2. Hydrogen as an Energy Carrier
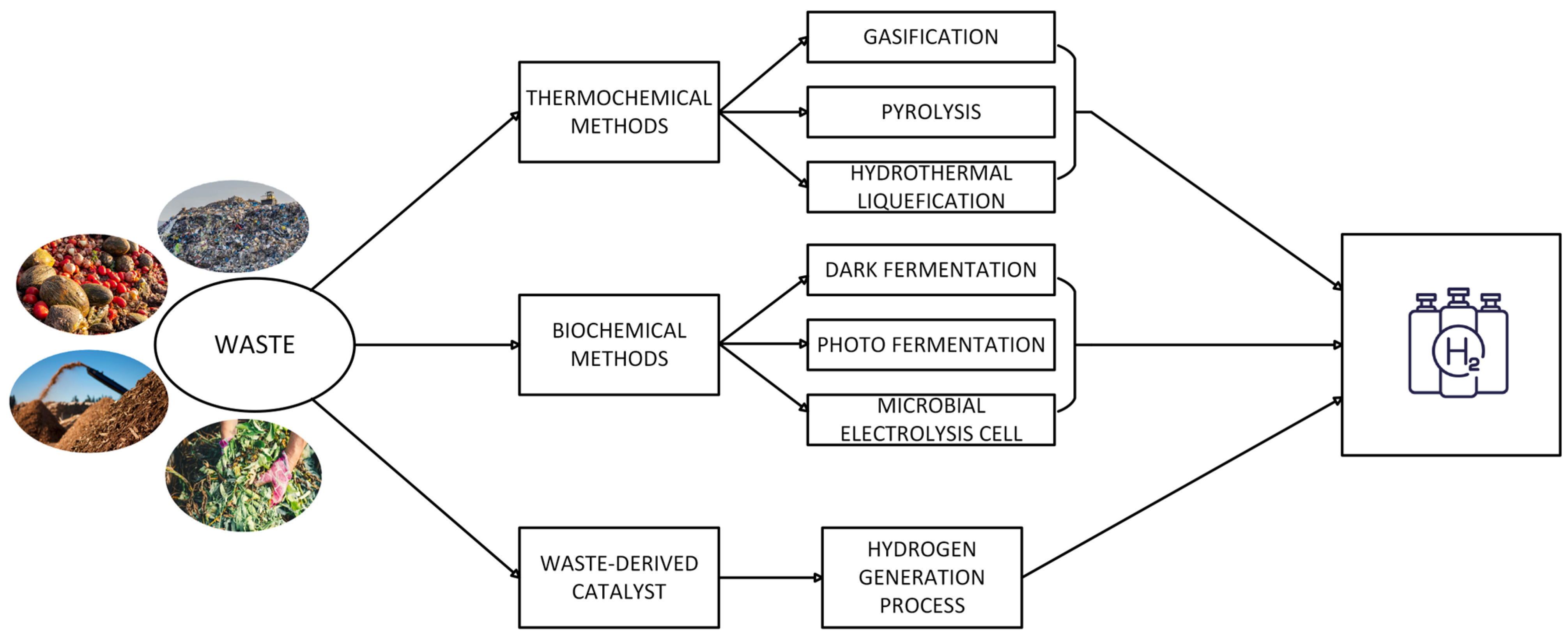
3. Waste Valorization in the Process of Hydrogen Production
3.1. Thermochemical Methods
3.2. Biochemical Methods
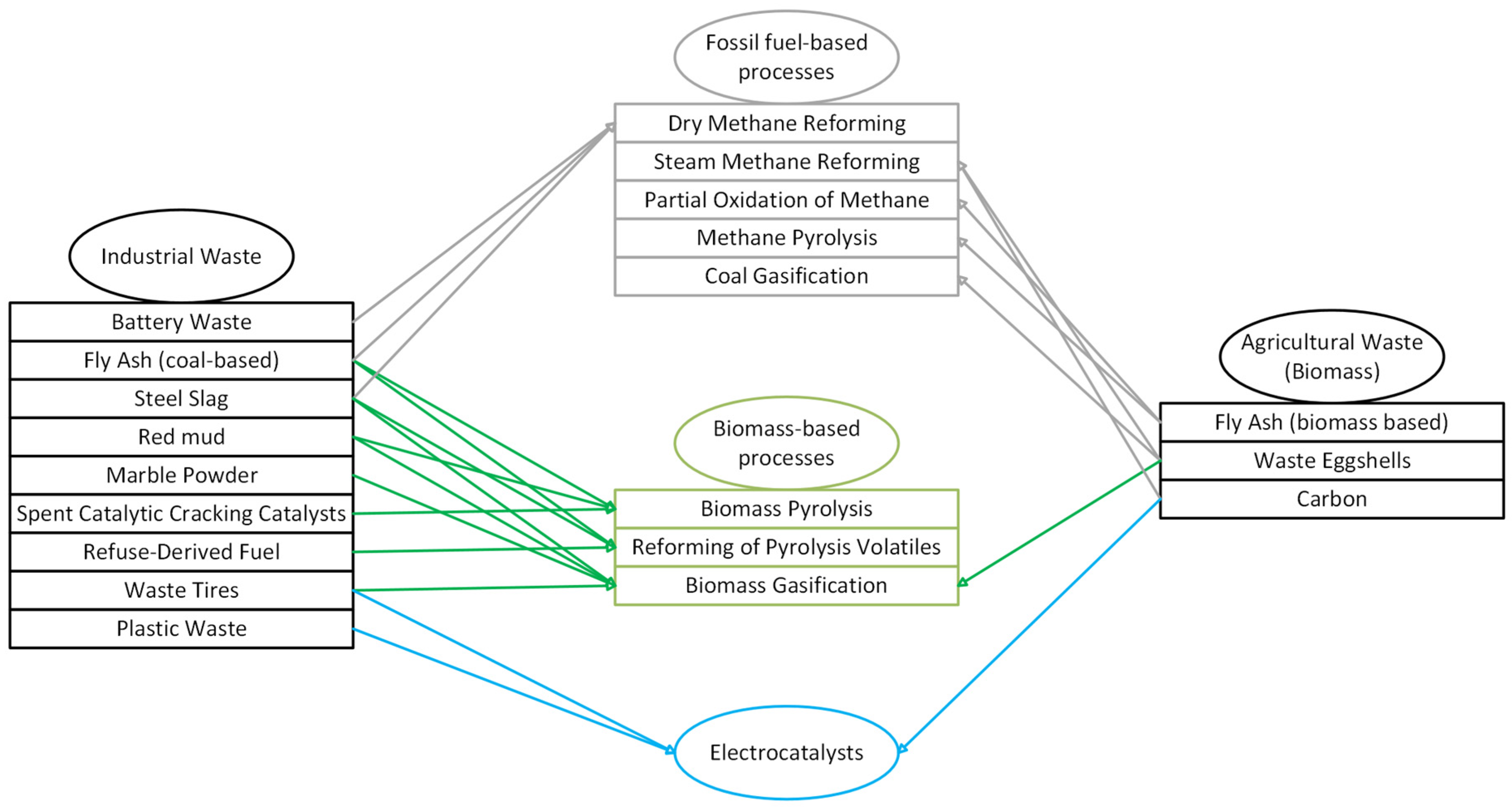
3.3. Waste as a Catalyst
4. Waste-Derived Catalysts for Fossil Fuel Processes
4.1. Waste-Derived Catalysts in Dry Methane Reforming
4.2. Waste-Derived Catalysts in Other Processes Utilizing Methane
4.3. Waste-Derived Catalysts in Coal Gasification
5. Waste-Derived Catalysts for Biomass-Based Processes
5.1. Waste-Derived Catalysts in Biomass Pyrolysis
5.2. Waste-Derived Catalysts in Reforming of Pyrolysis Volatiles
5.3. Waste-Derived Catalysts in Biomass Gasification
6. Waste-Derived Catalysts for Electrochemical-Based Processes
6.1. Pure Carbon Electrocatalysts
6.2. Self-Doped Carbon Electrocatalysts
6.3. Metal-Doped Carbon Electrocatalysts
6.4. Multiple-Doped Carbon Electrocatalysts
6.5. Comparison of Commercial and the Best Performing Carbon Electrocatalysts
7. Future Prospects
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AC | Activated carbon |
| BFA | Blast furnace ash |
| CE | Circular economy |
| CFA | Coal fly ash |
| CG | Coal gasification |
| CRM | Critical raw materials |
| DMR | Dry methane reforming |
| FA | Fly ash |
| GHG | Greenhouse gas |
| HER | Hydrogen evolution reaction |
| IRENA | International Renewable Energy Agency |
| LCA | Life cycle assessment |
| MP | Methane pyrolysis |
| MSW | Municipal solid waste |
| PGM | Platinum group metals |
| POM | Partial oxidation of methane |
| RDF | Refuse-derived fuel |
| REE | Rare earth elements |
| RM | Red mud |
| sFCC | Spent fluid catalytic cracking catalyst |
| SMR | Steam methane reforming |
| SS | Steel slag |
| UNEP | United Nations Environment Programme |
| WE | Waste eggshells |
| WMP | Waste marble powder |
| WTA | Waste tire ash |
| W-t-E | Waste to energy |
References
- Tashie-Lewis, B.C.; Nnabuife, S.G. Hydrogen Production, Distribution, Storage and Power Conversion in a Hydrogen Economy—A Technology Review. Chem. Eng. J. Adv. 2021, 8, 100172. [Google Scholar] [CrossRef]
- Osman, A.I.; Mehta, N.; Elgarahy, A.M.; Hefny, M.; Al-Hinai, A.; Al-Muhtaseb, A.H.; Rooney, D.W. Hydrogen production, storage, utilisation and environmental impacts: A review. Environ. Chem. Lett. 2022, 20, 153–188. [Google Scholar] [CrossRef]
- Gunathilake, C.; Soliman, I.; Panthi, D.; Tandler, P.; Fatani, O.; Ghulamullah, N.A.; Marasinghe, D.; Farhath, M.; Madhujith, T.; Conrad, K.; et al. A comprehensive review on hydrogen production, storage, and applications. Chem. Soc. Rev. 2024, 53, 10900–10969. [Google Scholar] [CrossRef]
- IEA. World Energy Outlook 2024; IEA: Paris, France, 2024; Available online: https://www.iea.org/reports/world-energy-outlook-2024 (accessed on 21 June 2025).
