An Overview of Biodiesel Production via Heterogeneous Catalysts: Synthesis, Current Advances, and Challenges
Abstract
1. Introduction
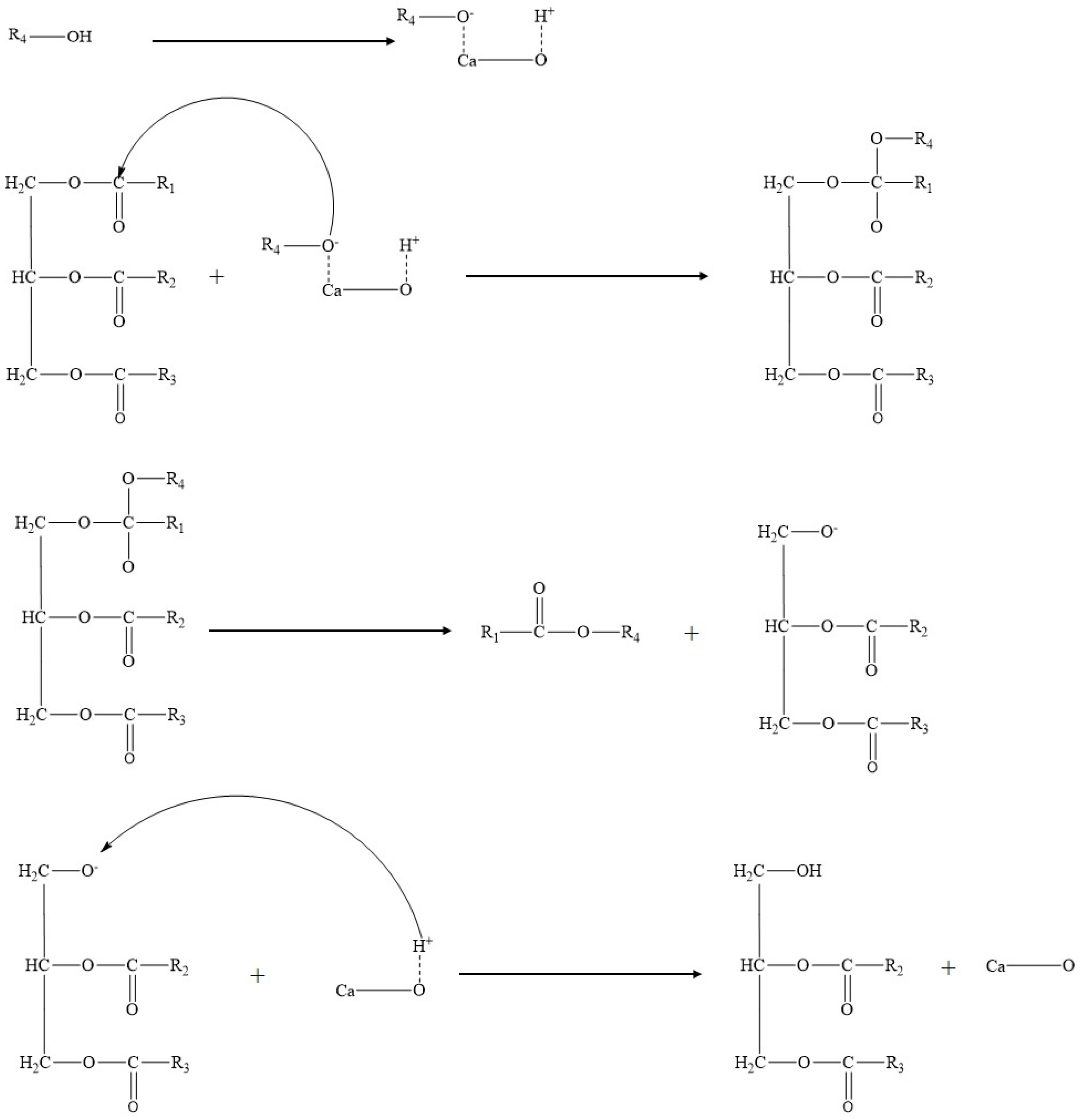
2. Homogeneous Catalysts
2.1. Homogeneous Acid Catalysts
2.2. Homogeneous Base Catalysts
3. Heterogeneous Catalysts
3.1. General Properties of Heterogenous Catalysts
3.2. Heterogeneous Acid Catalysts
3.2.1. Heteropoly Acid Catalysts (HPA)
3.2.2. Supported Acid Catalyst on the Basis of Support
Aluminum-Supported Acid Catalysts
Silicate-Supported Acid Catalysts
Zinc-Oxide-Supported Acid Catalysts
Zirconium-Oxide-Supported Acid Catalysts
3.2.3. Sulfated Waste Catalysts
3.2.4. Ion Exchange Resin
3.2.5. Mixed Metal Oxides
| Raw Material | Reaction | Catalyst | Preparation Methods | Reaction Parameters | Biodiesel (Y = Yield, C = Conversion) | Reuse | Reference | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Surface Area (m2/g) | Volume (cm3/g) | Pore Size (nm) | Acidity/Basicity (mmol/g) | T° | Time | Ratio of Alcohol:Oil | Catalyst wt% | ||||||
| Heteropoly Acids | |||||||||||||
| Oleic acid | Esterification with methanol | TPA/MCM-41 | Impregnation Tcalcination MCM-41= 555 °C for 5 h Tcalcination SBA-15= 500 °C for 6 h | 40 °C | 2–4 h | 40:1 | 0.1 g | TPA/MCM-41 C = 100% TPA/SBA-15 C = 98% | 4 | [77] | |||
| 360 | 0.5 | 3.01 | 1.41 acidity | ||||||||||
| TPA/SBA-15 | |||||||||||||
| 714 | 1.12 | 6.20 | 1.82 acidity | ||||||||||
| Water, cooking oil | Transesterification with ethanol | 12-tungstophosphoric acid (TPA) supported on MCM-48 | Impregnation Calcination at 550 °C for 6 h | 60 °C | 8 h | - | 0.1 g | C = 95% | 4 | [78] | |||
| 286 | 0.38 | - | 1.53 acidity | ||||||||||
| Levulinic acid (LA) | Esterification with butanol | 12-tungstophosphoric acid and zeolitic support, MCM-22 | Wet impregnation | 90 °C | 8 h | 2:1 | 1.86 | C = 68% | 6 | [35] | |||
| 258 | 0.25 | 5.31 | - | ||||||||||
| Glycerol | Esterification with methanol | 12-tungstophosphoric anchored to MCM-41 | MCM-41 calcination at 550 °C for 5 h ZrO2 incipient impregnation | 100 °C | 6 h | 6:1 | 0.15 | TPA/MCM-41 C = 87% TPA/ZrO2C = 80% | 4 | [79] | |||
| 360 | - | - | 0.08 acidity | ||||||||||
| 12-tungstophosphoric anchored to ZrO2 | |||||||||||||
| 146 | - | - | 0.08 acidity | ||||||||||
| Palmitic acid | Esterification with methanol | 12-tungstophosphoric heteropoly acid over zirconia | Sol–gel method Hydrothermal treatment | 60 °C | - | - | 30 | C = 95% | 5 | [36] | |||
| 365 | 0.33 | - | - | ||||||||||
| Oleic acid | Esterification with methanol | 12-tungstosilicicacid anchored to SBA-15 | Impregnation Calcination at 300 °C for 3 h | 60 °C | 8 h | 20:1 | 30 | Y = 89.7% | 5 | [80] | |||
| 537 | 0.594 | 5.322 | - | ||||||||||
| Spent frying oil | Esterification with methanol | TPA/bentonite | Impregnation Calcination at 500 °C for 5 h | 85 °C | 4.5 h | 10:1 | 10 | Y = 96% | 6 | [37] | |||
| - | - | - | 4.5 acidity | ||||||||||
| Wild olive oil | Transesterification with methanol | TPA/Cr–Al | Impregnation Calcination at 120 °C for 12 h | 80 °C | 5 h | 21:1 | 4 | Y = 93% | 5 | [81] | |||
| 56.168 | 0.014 | - | - | ||||||||||
| Oleic acid | Esterification with methanol | TPA@C-NiZr-MOF | Impregnation Calcination at 300 °C for 180 min | 140 °C | 4 h | 20:1 | 0.15 g | C = 91.9% | 6 | [82] | |||
| 441 | 0.3 | 2.72 | 8.82 acidity | ||||||||||
| Oleic acid | Esterification with methanol | Picolinic-acid-modified 12-tungstophosphoric acid | Impregnation Calcination at 300 °C for 180 min | 80 °C | 5 h | 10:1 | 7 | C = 100% | 4 | [83] | |||
| 27.6 | - | - | - | ||||||||||
| One-pot fungal biomass | Esterification and transesterification with ethanol | H3PMo/Al2O3 | Impregnation | 200 °C | 6 h | 120:1 | 15 | C = 96.6% | N/A | [84] | |||
| - | - | - | - | ||||||||||
| Molasses, vinasse | Esterification and transesterification with ethanol | H3PMo/Al2O3 | Impregnation | 200 °C | 6 h | 120:1 | - | C > 95% | N/A | [85] | |||
| - | - | - | - | ||||||||||
| Oleic acid, rapeseed oil | Esterification with methanol | H3PMo12O40@EB-COF | Impregnation | 70 °C | 8 h | 15:1 | 10 | C = 85% | 6 | [86] | |||
| 24.7 | 0.0698 | - | - | ||||||||||
| Soybean oil | Transesterification with methanol | H6PV3MoW8O40/AC-Ag | Impregnation Calcination at 300 °C for 3 h | 140 °C | 10 h | 30:1 | 8 | C = 91.3% | 5 | [33] | |||
| 309.8 | 0.23 | 2.98 | - | ||||||||||
| Supported Acid Catalyst on the Basis of Support | |||||||||||||
| Palm oil | Esterification and transesterification with methanol | Al-SBA-15 (stable silica) | Sol–gel method Calcination at 550 °C for 6 h | 215 °C | 4 h | 30:1 | 15 | Y = 40–87% | N/A | [87] | |||
| 844–938 | 1.284–1.292 | 5.5–6.1 | - | ||||||||||
| Jatropha oil | Esterification and transesterification with methanol | Monometallic catalysts (Ce/Al- MCM-41 and Zr/Al-MCM-41) and bimetallic catalyst (Ce-Zr/Al-MCM-41) | Impregnation/co-impregnation Calcination at 500 °C for 5 h | 90 °C | 4 h | 6:1 | 5 | C = 93% | N/A | [88] | |||
| 261 | 0.2 | 4.3 | - | ||||||||||
| Soybean oil | Transesterification with methanol | SBA-15 silica | Calcination 823 K for 6 h | 70 °C | 12 h | 15:1 | 5 | C = 91.7% | 5 | [89] | |||
| 325 | 0.52 | 5.56 | 1.91–2.95 | ||||||||||
| 2-aryl benzimidazoles and benzothiazoles | Transesterification with ethanol | SBA-15-SO3H | Hydrothermal treatment at 100 °C for 48 h Calcination at 450 °C for 8 h | Room | 24 h | 1:1 | 0.05 | C = 100% Y = 70–85% | 5 | [90] | |||
| 568 | 0.23 | 2.04 | - | ||||||||||
| Waste cooking oil | Esterification with methanol | S–TiO2/SBA-15 | Impregnation Calcination at 540 °C | 200 °C | 30 min | 15:1 | 1 | Y = 94.96% | 3 | [91] | |||
| 733.98 | 0.09 | 5.10 | 0.1 acidity | ||||||||||
| Municipal sewage sludge | Transesterification and esterification with methanol | Zr-SBA-15 | Hydrothermal process at 130 °C for 24 h Calcination at 450 °C for 5 h | 209 °C | 3 h | 0.25 g/mL | 15.5 | Y > 90% | N/A | [92] | |||
| 531 | 1.41 | 12.8 | - | ||||||||||
| Oleic acid | Esterification with methanol | Zr-SBA-15 | Zr-SBA-15 impregnation Calcination at 600 °C for 6 h SO42−/Zr-SBA-15 Hotplate at approximately 65 °C under constant stirring Oven drying at 110 °C for 15 h | 65 °C | 8 h | 20:1 | 5 | Y = 80.7% | N/A | [44] | |||
| 675.6 | 0.84 | 6.37 | 1.44 acidity | ||||||||||
| SO42−/Zr-SBA-15 | |||||||||||||
| 525.3 | 0.80 | 6.37 | 1.97 acidity | ||||||||||
| Acidified oil | Esterification with methanol | [Zr(SO4)2.H2O]/tubular carbon membranes (ZS/TCM) | Impregnation | 60 °C | 30 min | 6:1 | - | C = 99.9% | 5 | [45] | |||
| 109 | 0.21 | 7.12 | - | ||||||||||
| Neem oil | Esterification and transesterification with methanol | Sulfated zirconia | Calcination at 600 °C for 5 h - | 65 °C - | 2 h - | 9:1 | 1 | C = 95% | N/A | [93] | |||
| - | |||||||||||||
| Cerbera odollam (sea mango) oil | Transesterification with methanol | Sulfated zirconia alumina and montmorillonite KSF | Calcination at 400 °C for 2.5 h | 64.7 °C | 1 h | 6:1 | 0.1 | Y = 83.8% | N/A | [94] | |||
| - | - | - | - | ||||||||||
| Fatty acid | Esterification with methanol | Sulfated lanthanum oxide (SLO) SLO/HZSM-5 | Impregnation Calcination at 400 °C for 3 h | 100 °C | 4 h | 20:1 | 10 | SLO/HZSM-5 C = 100% | N/A | [95] | |||
| 217 | 0.1 | 2.5 | - | ||||||||||
| Waste cooking oil | Esterification with methanol and glycerol | Lanthanum-supported sulfated zirconia | Calcination at 600 °C | 60/150 °C | 3 h | 10:1 | 3 | C > 95% | N/A | [96] | |||
| 98 | 0.09 | - | |||||||||||
| Palm fatty acid distillate | Transesterification with methanol | SO3H-GO@TiO2 | Microwave-assisted hydrothermal acid treatment | 70 °C | 40 min | 9:1 | 3 | Y = 96.73% | 10 | [97] | |||
| 611 | 0.16 | 7.25 | 3.30 acidity | ||||||||||
| Mixed Oxides | |||||||||||||
| Oleic acid | Esterification with methanol | Zirconia–alumina nanocatalyst | Impregnation Calcination at 550 °C for 4 h | 90 °C | 4 h | 9:1 | 3 | C = 91.6% | 7 | [98] | |||
| 14.35 | 0.0017 | 4.9 | - | ||||||||||
| Oleic acid | Esterification with butanol and ethanol | Fe(III)-based MOF (MIL-53) | Prepared with composites Ultrasound | Room T° | 15 min | 16:1 | 30 | C = 96% (ethanol) C = 98% (butanol) | 5 | [99] | |||
| 569 | 127 | - | - | ||||||||||
| Oleic acid | Esterification with ethanol | Fe2(MoO4)3 | Sol–gel method Calcination at 500 °C for 2 h | 70 °C | - | 9:1 | 3 | C = 92.50% | 6 | [66] | |||
| - | - | - | - | ||||||||||
| Soybean oil | Transesterification and esterification with methanol | Fe3O4/SiO2 | Hydrothermal method Sol–gel method | 120 °C | 6 h | 35:1 | 9 | C = 93.3% | 5 | [12] | |||
| 58.99 | 0.14 | 9.1 | 1.89 acidity | ||||||||||
| Oleic acid | Esterification with ethanol | Fe3O4@SiO2 | N/A | 90 °C | 4 h | 11.5:1 | 9.5 | C = 92.10% Y = 92.1% | 6 | [67] | |||
| - | - | - | - | ||||||||||
| Fatty acids, non-food oil | Transesterification with methanol | Fe3O4@SiO2-SO3H | Sol–gel method Calcination at 1000 °C for 5 h | 65 °C | 4 h | 20:1 | 4 | Y = 97.8% | 5 | [68] | |||
| 4.5 | - | 8.9 | 0.0444 basicity 0.020 acidity | ||||||||||
| Jatropha curcas oil | Esterification and transesterification with methanol | Fe3O4@SiO2-SO3H core | Co-precipitation Coating Functionalization | 80 °C | 3.5 h | 9:1 | 8 | Y = 98% C = 98.7% | 10 | [100] | |||
| 32.88 | 0.07 | 3.48 | 0.76 acidity | ||||||||||
| Palm oil | Esterification with methanol | Ferric alginate | N/A | 60 °C | 3 h | 16:1 | 2 | C = 98% | N/A | [101] | |||
| - | - | - | - | ||||||||||
| Palm oil | Esterification and transesterification with methanol | Ferric hydrogen | N/A | 205 °C | 4 h | 15:1 | 1 | Y = 94.5% | 5 | [102] | |||
| 4.9 | - | 34.4 | 10.5 acidity | ||||||||||
| Mesua ferrea oil | Esterification and transesterification with methanol | Co-doped ZnO | N/A | 60 °C | 3 h | 9:1 | 2.5 | C= 98.03% | 4 | [103] | |||
| - | - | - | 0.91 | ||||||||||
| Oleic acid | Esterification with methanol | CoFe2O4-SL5 | Co-precipitation | 100 °C | 6 h | 10:1 | 5 | C = 79.5% C = 78.5% | N/A | [70] | |||
| 41.47 | 0.129 | 9.25 | - | ||||||||||
| CoFe2O4-SL7.5 | |||||||||||||
| 34.41 | 0.1128 | 9.39 | 1.8 acidity | ||||||||||
| Palm fatty acid distillate | Esterification with methanol | MnO-NiO-SO42-/ZrO2 | Wet impregnation Calcination at 600 °C for 3 h | 70 °C | 3 h | 15:1 | 3 | C = 97% | 5 | [72] | |||
| 15 | 0.13 | - | 2.7572 acidity | ||||||||||
| Water, cooking oil | Esterification and transesterification with methanol | Sr/ZrO2, Mg/ZrO2, Ca/ZrO2, and Ba/ZrO2 | Wet impregnation Calcination at 650 °C for 5 h | 115.5 °C | 169 min | 29:1 | 2.7 | Y = 79.70% | N/A | [104] | |||
| 12.97 | 0.083 | 25.51 | 1.067 acidity | ||||||||||
| Soybean oil, corn oil | Transesterification and esterification with methanol | ZnO–SiO2/ZrO2 | Sol–gel method | 190 °C | 1.15 h | 9.4:1 | - | C > 90% Y > 99% using two-stage packed bed reactor | N/A | [105] | |||
| 15 | - | - | - | ||||||||||
| ZnO–TiO2–Nd2O3/ZrO2 | |||||||||||||
| 15 | - | - | - | ||||||||||
| ZnO–SiO2–Yb2O3/ZrO2 | |||||||||||||
| 15 | - | - | - | ||||||||||
| ZnO–Yb2O3/ZrO2 | |||||||||||||
| 15 | - | - | - | ||||||||||
| N/A | Esterification with glycerol | Zr-modified hierarchical mordenite | Incipient wetness impregnation Calcination at 450 °C | 100 °C | 3 h | 10:1 | 0.1 g | Glycerol C = 90.6% | 3 | [46] | |||
| 289 | 0.18 | 1.2 | - | ||||||||||
| Brown grease | Esterification with methanol | ZrO2 | Impregnation | 200 °C | 2 h | 5.2:1 | 0.8 g | C = 78% | N/A | [106] | |||
| - | - | - | - | ||||||||||
| Palmitic acid | Esterification with methanol | ZrO2-TiO2 | Wet chemical deposition precipitation method Ultrasound Calcination at 400 °C for 3 h | 100 °C | 5 h | 20:1 | 5 | Y > 85% | 5 | [107] | |||
| 32.47 | - | - | 1.9 acidity | ||||||||||
| Palm oil | Transesterification with methanol | TiO2–ZnO | Calcination at 400 °C for 3 h | 60 °C | 5 h | 6:1 | 200 mg | C = 98% Y = 92% | N/A | [108] | |||
| - | - | - | - | ||||||||||
| Waste soybean oil | Esterification and transesterification with methanol | Titania–silica (S_TSC) | Impregnation Calcination at 450–800 °C for 2 h | 120 °C | 3 h | 20:1 | 10 | C = 93.8% | 3 | [109] | |||
| 381 | 0.27 | 120 °C | 120 °C | ||||||||||
| Low-grade palm oil | Esterification with methanol | Titanium oxysulphate sulphuric acid 5-sulfosalicylic acid dihydrate | N/A | 60 °C | 1 h | 8:1 | 1.5 | Y = 90% | N/A | [47] | |||
| - | - | - | - | ||||||||||
| Jatropha curcas, crude oil | Esterification with methanol | Mesoporous Ti–Mo Bi metal oxide | Calcination at 600 °C for 5 h | 180 °C | 2 h | 20:1 | 3 | Y = 95% C = 87.8% | 5 | [110] | |||
| 33 | 0.24 | 29 | 0.78 acidity | ||||||||||
| Sulphated Waste | |||||||||||||
| Waste cooking oil | Esterification and transesterification with methanol | Sulfonating pyrolyzed rice husk with concentrated sulfuric acid (RHC) | N/A | 110 °C | 15 h | 20:1 | 5 | Y = 87.57% C = 98.17% | N/A | [53] | |||
| 4 | - | 7.7 | - | ||||||||||
| Malaysian palm fatty acid distillate | Esterification and transesterification with methanol | Rice husk bioderived silica-supported Cu2S-FeS | Co-precipitation Calcination at 600 °C for 6 h | 70 °C | 3 h | 15:1 | 2 | C = 98% | 5 | [76] | |||
| 40 | 0.57 | 46 | 4.13329 acidity | ||||||||||
| Palm fatty acid distillate | Esterification with methanol | Sulphonated multi-walled carbon nanotubes (s-MWCNTs) | Purification Sulfonation | 170 °C | 2 h | 20:1 | 3 | Y = 93.5% | 5 | [111] | |||
| - | - | - | - | ||||||||||
| Palm fatty acid distillate | Esterification with methanol | Sulphonated sago pith waste | Carbonization Sulfonation | 70 °C | 90 min | 20:1 | 2.5 | Y = 99.34% C = 94.03% | N/A | [54] | |||
| 11.097 | 0.013 | 432 | - | ||||||||||
| Palmitic oil | Esterification with methanol | Sulfate (SIL-1) | Impregnation | 65 °C | 8 h | 9:1 | 15 | Y > 98% | 5 | [112] | |||
| 215.6 | 0.384 | 6.89 | - | ||||||||||
| Trifluoromethanesulfonate (SIL-2) | |||||||||||||
| 202 | 0.365 | 6.81 | - | ||||||||||
| Dihydrogen (SIL-3) | |||||||||||||
| 162.5 | 0.269 | 6.57 | - | ||||||||||
| Oleic acid | Esterification with methanol | Sulfonated-activated carbon from bamboo | Sulfonation | 85 °C | 3 h | 7:1 | 12 | C = 96% | 4 | [113] | |||
| 225 | 0.12 | - | 1.69 acidity | ||||||||||
| Palm fatty acid distillate | Esterification with methanol | Sulfonated beet pulp | Carbonization Sulfonation | 85 °C | 5 h | 5:1 | 3 g | Y = 92% C = 97.4% | N/A | [114] | |||
| 37.5 | - | - | - | ||||||||||
| Castor oil | Esterification with methanol | Sulfonated carbon | N/A | 50 °C | 1 h | 20:1 | 1 | C = 90.83% | N/A | [115] | |||
| - | - | - | - | ||||||||||
| Palm fatty acid distillate | Esterification with methanol | Chicken bone | Microwave irradiation Calcination at 900 °C for 5 h | 70 °C | 3 h | 20:1 | 5 | Chicken bone Y = 80.8% C = 98.2% Cow bone Y = 81.5% C = 97.7% | N/A | [49] | |||
| 0.3436 | 0.00374 | 5.6928 | 3.4 acidity | ||||||||||
| 0.5635 | 0.005 | 5.7253 | - | ||||||||||
| Palm fatty acid distillate | Esterification with methanol | Sulfonated glucose | Carbonization Sulfonation | 75 °C | 2 h | 10:1 | 2.5 | Y = 91.41% C = 92% | 6 | [116] | |||
| 16.94 | - | - | 25.65 acidity | ||||||||||
| Palm fatty acid distillate | Esterification with methanol | Sulfonated glucose-derived acid | Carbonization | 75 °C | 2 h | 10:1 | 2.5 | Y = 92.3% C = 95.4% | 6 | [117] | |||
| 10.67 | - | - | 4.23 acidity | ||||||||||
| Acidified oil | Esterification with methanol | Sulfonated Polyethersulfone (SPES)/Polyethersulfone (PES) | Sulfonation | 65 °C | 5 h | 13.5:1 | - | C = 97.60% | 5 | [118] | |||
| - | - | - | - | ||||||||||
| Tallow fat and canola oil | Esterification with methanol | Sulfonated polymer waste | Sulfonation | 75 °C | 2.5 h | - | - | C = 91% Y = 99% | N/A | [119] | |||
| - | - | - | - | ||||||||||
| Olive pomace oil | Esterification with methanol | Olive pomace activated carbon | Sulfonation Pyrolysis Steam activation | 60 °C | 5 h | 9:1 | 20% | Y = 97% | - | [48] | |||
| 618.18 | 0.328 | - | - | ||||||||||
| Palm oil | Esterification with methanol | Spent bleaching clay | Calcination at 600 °C Sulfonation Reflux method | 100 °C | 4 h | 10:1 | 10 | C = 93.18% | 3 | [55] | |||
| 57.3 | 0.18 | 10.14 | - | ||||||||||
| Ion Exchange Resin | |||||||||||||
| Chlorella protothecoides Scenedesmus obliquus microalga | Esterification with methanol | Amberlyst-15 | Co-precipitation Calcination at 650 °C for 5 h | 120 °C | 1 h | 5:1 | 2.5 | C > 90% | N/A | [120] | |||
| 53 | 0.46 | - | 1.60 acidity | ||||||||||
| Acrocomia aculeata (Macaúba) crude oil | Transesterification with ethanol | Amberlyst-15 | N/A | 130 °C | - | 9:1 | 16 | Y = 89.10% C = 86% | N/A | [62] | |||
| 31.3 | - | 14.3 | - | ||||||||||
| Macauba pulp oil | Transesterification with ethanol | Cation exchange resin: Purolite® CT275 | N/A | 85 °C | 9 h | 8.6:1 | 30.4 | C = 93% | 10 | [64] | |||
| 20–40 | 0.4–0.6 | 40–70 | - | ||||||||||
| Waste frying oil | Esterification with methanol | Cation exchange resin: NKC-9 | N/A | 65 °C | 3 h | 3:1 | 18 | C = 90% | 10 | [121] | |||
| 77 | - | 56 | - | ||||||||||
| Cation exchange resin: D61 | |||||||||||||
| 83.9 | - | 11.3 | - | ||||||||||
| Rapeseed oil | Transesterification with methanol | Purolite CT275DR | N/A | 140 °C | 8 h | 27.7 mol/mol | 10 | Y = 16.5–55% | N/A | [122] | |||
| 20–40 | 0.4–0.6 | 40–70 | - | ||||||||||
| Purolite CT169DR | |||||||||||||
| 35–50 | 0.3–0.5 | 25.42.5 | - | ||||||||||
| N. gaditana microalga oil | Esterification and transesterification with methanol | Putolite® CT-269 ion exchange resin | N/A | 95 °C | - | 33:1 | - | C = 81.6% | N/A | [123] | |||
| - | - | - | - | ||||||||||
| Used cooking oil | Esterification and transesterification with methanol | Purolite D5081 | N/A | 56 °C | - | 18:1 | 9 | C = 92% | N/A | [124] | |||
| 514.18 | 0.47 | 3.69 | - | ||||||||||
| Waste cooking oil | Esterification with ethanol | NKC-9 ion-exchange resin and H-beta zeolite | N/A | 80 °C | 6 h | 3:1 | 15 | C = 98.40% | 5 | [125] | |||
| - | - | - | - | ||||||||||
| Oil feedstock from waste fried oil | Esterification with methanol | NKC-9 resin | N/A | 65 °C | 500 h | 2.8:1 | - | C = 98% | N/A | [126] | |||
| - | - | - | - | ||||||||||
| Sunflower oil | Transesterification with methanol | Solid acid (SAC-13) | N/A | 200 °C | 2 min | 25:1 | 9 g | C = 88% | N/A | [63] | |||
| - | - | - | - | ||||||||||
3.2.6. Heterogeneous Base Catalysts
3.2.7. Alkaline Earth Metal Oxides
3.2.8. Alkali Metal Salts
3.2.9. Mixed Metal Oxides
| Raw Material | Reaction | Catalyst | Preparation Methods | Reaction Parameters | Biodiesel Yield | Reuse | Reference | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Surface Area (m2/g) | Volume (cm3/g) | Pore Size (nm) | Acidity/Basicity (mmol/g) | T° | Reaction Time | Ratio Alcohol:Oil | Catalyst wt% | ||||||
| Alkaline earth metal oxides | |||||||||||||
| Zanthoxylum bungeanum seed oil (ZSO) | Transesterification with methanol | Commercial CaO | Calcination at 900 °C for 1.5 h | 65 °C | 2 h 45 | 11.69:1 | 2.52 | C > 96% | N/A | [206] | |||
| - | - | - | - | ||||||||||
| Silk cotton seed oil | Transesterification with methanol | Commercial CaO | N/A | - | 114 s | 18:1 | 0.3 | Y = 97.40% | 10 | [138] | |||
| 20.29 | 0.029 | 3.117 | - | ||||||||||
| Sunflower oil | Transesterification with methanol | Commercial CaO | Calcination at 500 °C and 900 °C for 1.5–5.5 h | 100 °C | 5.5 h | 6:1 | 1 mass% | C = 91% | N/A | [207] | |||
| 4.6 | 0.01 | 14.6 | - | ||||||||||
| Soybean oil | Transesterification with methanol | Commercial CaO | Calcination at 900 °C for 3 h | 62 °C | - | 10:1 | 6 | Y = 89.36% | N/A | [208] | |||
| 7.114 | 0.0607 | - | - | ||||||||||
| Jatropha oil | Transesterification with methanol | Dolomite-bead-PB-20 (CaMg(CO3)2) | Calcination at 550 °C | 65 °C | 2 h | 10:1 | 1 | N/A | 2 | [140] | |||
| 57 | 0.43 | 58 | - | ||||||||||
| Rapeseed oil | Transesterification with methanol | Ca/Al composite oxide-based alkaline | Calcination at 120 °C, 400 °C, 600 °C, 800 °C, and 1000 °C for 8 h | 65 °C | 3 h | 15:1 | 6 | Y = 94% | N/A | [148] | |||
| 5.14 | - | - | - | ||||||||||
| Soybean oil | Transesterification with methanol | CaFeAl/LDO (layered double oxide) | Co-precipitation Tcalcination = 750 °C for 3 h | 60 °C | 1 h | 12:1 | 6 | Y = 90% | 8 | [149] | |||
| 117 | - | - | - | ||||||||||
| Babasssu oil (Attalea speciosa) | Transesterification with methanol | CaO/SnO2 | Calcination at 650 °C for 5 h | 54.1 °C | 2 h | 10:1 | 6 | Y = 89.58% | N/A | [150] | |||
| 7.80 | 0.064 | 33 | - | ||||||||||
| Olive, sunflower, corn oil | Transesterification with methanol | CaO–MgO | Calcination at 600 °C for 2 h | 60 °C | 1–6 h | 6:1 | 2 | C = 99% | N/A | [151] | |||
| - | - | - | - | ||||||||||
| Sunflower oil, soybean oil | Transesterification with methanol | Calcium zincate | Calcination at 800 °C | 60 °C | 45 min | 12:1 | 3 | Y > 90% | 3 | [209] | |||
| 76.7 | 0.144 | 4.3 | - | ||||||||||
| Canola oil | Transesterification with methanol | CaO-La2O3 (commercial CaO) | Co-precipitation Wet impregnation Tcalcination = 700 °C for 6 h | 65 °C | 2.5 h | 15:1 | 5 | Y = 96.30% | 5 | [152] | |||
| 18.23 | - | 80–120 | - | ||||||||||
| Jatropha Curcas oil | Transesterification with methanol | CaO-NiO | Co-precipitation Calcination T = 900 °C for 6 h | 65 °C | 6 h | 15:1 | 5 | C > 80% | 6 | [146] | |||
| 7.2 | - | 34.7 | 6.32 basicity | ||||||||||
| CaO-Nd2O3 | |||||||||||||
| 8 | - | 62.3 | 4.09 basicity | ||||||||||
| Soybean oil | Transesterification with methanol | Pineapple leaves ash | Calcination at 600 °C for 2 h and 900 °C for 1 h | 60 °C | 30 min | 40:1 | 4 | C > 98% | 4 | [153] | |||
| - | - | - | - | ||||||||||
| Palm oil | Esterification saponification with methanol | Rice husk ash (RHA) | Calcination at 800 °C for 16 h | 65 °C | 1 h | 5:1 | 5 | Y > 97% | 5 | [154] | |||
| 1.914 | 0.0005161 | - | - | ||||||||||
| Activated RHA (ARHA) | |||||||||||||
| 18.947 | 0.008109 | - | - | ||||||||||
| Huskcatbase | |||||||||||||
| 14.493 | 0.004985 | - | - | ||||||||||
| Huskcatacid | |||||||||||||
| 7.362 | 0.002726 | - | - | ||||||||||
| Waste cooking oil | Esterification and transesterification with methanol | Tectona grandis leaves ash | Calcination at 700 °C for 4 h | Room T° | 3 h | 6:1 | 2.5 | C = 100% | 5 | [155] | |||
| 116.833 | 0.185 | 11.221 | - | ||||||||||