- Megía, P.J.; Vizcaíno, A.J.; Calles, J.A.; Carrero, A. Hydrogen Production Technologies: From Fossil Fuels toward Renewable Sources. A Mini Review. Energy Fuels 2021, 35, 16403–16415. [Google Scholar] [CrossRef]
- Afanasev, P.; Askarova, A.; Alekhina, T.; Popov, E.; Markovic, S.; Mukhametdinova, A.; Cheremisin, A.; Mukhina, E. An overview of hydrogen production methods: Focus on hydrocarbon feedstock. Int. J. Hydrogen Energy 2024, 78, 805–828. [Google Scholar] [CrossRef]
- IEA. CO2 Emissions in 2023; IEA: Paris, France, 2024; Available online: https://www.iea.org/reports/co2-emissions-in-2023 (accessed on 17 June 2025).
- Dash, S.K.; Chakraborty, S.; Elangovan, D. A Brief Review of Hydrogen Production Methods and Their Challenges. Energies 2023, 16, 1141. [Google Scholar] [CrossRef]
- Ishaq, H.; Dincer, I.; Crawford, C. A review on hydrogen production and utilization: Challenges and opportunities. Int. J. Hydrogen Energy 2022, 47, 26238–26264. [Google Scholar] [CrossRef]
- Acar, C.; Dincer, I. Review and evaluation of hydrogen production options for better environment. J. Clean. Prod. 2019, 218, 835–849. [Google Scholar] [CrossRef]
- Chandrasekhar, K.; Kumar, S.; Lee, B.-D.; Kim, S.-H. Waste based hydrogen production for circular bioeconomy: Current status and future directions. Bioresour. Technol. 2020, 302, 122920. [Google Scholar] [CrossRef]
- Rasmussen, K.D.; Wenzel, H.; Bangs, C.; Petavratzi, E.; Liu, G. Platinum Demand and Potential Bottlenecks in the Global Green Transition: A Dynamic Material Flow Analysis. Environ. Sci. Technol. 2019, 53, 11541–11551. [Google Scholar] [CrossRef] [PubMed]
- National Research Council. The Role of the Chemical Sciences in Finding Alternatives to Critical Resources; National Academies Press: Washington, DC, USA, 2012. [Google Scholar] [CrossRef]
- Kamran, M.; Raugei, M.; Hutchinson, A. Critical elements for a successful energy transition: A systematic review. Renew. Sustain. Energy Transit. 2023, 4, 100068. [Google Scholar] [CrossRef]
- van Gaalen, J.M.; Slootweg, J.C. From Critical Raw Materials to Circular Raw Materials. ChemSusChem 2025, 18, e202401170. [Google Scholar] [CrossRef]
- Weng, Z.; Jowitt, S.M.; Mudd, G.M.; Haque, N. A Detailed Assessment of Global Rare Earth Element Resources: Opportunities and Challenges. Econ. Geol. 2015, 110, 1925–1952. [Google Scholar] [CrossRef]
- Girtan, M.; Wittenberg, A.; Grilli, M.L.; de Oliveira, D.P.S.; Giosuè, C.; Ruello, M.L. The Critical Raw Materials Issue between Scarcity, Supply Risk, and Unique Properties. Materials 2021, 14, 1826. [Google Scholar] [CrossRef] [PubMed]
- Blengini, G.A.; El Latunussa, C.; Eynard, U.; Torres De Matos, C.; Wittmer, D.; Georgitzikis, K.; Pavel, C.; Carrara, S.; Mancini, L.; Unguru, M.; et al. Study on the EU’s List of Critical Raw Materials (2020): Final Report; Publications Office of the European Union: Luxembourg, 2020; Available online: https://data.europa.eu/doi/10.2873/11619 (accessed on 1 July 2025).
- Korhonen, J.; Honkasalo, A.; Seppälä, J. Circular Economy: The Concept and its Limitations. Ecol. Econ. 2018, 143, 37–46. [Google Scholar] [CrossRef]
- Rhodes, C.J. Endangered elements, critical raw materials and conflict minerals. Sci. Prog. 2019, 102, 304–350. [Google Scholar] [CrossRef] [PubMed]
- Shchegolkov, A.; Shchegolkov, A.; Zemtsova, N.; Stanishevskiy, Y.; Vetcher, A. Recent Advantages on Waste Management in Hydrogen Industry. Polymers 2022, 14, 4992. [Google Scholar] [CrossRef] [PubMed]
- Chioatto, E.; Sospiro, P. Transition from waste management to circular economy: The European Union roadmap. Environ. Dev. Sustain. 2023, 25, 249–276. [Google Scholar] [CrossRef]
- Mishra, R.; Ong, H.C.; Lin, C.-W. Progress on co-processing of biomass and plastic waste for hydrogen production. Energy Convers. Manag. 2023, 284, 116983. [Google Scholar] [CrossRef]
- Campitelli, A.; Kannengießer, J.; Schebek, L. Approach to assess the performance of waste management systems towards a circular economy: Waste management system development stage concept (WMS-DSC). MethodsX 2022, 9, 101634. [Google Scholar] [CrossRef] [PubMed]
- Kabir, M.M.; Akter, M.M.; Huang, Z.; Tijing, L.; Shon, H.K. Hydrogen production from water industries for a circular economy. Desalination 2023, 554, 116448. [Google Scholar] [CrossRef]
- Salmenperä, H.; Pitkänen, K.; Kautto, P.; Saikku, L. Critical factors for enhancing the circular economy in waste management. J. Clean. Prod. 2021, 280, 124339. [Google Scholar] [CrossRef]
- Alao, M.A.; Popoola, O.M.; Ayodele, T.R. Waste-to-energy nexus: An overview of technologies and implementation for sustainable development. Clean. Energy Syst. 2022, 3, 100034. [Google Scholar] [CrossRef]
- Hsu, H.-W.; Binyet, E.; Nugroho, R.A.A.; Wang, W.-C.; Srinophakun, P.; Chein, R.-Y.; Demafelis, R.; Chiarasumran, N.; Saputro, H.; Alhikami, A.F.; et al. Toward sustainability of Waste-to-Energy: An overview. Energy Convers. Manag. 2024, 321, 119063. [Google Scholar] [CrossRef]
- Aziz, M.; Darmawan, A.; Juangsa, F.B. Hydrogen production from biomasses and wastes: A technological review. Int. J. Hydrogen Energy 2021, 46, 33756–33781. [Google Scholar] [CrossRef]
- Lisbona, P.; Pascual, S.; Pérez, V. Waste to energy: Trends and perspectives. Chem. Eng. J. Adv. 2023, 14, 100494. [Google Scholar] [CrossRef]
- Sampath, P.; Brijesh Reddy, K.R.; Reddy, C.V.; Shetti, N.P.; Kulkarni, R.V.; Raghu, A.V. Biohydrogen Production from Organic Waste—A Review. Chem. Eng. Technol. 2020, 43, 1240–1248. [Google Scholar] [CrossRef]
- Taqvi, S.A.A.; Kazmi, B.; Naqvi, S.R.; Juchelková, D.; Bokhari, A. State-of-the-Art Review of Biomass Gasification: Raw to Energy Generation. ChemBioEng Rev. 2024, 11, e202400003. [Google Scholar] [CrossRef]
- Xie, W.; Zhang, Y.; Zhang, Y.; Liu, C.; Wang, Y.; Xie, Y.; Ji, G.; Li, A. Fast pyrolysis of biomass with diverse properties to produce liquid hydrogen storage molecules. Carbon Capture Sci. Technol. 2024, 12, 100230. [Google Scholar] [CrossRef]
- Qiu, B.; Tao, X.; Wang, Y.; Zhang, D.; Chu, H. Hydrothermal liquefaction for producing liquid fuels and chemicals from biomass-derived platform compounds: A review. Environ. Chem. Lett. 2025, 23, 81–115. [Google Scholar] [CrossRef]
- Gbiete, D.; Narra, S.; Kongnine, D.M.; Narra, M.-M.; Nelles, M. Insights into Biohydrogen Production Through Dark Fermentation of Food Waste: Substrate Properties, Inocula, and Pretreatment Strategies. Energies 2024, 17, 6350. [Google Scholar] [CrossRef]
- Xie, D.; Kong, L.; Hu, J.; Li, H.; Wang, Y. A comparative review of biohydrogen and biomethane production from biowaste through photo-fermentation. Green Chem. 2025, 27, 1331–1347. [Google Scholar] [CrossRef]
- Swaminathan, P.; Ghosh, A.; Sunantha, G.; Sivagami, K.; Mohanakrishna, G.; Aishwarya, S.; Shah, S.; Sethumadhavan, A.; Ranjan, P.; Prajapat, R. A comprehensive review of microbial electrolysis cells: Integrated for wastewater treatment and hydrogen generation. Process. Saf. Environ. Prot. 2024, 190, 458–474. [Google Scholar] [CrossRef]