| Crude palm oil | Transesterification with methanol | CaO/CaCO3 + bottom ash | Calcination at 900 °C for 5 h | 60 °C | 3 h | 12:1 | 2 | C = 94.48% | N/A | [210] | |||
| 1.133 | - | 2–50 | - | ||||||||||
| CaO/CaCO3 + fly ash | |||||||||||||
| 1.719 | - | 2–50 | - | ||||||||||
| Fish oil | Esterification with methanol, ethanol, and isopropanol | Calcinated eggshell and copper oxide ([CaCu(OCH3)2]) | Calcination at 800 °C for 6 h | 65 °C 80 °C 85 °C | 1.5 h | 16:1 | 3 | Y = 93% | N/A | [211] | |||
| - | - | >100 | - | ||||||||||
| Palm oil | Transesterification with methanol | Calcined milled animal bone | Calcination at 800 °C | 65 °C | 4 h | 18:1 | 20 | C = 96.78% | 5 | [156] | |||
| 88.3 | - | - | - | ||||||||||
| Macauba oil | Transesterification–esterification with methanol | Eggshell | Calcination at 900 °C for 3.5 h | 80 °C | 1 h | 10:1 | 15 | C = 91.60% | N/A | [170] | |||
| 4 | 0.03 | 27.7 | - | ||||||||||
| Frying residual oil | Transesterification with methanol | Eggshell calcined and enriched with glycerin (ECEG) | Calcination Wet impregnation 10 °C/min until 150 °C for 120 min 10 °C/min until 150 °C for 240 min 10 °C/min until 800 °C for 240 min | 63 °C | 3 h | 6:1 | 15 | Oil mass 15% Y = 97.39% Oil mass 5% Y = 96.97% Oil mass 3% Y = 97.75% Oil mass 1% Y = 92.96% | 4 | [163] | |||
| 1.09 | - | 4.15 | - | ||||||||||
| Waste chicken fat | Transesterification with methanol | Eggshell | Calcination at 900 °C for 4 h | 57.5 °C | 5 h | 13:1 | 8.5 | Y = 90.41% C = 92.9% | 5 | [167] | |||
| 0.10806 | 0.001217 | 5.66399 | - | ||||||||||
| Waste cooking oil | Transesterification with methanol | Supported eggshell AEC-10 | Calcination at 900 °C for 2 h Wet impregnation | 65 °C | 3 h | 12:1 | 10 | C = >95% | N/A | [168] | |||
| 23.48 | 0.1 | 20.4 | - | ||||||||||
| Supported eggshell AEC-20 | |||||||||||||
| 10.36 | 0.02 | 10.8 | - | ||||||||||
| Supported eggshell AEC-30 | |||||||||||||
| 1.63 | 0.009 | 22.3 | - | ||||||||||
| Palm oil | Transesterification with methanol | Waste mud crab (Scylla serrata) shell | Calcination at 900 °C for 2 h | 65 °C | 3 h | 0.5:1 | 5 | C = 98.80% | 11 | [101] | |||
| 13 | - | - | - | ||||||||||
| Palm olein oil | Transesterification with methanol | Waste shells of egg | Calcination at 800 °C for 4 h | 60 °C | 2 h | 18:1 | 10 | Y = >90% | N/A | [157] | |||
| 1.1 | 0.005 | - | - | ||||||||||
| Golden apple snail | |||||||||||||
| 0.9 | 0.004 | - | - | ||||||||||
| Meretrix venus | |||||||||||||
| 0.5 | 0.002 | - | - | ||||||||||
| Rapeseed oil | Transesterification with methanol | CaO-loaded microcapsules | Co-extrusion | 65 °C | 4 h | 8:1 | 20 | 1st cycle Y = 95.5% 2nd cycle Y = 97.7% 3rd cycle Y = 94.5% 4th cycle Y = 80.9% | 3 | [172] | |||
| 4.26 | - | - | - | ||||||||||
| Refined, bleached, and deodorized palm oil (RBD palm oil) | Transesterification with methanol | CaO/γ-Al2O3 | Calcination at 718 °C for 5 h Impregnation | 60 °C | 3 h | 15:1 | 9 | C = 86.38% Y = 79.32% | N/A | [212] | |||
| - | - | - | - | ||||||||||
| Canola oil | Transesterification with methanol | CaO with K2CO3 | Calcination 499.85 °C for 3 h Impregnation with 3–10% of K2CO3 | 65 °C | 8 h | 9:1 | - | Y = 97.67% | 5 | [213] | |||
| 10.24–14.65 | - | 2–300 | 0.88–1.64 basicity | ||||||||||
| Waste palm oil (WPO), waste sunflower oil (WSO) | Esterification and transesterification with methanol | CaO/Al2O3 | Co-precipitation Dissolution Calcination at 750 °C for 6 h | 65 °C | 4 h | 9:1 | - | WPO Y = 89% WCO Y = 98% | 2 | [173] | |||
| 8.5683 | 0.045008 | 210.1157 | - | ||||||||||
| Waste frying sunflower oil | Transesterification with methanol | CaO/ZnFe2O4 | Co-precipitation Calcination at 800 °C for 2.5 h | 65 °C | 3 h | 12:1 | 6 | Y = 98% | 5 | [214] | |||
| 141–198 | 0.39–0.65 | 1.5–25 | - | ||||||||||
| Sunflower oil | Transesterification with methanol | CaO/ZM (zeolitic material) | Ultrasound-assisted impregnation Calcination at 450–600 °C for 6 h | 60 °C | 2 h | 12:1 | 4 | C = 96.50% | N/A | [176] | |||
| 22.6 | 2.162 | - | 0–0.8645 basicity | ||||||||||
| Palm oil | Transesterification with methanol | CaO-loaded unimodal porous silica (CaO/U) | Calcination at 800 °C for 4 h Impregnation with calcium nitrate tetrahydrate | 60 °C | 6 h | 12:1 | 5 | 1st cycle Y = 94.15% 5th cycle Y = 88.87% | 5 | [215] | |||
| 22–371 | - | 12.2 | - | ||||||||||
| CaO-loaded bimodal porous silica (CaO/B) | |||||||||||||
| 24–415 | - | 12.2 | - | ||||||||||
| Cotton seed oil (CSO), waste frying oil (WFO) | Transesterification with methanol | Sr(NO3)2 on CaO | Calcination at 750 °C for 6 h Impregnation | 30–80 °C | 2 h | 12:1 | 3.5 | CSO C = 97.3% UFO C = 96.7% | 3 | [216] | |||
| 90 | - | - | - | ||||||||||
| Oleic acid | Esterification with methanol | Metal–organic framework Mg-MOF | N/A | 70 °C | 3 h | 15:1 | 0.15 | C = 97% | 5 | [179] | |||
| 162 | - | - | - | ||||||||||
| Waste cooking oil | Transesterification with methanol | Mg-Al hydrotalcite | Calcination at 450 °C for 3 h | 65 °C | 24 h | 30:1 | 5 | Y = 87.23% | N/A | [180] | |||
| - | - | - | - | ||||||||||
| Stearic acid monoethanolamide | Transesterification with methanol | MgAl-layered double hydroxide (MgAl-LDH) | Calcination at 500 °C for 8 h Rehydration | 109 °C | 4 h | 1.1:1 | 5 | C = 87% | N/A | [217] | |||
| 44 | - | - | - | ||||||||||
| Soybean oil | Transesterification with methanol | Strontium zirconate | Calcination at 900 °C for 1 h | 60 °C | 3 h | 12:1 | 3 | Y = 98% | N/A | [218] | |||
| - | - | - | - | ||||||||||
| Cotton seed oil (CSO) Waste frying oil (WFO) | Transesterification with methanol | Sr(NO3)2 on CaO | Impregnation Calcination at 750 °C for 6 h | 60 °C | 2 h | 12:1 | 3.5 | CSO C = 97.3% WFO C = 96.7% | 3 | [193] | |||
| 90 | - | - | - | ||||||||||
| Soybean oil | Transesterification with methanol | Sr3Al2O6 | Calcination to up to 1200 °C | 60 °C | 61 min | 25:1 | 1.3 | C = 95.70% | 4 | [219] | |||
| 4.355 | - | - | - | ||||||||||
| Waste cooking oil | Transesterification with methanol | Sr-Ce | Gel combustion Calcination at 900 °C for 4 h | 65 °C | 2 h | 14:1 | 2 | C = 99.5% | 4 | [181] | |||
| 66 | 0.0058 | 8.1 | 1.54 basicity | ||||||||||
| Corn oil and oleic acid | Esterification and transesterification with ethanol | SrO-ZnO/Al2O3 | Calcination at 900 °C for 6 h | 70 °C | 6 h | 5:1 | 10 | C = 95.1% | N/A | [182] | |||
| 3.515 | 0.054 | 3.516 | - | ||||||||||
| Waste cooking oil | Transesterification with methanol | Sr-Ti mixed metal oxide | Calcination at 880 °C for 8 h | 65 °C | 80 min | 11:1 | 1 | C = 97.90% | 8 | [183] | |||
| 43.6 | 0.0811 | 8.7125 | 2.89 basicity | ||||||||||
| Palm oil | Transesterification with methanol | SrTiO3 | Sol–gel method Calcination at 1050 °C for 4 h | 170 °C | 3 h | 15:1 | 6 | Y = 93.14% | 3 | [53] | |||
| 13.07 | 0.096 | - | 0.150 basicity 0.078 acidity | ||||||||||
| Olive oil | Transesterification with methanol | SrO | Calcination at 900 °C for 5 h | 45 °C | 30 min | 1:6 | 3.23 SrO 3.14 SrO/SiO2 | C = >80% | 4 | [220] | |||
| 4.743 | - | - | - | ||||||||||
| SrO/CaO | |||||||||||||
| 2.272 | - | - | - | ||||||||||
| SrO/SiO2 | |||||||||||||
| 1.966 | - | - | - | ||||||||||
| Corn oil | Transesterification with methanol | Ba(OH)2 | Calcination | - | 118 min | 11:32 | 3.6 | Y = 99.15% | 4 | [185] | |||
| 1.24 | - | - | - | ||||||||||
| Karanja oil | Transesterification with methanol | Ba(OH)2·8H2O | N/A | 30 °C | 1.5 h | 9:1 | 0.5 | C = 84% | N/A | [186] | |||
| - | - | - | - | ||||||||||
| Microalgae Anabaena PCC 7120 | Transesterification with methanol | Ba2TiO4 | Wet impregnation Calcination at 800 °C for 4 h | 60 °C | 4 h | 15:1 | 3.5 | C = 98.41% | 6 | [221] | |||
| 6.94 | - | - | 2.75 basicity | ||||||||||
| Alkali metal salts | |||||||||||||
| Safflower oil | Esterification with methanol | Potassium Titanate | Hydrothermal treatment at 200 °C for 20 h Calcination | 50 °C | 1 h | 1:1 | 3 | Y = approx. 100% | 3 | [84] | |||
| - | - | - | - | ||||||||||
| Bauhinia monandra seed oil | Esterification and transesterification with methanol | Banana peels | Calcination at 700 °C for 4 h | 65 °C | 69.02 min | 7.6:1 | 2.75 | C = 98.5% | N/A | [222] | |||
| 4.442 | 0.020 | 17.864 | - | ||||||||||
| Madhuca indica | Transesterification with methanol | Banana pseudo stem | Calcination at 700 °C for 4 h | 65 °C | 178.1 min | 14.9:1 | 5.9 | C = 98.8% | 3 | [188] | |||
| 4.580 | 0.006 | 2.245 | - | ||||||||||
| Soybean oil | Transesterification with methanol | KF/c-Al2O3 | Impregnation Calcination at 500 °C for 3 h | 50 °C | 40 min | 12:1 | 2 | C = 95% | N/A | [223] | |||
| - | - | 50 | - | ||||||||||
| Jatropha oil | Transesterification with methanol | KF-loaded nano-g-Al2O3 | Impregnation Calcination at 500 °C for 3 h | 65 °C | 8 h | 15:1 | 3 | C = 97.7% | N/A | [224] | |||
| 41.7 | - | 7–40 | 1.68 basicity | ||||||||||
| Madhuca indica | Transesterification with methanol | KI/mesoporous silica | Impregnation Calcination at 600 °C for 3 h | 70 °C | 8 h | 5 wt% | 15 | C = 90.09% | N/A | [189] | |||
| 801 | 1.05 | 5.23 | - | ||||||||||
| Jatropha oil | Transesterification with methanol | KNO3/Al2O3 | Impregnation Calcination at 500 °C for 4 h | 70 °C | 6 h | 12:1 | 6 | C > 84% | 3 | [225] | |||
| 126 | - | - | - | ||||||||||
| Waste cooking oil | Transesterification with methanol | KOH/corncob-derived activated carbon | Wet impregnation Calcination at 450 °C for 2 h | 45 °C | 1 h | 18:1 | 1 | Y = 97.80% C = 92% | 2 | [190] | |||
| 627 | 0.637 | - | 9.903 basicity | ||||||||||
| Castor seed oil | Transesterification with methanol | K-promoted La2O3 | Sol–gel method Combustion method Calcination 900 °C for 5 h | 65 °C | 2.5 h | 16:1 | 2 | C = 97.5% | 5 | [191] | |||
| 2.18 | 0.0052 | >5 | 10.12 basicity | ||||||||||
| Palm, sunflower, and soybean oil | Transesterification with methanol | K/SnO2 | Calcination at 700 °C for 5 h | 65 °C | 1.5 h | 12:1 | 3 | Y = 97.5% | N/A | [192] | |||
| 19.7 | 0.074 | - | - | ||||||||||
| Soybean oil | Transesterification with methanol | K2CO3 supported on MgO | Calcination at 600 °C for 3 h | 70 °C | 45 min | 4:1 | 1.3 | Y = 99% | 6 | [226] | |||
| - | - | - | - | ||||||||||
| Waste cooking oil | Transesterification with methanol | K3PO4 | N/A | 50 °C | 1.5 h | 6:1 | 3 | Y = 92% | 4 | [227] | |||
| - | - | - | - | ||||||||||
| Waste cooking oil | Esterification and transesterification with ethanol | K3PO4/seashell | Calcination 800 °C for 4 h | 60 °C | 5 h | 12:1 | 5–25 | Y = 95% | N/A | [228] | |||
| 22.5 | 8.5 | - | - | ||||||||||
| Sunflower oil (SO), used frying oil (UFO) | Transesterification with methanol | LiNO3/CaO | Calcination at 750 °C for 6 h | 60 °C | 2 h | 12:1 | 3.5 | SO C = 97.8% UFO C = 96.7% | 6 | [193] | |||
| 90 | - | - | - | ||||||||||
| Soybean oil | Transesterification with methanol | Sodium geopolymer powder | Heat treatment at 110–700 °C | - | - | - | - | Y = 89.9% | N/A | [194] | |||
| 6.34–32.62 | - | 17 | - | ||||||||||
| Potassium geopolymer powder | |||||||||||||
| 28.64–62.54 | - | 9 | - | ||||||||||
| Sunflower oil | Transesterification with methanol | Sodium titanate nanotubes | N/A | 80 °C | 2 h | 40:1 | 1.5 | C = 95.9% | 3 | [229] | |||