- Varjani, S.; Shahbeig, H.; Popat, K.; Patel, Z.; Vyas, S.; Shah, A.V.; Barceló, D.; Hao Ngo, H.; Sonne, C.; Shiung Lam, S.; et al. Sustainable management of municipal solid waste through waste-to-energy technologies. Bioresour. Technol. 2022, 355, 127247. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, M.; Batra, V.S.; Hargreaves, J.S.J.; Pulford, I.D. Waste materials—Catalytic opportunities: An overview of the application of large scale waste materials as resources for catalytic applications. Green Chem. 2011, 13, 16–24. [Google Scholar] [CrossRef]
- Devarajan, B.; Saravanakumar, R.; Sivalingam, S.; Bhuvaneswari, V.; Karimi, F.; Rajeshkumar, L. Catalyst derived from wastes for biofuel production: A critical review and patent landscape analysis. Appl. Nanosci. 2022, 12, 3677–3701. [Google Scholar] [CrossRef]
- Kumar, S.S.; Lim, H. An overview of water electrolysis technologies for green hydrogen production. Energy Rep. 2022, 8, 13793–13813. [Google Scholar] [CrossRef]
- El-Shafie, M. Hydrogen production by water electrolysis technologies: A review. Results Eng. 2023, 20, 101426. [Google Scholar] [CrossRef]
- Dash, S.K.; Chakraborty, S.; Roccotelli, M.; Sahu, U.K. Hydrogen Fuel for Future Mobility: Challenges and Future Aspects. Sustainability 2022, 14, 8285. [Google Scholar] [CrossRef]
- Hassan, Q.; Algburi, S.; Sameen, A.Z.; Jaszczur, M.; Salman, H.M. Hydrogen as an energy carrier: Properties, storage methods, challenges, and future implications. Environ. Syst. Decis. 2024, 44, 327–350. [Google Scholar] [CrossRef]
- Teoh, Y.H.; How, H.G.; Le, T.D.; Nguyen, H.T.; Loo, D.L.; Rashid, T.; Sher, F. A review on production and implementation of hydrogen as a green fuel in internal combustion engines. Fuel 2023, 333, 126525. [Google Scholar] [CrossRef]
- Bansal, P.; Meena, R. Methanol as an Alternative Fuel in Internal Combustion Engine: Scope, Production, and Limitations. In Methanol; Springer: Singapore, 2021; pp. 11–36. [Google Scholar] [CrossRef]
- Purayil, S.T.P.; Hamdan, M.O.; Al-Omari, S.A.B.; Selim, M.Y.E.; Elnajjar, E. Review of hydrogen–gasoline SI dual fuel engines: Engine performance and emission. Energy Rep. 2023, 9, 4547–4573. [Google Scholar] [CrossRef]
- Aziz, M.; Juangsa, F.B.; Irhamna, A.R.; Irsyad, A.R.; Hariana, H.; Darmawan, A. Ammonia utilization technology for thermal power generation: A review. J. Energy Inst. 2023, 111, 101365. [Google Scholar] [CrossRef]
- Lu, H.T.; Li, W.; Miandoab, E.S.; Kanehashi, S.; Hu, G. The opportunity of membrane technology for hydrogen purification in the power to hydrogen (P2H) roadmap: A review. Front. Chem. Sci. Eng. 2021, 15, 464–482. [Google Scholar] [CrossRef]
- Irani, R.S. Hydrogen Storage: High-Pressure Gas Containment. MRS Bull. 2002, 27, 680–682. [Google Scholar] [CrossRef]
- Zohuri, B. Cryogenics and Liquid Hydrogen Storage. In Hydrogen Energy; Springer International Publishing: Cham, Switzerland, 2019; pp. 121–139. [Google Scholar] [CrossRef]
- Scarpati, G.; Frasci, E.; Di Ilio, G.; Jannelli, E. A comprehensive review on metal hydrides-based hydrogen storage systems for mobile applications. J. Energy Storage 2024, 102, 113934. [Google Scholar] [CrossRef]
- Lan, R.; Irvine, J.T.S.; Tao, S. Ammonia and related chemicals as potential indirect hydrogen storage materials. Int. J. Hydrogen Energy 2012, 37, 1482–1494. [Google Scholar] [CrossRef]
- Moioli, E.; Mutschler, R.; Züttel, A. Renewable energy storage via CO2 and H2 conversion to methane and methanol: Assessment for small scale applications. Renew. Sustain. Energy Rev. 2019, 107, 497–506. [Google Scholar] [CrossRef]
- Eijk, C.; Dalaker, H.; Safarian, J. Possibilities and Limitations of the Use of Hydrogen in Different Metallurgical Sectors. Mater. Proc. 2023, 15, 63. [Google Scholar] [CrossRef]
- Sollai, S.; Porcu, A.; Tola, V.; Ferrara, F.; Pettinau, A. Renewable methanol production from green hydrogen and captured CO2: A techno-economic assessment. J. CO2 Util. 2023, 68, 102345. [Google Scholar] [CrossRef]
- Faria, J.A. Renaissance of ammonia synthesis for sustainable production of energy and fertilizers. Curr. Opin. Green Sustain. Chem. 2021, 29, 100466. [Google Scholar] [CrossRef]
- International Energy Agency. Global Hydrogen Review 2024. Available online: www.iea.org (accessed on 22 June 2025).
- Kumar, R.; Singh, R.; Dutta, S. Review and Outlook of Hydrogen Production through Catalytic Processes. Energy Fuels 2024, 38, 2601–2629. [Google Scholar] [CrossRef]
- Lui, J.; Chen, W.-H.; Tsang, D.C.W.; You, S. A critical review on the principles, applications, and challenges of waste-to-hydrogen technologies. Renew. Sustain. Energy Rev. 2020, 134, 110365. [Google Scholar] [CrossRef]
- Ajorloo, M.; Ghodrat, M.; Scott, J.; Strezov, V. Evaluating the role of feedstock composition and component interactions on biomass gasification. Fuel 2025, 381, 133528. [Google Scholar] [CrossRef]
- Tian, T.; Li, Q.; He, R.; Tan, Z.; Zhang, Y. Effects of biochemical composition on hydrogen production by biomass gasification. Int. J. Hydrogen Energy 2017, 42, 19732. [Google Scholar] [CrossRef]
- Chai, Y.H.; Mohamed, M.; Cheng, Y.W.; Chin, B.L.F.; Yiin, C.L.; Yusup, S.; Lam, M.K. A review on potential of biohydrogen generation through waste decomposition technologies. Biomass Convers. Biorefin. 2023, 13, 8549–8574. [Google Scholar] [CrossRef]
- Fu, Q.; Wang, D.; Li, X.; Yang, Q.; Xu, Q.; Ni, B.-J.; Wang, Q.; Liu, X. Towards hydrogen production from waste activated sludge: Principles, challenges and perspectives. Renew. Sustain. Energy Rev. 2021, 135, 110283. [Google Scholar] [CrossRef]
- Bennett, J.A.; Wilson, K.; Lee, A.F. Catalytic applications of waste derived materials. J. Mater. Chem. A 2016, 4, 3617–3637. [Google Scholar] [CrossRef]
- Hargreaves, J.S.J. Catalysts derived from waste materials. In Catalysis; The Royal Society of Chemistry: London, UK, 2018; pp. 1–20. [Google Scholar] [CrossRef]
- Sushil, S.; Batra, V.S. Catalytic applications of red mud, an aluminium industry waste: A review. Appl. Catal. B Environ. 2008, 81, 64–77. [Google Scholar] [CrossRef]
- Wang, F.-P.; Liu, T.-J.; Cai, S.; Gao, D.; Yu, Q.; Wang, X.-M.; Wang, Y.-T.; Zeng, Y.-N.; Li, J.-G. A Review of Modified Steel Slag Application in Catalytic Pyrolysis, Organic Degradation, Electrocatalysis, Photocatalysis, Transesterification and Carbon Capture and Storage. Appl. Sci. 2021, 11, 4539. [Google Scholar] [CrossRef]
- Laca, A.; Laca, A.; Díaz, M. Eggshell waste as catalyst: A review. J. Environ. Manag. 2017, 197, 351–359. [Google Scholar] [CrossRef]
- Jan, B.M.; Dahari, M.B.; Abro, M.; Ikram, R. Exploration of waste-generated nanocomposites as energy-driven systems for various methods of hydrogen production; A review. Int. J. Hydrogen Energy 2022, 47, 16398–16423. [Google Scholar] [CrossRef]
- Jiang, T.; Wang, B.; Hassan, M.; Zou, Q. Solid waste-derived carbonaceous catalysts for environmental and energy applications. Carbon Res. 2024, 3, 78. [Google Scholar] [CrossRef]
- Murugesan, R.; Yesupatham, M.S.; Agamendran, N.; Sekar, K.; Neethinathan, C.S.S.; Maruthapillai, A. Recent Advances in Biomass-Derived Carbon-Based Nanostructures for Electrocatalytic Reduction Reactions: Properties–Performance Correlations. Energy Technol. 2024, 12, 2400882. [Google Scholar] [CrossRef]
- Feng, Y.; Jiang, J.; Xu, Y.; Wang, S.; An, W.; Chai, Q.; Prova, U.H.; Wang, C.; Huang, G. Biomass derived diverse carbon nanostructure for electrocatalysis, energy conversion and storage. Carbon 2023, 211, 118105. [Google Scholar] [CrossRef]
- Li, L.; Lu, S.; Fang, L.; Wei, Y.; Yang, S. Review on Biomass-Derived Carbon-Based Materials for Electrocatalytic Hydrogen Production: State of the Art and Outlook. Energy Fuels 2023, 37, 18485–18501. [Google Scholar] [CrossRef]
- Teh, L.P.; Setiabudi, H.D.; Timmiati, S.N.; Aziz, M.A.A.; Annuar, N.H.R.; Ruslan, N.N. Recent progress in ceria-based catalysts for the dry reforming of methane: A review. Chem. Eng. Sci. 2021, 242, 116606. [Google Scholar] [CrossRef]
- Alhassan, M.; Jalil, A.A.; Nabgan, W.; Hamid, M.Y.S.; Bahari, M.B.; Ikram, M. Bibliometric studies and impediments to valorization of dry reforming of methane for hydrogen production. Fuel 2022, 328, 125240. [Google Scholar] [CrossRef]
- de Medeiros, F.G.M.; Lopes, F.W.B.; de Vasconcelos, B.R. Prospects and Technical Challenges in Hydrogen Production through Dry Reforming of Methane. Catalysts 2022, 12, 363. [Google Scholar] [CrossRef]
- Hussien, A.G.S.; Polychronopoulou, K. A Review on the Different Aspects and Challenges of the Dry Reforming of Methane (DRM) Reaction. Nanomaterials 2022, 12, 3400. [Google Scholar] [CrossRef]
- Kanamori, T.; Matsuda, M.; Miyake, M. Recovery of rare metal compounds from nickel–metal hydride battery waste and their application to CH4 dry reforming catalyst. J. Hazard. Mater. 2009, 169, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, Q.; Zhao, T.; Zhu, X.; Wang, Z. The dry reforming of methane over fly ash modified with different content levels of MgO. RSC Adv. 2021, 11, 14154–14160. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Jiang, J.; Meng, Y.; Aihemaiti, A.; Ju, T.; Chen, X.; Yan, F. A novel nickel catalyst supported on activated coal fly ash for syngas production via biogas dry reforming. Renew. Energy 2020, 149, 786–793. [Google Scholar] [CrossRef]
- Chen, X.; Jiang, J.; Tian, S.; Li, K. Biogas dry reforming for syngas production: Catalytic performance of nickel supported on waste-derived SiO2 . Catal. Sci. Technol. 2015, 5, 860–868. [Google Scholar] [CrossRef]
- Dega, F.B.; Chamoumi, M.; Braidy, N.; Abatzoglou, N. Autothermal dry reforming of methane with a nickel spinellized catalyst prepared from a negative value metallurgical residue. Renew. Energy 2019, 138, 1239–1249. [Google Scholar] [CrossRef]
- Manabayeva, A.; Mäki-Arvela, P.; Vajglová, Z.; Martinez-Klimov, M.; Yevdokimova, O.; Peuronen, A.; Lastusaari, M.; Tirri, T.; Kassymkan, K.; Baizhumanova, T.S.; et al. Dry Reforming of Methane over Rare-Earth Metal Oxide Ni–M–Al (M = Ce, La) Catalysts. Ind. Eng. Chem. Res. 2023, 62, 20588–20607. [Google Scholar] [CrossRef]
- Yusuf, B.O.; Umar, M.; Kotob, E.; Abdulhakam, A.; Taialla, O.A.; Awad, M.M.; Hussain, I.; Alhooshani, K.R.; Ganiyu, S.A. Recent Advances in Bimetallic Catalysts for Methane Steam Reforming in Hydrogen Production: Current Trends, Challenges, and Future Prospects. Chem. Asian J. 2024, 19, e202300641. [Google Scholar] [CrossRef]
- Chau, K.; Djire, A.; Khan, F. Review and analysis of the hydrogen production technologies from a safety perspective. Int. J. Hydrogen Energy 2022, 47, 13990–14007. [Google Scholar] [CrossRef]
- Chen, L.; Qi, Z.; Zhang, S.; Su, J.; Somorjai, G.A. Catalytic Hydrogen Production from Methane: A Review on Recent Progress and Prospect. Catalysts 2020, 10, 858. [Google Scholar] [CrossRef]
- Meloni, E.; Martino, M.; Palma, V. A Short Review on Ni Based Catalysts and Related Engineering Issues for Methane Steam Reforming. Catalysts 2020, 10, 352. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, Z.; Hu, Y.H. Steam reforming of methane: Current states of catalyst design and process upgrading. Renew. Sustain. Energy Rev. 2021, 149, 111330. [Google Scholar] [CrossRef]
- Ayesha, M.; Khoja, A.H.; Butt, F.A.; Sikandar, U.; Javed, A.H.; Naqvi, S.R.; din Iud Mehran, M.T. Sorption enhanced steam reforming of methane over waste-derived CaO promoted MgNiAl hydrotalcite catalyst for sustainable H2 production. J. Environ. Chem. Eng. 2022, 10, 107651. [Google Scholar] [CrossRef]
- Minhas, R.; Khoja, A.H.; Naeem, N.; Anwar, M.; Shakir, S.; Liaquat, R.; Din, I.U. Thermal steam methane reforming over bimetal-loaded hemp-derived activated carbon-based catalyst for hydrogen production. Res. Chem. Intermed. 2023, 49, 3181–3203. [Google Scholar] [CrossRef]
- De Freitas Silva, T.; Dias, J.A.C.; Maciel, C.G.; Assaf, J.M. Ni/Al2O3 catalysts: Effects of the promoters Ce, La and Zr on the methane steam and oxidative reforming reactions. Catal. Sci. Technol. 2013, 3, 635–643. [Google Scholar] [CrossRef]
- Al-Sayari, S.A. Recent Developments in the Partial Oxidation of Methane to Syngas. Open Catal. J. 2013, 6, 17–28. [Google Scholar] [CrossRef]
- Makaryan, I.A.; Salgansky, E.A.; Arutyunov, V.S.; Sedov, I.V. Non-Catalytic Partial Oxidation of Hydrocarbon Gases to Syngas and Hydrogen: A Systematic Review. Energies 2023, 16, 2916. [Google Scholar] [CrossRef]
- Siang, T.J.; Jalil, A.A.; Liew, S.Y.; Owgi, A.H.K.; Rahman, A.F.A. A review on state-of-the-art catalysts for methane partial oxidation to syngas production. Catal. Rev. 2024, 66, 343–399. [Google Scholar] [CrossRef]
- Hasanat, A.U.; Khoja, A.H.; Naeem, N.; Al-Anazi, A.; Liaquat, R.; Khan, B.A.; Din, I.U. Thermocatalytic partial oxidation of methane to syngas (H2, CO) production using Ni/La2O3 modified biomass fly ash supported catalyst. Results Eng. 2023, 19, 101333. [Google Scholar] [CrossRef]
- Vermeiren, W.J.M.; Blomsma, E.; Jacobs, P.A. Catalytic and thermodynamic approach of the oxyreforming reaction of methane. Catal. Today 1992, 13, 427–436. [Google Scholar] [CrossRef]
- Shokrollahi, M.; Teymouri, N.; Ashrafi, O.; Navarri, P.; Khojasteh-Salkuyeh, Y. Methane pyrolysis as a potential game changer for hydrogen economy: Techno-economic assessment and GHG emissions. Int. J. Hydrogen Energy 2024, 66, 337–353. [Google Scholar] [CrossRef]
- Pangestu, M.R.G.; Malaibari, Z.; Muhammad, A.; Al-Rowaili, F.N.; Zahid, U. Comprehensive Review on Methane Pyrolysis for Sustainable Hydrogen Production. Energy Fuels 2024, 38, 13514–13538. [Google Scholar] [CrossRef]