| 120 | 0.29 | 5.37 | - | ||||||||||
| Mixture of soybean and sunflower oils | Esterification and transesterification with methanol | Mixture of sodium alginate, k-carrageenan, and sodium bentonite. | N/A | 65 °C | 0.5 h | 21:1 | 3 | C = 97% | 25 | [195] | |||
| 151 | - | 1.5mm | 1.07 acidity 5.84 basicity | ||||||||||
| Rapeseed oil | Transesterification with methanol | NaNO3/SiAl | Impregnation Calcination at 600 °C for 25 h | 65 °C | 3 h | 9:1 | 5 | C > 99% | 3 | [196] | |||
| 0.410–2.406 | - | - | - | ||||||||||
| Wild olive oil | Transesterification with methanol | Na-SiO2@CeO2 | Wet impregnation Calcination at 500 °C for 5 h | 65 °C | 2 h | 10:1 | 2.5 | Y = 97% | 5 | [230] | |||
| 88.4 | 0.0122 | 2.15 | - | ||||||||||
| Waste cooking oil | Transesterification with methanol | Na-SiO2@TiO2 | Wet impregnation Ultrasound Calcination at 500 °C for 5 h | 65 °C | 2 h | 25:1 | 2.5 | Y = 98% | 5 | [190] | |||
| 107.26 | 0.133 | 6.16 | - | ||||||||||
| Mixed metal oxides | |||||||||||||
| Nannochloropsis oculate microalga | Transesterification with methanol | Al2O3-supported CaO and MgO | Sol–gel method Calcination at 500 °C for 6 h | 50 °C | 4 h | 30:1 | 2 | Y = 97.5% | 2 | [231] | |||
| - | - | - | - | ||||||||||
| Calophyllum inophyllum oil | Esterification with methanol | Al2O3 | Co-precipitation | 160 °C | 1 h | 4 g of methanol | 0.1 | Y = 89% | 10 | [232] | |||
| 119 | - | - | - | ||||||||||
| SnO | |||||||||||||
| 15 | - | - | - | ||||||||||
| (Al2O3)8(SnO)2 | |||||||||||||
| 22 | - | - | - | ||||||||||
| (Al2O3)8(ZnO)2 | |||||||||||||
| 33 | - | - | - | ||||||||||
| Oleic acid | Esterification with methanol | γ-Al2O3 | Calcination at 450 °C for 4 h | 275 °C | 1 min | 20:1 | - | C = 90% | N/A | [233] | |||
| 85.98 | 0.16 | - | - | ||||||||||
| Palm kernel oil and coconut oil | Transesterification with methanol | LiNO3/Al2O3 | Incipient wetness impregnation Sol–gel method Calcination at 450 °C, 550 °C, 650 °C | 60 °C | 3 h | 65:1 | 10–15–20 | Palm oil C = 94% Coconut oil C = 99.8% | N/A | [234] | |||
| - | - | - | - | ||||||||||
| NaNO3/Al2O3 KNO3/Al2O3 | |||||||||||||
| - | - | - | - | ||||||||||
| Ca(NO3)2/Al2O3 | |||||||||||||
| - | - | - | - | ||||||||||
| Mg(NO3)2/Al2O3 | |||||||||||||
| - | - | - | - | ||||||||||
| Waste cooking oil | Esterification and transesterification with methanol | Cu-MOF | Solvothermal and hydrothermal methods | 60 °C | 1 h | 20:1 | 1 g/100 mL | Y = 85% | 3 | [198] | |||
| 118 | 0.087 | <6 | - | ||||||||||
| Ca-MOF | |||||||||||||
| 101 | 0.035 | <6 | - | ||||||||||
| Waste cooking oil | Transesterification with methanol | Copper-doped zinc oxide nanocomposite (CZO) | Calcination 500 °C for 2 h | 55 °C | 50 min | 8:1 | 12 | Y = 97.71% | 5 | [235] | |||
| - | - | 80 | - | ||||||||||
| Oleic acid | Esterification with methanol | Copper(II)-alginate beads | N/A | 70 °C | 3 h | 10:1 | 250 mg | C = 71.80% | N/A | [236] | |||
| - | - | - | - | ||||||||||
| Waste edible oil | Transesterification with methanol | CoFe2O4 | Ultrasound | 64.75 °C | 55.75 min | 16.05:1 | 5.22 | Y = 91.64% Y = 98.17% | 5 | [199] | |||
| - | 0.220 | 12.47 | - | ||||||||||
| CoFe2O4@GO | |||||||||||||
| - | 0.164 | 16.61 | 0.91 basicity | ||||||||||
| Phoenix dactylifera L. kernel oil | Esterification and transesterification with methanol | Mn@MgO-ZrO2 | Co-precipitation Impregnation Calcination at 650 °C for 4 h | 90 °C | 4 h | 15:1 | 3 | Y = 96.4% | 6 | [237] | |||
| 45 | 0.120 | 17.06 | - | ||||||||||
| Soybean oil | Transesterification and esterification with methanol | MnO and TiO | N/A | 260 °C | 0.35 h | 30:1 | 28.1g | Y = 96–99% | N/A | [238] | |||
| - | - | - | - | ||||||||||
| Oleic acid | Esterification with ethanol | MnO2@Mn (btc) | N/A | 100 °C | 12 h | 12:1 | 3 | Y = 98% | 5 | [71] | |||
| 10.867 | 0.039 | - | - | ||||||||||
| Nannochloropsis oculate oil | Transesterification with methanol | Mn-ZnO capped with Polyethylene | Precipitation method Calcination at 700 °C for 3 h. | 60 °C | 4 h | 15:1 | 3.5 | Y = 87.5% | 4 | [203] | |||
| - | - | - | - | ||||||||||
| Glycerol (PEG) | |||||||||||||
| - | - | - | - | ||||||||||
| Soybean oil | Transesterification with methanol | MoO3/ZrO2/KIT-6 | Calcination 500 °C for 6 h | 130 °C | 12 h | 20:1 | 12 | C = 92.7% | 5 | [15] | |||
| - | - | - | - | ||||||||||
| Castor oil | Transesterification with methanol | Ni-doped ZnO | Calcination at 800 °C for 3 h | 55 °C | 1 h | 8:1 | 11 | Y = 95.2% | 3 | [200] | |||
| - | - | - | - | ||||||||||
| Glycerol carbonate | Transesterification with methanol | Ni/CaO | Calcination at 800 °C for 4 h | 90 °C | 1.5 h | 3:1 | 3 | C = 99.2% Y = 94.02% | 5 | [239] | |||
| - | - | - | 35.65 basicity | ||||||||||
| Soybean oil | Esterification and transesterification with methanol | Ni0.5Zn0.5Fe2O4 | Sol–gel method | 180 °C | 1 h | 12–15:1 | 2–3 | C = 99.54% | 3 | [202] | |||
| 65.289 | 0.167 | 4.27 | - | ||||||||||
| Calophyllum inophyllum oil | Transesterification with methanol | Zn/CaO | N/A | 56.71 °C | 81.31 min | 9.66:1 | 5 | C = 91.95% | N/A | [93] | |||
| - | - | - | - | ||||||||||
| Waste frying oil | Transesterification with methanol | ZnAl–LDH@SiO2 | Titration method | 25 °C | 1.5 h | 6:1 | 3.3 | Y = 98.4% | 5 | [240] | |||
| - | - | - | - | ||||||||||
| Waste coconut oil | Pre-esterification | ZnO/CuO | Calcination at 300 °C for 3 h Wet impregnation with zinc sulphate | 55 °C | 113 min | 10.5:1 | 1.66 | C = 90.26% | 4 | [241] | |||
| - | - | - | - | ||||||||||
| Jatropha curcas crude oil | Esterification and transesterification with methanol | ZnO/SiO2 | Impregnation Calcination at 500 °C for 12 h | 60 °C | 20 min | 12:1 | 2 | C = 96% | 10 | [242] | |||
| 160 | - | - | - | ||||||||||
| Jatropha oil | Esterification with methanol | ZnO/zeolite, PbO/zeolite, and MgO | Hydrothermal impregnation precipitation (HIP) method | 60–65 °C | 1.4 h | 12:1 | 5 | Y = 98.5% | 4 | [174] | |||
| 422.50 | 0.2655 | 2.593 | - | ||||||||||
| Chlorella vulgaris | Esterification and transesterification with methanol | SiC/NaOH-GO | Impregnation Calcination at 400 °C for 5 h | 85 °C | 5 min | 48:1 | 4 | Transesterification Y = 96% Esterification Y = 92% | 4 | [243] | |||
| 1.60 | - | 27.1 | - | ||||||||||
3.3. Influence of Physico-Chemical Properties on Biodiesel Yield
3.4. An Analytical Review of Variability and Its Implications
4. Advantages and Disadvantages of the Different Types of Heterogeneous Catalysts
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AC | Activated Carbon |
| AMO | Alkaline Earth Metal Oxides |
| ANN | Artificial Neural Network |
| BET | Brunauer–Emmett–Teller |
| BJH | Barrett–Joyner |
| CELO | Crude Euphorbia lathyrism seed oil |
| CI | Compression Ignition |
| CPO | Ceiba Pentandra Oil |
| CPO | Crude Palm Oil |
| DMC | Dimethyl Carbonate |
| FAME | Fatty Acid Methyl Ester |
| FFA | Free Fatty Acid |
| GO | Graphene Oxide |
| HPA | Heteropoly Acid |
| IL | Ionic Liquid |
| KI | Potassium Iodide |
| LDH | Layered Double Hydroxide |
| LDO | Layered Double Oxide |
| LGCPO | Low-Grade Crude Palm Oil |
| MATP | Microwave-Assisted Transesterification Process |
| MCM | Mobil Composition of Matter |
| MFL | Mesuaferrea Linn |
| MNP | Magnetic Nanoparticles |
| MOF | Metal–Organic Framework |
| MW | Microwave |
| NBPA | Nendran Banan Peduncle Ash |
| OA | Oleic Acid |
| PBPS | Poovan Banan Pseudostem |
| PCC | Pasteur Culture Collection of Cyanobacteria |
| PEG | Polyethylene Glycols |
| PFAD | Palm Fatty Acid Distillate |
| SBA | Santa Barbara Amorphous |
| SBC | Spent Bleaching Clay |
| SEM | Scanning Electron Microscopy |
| SL | Sulfonated Lignin |
| SPW | Sulfonated Sago Pith Waste |
| SSA | Specific Surface Area |
| TCM | Tubular Carbon Membrane |
| TG | Triglyceride |
| TGA | Thermogravimetric Analysis |
| TPA | Tungstophosphoric Acid |
| WCO | Waste Cooking Oil |
| WEO | Waste Edible Oil |
| WPO | Waste Palm Oil |
| WSO | Waste Sunflower Oil |
| Wt | Weight |
| w/v | Weight in Volume |
| w/w | Weigh in Weight |
| XPS | X-Ray Photoelectron Spectroscopy |
| XRD | X-Ray Diffraction |
| ZSO | Zanthoxylum bungeanum Seed Oil |
References
- Singh, N.; Saluja, R.K.; Rao, H.J.; Kaushal, R.; Gahlot, N.K.; Suyambulingam, I.; Sanjay, M.R.; Divakaran, D.; Siengchin, S. Progress and facts on biodiesel generations, production methods, influencing factors, and reactors: A comprehensive review from 2000 to 2023. Energy Convers. Manag. 2024, 302, 118157. [Google Scholar] [CrossRef]
- Athar, M.; Zaidi, S. A review of the feedstocks, catalysts, and intensification techniques for sustainable biodiesel production. J. Environ. Chem. Eng. 2020, 8, 104523. [Google Scholar] [CrossRef]
- Vasić, K.; Hojnik Podrepšek, G.; Knez, Ž.; Leitgeb, M. Biodiesel Production Using Solid Acid Catalysts Based on Metal Oxides. Catalysts 2020, 10, 237. [Google Scholar] [CrossRef]
- Abedin, M.J.; Masjuki, H.H.; Kalam, M.A.; Sanjid, A.; Rahman, S.M.A.; Fattah, I.M.R. Performance, emissions, and heat losses of palm and jatropha biodiesel blends in a diesel engine. Ind. Crops Prod. 2014, 59, 96–104. [Google Scholar] [CrossRef]
- Hoekman, S.K.; Robbins, C. Review of the effects of biodiesel on NOx emissions. Fuel Process. Technol. 2012, 96, 237–249. [Google Scholar] [CrossRef]
- Fattah, I.M.R.; Ong, H.C.; Mahlia, T.M.I.; Mofijur, M.; Silitonga, A.S.; Rahman, S.M.A.; Ahmad, A. State of the Art of Catalysts for Biodiesel Production. Front. Energy Res. 2020, 8, 101. [Google Scholar] [CrossRef]
- Hamza, M.; Ayoub, M.; Shamsuddin, R.B.; Mukhtar, A.; Saqib, S.; Zahid, I.; Ameen, M.; Ullah, S.; Al-Sehemi, A.G.; Ibrahim, M. A review on the waste biomass derived catalysts for biodiesel production. Environ. Technol. Innov. 2021, 21, 101200. [Google Scholar] [CrossRef]
- Jayakumar, M.; Karmegam, N.; Gundupalli, M.P.; Bizuneh Gebeyehu, K.; Tessema Asfaw, B.; Chang, S.W.; Ravindran, B.; Kumar Awasthi, M. Heterogeneous base catalysts: Synthesis and application for biodiesel production—A review. Bioresour. Technol. 2021, 331, 125054. [Google Scholar] [CrossRef] [PubMed]
- Gupta, J.; Agarwal, M.; Dalai, A.K. Optimization of biodiesel production from mixture of edible and nonedible vegetable oils. Biocatal. Agric. Biotechnol. 2016, 8, 112–120. [Google Scholar] [CrossRef]
- Nahas, L.; Dahdah, E.; Aouad, S.; El Khoury, B.; Gennequin, C.; Abi Aad, E.; Estephane, J. Highly efficient scallop seashell-derived catalyst for biodiesel production from sunflower and waste cooking oils: Reaction kinetics and effect of calcination temperature studies. Renew. Energy 2023, 202, 1086–1095. [Google Scholar] [CrossRef]
- Vera-Rozo, J.R.; Sáez-Bastante, J.; Carmona-Cabello, M.; Riesco-Ávila, J.M.; Avellaneda, F.; Pinzi, S.; Dorado, M.P. Cetane index prediction based on biodiesel distillation curve. Fuel 2022, 321, 124063. [Google Scholar] [CrossRef]
- Xie, W.; Wang, H. Immobilized polymeric sulfonated ionic liquid on core-shell structured Fe3O4/SiO2 composites: A magnetically recyclable catalyst for simultaneous transesterification and esterifications of low-cost oils to biodiesel. Renew. Energy 2020, 145, 1709–1719. [Google Scholar] [CrossRef]