- Chan, Y.H.; Chan, Z.P.; Lock, S.S.M.; Yiin, C.L.; Foong, S.Y.; Wong, M.K.; Ishak, M.A.; Quek, V.C.; Ge, S.; Lam, S.S. Thermal pyrolysis conversion of methane to hydrogen (H2): A review on process parameters, reaction kinetics and techno-economic analysis. Chin. Chem. Lett. 2024, 35, 109329. [Google Scholar] [CrossRef]
- Patlolla, S.R.; Katsu, K.; Sharafian, A.; Wei, K.; Herrera, O.E.; Mérida, W. A review of methane pyrolysis technologies for hydrogen production. Renew. Sustain. Energy Rev. 2023, 181, 113323. [Google Scholar] [CrossRef]
- Cheon, S.; Byun, M.; Lim, D.; Lee, H.; Lim, H. Parametric Study for Thermal and Catalytic Methane Pyrolysis for Hydrogen Production: Techno-Economic and Scenario Analysis. Energies 2021, 14, 6102. [Google Scholar] [CrossRef]
- Sánchez-Bastardo, N.; Schlögl, R.; Ruland, H. Methane Pyrolysis for Zero-Emission Hydrogen Production: A Potential Bridge Technology from Fossil Fuels to a Renewable and Sustainable Hydrogen Economy. Ind. Eng. Chem. Res. 2021, 60, 11855–11881. [Google Scholar] [CrossRef]
- Sánchez-Bastardo, N.; Schlögl, R.; Ruland, H. Methane Pyrolysis for CO2-Free H2 Production: A Green Process to Overcome Renewable Energies Unsteadiness. Chem. Ing. Tech. 2020, 92, 1596–1609. [Google Scholar] [CrossRef]
- Raza, J.; Khoja, A.H.; Naqvi, S.R.; Mehran, M.T.; Shakir, S.; Liaquat, R.; Tahir, M.; Ali, G. Methane decomposition for hydrogen production over biomass fly ash-based CeO2 nanowires promoted cobalt catalyst. J. Environ. Chem. Eng. 2021, 9, 105816. [Google Scholar] [CrossRef]
- Ramasubramanian, V.; Ramsurn, H.; Price, G.L. Hydrogen production by catalytic decomposition of methane over Fe based bi-metallic catalysts supported on CeO2–ZrO2. Int. J. Hydrogen Energy 2020, 45, 12026–12036. [Google Scholar] [CrossRef]
- Sutardi, T.; Paul, M.C.; Karimi, N. Investigation of coal particle gasification processes with application leading to underground coal gasification. Fuel 2019, 237, 1186–1202. [Google Scholar] [CrossRef]
- Bell, D.A.; Towler, B.F.; Fan, M. Gasification Fundamentals. In Coal Gasification and Its Applications; Elsevier: Amsterdam, The Netherlands, 2011; pp. 35–71. [Google Scholar] [CrossRef]
- Midilli, A.; Kucuk, H.; Topal, M.E.; Akbulut, U.; Dincer, I. A comprehensive review on hydrogen production from coal gasification: Challenges and Opportunities. Int. J. Hydrogen Energy 2021, 46, 25385–25412. [Google Scholar] [CrossRef]
- Fan, S.; Xu, L.-H.; Kang, T.-J.; Kim, H.-T. Application of eggshell as catalyst for low rank coal gasification: Experimental and kinetic studies. J. Energy Inst. 2017, 90, 696–703. [Google Scholar] [CrossRef]
- Fan, S.; Yuan, X.; Zhao, L.; Xu, L.-H.; Kang, T.-J.; Kim, H.-T. Experimental and kinetic study of catalytic steam gasification of low rank coal with an environmentally friendly, inexpensive composite K2CO3–Eggshell derived CaO catalyst. Fuel 2016, 165, 397–404. [Google Scholar] [CrossRef]
- Zhao, Z.; Kong, W.; Zhang, X.; Liu, M.; Cui, P. Catalytic steam gasification of bituminous coal for syngas production with catalyst derived from calcium-rich construction demolition waste. Fuel 2024, 359, 130428. [Google Scholar] [CrossRef]
- Zhou, X.; Yang, X.; Li, J.; Zhao, J.; Li, C.; Du, M.; Yu, Z.; Fang, Y. Pressurized catalytic calcium looping hydrogen generation from coal with in-situ CO2 capture. Energy Convers. Manag. 2019, 198, 111899. [Google Scholar] [CrossRef]
- Wang, F.; Wang, P.; Raheem, A.; Ji, G.; Memon, M.Z.; Song, Y.; Zhao, M. Enhancing hydrogen production from biomass pyrolysis by dental-wastes-derived sodium zirconate. Int. J. Hydrogen Energy 2019, 44, 23846–23855. [Google Scholar] [CrossRef]
- Lan, K.; Shang, S.; Guo, C.; Xiong, T.; Qin, Z.; He, W.; Li, J. Preparation of Fly Ash Nickel Catalyst and its Application in Catalytic Pyrolysis of Rice Straw for Syngas Production. BioResources 2019, 14, 6983–7000. [Google Scholar] [CrossRef]
- Zhang, Y.; Guan, J.; Qiao, P.; Li, G.; Li, J.; Zhang, W.; Liu, M. Study on the pyrolysis characteristics of sawdust catalyzed by spent FCC catalyst and blast furnace ash. J. Fuel Chem. Technol. 2022, 50, 1524–1534. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, L.; Chen, Q.; Xiao, X.; Wang, T.; Cheng, S.; Li, J. Study on the mechanism and reaction characteristics of red-mud-catalyzed pyrolysis of corn stover. Fuel 2023, 338, 127290. [Google Scholar] [CrossRef]
- Hao, Q.; Wang, C.; Lu, D.; Wang, Y.; Li, D.; Li, G. Production of hydrogen-rich gas from plant biomass by catalytic pyrolysis at low temperature. Int. J. Hydrogen Energy 2010, 35, 8884–8890. [Google Scholar] [CrossRef]
- Arregi, A.; Barbarias, I.; Alvarez, J.; Erkiaga, A.; Artetxe, M.; Amutio, M.; Olazar, M. Hydrogen production from biomass pyrolysis and in-line catalytic steam reforming. Chem. Eng. Trans. 2015, 43, 547–552. [Google Scholar] [CrossRef]
- Santamaria, L.; Lopez, G.; Fernandez, E.; Cortazar, M.; Arregi, A.; Olazar, M.; Bilbao, J. Progress on Catalyst Development for the Steam Reforming of Biomass and Waste Plastics Pyrolysis Volatiles: A Review. Energy Fuels 2021, 35, 17051–17084. [Google Scholar] [CrossRef]
- Arregi, A.; Lopez, G.; Amutio, M.; Barbarias, I.; Bilbao, J.; Olazar, M. Hydrogen production from biomass by continuous fast pyrolysis and in-line steam reforming. RSC Adv. 2016, 6, 25975–25985. [Google Scholar] [CrossRef]
- Al-Rahbi, A.S.; Williams, P.T. Waste ashes as catalysts for the pyrolysis–catalytic steam reforming of biomass for hydrogen-rich gas production. J. Mater. Cycles Waste Manag. 2019, 21, 1224–1231. [Google Scholar] [CrossRef]
- Li, Y.; Williams, P.T. Waste derived ash as catalysts for the pyrolysis-catalytic steam reforming of waste plastics for hydrogen-rich syngas production. J. Anal. Appl. Pyrolysis 2024, 177, 106374. [Google Scholar] [CrossRef]
- Ryczkowski, R.; Goscianska, J.; Panek, R.; Franus, W.; Przybysz, K.; Grams, J. Sustainable nickel catalyst for the con-version of lignocellulosic biomass to H2-rich gas. Int. J. Hydrogen Energy 2021, 46, 10708–10722. [Google Scholar] [CrossRef]
- Wu, Y.; Yu, H.; Chao, H.; Chen, D. A novel nickel catalyst supported on activated steel slags for syngas production and tar removal from biomass pyrolysis. Int. J. Hydrogen Energy 2021, 46, 37268–37280. [Google Scholar] [CrossRef]
- Guo, F.; Liang, S.; Zhao, X.; Jia, X.; Peng, K.; Jiang, X.; Qian, L. Catalytic reforming of biomass pyrolysis tar using the low-cost steel slag as catalyst. Energy 2019, 189, 116161. [Google Scholar] [CrossRef]
- Guo, F.; Zhao, X.; Peng, K.; Liang, S.; Jia, X.; Qian, L. Catalytic reforming of biomass primary tar from pyrolysis over waste steel slag based catalysts. Int. J. Hydrogen Energy 2019, 44, 16224–16233. [Google Scholar] [CrossRef]
- Rubinsin, N.J.; Karim, N.A.; Timmiati, S.N.; Lim, K.L.; Isahak, W.N.R.W.; Pudukudy, M. An overview of the enhanced biomass gasification for hydrogen production. Int. J. Hydrogen Energy 2024, 49, 1139–1164. [Google Scholar] [CrossRef]
- Castro, J.; Leaver, J.; Pang, S. Simulation and Techno-Economic Assessment of Hydrogen Production from Biomass Gasification-Based Processes: A Review. Energies 2022, 15, 8455. [Google Scholar] [CrossRef]
- Hosseinzadeh, A.; Zhou, J.L.; Li, X.; Afsari, M.; Altaee, A. Techno-economic and environmental impact assessment of hydrogen production processes using bio-waste as renewable energy resource. Renew. Sustain. Energy Rev. 2022, 156, 111991. [Google Scholar] [CrossRef]
- Tezer, Ö.; Karabağ, N.; Öngen, A.; Çolpan, C.Ö.; Ayol, A. Biomass gasification for sustainable energy production: A review. Int. J. Hydrogen Energy 2022, 47, 15419–15433. [Google Scholar] [CrossRef]
- Raheem, A.; Liu, H.; Ji, G.; Zhao, M. Gasification of lipid-extracted microalgae biomass promoted by waste eggshell as CaO catalyst. Algal Res. 2019, 42, 101601. [Google Scholar] [CrossRef]
- Chen, A.; Tian, Z.; Han, R.; Wei, X.; Hu, R.; Chen, Y. Preparation of Ni-based steel slag catalyst by impregnation method for sludge steam gasification. Sustain. Energy Technol. Assess. 2021, 47, 101553. [Google Scholar] [CrossRef]
- Dong, W.; Li, S.; Wang, M.; Yuan, X.; Cao, Y.; Ao, X. Nickel-loaded red mud catalyst for steam gasification of bamboo sawdust to produce hydrogen-rich syngas. Int. J. Hydrogen Energy 2023, 48, 21624–21635. [Google Scholar] [CrossRef]
- Vamvuka, D.; Panagiotidou, S.; Orfanoudaki, A. Use of building wastes and red mud as CO2 sorbent and catalyst for the production of hydrogen. Acad. Green Energy 2024, 1. [Google Scholar] [CrossRef]
- Irfan, M.; Li, A.; Zhang, L.; Wang, M.; Chen, C.; Khushk, S. Production of hydrogen enriched syngas from municipal solid waste gasification with waste marble powder as a catalyst. Int. J. Hydrogen Energy 2019, 44, 8051–8061. [Google Scholar] [CrossRef]
- Amin, N.; Khan, Z.; Razzaq, A.; Ghauri, M.; Khurram, S.; Inayat, A.; Jaffery, M.; Hameed, Z. Municipal solid waste air gasification using waste marble powder as a catalyst for syngas production. J. Energy Inst. 2024, 113, 101496. [Google Scholar] [CrossRef]
- Laghari, A.A.; Jamro, I.A.; Kumar, A.; Chen, G.; Sajnani, S.; Guo, Z.; Shen, Y.; Zhang, J.; Khoso, S.; Guo, Q.; et al. Catalytic gasification of municipal solid waste using eggshell-derived CaO catalyst: An investigation of optimum H2 production, production distribution, and tar compounds. Next Sustain. 2024, 4, 100038. [Google Scholar] [CrossRef]
- Peng, W.X.; Wang, L.S.; Mirzaee, M.; Ahmadi, H.; Esfahani, M.J.; Fremaux, S. Hydrogen and syngas production by catalytic biomass gasification. Energy Convers. Manag. 2017, 135, 270–273. [Google Scholar] [CrossRef]
- Wang, S.; Lu, A.; Zhong, C.-J. Hydrogen production from water electrolysis: Role of catalysts. Nano Converg. 2021, 8, 4. [Google Scholar] [CrossRef]
- Qadeer, M.A.; Zhang, X.; Farid, M.A.; Tanveer, M.; Yan, Y.; Du, S.; Huang, Z.-F.; Tahir, M.; Zou, J.-J. A review on fundamentals for designing hydrogen evolution electrocatalyst. J. Power Sources 2024, 613, 234856. [Google Scholar] [CrossRef]
- Raveendran, A.; Chandran, M.; Dhanusuraman, R. A comprehensive review on the electrochemical parameters and recent material development of electrochemical water splitting electrocatalysts. RSC Adv. 2023, 13, 3843–3876. [Google Scholar] [CrossRef] [PubMed]
- van der Heijden, O.; Park, S.; Vos, R.E.; Eggebeen, J.J.J.; Koper, M.T.M. Tafel Slope Plot as a Tool to Analyze Electro-catalytic Reactions. ACS Energy Lett. 2024, 9, 1871–1879. [Google Scholar] [CrossRef] [PubMed]
- Sekar, S.; Ahmed, A.T.A.; Sim, D.H.; Lee, S. Extraordinarily high hydrogen-evolution-reaction activity of corrugated graphene nanosheets derived from biomass rice husks. Int. J. Hydrogen Energy 2022, 47, 40317–40326. [Google Scholar] [CrossRef]
- Prabu, N.; Saravanan, R.S.A.; Kesavan, T.; Maduraiveeran, G.; Sasidharan, M. An efficient palm waste derived hierarchical porous carbon for electrocatalytic hydrogen evolution reaction. Carbon 2019, 152, 188–197. [Google Scholar] [CrossRef]
- Pandey, K.; Jeong, H.K. Coffee waste-derived porous carbon for hydrogen and oxygen evolution reaction. Chem. Phys. Impact 2023, 6, 100175. [Google Scholar] [CrossRef]
- Thirumal, V.; Yuvakkumar, R.; Saravanakumar, B.; Ravi, G.; Isacfranklin, M.; Shobana, M.; Al-Sehemi, A.G.; Velauthapillai, D. Carbonization and optimization of biomass waste for HER application. Fuel 2022, 324, 124466. [Google Scholar] [CrossRef]
- Fu, H.; Chen, L.; Gao, H.; Yu, X.; Hou, J.; Wang, G.; Yu, F.; Li, H.; Fan, C.; Shi, Y.; et al. Walnut shell-derived hierarchical porous carbon with high performances for electrocatalytic hydrogen evolution and symmetry supercapacitors. Int. J. Hydrogen Energy 2020, 45, 443–451. [Google Scholar] [CrossRef]
- Saravanan, K.R.A.; Prabu, N.; Sasidharan, M.; Maduraiveeran, G. Nitrogen-self doped activated carbon nanosheets derived from peanut shells for enhanced hydrogen evolution reaction. Appl. Surf. Sci. 2019, 489, 725–733. [Google Scholar] [CrossRef]
- Zhu, G.; Ma, L.; Lv, H.; Hu, Y.; Chen, T.; Chen, R.; Liang, J.; Wang, X.; Wang, Y.; Yan, C.; et al. Pine needle-derived microporous nitrogen-doped carbon frameworks exhibit high performances in electrocatalytic hydrogen evolution reaction and supercapacitors. Nanoscale 2017, 9, 1237–1243. [Google Scholar] [CrossRef]
- Sekar, S.; Sim, D.H.; Lee, S. Excellent Electrocatalytic Hydrogen Evolution Reaction Performances of Partially Graphitized Activated-Carbon Nanobundles Derived from Biomass Human Hair Wastes. Nanomaterials 2022, 12, 531. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Leng, Y.; Zhou, W.; Huang, J.; Zhao, M.; Zhan, J.; Feng, C.; Tang, Z.; Chen, S.; Liu, H. Sulfur and nitrogen self-doped carbon nanosheets derived from peanut root nodules as high-efficiency non-metal electrocatalyst for hydrogen evolution reaction. Nano Energy 2015, 16, 357–366. [Google Scholar] [CrossRef]
- Nikitin, D.S.; Shanenkov, I.I.; Yeletsky, P.M.; Nassyrbayev, A.; Tabakaev, R.B.; Shanenkova, Y.L.; Ryskulov, D.N.; Tsimmerman, A.I.; Sivkov, A.A. Agricultural waste derived silicon carbide composite nanopowders as efficient coelectrocatalysts for water splitting. J. Clean. Prod. 2024, 442, 140890. [Google Scholar] [CrossRef]
- Yang, Z.; Yang, R.; Dong, G.; Xiang, M.; Hui, J.; Ou, J.; Qin, H. Biochar Nanocomposite Derived from Watermelon Peels for Electrocatalytic Hydrogen Production. ACS Omega 2021, 6, 2066–2073. [Google Scholar] [CrossRef] [PubMed]
- Min, S.; Duan, Y.; Li, Y.; Wang, F. Biomass-derived self-supported porous carbon membrane embedded with Co nanoparticles as an advanced electrocatalyst for efficient and robust hydrogen evolution reaction. Renew. Energy 2020, 155, 447–455. [Google Scholar] [CrossRef]
- Lu, S.; Hummel, M.; Gu, Z.; Gu, Y.; Cen, Z.; Wei, L.; Zhou, Y.; Zhang, C.; Yang, C. Trash to treasure: A novel chemical route to synthesis of NiO/C for hydrogen production. Int. J. Hydrogen Energy 2019, 44, 16144–16153. [Google Scholar] [CrossRef]