- Changmai, B.; Vanlalveni, C.; Prabhakar Ingle, A.; Bhagat, R.; Lalthazuala Rokhum, S. Widely used catalysts in biodiesel production: A review. RSC Adv. 2020, 10, 41625–41679. [Google Scholar] [CrossRef] [PubMed]
- Leung, D.Y.C.; Wu, X.; Leung, M.K.H. A review on biodiesel production using catalyzed transesterification. Appl. Energy 2010, 87, 1083–1095. [Google Scholar] [CrossRef]
- Wang, L.; Wang, H.; Fan, J.; Han, Z. Synthesis, catalysts and enhancement technologies of biodiesel from oil feedstock—A review. Sci. Total Environ. 2023, 904, 166982. [Google Scholar] [CrossRef] [PubMed]
- Gautam, A.; Khajone, V.B.; Bhagat, P.R.; Kumar, S.; Patle, D.S. Metal- and ionic liquid-based photocatalysts for biodiesel production: A review. Environ. Chem. Lett. 2023, 21, 3105–3126. [Google Scholar] [CrossRef]
- Sulaiman, S. Overview of Catalysts In Biodiesel Production. J. Eng. Appl. Sci. 2016, 11, 439–442. [Google Scholar]
- Orege, J.I.; Oderinde, O.; Kifle, G.A.; Ibikunle, A.A.; Raheem, S.A.; Ejeromedoghene, O.; Okeke, E.S.; Olukowi, O.M.; Orege, O.B.; Fagbohun, E.O.; et al. Recent advances in heterogeneous catalysis for green biodiesel production by transesterification. Energy Convers. Manag. 2022, 258, 115406. [Google Scholar] [CrossRef]
- Atadashi, I.M.; Aroua, M.K.; Abdul Aziz, A.R.; Sulaiman, N.M.N. The effects of catalysts in biodiesel production: A review. J. Ind. Eng. Chem. 2013, 19, 14–26. [Google Scholar] [CrossRef]
- Oyekunle, D.T.; Barasa, M.; Gendy, E.A.; Tiong, S.K. Heterogeneous catalytic transesterification for biodiesel production: Feedstock properties, catalysts and process parameters. Process Saf. Environ. Prot. 2023, 177, 844–867. [Google Scholar] [CrossRef]
- Mendonça, I.M.; Paes, O.A.R.L.; Maia, P.J.S.; Souza, M.P.; Almeida, R.A.; Silva, C.C.; Duvoisin, S.; de Freitas, F.A. New heterogeneous catalyst for biodiesel production from waste tucumã peels (Astrocaryum aculeatum Meyer): Parameters optimization study. Renew. Energy 2019, 130, 103–110. [Google Scholar] [CrossRef]
- Goldsmith, B.R.; Esterhuizen, J.; Liu, J.; Bartel, C.J.; Sutton, C. Machine learning for heterogeneous catalyst design and discovery. Aiche J. 2018, 64, 2311–2323. [Google Scholar] [CrossRef]
- Gupta, J.; Agarwal, M.; Dalai, A.K. An overview on the recent advancements of sustainable heterogeneous catalysts and prominent continuous reactor for biodiesel production. J. Ind. Eng. Chem. 2020, 88, 58–77. [Google Scholar] [CrossRef]
- Esmi, F.; Borugadda, V.B.; Dalai, A.K. Heteropoly acids as supported solid acid catalysts for sustainable biodiesel production using vegetable oils: A review. Catal. Today 2022, 404, 19–34. [Google Scholar] [CrossRef]
- Luo, X.; Wu, H.; Li, C.; Li, Z.; Li, H.; Zhang, H.; Li, Y.; Su, Y.; Yang, S. Heteropoly Acid-Based Catalysts for Hydrolytic Depolymerization of Cellulosic Biomass. Front. Chem. 2020, 8, 580146. [Google Scholar] [CrossRef] [PubMed]
- Bhat, N.S.; Mal, S.S.; Dutta, S. Recent advances in the preparation of levulinic esters from biomass-derived furanic and levulinic chemical platforms using heteropoly acid (HPA) catalysts. Mol. Catal. 2021, 505, 111484. [Google Scholar] [CrossRef]
- Palacios, E.A.; Palermo, V.; Sathicq, A.; Pizzio, L.; Romanelli, G. A New Series of Tungstophosphoric Acid-Polymeric Matrix Catalysts: Application in the Green Synthesis of 2-Benzazepines and Analogous Rings. Catalysts 2022, 12, 1155. [Google Scholar] [CrossRef]
- Mansir, N.; Taufiq-Yap, Y.H.; Rashid, U.; Lokman, I.M. Investigation of heterogeneous solid acid catalyst performance on low grade feedstocks for biodiesel production: A review. Energy Convers. Manag. Sustain. Biofuel. 2017, 141, 171–182. [Google Scholar] [CrossRef]
- Sani, Y.M.; Daud, W.M.A.W.; Aziz, A.A. Activity of solid acid catalysts for biodiesel production: A critical review. Appl. Catal. A Gen. 2014, 470, 140–161. [Google Scholar] [CrossRef]
- Hanif, M.A.; Nisar, S.; Rashid, U. Supported solid and heteropoly acid catalysts for production of biodiesel. Catal. Rev. 2017, 59, 165–188. [Google Scholar] [CrossRef]
- Su, F.; Guo, Y. Advancements in solid acid catalysts for biodiesel production. Green Chem. 2014, 16, 2934–2957. [Google Scholar] [CrossRef]
- Bento, H.B.S.; Reis, C.E.R.; Cunha, P.G.; Carvalho, A.K.F.; De Castro, H.F. One-pot fungal biomass-to-biodiesel process: Influence of the molar ratio and the concentration of acid heterogenous catalyst on reaction yield and costs. Fuel 2021, 300, 120968. [Google Scholar] [CrossRef]
- Guo, L.; Xie, W.; Gao, C. Heterogeneous H6PV3MoW8O40/AC-Ag catalyst for biodiesel production: Preparation, characterization and catalytic performance. Fuel 2022, 316, 123352. [Google Scholar] [CrossRef]
- Kaur, M.; Sharma, S.; Bedi, P.M.S. Silica supported Brönsted acids as catalyst in organic transformations: A comprehensive review. Chin. J. Catal. 2015, 36, 520–549. [Google Scholar] [CrossRef]
- Pithadia, D.; Patel, A.; Hatiya, V. 12-Tungstophosphoric acid anchored to MCM-22, as a novel sustainable catalyst for the synthesis of potential biodiesel blend, levulinate ester. Renew. Energy 2022, 187, 933–943. [Google Scholar] [CrossRef]
- Alcañiz-Monge, J.; Bakkali, B.E.; Trautwein, G.; Reinoso, S. Zirconia-supported tungstophosphoric heteropolyacid as heterogeneous acid catalyst for biodiesel production. Appl. Catal. B Environ. 2018, 224, 194–203. [Google Scholar] [CrossRef]
- Khan, I.W.; Naeem, A.; Farooq, M.; Mahmood, T.; Ahmad, B.; Hamayun, M.; Ahmad, Z.; Saeed, T. Catalytic conversion of spent frying oil into biodiesel over raw and 12-tungsto-phosphoric acid modified clay. Renew. Energy 2020, 155, 181–188. [Google Scholar] [CrossRef]
- Kaita, J.; Mimura, T.; Fukuoka, N.; Hattori, Y. Catalyst for Transesterification. U.S. Patent 6407269B2, 18 June 2002. [Google Scholar]
- Zabeti, M.; Daud, W.M.A.W.; Aroua, M.K. Activity of solid catalysts for biodiesel production: A review. Fuel Process. Technol. 2009, 90, 770–777. [Google Scholar] [CrossRef]
- Hegde, V.; Pandit, P.; Rananaware, P.; Brahmkhatri, V.P. Sulfonic acid-functionalized mesoporous silica catalyst with different morphology for biodiesel production. Front. Chem. Sci. Eng. 2022, 16, 1198–1210. [Google Scholar] [CrossRef]
- Lin, V.S.-Y.; Radu, D.R. Use of Functionalized Mesoporous Silicates to Esterify Fatty Acids and Transesterify Oils. U.S. Patent 7122688B2, 17 October 2006. [Google Scholar]
- Istadi, I.; Anggoro, D.D.; Buchori, L.; Rahmawati, D.A.; Intaningrum, D. Active Acid Catalyst of Sulphated Zinc Oxide for Transesterification of Soybean Oil with Methanol to Biodiesel. Procedia Environ. Sci. 2015, 23, 385–393. [Google Scholar] [CrossRef]
- Thanh, L.T.; Okitsu, K.; Boi, L.V.; Maeda, Y. Catalytic technologies for biodiesel fuel production and utilization of glycerol: A review. Catalysis 2012, 2, 191–222. [Google Scholar] [CrossRef]
- Evangelista, J.P.D.C.; De Morais Araújo, A.M.; Gondim, A.D.; De Araujo, A.S. The role of acidity in fatty acid esterification with heterogeneous silica catalysts impregnated with Zr and sulfates. Energy Environ. 2025, 36, 786–806. [Google Scholar] [CrossRef]
- Ma, L.; Lv, E.; Du, L.; Han, Y.; Lu, J.; Ding, J. A flow-through tubular catalytic membrane reactor using zirconium sulfate tetrahydrate-impregnated carbon membranes for acidified oil esterification. J. Energy Inst. 2017, 90, 875–883. [Google Scholar] [CrossRef]
- Popova, M.; Lazarova, H.; Kalvachev, Y.; Todorova, T.; Szegedi, Á.; Shestakova, P.; Mali, G.; Dasireddy, V.D.B.C.; Likozar, B. Zr-modified hierarchical mordenite as heterogeneous catalyst for glycerol esterification. Catal. Commun. 2017, 100, 10–14. [Google Scholar] [CrossRef]
- Hayyan, A.; Yeow, A.T.H.; Abed, K.M.; Jeffrey Basirun, W.; Boon Kiat, L.; Saleh, J.; Wen Han, G.; Chia Min, P.; Aljohani, A.S.M.; Zulkifli, M.Y.; et al. The development of new homogenous and heterogeneous catalytic processes for the treatment of low grade palm oil. J. Mol. Liq. 2021, 344, 117574. [Google Scholar] [CrossRef]
- Ayadi, M.; Awad, S.; Villot, A.; Abderrabba, M.; Tazerout, M. Heterogeneous acid catalyst preparation from olive pomace and its use for olive pomace oil esterification. Renew. Energy 2021, 165, 1–13. [Google Scholar] [CrossRef]
- Kader, S.S.; Jusoh, M.; Zakaria, Z.Y. Palm fatty acid distillate-based biodiesel with sulfonated chicken and cow bone catalyst. Mater. Today Proc. 2022, 57, 1053–1060. [Google Scholar] [CrossRef]
- Shan, R.; Lu, L.; Shi, Y.; Yuan, H.; Shi, J. Catalysts from renewable resources for biodiesel production. Energy Convers. Manag. 2018, 178, 277–289. [Google Scholar] [CrossRef]
- Tan, X.; Sudarsanam, P.; Tan, J.; Wang, A.; Zhang, H.; Li, H.; Yang, S. Sulfonic acid-functionalized heterogeneous catalytic materials for efficient biodiesel production: A review. J. Environ. Chem. Eng. 2021, 9, 104719. [Google Scholar] [CrossRef]
- Zavarize, D.G.; De Oliveira, J.D. Brazilian açaí berry seeds: An abundant waste applied in the synthesis of carbon-based acid catalysts for transesterification of low free fatty acid waste cooking oil. Environ. Sci. Pollut. Res. 2021, 28, 21285–21302. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zheng, Y.; Chen, Y.; Zhu, X. Biodiesel production from waste cooking oil using a heterogeneous catalyst from pyrolyzed rice husk. Bioresour. Technol. 2014, 154, 345–348. [Google Scholar] [CrossRef] [PubMed]
- Zailan, Z.; Ali, H.; Chau, J.W.; Jusoh, M.; Tahir, M.; Zakaria, Z.Y. Development of Sulphonated Sago Pith Waste Catalyst for Esterification of Palm Fatty Acid Distillate to Methyl Ester. Chem. Eng. Trans. 2020, 78, 31–36. [Google Scholar] [CrossRef]
- Esan, A.O.; Smith, S.M.; Ganesan, S. Dimethyl carbonate assisted catalytic esterification of palm fatty acid distillate using catalyst derived from spent bleaching clay. J. Clean. Prod. 2022, 337, 130574. [Google Scholar] [CrossRef]
- Zhang, B.; Gao, M.; Tang, W.; Wang, X.; Wu, C.; Wang, Q.; Xie, H. Reduced surface sulphonic acid concentration Alleviates carbon-based solid acid catalysts deactivation in biodiesel production. Energy 2023, 271, 127079. [Google Scholar] [CrossRef]
- Karmakar, B.; Ghosh, B.; Halder, G. Sulfonated Mesua ferrea Linn Seed Shell Catalyzed Biodiesel Synthesis From Castor Oil—Response Surface Optimization. Front. Energy Res. 2020, 8, 576792. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Gurav, R.; Choi, T.-R.; Kim, H.J.; Yang, S.-Y.; Song, H.-S.; Park, J.Y.; Park, Y.-L.; Han, Y.-H.; Choi, Y.-K.; et al. Conversion of waste cooking oil into biodiesel using heterogenous catalyst derived from cork biochar. Bioresour. Technol. 2020, 302, 122872. [Google Scholar] [CrossRef] [PubMed]
- Falowo, O.A.; Betiku, E. A novel heterogeneous catalyst synthesis from agrowastes mixture and application in transesterification of yellow oleander-rubber oil: Optimization by Taguchi approach. Fuel 2022, 312, 122999. [Google Scholar] [CrossRef]
- El Yaakouby, I.; Rhrissi, I.; Abouliatim, Y.; Hlaibi, M.; Kamil, N. Moroccan sardine scales as a novel and renewable source of heterogeneous catalyst for biodiesel production using palm fatty acid distillate. Renew. Energy 2023, 217, 119223. [Google Scholar] [CrossRef]
- Soltani, S.; Rashid, U.; Al-Resayes, S.I.; Nehdi, I.A. Recent progress in synthesis and surface functionalization of mesoporous acidic heterogeneous catalysts for esterification of free fatty acid feedstocks: A review. Energy Convers. Manag. 2017, 141, 183–205. [Google Scholar] [CrossRef]
- Pasa, T.L.B.; Souza, G.K.; Diório, A.; Arroyo, P.A.; Pereira, N.C. Assessment of commercial acidic ion-exchange resin for ethyl esters synthesis from Acrocomia aculeata (Macaúba) crude oil. Renew. Energy 2020, 146, 469–476. [Google Scholar] [CrossRef]
- Maçaira, J.; Santana, A.; Recasens, F.; Angeles Larrayoz, M. Biodiesel production using supercritical methanol/carbon dioxide mixtures in a continuous reactor. Fuel 2011, 90, 2280–2288. [Google Scholar] [CrossRef]
- Rodrigues, K.L.T.; Pasa, V.M.D.; Cren, É.C. Kinetic modeling of catalytic esterification of non-edible macauba pulp oil using macroporous cation exchange resin. J. Environ. Chem. Eng. 2018, 6, 4531–4537. [Google Scholar] [CrossRef]
- Chang, F.; Zhou, Q.; Pan, H.; Liu, X.-F.; Zhang, H.; Xue, W.; Yang, S. Solid Mixed-Metal-Oxide Catalysts for Biodiesel Production: A Review. Energy Technol. 2014, 2, 865–873. [Google Scholar] [CrossRef]
- AlKahlaway, A.A.; Betiha, M.A.; Aman, D.; Rabie, A.M. Facial synthesis of ferric molybdate (Fe2(MoO4)3) nanoparticle and its efficiency for biodiesel synthesis via oleic acid esterification. Environ. Technol. Innov. 2021, 22, 101386. [Google Scholar] [CrossRef]
- Ding, J.; Zhou, C.; Wu, Z.; Chen, C.; Feng, N.; Wang, L.; Wan, H.; Guan, G. Core-shell magnetic nanomaterial grafted spongy-structured poly (ionic liquid): A recyclable brönsted acid catalyst for biodiesel production. Appl. Catal. A Gen. 2021, 616, 118080. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, L.-L.; Tan, X.; Li, H.; Yang, S. Catalytic high-yield biodiesel production from fatty acids and non-food oils over a magnetically separable acid nanosphere. Ind. Crops Prod. 2021, 173, 114126. [Google Scholar] [CrossRef]
- Changmai, B.; Rano, R.; Vanlalveni, C.; Rokhum, S.L. A novel Citrus sinensis peel ash coated magnetic nanoparticles as an easily recoverable solid catalyst for biodiesel production. Fuel 2021, 286, 119447. [Google Scholar] [CrossRef]
- Varão, L.H.R.; Silva, T.A.L.; Zamora, H.D.Z.; De Morais, L.C.; Pasquini, D. Synthesis of methyl biodiesel by esterification using magnetic nanoparticles coated with sulfonated lignin. Biomass Conv. Bioref. 2023, 13, 12277–12290. [Google Scholar] [CrossRef]
- Amouhadi, E.; Fazaeli, R.; Aliyan, H. Biodiesel production via esterification of oleic acid catalyzed by MnO2@Mn(btc) as a novel and heterogeneous catalyst. J. Chin. Chem. Soc. 2019, 66, 608–613. [Google Scholar] [CrossRef]
- Al-Jaberi, S.H.H.; Rashid, U.; Al-Doghachi, F.A.J.; Abdulkareem-Alsultan, G.; Taufiq-Yap, Y.H. Synthesis of MnO-NiO-SO4−2/ZrO2 solid acid catalyst for methyl ester production from palm fatty acid distillate. Energy Convers. Manag. 2017, 139, 166–174. [Google Scholar] [CrossRef]
- Wang, Q.; Xie, W.; Guo, L. Molybdenum and zirconium oxides supported on KIT-6 silica: A recyclable composite catalyst for one–pot biodiesel production from simulated low-quality oils. Renew. Energy 2022, 187, 907–922. [Google Scholar] [CrossRef]
- Vieira, S.S.; Magriotis, Z.M.; Santos, N.A.V.; Saczk, A.A.; Hori, C.E.; Arroyo, P.A. Biodiesel production by free fatty acid esterification using lanthanum (La3+) and HZSM-5 based catalysts. Bioresour. Technol. 2013, 133, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Rezania, S.; Mahdinia, S.; Oryani, B.; Cho, J.; Kwon, E.E.; Bozorgian, A.; Rashidi Nodeh, H.; Darajeh, N.; Mehranzamir, K. Biodiesel production from wild mustard (Sinapis Arvensis) seed oil using a novel heterogeneous catalyst of LaTiO3 nanoparticles. Fuel 2022, 307, 121759. [Google Scholar] [CrossRef]
- Albalushi, M.Y.; Abdulkreem-Alsultan, G.; Asikin-Mijan, N.; Bin Saiman, M.I.; Tan, Y.P.; Taufiq-Yap, Y.H. Efficient and Stable Rice Husk Bioderived Silica Supported Cu2S-FeS for One Pot Esterification and Transesterification of a Malaysian Palm Fatty Acid Distillate. Catalysts 2022, 12, 1537. [Google Scholar] [CrossRef]
- Patel, A.; Brahmkhatri, V. Kinetic study of oleic acid esterification over 12-tungstophosphoric acid catalyst anchored to different mesoporous silica supports. Fuel. Technol. 2013, 113, 141–149. [Google Scholar] [CrossRef]
- Singh, S.; Patel, A. 12-Tungstophosphoric acid supported on mesoporous molecular material: Synthesis, characterization and performance in biodiesel production. J. Clean. Prod. 2014, 72, 46–56. [Google Scholar] [CrossRef]
- Patel, A.; Singh, S. A green and sustainable approach for esterification of glycerol using 12-tungstophosphoric acid anchored to different supports: Kinetics and effect of support. Fuel 2014, 118, 358–364. [Google Scholar] [CrossRef]
- Chen, Y.; Cao, Y.; Suo, Y.; Zheng, G.-P.; Guan, X.-X.; Zheng, X.-C. Mesoporous solid acid catalysts of 12-tungstosilicic acid anchored to SBA-15: Characterization and catalytic properties for esterification of oleic acid with methanol. J. Taiwan Inst. Chem. Eng. 2015, 51, 186–192. [Google Scholar] [CrossRef]
- Islam, M.G.U.; Jan, M.T.; Farooq, M.; Naeem, A.; Khan, I.W.; Khattak, H.U. Biodiesel production from wild olive oil using TPA decorated Cr–Al acid heterogeneous catalyst. Chem. Eng. Res. Des. 2022, 178, 540–549. [Google Scholar] [CrossRef]
- Zhang, Q.; Luo, L.; Jin, J.; Wu, Y.; Zhang, Y. Facile Preparation of Bimetallic MOF-derived Supported Tungstophosphoric Acid Composites for Biodiesel Production. Period. Polytech. Chem. Eng. 2023, 67, 337–344. [Google Scholar] [CrossRef]
- Gong, S.; Lu, J.; Wang, H.; Liu, L.; Zhang, Q. Biodiesel production via esterification of oleic acid catalyzed by picolinic acid modified 12-tungstophosphoric acid. Appl. Energy 2014, 134, 283–289. [Google Scholar] [CrossRef]
- González, E.A.Z.; García-Guaderrama, M.; Villalobos, M.R.; Dellamary, F.L.; Kandhual, S.; Rout, N.P.; Tiznado, H.; Arizaga, G.G.C. Potassium titanate as heterogeneous catalyst for methyl transesterification. Powder Technol. 2015, 280, 201–206. [Google Scholar] [CrossRef]
- Reis, C.E.R.; Valle, G.F.; Bento, H.B.S.; Carvalho, A.K.F.; Alves, T.M.; De Castro, H.F. Sugarcane by-products within the biodiesel production chain: Vinasse and molasses as feedstock for oleaginous fungi and conversion to ethyl esters. Fuel 2020, 277, 118064. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, G. Construction of H3PMo12O40@EB-COF for Biodiesel Preparation by Heterogeneous Catalytical Esterification of Oleic Acid and Rapeseed Oil. J. Inorg. Organomet. Polym. 2023, 35, 46–57. [Google Scholar] [CrossRef]
- Cabrera-Munguia, D.A.; González, H.; Gutiérrez-Alejandre, A.; Rico, J.L.; Huirache-Acuña, R.; Maya-Yescas, R.; Del Río, R.E. Heterogeneous acid conversion of a tricaprylin-palmitic acid mixture over Al-SBA-15 catalysts: Reaction study for biodiesel synthesis. Catal. Today 2017, 282, 195–203. [Google Scholar] [CrossRef]
- Jibril, Z.I.; Ramli, A.; Jumbri, K. Al-MCM-41 Based Catalysts for Transesterification of Jatropha Oil to Biodiesel: Effect of Ce and Zr. J. Jpn. Inst. Energy 2018, 97, 200–204. [Google Scholar] [CrossRef]
- Xie, W.; Fan, M. Immobilization of tetramethylguanidine on mesoporous SBA-15 silica: A heterogeneous basic catalyst for transesterification of soybean oil. Bioresour. Technol. 2013, 139, 388–392. [Google Scholar] [CrossRef] [PubMed]
- Arun, R.; Athira, M.P.; Remello, S.N.; Haridas, S. SBA-15-SO3H catalysed room temperature synthesis of 2-aryl benzimidazoles and benzothiazoles. React. Kinet. Mech. Catal. 2023, 136, 2277–2294. [Google Scholar] [CrossRef]
- Hossain, M.; Siddik Bhuyan, M.; Md Ashraful Alam, A.; Seo, Y. Optimization of Biodiesel Production from Waste Cooking Oil Using S–TiO2/SBA-15 Heterogeneous Acid Catalyst. Catalysts 2019, 9, 67. [Google Scholar] [CrossRef]
- Melero, J.A.; Sánchez-Vázquez, R.; Vasiliadou, I.A.; Martínez Castillejo, F.; Bautista, L.F.; Iglesias, J.; Morales, G.; Molina, R. Municipal sewage sludge to biodiesel by simultaneous extraction and conversion of lipids. Energy Convers. Manag. 2015, 103, 111–118. [Google Scholar] [CrossRef]
- Naveenkumar, R.; Baskar, G. Optimization and techno-economic analysis of biodiesel production from Calophyllum inophyllum oil using heterogeneous nanocatalyst. Bioresour. Technol. 2020, 315, 123852. [Google Scholar] [CrossRef]
- Kansedo, J.; Lee, K.T.; Bhatia, S. Cerbera odollam (sea mango) oil as a promising non-edible feedstock for biodiesel production. Fuel 2009, 88, 1148–1150. [Google Scholar] [CrossRef]
- Vieira, S.S.; Magriotis, Z.M.; Ribeiro, M.F.; Graça, I.; Fernandes, A.; Lopes, J.M.F.M.; Coelho, S.M.; Santos, N.A.p.V.; Saczk, A.A. Use of HZSM-5 modified with citric acid as acid heterogeneous catalyst for biodiesel production via esterification of oleic acid. Micropor. Mesopor. Mat. 2015, 201, 160–168. [Google Scholar] [CrossRef]
- Subbiah, V.; Van Zwol, P.; Dimian, A.C.; Gitis, V.; Rothenberg, G. Glycerol Esters from Real Waste Cooking Oil Using a Robust Solid Acid Catalyst. Top. Catal. 2014, 57, 1545–1549. [Google Scholar] [CrossRef]
- Soltani, S.; Khanian, N.; Shean Yaw Choong, T.; Asim, N.; Zhao, Y. Microwave-assisted hydrothermal synthesis of sulfonated TiO2-GO core–shell solid spheres as heterogeneous esterification mesoporous catalyst for biodiesel production. Energy Convers. Manag. 2021, 238, 114165. [Google Scholar] [CrossRef]
- Nayebzadeh, H.; Saghatoleslami, N.; Tabasizadeh, M. Application of microwave irradiation for fabrication of sulfated ZrO2–Al2O3 nanocomposite via combustion method for esterification reaction: Process condition evaluation. J. Nanostruct. Chem. 2019, 9, 141–152. [Google Scholar] [CrossRef]
- Nikseresht, A.; Daniyali, A.; Ali-Mohammadi, M.; Afzalinia, A.; Mirzaie, A. Ultrasound-assisted biodiesel production by a novel composite of Fe(III)-based MOF and phosphotangestic acid as efficient and reusable catalyst. Ultrason. Sonochem. 2017, 37, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Changmai, B.; Wheatley, A.E.H.; Rano, R.; Halder, G.; Selvaraj, M.; Rashid, U.; Rokhum, S.L. A magnetically separable acid-functionalized nanocatalyst for biodiesel production. Fuel 2021, 305, 121576. [Google Scholar] [CrossRef]
- Boey, P.-L.; Ganesan, S.; Maniam, G.P.; Khairuddean, M.; Lee, S.-E. A new heterogeneous acid catalyst system for esterification of free fatty acids into methyl esters. Appl. Catal. A Gen. 2012, 433–434, 12–17. [Google Scholar] [CrossRef]
- Alhassan, F.H.; Yunus, R.; Rashid, U.; Sirat, K.; Islam, A.; Lee, H.V.; Taufiq-Yap, Y.H. Production of biodiesel from mixed waste vegetable oils using Ferric hydrogen sulphate as an effective reusable heterogeneous solid acid catalyst. Appl. Catal. A Gen. 2013, 456, 182–187. [Google Scholar] [CrossRef]
- Borah, M.J.; Devi, A.; Borah, R.; Deka, D. Synthesis and application of Co doped ZnO as heterogeneous nanocatalyst for biodiesel production from non-edible oil. Renew. Energy 2019, 133, 512–519. [Google Scholar] [CrossRef]