- Chai, D.-F.; Han, Y.; Zhang, W.; Dong, G.; Zhang, Z.; Bai, L.; Guo, D. Ni nanoparticles assembled on the surface of biomass-derived porous carbon as competitive candidates for the hydrogen evolution reaction. CrystEngComm 2023, 25, 2298–2306. [Google Scholar] [CrossRef]
- Zhou, B.; Zhang, M.; He, W.; Wang, H.; Jian, M.; Zhang, Y. Blue rose-inspired approach towards highly graphitic carbons for efficient electrocatalytic water splitting. Carbon 2019, 150, 21–26. [Google Scholar] [CrossRef]
- Fakayode, O.A.; Yusuf, B.A.; Zhou, C.; Xu, Y.; Ji, Q.; Xie, J.; Ma, H. Simplistic two-step fabrication of porous carbon-based biomass-derived electrocatalyst for efficient hydrogen evolution reaction. Energy Convers. Manag. 2021, 227, 113628. [Google Scholar] [CrossRef]
- Humagain, G.; MacDougal, K.; MacInnis, J.; Lowe, J.M.; Coridan, R.H.; MacQuarrie, S.; Dasog, M. Highly Efficient, Biochar-Derived Molybdenum Carbide Hydrogen Evolution Electrocatalyst. Adv. Energy Mater. 2018, 8, 1801461. [Google Scholar] [CrossRef]
- Mir, R.A.; Pandey, O.P. Waste plastic derived carbon supported Mo2C composite catalysts for hydrogen production and energy storage applications. J. Clean. Prod. 2019, 218, 644–655. [Google Scholar] [CrossRef]
- Sekar, S.; Aqueel Ahmed, A.T.; Pawar, S.M.; Lee, Y.; Im, H.; Kim, D.Y.; Lee, S. Enhanced water splitting performance of biomass activated carbon-anchored WO3 nanoflakes. Appl. Surf. Sci. 2020, 508, 145127. [Google Scholar] [CrossRef]
- Mulyadi, A.; Zhang, Z.; Dutzer, M.; Liu, W.; Deng, Y. Facile approach for synthesis of doped carbon electrocatalyst from cellulose nanofibrils toward high-performance metal-free oxygen reduction and hydrogen evolution. Nano Energy 2017, 32, 336–346. [Google Scholar] [CrossRef]
- Deng, L.; Zhang, Y.; Wang, Y.; Yuan, H.; Chen, Y.; Wu, Y. In situ N-, P- and Ca-codoped biochar derived from animal bones to boost the electrocatalytic hydrogen evolution reaction. Resour. Conserv. Recycl. 2021, 170, 105568. [Google Scholar] [CrossRef]
- Yang, Z.; Yan, C.; Xiang, M.; Shi, Y.; Ding, M.; Hui, J. Biomass carbon dual-doped with iron and nitrogen for high-performance electrocatalyst in water splitting. Int. J. Energy Res. 2021, 45, 8474–8483. [Google Scholar] [CrossRef]
- Wang, J.; Gao, Y.; You, T.L.; Ciucci, F. Bimetal-decorated nanocarbon as a superior electrocatalyst for overall water splitting. J. Power Sources 2018, 401, 312–321. [Google Scholar] [CrossRef]
- Abdolahi, B.; Gholivand, M.B.; Shamsipur, M.; Amiri, M. Engineering of nickel-cobalt oxide nanostructures based on biomass material for high performance supercapacitor and catalytic water splitting. Int. J. Energy Res. 2021, 45, 12879–12897. [Google Scholar] [CrossRef]
- Jiang, E.; Song, N.; Hong, S.; She, C.; Li, C.; Fang, L.; Dong, H. Zn, S, N self-doped carbon material derived from waste tires for electrocatalytic hydrogen evolution. Int. J. Hydrogen Energy 2022, 47, 16544–16551. [Google Scholar] [CrossRef]
- Sun, H.; Xue, L.; Shi, Y.; Dong, J.; Wu, Q.; Yao, W. Waste paper derived Co, N co-doped carbon as an efficient electro-catalyst for hydrogen evolution. React. Kinet. Catal. Lett. 2021, 132, 1137–1150. [Google Scholar] [CrossRef]
- Zhao, P.; Zhao, Y.; Liang, H.; Song, X.; Yu, B.; Liu, F.; Ragauskas, A.J.; Wang, C. Novel waste-derived non-noble metal catalysts to accelerate acidic and alkaline hydrogen evolution reaction. Chem. Eng. J. 2023, 466, 143140. [Google Scholar] [CrossRef]
- Zheng, A.; Wang, Y.; Zhang, F.; He, C.; Zhu, S.; Zhao, N. Data-driven design and controllable synthesis of Pt/carbon electrocatalysts for H2 evolution. iScience 2021, 24, 103430. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Liu, Q.; Lu, B.; He, T.; Nichols, F.; Hu, X.; Huang, T.; Huang, G.; Guzman, L.; Ping, Y.; et al. Organically Capped Iridium Nanoparticles as High-Performance Bifunctional Electrocatalysts for Full Water Splitting in Both Acidic and Alkaline Media: Impacts of Metal–Ligand Interfacial Interactions. ACS Catal. 2021, 11, 1179–1188. [Google Scholar] [CrossRef]
| Parameter | Hydrogen | Methanol | Ammonia | Gasoline | Methane | Diesel |
|---|---|---|---|---|---|---|
| Higher heating value (MJ/kg) | 142 | 22.7 | 22.5 | 48.29 | 55 | 44.8 |
| Lower heating value (MJ/kg) | 119.9 | 18.0 | 18.8 | 43.9 | 50 | 42.5 |
| Density at STP (kg/m3) | 0.089 | 790 | 0.730 | 730–780 | 0.720 | 830.0 |
| Auto-ignition temperature (K) | 853 | 733 | 931 | 623 | 813 | 523 |
| Flammability limits in air (%) | 4–76 | 6.7–36 | 15–28 | 1–7.6 | 5.3–15 | 0.6–5.5 |
| Flame temperature (K) | 2480 | 2143 | 1850 | 2580 | 2187 | 2600 |
| Catalyst | Preparation Method | Process Parameters | Substrate Conversion | H2:CO Ratio | Reference |
|---|---|---|---|---|---|
| Non-waste 12% Ni-Al2O3 | Wet impregnation Calcination | 850 °C CH4:CO2 = 1 10 h | 94% | 1.2 | [84] |
| NiO | Acid/Base treatment Precipitation Calcination | 780 °C CH4:CO2 = 1 24 h | 100% | Slightly over 1 | [79] |
| 20% Ni/MgO-FA | Alkali treatment Sol-gel synthesis | 750 °C CH4:CO2 = 1 9 h | 75–80% | Slightly below 1 | [80] |
| 10% Ni-CFA | Alkali/acid treatment Wet impregnation Calcination | 850 °C CH4:CO2 = 1 1 h | 96% | Slightly below 1 | [81] |
| 10% Ni-SiO2 | Wet impregnation Calcination | 800 °C CH4:CO2 = 1 1 h | 92.3% | 0.95 | [82] |
| 13% Ni-SS oxide | Ni doping Calcination | 850 °C CH4:CO2 = 3 24 h | 95% | 1.62 | [83] |
| Process | Catalyst | Preparation Method | Process Parameters | Substrate Conversion | H2:CO Ratio | Reference |
|---|---|---|---|---|---|---|
| SMR | Non-waste NiO/γ-Al2O3 | Wet impregnation Calcination | 700 °C S/C = 4 | 78% | 6.8 | [92] |
| Sorption-enhanced SMR | 10% CaO-Mg/Ni/Al | Co-precipitation Wet impregnation Calcination | 650 °C S/C = 2 | 77% | / | [90] |
| SMR | 5% Co-AC | Wet impregnation Calcination | 750 °C S/C = 2 | 97.7% | 2.7 | [91] |
| POM | Non-waste 5% Ni/Al2O3 | Wet impregnation Calcination | 780 °C CH4:O2 = 2 | 95.7% | 2 | [97] |
| POM | 5% Ni/La2O3-FA | Wet impregnation Calcination | 850 °C CH4:O2 = 2 | 85% | 2 | [96] |
| MP | Non-waste FeCo/CeZrO2 | Wet impregnation Calcination | 700 °C | 90% | / | [106] |
| MP | 10% Co/CeO2-FA | Hydrothermal processes Wet impregnation Calcination | 850 °C | 76% | / | [105] |
| Catalyst | Preparation Method | Process Parameters | Hydrogen Yield | H2:CO Ratio | Reference |
|---|---|---|---|---|---|
| Non-waste K2CO3 + CaO | Mechanical mixing | 700 °C | ≈1.53–1.58 mol H2/mol C | Not reported | [113] |
| 20% WE CaO | Calcination | 900 °C | 1.24 mol H2/mol C | 3.65 | [110] |
| 5% WE CaO + 15% K2CO3 | Calcination | 800 °C | 1.34 mol H2/mol C | 4.19 | [111] |
| CaO-rich catalyst from demolition waste | Calcination | 900 °C | 1.64 mol H2/mol C | 4.25 | [112] |
| Catalyst | Preparation Method | Substrate | Process Parameters | Hydrogen Yield | H2:CO Ratio | Reference |
|---|---|---|---|---|---|---|
| Non-waste NiMo/Al2O3 | / | Pine chips | 450 °C 40 min | 33.6 g/kgbiomass (374 mL/gbiomss) | 1.44 | [118] |