- Wan Omar, W.N.N.; Amin, N.A.S. Biodiesel production from waste cooking oil over alkaline modified zirconia catalyst. Fuel Process. Technol. 2011, 92, 2397–2405. [Google Scholar] [CrossRef]
- Kim, M.; DiMaggio, C.; Salley, S.O.; Simon Ng, K.Y. A new generation of zirconia supported metal oxide catalysts for converting low grade renewable feedstocks to biodiesel. Bioresour. Technol. 2012, 118, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; DiMaggio, C.; Yan, S.; Wang, H.; Salley, S.O.; Simon Ng, K.Y. Performance of heterogeneous ZrO2 supported metaloxide catalysts for brown grease esterification and sulfur removal. Bioresour. Technol. 2011, 102, 2380–2386. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.; Si, Z.; Sun, W.; Zhang, P. Sulfonated ZrO2-TiO2 nanorods as efficient solid acid catalysts for heterogeneous esterification of palmitic acid. Fuel 2019, 252, 254–261. [Google Scholar] [CrossRef]
- Madhuvilakku, R.; Piraman, S. Biodiesel synthesis by TiO2–ZnO mixed oxide nanocatalyst catalyzed palm oil transesterification process. Bioresour. Technol. 2013, 150, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Shao, G.N.; Sheikh, R.; Hilonga, A.; Lee, J.E.; Park, Y.-H.; Kim, H.T. Biodiesel production by sulfated mesoporous titania–silica catalysts synthesized by the sol–gel process from less expensive precursors. Chem. Eng. J. 2013, 215–216, 600–607. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, H.; Yang, S. Facile and Low-cost Synthesis of Mesoporous Ti–Mo Bi-metal Oxide Catalysts for Biodiesel Production from Esterification of Free Fatty Acids in Jatropha curcas/Crude Oil. J. Oleo Sci. 2018, 67, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Shuit, S.H.; Tan, S.H. Biodiesel Production via Esterification of Palm Fatty Acid Distillate Using Sulphonated Multi-walled Carbon Nanotubes as a Solid Acid Catalyst: Process Study, Catalyst paper and Kinetic Study. Bioenerg. Res. 2015, 8, 605–617. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, D.; Wang, L.; Wang, X.; Li, L.; Xing, Z.; Ji, N.; Liu, S.; Ding, H. Immobilized phosphotungstic acid based ionic liquid: Application for heterogeneous esterification of palmitic acid. Fuel 2018, 216, 364–370. [Google Scholar] [CrossRef]
- Niu, S.; Ning, Y.; Lu, C.; Han, K.; Yu, H.; Zhou, Y. Esterification of oleic acid to produce biodiesel catalyzed by sulfonated activated carbon from bamboo. Energy Convers. Manag. 2018, 163, 59–65. [Google Scholar] [CrossRef]
- Babadi, F.E.; Hosseini, S.; Soltani, S.M.; Aroua, M.K.; Shamiri, A.; Samadi, M. Sulfonated Beet Pulp as Solid Catalyst in One-Step Esterification of Industrial Palm Fatty Acid Distillate. J. Am. Oil Chem. Soc. 2016, 93, 319–327. [Google Scholar] [CrossRef]
- Karmakar, B.; Dhawane, S.H.; Halder, G. Optimization of biodiesel production from castor oil by Taguchi design. J. Environ. Chem. Eng. 2018, 6, 2684–2695. [Google Scholar] [CrossRef]
- Saimon, N.N.; Jusoh, M.; Ngadi, N.; Zakaria, Z.Y. Development of Microwave-Assisted Sulfonated Glucose Catalyst for Biodiesel Production from Palm Fatty Acid Distillate (PFAD). Bull. Chem. React. Eng. Catal. 2021, 16, 601–622. [Google Scholar] [CrossRef]
- Lokman, I.M.; Rashid, U.; Taufiq-Yap, Y.H.; Yunus, R. Methyl ester production from palm fatty acid distillate using sulfonated glucose-derived acid catalyst. Renew. Energy 2015, 81, 347–354. [Google Scholar] [CrossRef]
- Shi, W.; He, B.; Li, J. Esterification of acidified oil with methanol by SPES/PES catalytic membrane. Bioresour. Technol. 2011, 102, 5389–5393. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Garnica, E.; Paredes-Casillas, M.; Herrera-Larrasilla, T.E.; Rodríguez-Palomera, F.; Ramírez-Arreola, D.E. Analysis of recycled poly (styrene-co-butadiene) sulfonation: A new approach in solid catalysts for biodiesel production. SpringerPlus 2013, 2, 475. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Veillette, M.; Giroir-Fendler, A.; Faucheux, N.; Heitz, M. Esterification of free fatty acids with methanol to biodiesel using heterogeneous catalysts: From model acid oil to microalgae lipids. Chem. Eng. J. 2017, 308, 101–109. [Google Scholar] [CrossRef]
- Feng, Y.; He, B.; Cao, Y.; Li, J.; Liu, M.; Yan, F.; Liang, X. Biodiesel production using cation-exchange resin as heterogeneous catalyst. Bioresour. Technol. 2010, 101, 1518–1521. [Google Scholar] [CrossRef] [PubMed]
- Galia, A.; Scialdone, O.; Tortorici, E. Transesterification of rapeseed oil over acid resins promoted by supercritical carbon dioxide. J. Supercrit. Fluid. 2011, 56, 186–193. [Google Scholar] [CrossRef][Green Version]
- Vicente, G.; Carrero, A.; Rodríguez, R.; Del Peso, G.L. Heterogeneous-catalysed direct transformation of microalga biomass into Biodiesel-Grade FAMEs. Fuel 2017, 200, 590–598. [Google Scholar] [CrossRef]
- Abidin, S.Z.; Mohammed, M.L.; Saha, B. Two-Stage Conversion of Used Cooking Oil to Biodiesel Using Ion Exchange Resins as Catalysts. Catalysts 2023, 13, 1209. [Google Scholar] [CrossRef]
- Liu, L.; Liu, Z.; Tang, G.; Tan, W. Esterification of free fatty acids in waste cooking oil by heterogeneous catalysts. Trans. Tianjin Univ. 2014, 20, 266–272. [Google Scholar] [CrossRef]
- Feng, Y.; Zhang, A.; Li, J.; He, B. A continuous process for biodiesel production in a fixed bed reactor packed with cation-exchange resin as heterogeneous catalyst. Bioresour. Technol. 2011, 102, 3607–3609. [Google Scholar] [CrossRef] [PubMed]
- Rozina; Chia, S.R.; Ahmad, M.; Sultana, S.; Zafar, M.; Asif, S.; Bokhari, A.; Nomanbhay, S.; Mubashir, M.; Khoo, K.S.; et al. Green synthesis of biodiesel from Citrus medica seed oil using green nanoparticles of copper oxide. Fuel 2022, 323, 124285. [Google Scholar] [CrossRef]
- Su, M.; Yang, R.; Li, M. Biodiesel production from hempseed oil using alkaline earth metal oxides supporting copper oxide as bi-functional catalysts for transesterification and selective hydrogenation. Fuel 2013, 103, 398–407. [Google Scholar] [CrossRef]
- Pozos, J.A.; Esquivel, G.C.; Toriello, V.A.S.; Vargas, C.E.S.; Rivas, O.A.; Martínez, O.U.V.; López, A.T.; Rodriguez, J.A. State of Art of Alkaline Earth Metal Oxides Catalysts Used in the Transesterification of Oils for Biodiesel Production. Energies 2021, 14, 1031. [Google Scholar] [CrossRef]
- Boey, P.-L.; Maniam, G.P.; Hamid, S.A. Performance of calcium oxide as a heterogeneous catalyst in biodiesel production: A review. Chem. Eng. J. 2011, 168, 15–22. [Google Scholar] [CrossRef]
- Zul, N.A.; Ganesan, S.; Hamidon, T.S.; Oh, W.-D.; Hussin, M.H. A review on the utilization of calcium oxide as a base catalyst in biodiesel production. J. Environ. Chem. Eng. 2021, 9, 105741. [Google Scholar] [CrossRef]
- Kesic, Z.; Lukic, I.; Zdujic, M.; Mojovic, L.; Skala, D. Calcium oxide based catalysts for biodiesel production: A review. Chem. Ind. Chem. Eng. Q. 2016, 22, 391–408. [Google Scholar] [CrossRef]
- Ling, J.S.J.; Tan, Y.H.; Mubarak, N.M.; Kansedo, J.; Saptoro, A.; Nolasco-Hipolito, C. A review of heterogeneous calcium oxide based catalyst from waste for biodiesel synthesis. SN Appl. Sci. 2019, 1, 810. [Google Scholar] [CrossRef]
- Mazaheri, H.; Ong, H.C.; Amini, Z.; Masjuki, H.H.; Mofijur, M.; Su, C.H.; Anjum Badruddin, I.; Khan, T.M.Y. An Overview of Biodiesel Production via Calcium Oxide Based Catalysts: Current State and Perspective. Energies 2021, 14, 3950. [Google Scholar] [CrossRef]
- Shan, R.; Zhao, C.; Lv, P.; Yuan, H.; Yao, J. Catalytic applications of calcium rich waste materials for biodiesel: Current state and perspectives. Energy Convers. Manag. 2016, 127, 273–283. [Google Scholar] [CrossRef]
- Granados, M.L.; Poves, M.D.Z.; Alonso, D.M.; Mariscal, R.; Galisteo, F.C.; Moreno-Tost, R.; Santamaría, J.; Fierro, J.L.G. Biodiesel from sunflower oil by using activated calcium oxide. Appl. Catal. B Environ. 2007, 73, 317–326. [Google Scholar] [CrossRef]
- Sringam, S.; Witoon, T.; Wattanakit, C.; Donphai, W.; Chareonpanich, M.; Rupprechter, G.; Seubsai, A. Effect of calcination temperature on the performance of K-Co/Al2O3 catalyst for oxidative coupling of methane. Carbon Resour. Convers. 2024, 8, 100261. [Google Scholar] [CrossRef]
- Soosai, M.R.; Moorthy, I.M.G.; Varalakshmi, P.; Yonas, C.J. Integrated global optimization and process modelling for biodiesel production from non-edible silk-cotton seed oil by microwave-assisted transesterification with heterogeneous calcium oxide catalyst. J. Clean. Prod. 2022, 367, 132946. [Google Scholar] [CrossRef]
- Olubunmi, B.E.; Alade, A.F.; Ebhodaghe, S.O.; Oladapo, O.T. Optimization and kinetic study of biodiesel production from beef tallow using calcium oxide as a heterogeneous and recyclable catalyst. Energy Convers. Manag. X 2022, 14, 100221. [Google Scholar] [CrossRef]
- Woo, J.; Joshi, R.; Park, Y.-K.; Jeon, J.-K. Biodiesel production from jatropha seeds with bead-type heterogeneous catalyst. Korean J. Chem. Eng. 2021, 38, 763–770. [Google Scholar] [CrossRef]
- Widiarti, N.; Lailun Ni’mah, Y.; Bahruji, H.; Prasetyoko, D. Development of CaO From Natural Calcite as a Heterogeneous Base Catalyst in the Formation of Biodiesel: Review. J. Renew. Mater. 2019, 7, 915–939. [Google Scholar] [CrossRef]
- Pasupulety, N.; Gunda, K.; Liu, Y.; Rempel, G.L.; Ng, F.T.T. Production of biodiesel from soybean oil on CaO/Al2O3 solid base catalysts. Appl. Catal. A Gen. 2013, 452, 189–202. [Google Scholar] [CrossRef]
- Ooi, H.K.; Koh, X.N.; Ong, H.C.; Lee, H.V.; Mastuli, M.S.; Taufiq-Yap, Y.H.; Alharthi, F.A.; Alghamdi, A.A.; Asikin Mijan, N. Progress on Modified Calcium Oxide Derived Waste-Shell Catalysts for Biodiesel Production. Catalysts 2021, 11, 194. [Google Scholar] [CrossRef]
- Xu, Y.; Nordblad, M.; Woodley, J.M. A two-stage enzymatic ethanol-based biodiesel production in a packed bed reactor. J. Biotechnol. Curr. Res. Future Perspect. Appl. Biotechnol. 2012, 162, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Basumatary, S.F.; Brahma, S.; Hoque, M.; Das, B.K.; Selvaraj, M.; Brahma, S.; Basumatary, S. Advances in CaO-based catalysts for sustainable biodiesel synthesis. Green Energy Resour. 2023, 1, 100032. [Google Scholar] [CrossRef]
- Teo, S.H.; Rashid, U.; Taufiq-Yap, Y.H. Biodiesel is produced from crude Jatropha Curcas oil using calcium based mixed oxide catalysts. Fuel 2014, 136, 244–252. [Google Scholar] [CrossRef]
- Reyero, I.; Arzamendi, G.; Gandía, L.M. Heterogenization of the biodiesel synthesis catalysis: CaO and novel calcium compounds as transesterification catalysts. Chem. Eng. Res. Des. Green Process. Eco-Technol.–Focus Biofuels 2014, 92, 1519–1530. [Google Scholar] [CrossRef]
- Meng, Y.-L.; Wang, B.-Y.; Li, S.-F.; Tian, S.-J.; Zhang, M.-H. Effect of calcination temperature on the activity of solid Ca/Al composite oxide-based alkaline catalyst for biodiesel production. Bioresour. Technol. 2013, 128, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhang, Z.; Xu, Y.; Liu, Q.; Qian, G. CaFeAl mixed oxide derived heterogeneous catalysts for transesterification of soybean oil to biodiesel. Bioresour. Technol. 2015, 190, 438–441. [Google Scholar] [CrossRef] [PubMed]