| Na2ZrO3 | Calcination | Spirulina algae | 900 °C 40 °C/min | 205 mL/gbiomass | 1.6 | [114] |
| 20% NiO-FA | Homogeneous precipitation Calcination | Rice straw | 600 °C 20 min | 41 vol% | 1.2 | [115] |
| 10% BFA | Calcination | Sawdust | 700 °C 20 min | 43 mL/gbiomass | 0.2 | [116] |
| 10% sFCC | Calcination | Sawdust | 700 °C 20 min | 40 mL/gbiomass | 0.2 | [116] |
| 40% RM | Direct use | Corn stover | 800 °C 1 h | 107.7 mL/gbiomass | 0.63 | [117] |
| Catalyst | Preparation Method | Substrate | Process Parameters | Hydrogen Yield | H2:CO Ratio | Reference |
|---|---|---|---|---|---|---|
| Non-waste Ni/Al2O3 | / | Pine wood sawdust | Pyrolysis: 500 °C Reforming: 600 °C | 117 g/kgbiomass (1300 mL/gbiomass) | / | [121] |
| 10% Ni-WTA | Ashing Ni impregnation | Waste wood pellets | Pyrolysis: 600 °C Reforming: 800 °C | 10.5 mmol/gbiomass (235.35 mL/gbiomass) | 1 | [122] |
| WTA | Oxidation | High Density Polyethylene | Pyrolysis: 600 °C Reforming:1000 °C | 83.2 mmol/gplastic (1865.89 mL/gbiomass) | 1.4 | [123] |
| 20% Ni/La-FA | Impregnation Calcination | Cellulose | Pyrolysis: 500 °C Reforming: 700 °C | 15 mmol/gbiomass (336.21 mL/gbiomass) | 1.43 | [124] |
| 20% Ni/La-FA | Impregnation Calcination | Pine Pulp | Pyrolysis: 500 °C Reforming: 700 °C | 10.8 mmol/gbiomass (242.07 mL/gbiomass) | 1.24 | [124] |
| Ni-SS | Impregnation Calcination | Pine sawdust volatiles | Reforming: 800 °C | 386.5 mL/gbiomass | / | [125] |
| SS | Calcination | Pine sawdust tar | Reforming: 800 °C | 91.3 mL/gbiomass | 0.34 | [126] |
| 10% Ni-SS | Impregnation Calcination | Pine sawdust tar | Reforming: 800 °C | 86 mL/gbiomass | 0.39 | [127] |
| Catalyst | Preparation Method | Substrate | Temperature | Hydrogen Yield | H2:CO Ratio | Reference |
|---|---|---|---|---|---|---|
| Non-waste Ni/CeO2/Al2O3 | Impregnation Calcination | Wood residue | 823 °C | 0.706 Nm3/kgbiomass (706 mL/gbiomass) | 1.84 | [139] |
| WE | Calcination | Spirulina platensis | 800 °C | 252 mL/gbiomass | 1.21 | [132] |
| WE | Calcination | Chlorella vulgaris | 800 °C | 344 mL/gbiomass | 2.28 | [132] |
| 20% Ni-SS | Impregnation Calcination | Sewage sludge | 900 °C | 15.7 mmol/gbiomass (351.1 mL/gbiomass) | 2.05 | [133] |
| 10% Ni-RM | Impregnation Calcination | Bamboo sawdust | 800 °C | 135 mmol/gbiomass (3025.9 mL/gbiomass) | 7.82 | [134] |
| 30% RM | Calcination | Acacia pruning | 850 °C | 1.5 m3/kgbiomass (1500 mL/gbiomass) | 13.9 | [135] |
| 20% RM | Calcination | Helianthus residues | 850 °C | 2.35 m3/kgbiomass (2350 mL/gbiomass) | 9.5 | [135] |
| 50% WMP | Calcination | MSW | 900 °C | 0.55 Nm3/kgbiomass (550 mL/gbiomass) | 2.3 | [136] |
| 10% WMP | Calcination | MSW | 700 °C | 0.12 Nm3/kgbiomass (130 mL/gbiomass) | 0.66 | [137] |
| 40% CaO (WE) | Calcination | MSW | 950 °C | 19.4 mmol/gbiomass (435.83 mL/gbiomass) | 2.5 | [138] |
| Waste Material | Catalyst | Overpotential (mV at 10 mA/cm2) | Tafel Slope (mV/dec) | Stability | Reference |
|---|---|---|---|---|---|
| Rice husk | Graphene nanosheets | 9 | 31 | 10 h | [144] |
| Palm spathe Pollen waste | Porous carbon nanosheets | 330 | 63 | 10 h | [145] |
| Coffe waste | Porous carbon | 210 | 120 | 24 h | [146] |
| Tamarind shells | AC | 221 | 204 | 51.7% after 2 h | [147] |
| Walnut shells | Porous carbon nanosheets | 170 | 69.8 | 15 h | [148] |
| Waste Material | Catalyst | Overpotential (mV at 10 mA/cm2) | Tafel Slope (mV/dec) | Stability | Reference |
|---|---|---|---|---|---|
| Peanut shells | N-doped carbon | 80 (onset) | 75.7 | 10 h | [149] |
| Pine needles | N-doped carbon | 62 | 45.9 | 100 h 1000 cycles | [150] |
| Human hair | N-doped nanobundles | 16 | 51 | / | [151] |
| Peanut root nodules | S, N-doped carbon | 116 | 67.8 | 12 h 1000 cycles | [152] |
| Waste Material | Catalyst | Overpotential (mV at 10 mA/cm2) | Tafel Slope (mV/dec) | Stability | Reference |
|---|---|---|---|---|---|
| Rice and oat husks | 5% Pt-SiC | 22–24 | 34–60 | 1500 cycles | [153] |
| Watermelon peels | CoO-porous carbon | 111 | 93.9 | 20 h | [154] |
| Pomelo peel | Co-carbon | 154 | 106.4 | 12 h 2000 cycles | [155] |
| Eggshell membranes | NiO-carbon | 565 | 77.8 | 500 cycles | [156] |
| leaves | Ni-carbon | 32 | 125.6 | 48 h 2000 cycles | [157] |
| Rose petals | Ni-carbon | 220 | 64 | 24 h | [158] |
| Watermelon rind | Mo2C-porous carbon | 133 | 25 | 300 h | [159] |
| Birch wood | Mo2C-porous carbon | 35 | 25 | 100 h | [160] |
| Waste plastic | Mo2C-carbon | 179 | 80 | 10 h 2000 cycles | [161] |
| Neem leaves | WO3-carbon | 360 | 14 | 12 h | [162] |
| Waste Material | Catalyst | Overpotential (mV at 10 mA/cm2) | Tafel Slope (mV/dec) | Stability | Reference |
|---|---|---|---|---|---|
| Softwood pulp | N, S, P-carbon nanofibers | 331 | 99 | / | [163] |
| Animal bones | N, P, Ca-biochar | 162 | 80 | 2000 cycles | [164] |
| Amaranth | Fe3O4, N-carbon | 92 | 95.8 | 10 h | [165] |
| Alfalfa | NiFe-N, P, S-nanocarbon | 250 | 84 | 50 h 1000 cycles | [166] |
| Chicken feathers | NiCoO-S, N-porous carbon | 87 | 50 | 20 h | [167] |
| Waste tires | Zn, S, N-carbon | 50 | 78 | 110 h | [168] |
| Office paper | Co, N-carbon | 226 | 91 | 14 h 3000 cycles | [169] |
| Cotton textiles | P, CoNiO2, N-carbon | 247.6 | 120.8 | 50 h | [170] |
| Catalyst Type | Catalyst | Overpotential (mV at 10 mA/cm2) | Tafel Slope (mV/dec) | Stability | Reference |
|---|---|---|---|---|---|
| Commercial | Pt/C | 29 | 46 | 10 h | [171] |
| / | Ir/C | 28 | 55 | 8 h | [172] |
| Pure carbon | Rice husk-based Graphene nanosheets | 9 | 31 | 10 h | [144] |
| Self-doped | Pine needle-based N-doped carbon | 62 | 45.9 | 100 h 1000 cycles | [150] |
| / | Human hair-based N-doped nanobundles | 16 | 51 | / | [151] |
| Metal-doped | Rice and oat husk-based 5% Pt-SiC | 22–24 | 34–60 | 1500 cycles | [153] |
| / | Birch wood-based Mo2C-porous carbon | 35 | 25 | 100 h | [160] |
| Multi-doped | Chicken feather-based NiCoO-S, N-porous carbon | 87 | 50 | 20 h | [167] |
| / | Waste tire-based Zn, S, N-carbon | 50 | 78 | 110 h | [168] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hren, D.T.; Nemet, A.; Urbancl, D. From Waste to Hydrogen: Utilizing Waste as Feedstock or Catalysts for Hydrogen Generation. Clean Technol. 2025, 7, 76. https://doi.org/10.3390/cleantechnol7030076
Hren DT, Nemet A, Urbancl D. From Waste to Hydrogen: Utilizing Waste as Feedstock or Catalysts for Hydrogen Generation. Clean Technologies. 2025; 7(3):76. https://doi.org/10.3390/cleantechnol7030076
Chicago/Turabian StyleHren, David Tian, Andreja Nemet, and Danijela Urbancl. 2025. "From Waste to Hydrogen: Utilizing Waste as Feedstock or Catalysts for Hydrogen Generation" Clean Technologies 7, no. 3: 76. https://doi.org/10.3390/cleantechnol7030076
APA StyleHren, D. T., Nemet, A., & Urbancl, D. (2025). From Waste to Hydrogen: Utilizing Waste as Feedstock or Catalysts for Hydrogen Generation. Clean Technologies, 7(3), 76. https://doi.org/10.3390/cleantechnol7030076