- Solis, J.L.; Alejo, L.; Kiros, Y. Calcium and tin oxides for heterogeneous transesterification of Babasssu oil (Attalea speciosa). J. Environ. Chem. Eng. 2016, 4, 4870–4877. [Google Scholar] [CrossRef]
- Korbag, S.; Korbag, I. Trans-esterification of Non-Edible Oil with a CaO-MgO heterogeneous Catalyst to Produce Biodiesel. Eurasian Chem. Commun. 2023, 285, 10.22034. [Google Scholar] [CrossRef]
- Maleki, H.; Kazemeini, M.; Larimi, A.S.; Khorasheh, F. Transesterification of canola oil and methanol by lithium impregnated CaO–La2O3 mixed oxide for biodiesel synthesis. J. Ind. Eng. Chem. 2017, 47, 399–404. [Google Scholar] [CrossRef]
- De S Barros, S.; Pessoa Junior, W.A.G.; Sá, I.S.C.; Takeno, M.L.; Nobre, F.X.; Pinheiro, W.; Manzato, L.; Iglauer, S.; De Freitas, F.A. Pineapple (Ananás comosus) leaves ash as a solid base catalyst for biodiesel synthesis. Bioresour. Technol. 2020, 312, 123569. [Google Scholar] [CrossRef] [PubMed]
- Ngaini, Z.; Jamil, N.; Wahi, R.; Shahrom, F.D.; Ahmad, Z.A.; Farooq, S. Convenient Conversion of Palm Fatty Acid Distillate to Biodiesel via Rice Husk Ash Catalyst. BioEnergy Res. 2022, 15, 1316–1326. [Google Scholar] [CrossRef]
- Gohain, M.; Laskar, K.; Phukon, H.; Bora, U.; Kalita, D.; Deka, D. Towards sustainable biodiesel and chemical production: Multifunctional use of heterogeneous catalyst from littered Tectona grandis leaves. Waste Manag. 2020, 102, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Obadiah, A.; Swaroopa, G.A.; Kumar, S.V.; Jeganathan, K.R.; Ramasubbu, A. Biodiesel production from Palm oil using calcined waste animal bone as catalyst. Bioresour. Technol. 2012, 116, 512–516. [Google Scholar] [CrossRef] [PubMed]
- Viriya-empikul, N.; Krasae, P.; Puttasawat, B.; Yoosuk, B.; Chollacoop, N.; Faungnawakij, K. Waste shells of mollusk and egg as biodiesel production catalysts. Bioresour. Technol. 2010, 101, 3765–3767. [Google Scholar] [CrossRef] [PubMed]
- Boey, P.-L.; Maniam, G.P.; Hamid, S.A. Biodiesel production via transesterification of palm olein using waste mud crab (Scylla serrata) shell as a heterogeneous catalyst. Bioresour. Technol. 2009, 100, 6362–6368. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.M.; Oopathum, C.; Weeramongkhonlert, V.; Smith, C.B.; Chaveanghong, S.; Ketwong, P.; Boonyuen, S. Transesterification of soybean oil using bovine bone waste as new catalyst. Bioresour. Technol. 2013, 143, 686–690. [Google Scholar] [CrossRef] [PubMed]
- Putra, M.D.; Irawan, C.; Udiantoro; Ristianingsih, Y.; Nata, I.F. A cleaner process for biodiesel production from waste cooking oil using waste materials as a heterogeneous catalyst and its kinetic study. J. Clean. Prod. 2018, 195, 1249–1258. [Google Scholar] [CrossRef]
- Madhu, D.; Chavan, S.B.; Singh, V.; Singh, B.; Sharma, Y.C. An economically viable synthesis of biodiesel from a crude Millettia pinnata oil of Jharkhand, India as feedstock and crab shell derived catalyst. Bioresour. Technol. 2016, 214, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Roschat, W.; Siritanon, T.; Kaewpuang, T.; Yoosuk, B.; Promarak, V. Economical and green biodiesel production process using river snail shells-derived heterogeneous catalyst and co-solvent method. Bioresour. Technol. 2016, 209, 343–350. [Google Scholar] [CrossRef] [PubMed]
- De Jesus De Oliveira, C.; Teleken, J.G.; Alves, H.J. Catalytic efficiency of the eggshell calcined and enriched with glycerin in the synthesis of biodiesel from frying residual oil. Environ. Sci. Pollut. Res. 2020, 27, 17878–17890. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Hossain, A.K.; Griffiths, G.; Duraisamy, G.; Jacob Thomas, J. Investigation on yield, fuel properties, ageing and low temperature flow of fish oil esters. Energy Convers. Manag. X 2022, 14, 100217. [Google Scholar] [CrossRef]
- Wei, Z.; Xu, C.; Li, B. Application of waste eggshell as low-cost solid catalyst for biodiesel production. Bioresour. Technol. 2009, 100, 2883–2885. [Google Scholar] [CrossRef] [PubMed]
- Birla, A.; Singh, B.; Upadhyay, S.N.; Sharma, Y.C. Kinetics studies of synthesis of biodiesel from waste frying oil using a heterogeneous catalyst derived from snail shell. Bioresour. Technol. 2012, 106, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Kirubakaran, M.; Arul Mozhi Selvan, V. Eggshell as heterogeneous catalyst for synthesis of biodiesel from high free fatty acid chicken fat and its working characteristics on a CI engine. J. Environ. Chem. Eng. 2018, 6, 4490–4503. [Google Scholar] [CrossRef]
- Gollakota, A.R.K.; Volli, V.; Shu, C.-M. Transesterification of waste cooking oil using pyrolysis residue supported eggshell catalyst. Sci. Total Environ. 2019, 661, 316–325. [Google Scholar] [CrossRef] [PubMed]
- Rahman, W.U.; Fatima, A.; Anwer, A.H.; Athar, M.; Khan, M.Z.; Khan, N.A.; Halder, G. Biodiesel synthesis from eucalyptus oil by utilizing waste egg shell derived calcium based metal oxide catalyst. Process Saf. Environ. Prot. 2019, 122, 313–319. [Google Scholar] [CrossRef]
- Proença, B.D.S.G.; Fioroto, P.O.; Heck, S.C.; Duarte, V.A.; Cardozo Filho, L.; Feihrmann, A.C.; Beneti, S.C. Obtention of methyl esters from macauba oil using egg shell catalyst. Chem. Eng. Res. Des. 2021, 169, 288–296. [Google Scholar] [CrossRef]
- Marinković, D.M.; Stanković, M.V.; Veličković, A.V.; Avramović, J.M.; Miladinović, M.R.; Stamenković, O.O.; Veljković, V.B.; Jovanović, D.M. Calcium oxide as a promising heterogeneous catalyst for biodiesel production: Current state and perspectives. Renew. Sust. Energy Rev. 2016, 56, 1387–1408. [Google Scholar] [CrossRef]
- Kurayama, F.; Yoshikawa, T.; Furusawa, T.; Bahadur, N.M.; Handa, H.; Sato, M.; Suzuki, N. Microcapsule with a heterogeneous catalyst for the methanolysis of rapeseed oil. Bioresour. Technol. 2013, 135, 652–658. [Google Scholar] [CrossRef] [PubMed]
- Elias, S.; Rabiu, A.M.; Okeleye, B.I.; Okudoh, V.; Oyekola, O. Bifunctional Heterogeneous Catalyst for Biodiesel Production from Waste Vegetable Oil. Appl. Sci. 2020, 10, 3153. [Google Scholar] [CrossRef]
- Kataria, J.; Mohapatra, S.K.; Kundu, K. Biodiesel production from waste cooking oil using heterogeneous catalysts and its operational characteristics on variable compression ratio CI engine. J. Energy Inst. 2019, 92, 275–287. [Google Scholar] [CrossRef]
- Chouhan, A.P.S.; Sarma, A.K. Modern heterogeneous catalysts for biodiesel production: A comprehensive review. Renew. Sustain. Energy Rev. 2011, 15, 4378–4399. [Google Scholar] [CrossRef]
- Pavlović, S.M.; Marinković, D.M.; Kostić, M.D.; Lončarević, D.R.; Mojović, L.V.; Stanković, M.V.; Veljković, V.B. The chicken eggshell calcium oxide ultrasonically dispersed over lignite coal fly ash-based cancrinite zeolite support as a catalyst for biodiesel production. Fuel 2021, 289, 119912. [Google Scholar] [CrossRef]
- Dong, S.; Liu, L.; Xu, W.; Cheng, H.; He, Z.; Dong, F.; Wang, D.; Wang, L.; Song, S.; Ma, J. Explore synergistic catalytic ozonation by dual active sites of oxygen vacancies and defects in MgO/biochar for atrazine degradation. J. Environ. Chem. Eng. 2024, 12, 114221. [Google Scholar] [CrossRef]
- Zhu, Z.; Shi, X.; Rao, Y.; Huang, Y. Recent progress of MgO-based materials in CO2 adsorption and conversion: Modification methods, reaction condition, and CO2 hydrogenation. Chin. Chem. Lett. 2024, 35, 108954. [Google Scholar] [CrossRef]
- AbdelSalam, H.; El-Maghrbi, H.H.; Zahran, F.; Zaki, T. Microwave-assisted production of biodiesel using metal-organic framework Mg3(bdc)3(H2O)2. Korean J. Chem. Eng. 2020, 37, 670–676. [Google Scholar] [CrossRef]
- Tajuddin, N.A. MgAl Mixed Oxide Derived Alkali-free Hydrotalcite for Transesterification of Waste Cooking Oil to Biodiesel. ASM Sci. J. 2020, 13, 1–7. [Google Scholar] [CrossRef]
- Banerjee, S.; Sahani, S.; Chandra Sharma, Y. Process dynamic investigations and emission analyses of biodiesel produced using Sr–Ce mixed metal oxide heterogeneous catalyst. J. Environ. Manag. 2019, 248, 109218. [Google Scholar] [CrossRef] [PubMed]
- Al-Saadi, A.; Mathan, B.; He, Y. Esterification and transesterification over SrO–ZnO/Al2O3 as a novel bifunctional catalyst for biodiesel production. Renew. Energy 2020, 158, 388–399. [Google Scholar] [CrossRef]
- Sahani, S.; Roy, T.; Sharma, Y.C. Smart Waste Manag.of waste cooking oil for large scale high quality biodiesel production using Sr-Ti mixed metal oxide as solid catalyst: Optimization and E-metrics studies. Waste Manag. 2020, 108, 189–201. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Niu, S.; Wang, J.; Zhou, W.; Wang, Y.; Han, K.; Lu, C. Mesoporous SrTiO3 perovskite as a heterogeneous catalyst for biodiesel production: Experimental and DFT studies. Renew. Energy 2022, 184, 164–175. [Google Scholar] [CrossRef]
- Mustata, F.; Bicu, I. The Optimization of the Production of Methyl Esters from Corn Oil Using Barium Hydroxide as a Heterogeneous Catalyst. J. Am. Oil. Chem. Soc. 2014, 91, 839–847. [Google Scholar] [CrossRef]
- Saha, R.; Goud, V.V. Ultrasound assisted transesterification of high free fatty acids karanja oil using heterogeneous base catalysts. Biomass Conv. Bioref. 2015, 5, 195–207. [Google Scholar] [CrossRef]
- Endalew, A.K.; Kiros, Y.; Zanzi, R. Inorganic heterogeneous catalysts for biodiesel production from vegetable oils. Biomass Bioenergy 2011, 35, 3787–3809. [Google Scholar] [CrossRef]
- Niju, S.; Janaranjani, A.; Nanthini, R.; Sindhu, P.A.; Balajii, M. Valorization of banana pseudostem as a catalyst for transesterification process and its optimization studies. Biomass Conv. Bioref. 2023, 13, 1805–1818. [Google Scholar] [CrossRef]
- Samart, C.; Sreetongkittikul, P.; Sookman, C. Heterogeneous catalysis of transesterification of soybean oil using KI/mesoporous silica. Fuel Process. Technol. 2009, 90, 922–925. [Google Scholar] [CrossRef]
- Naeem, M.M.; Al-Sakkari, E.G.; Boffito, D.C.; Gadalla, M.A.; Ashour, F.H. One-pot conversion of highly acidic waste cooking oil into biodiesel over a novel bio-based bi-functional catalyst. Fuel 2021, 283, 118914. [Google Scholar] [CrossRef]
- Roy, T.; Sahani, S.; Sharma, Y.C. Green synthesis of biodiesel from Ricinus communis oil (castor seed oil) using potassium promoted lanthanum oxide catalyst: Kinetic, thermodynamic and environmental studies. Fuel 2020, 274, 117644. [Google Scholar] [CrossRef]
- Goudarzi, F.; Izadbakhsh, A. Evaluation of K/SnO2 performance as a solid catalyst in the trans-esterification of a mixed plant oil. Reac. Kinet. Mech. Cat. 2017, 121, 539–553. [Google Scholar] [CrossRef]
- Anastopoulos, G.; Dodos, G.S.; Kalligeros, S.; Zannikos, F. Methanolysis of sunflower oil and used frying oil using LiNO3/CaO as a solid base catalyst. Int. J. Ambient. Energy 2013, 34, 73–82. [Google Scholar] [CrossRef]
- Botti, R.F.; Innocentini, M.D.M.; Faleiros, T.A.; Mello, M.F.; Flumignan, D.L.; Santos, L.K.; Franchin, G.; Colombo, P. Biodiesel Processing Using Sodium and Potassium Geopolymer Powders as Heterogeneous Catalysts. Molecules 2020, 25, 2839. [Google Scholar] [CrossRef] [PubMed]
- Al-Sakkari, E.G.; Attia, N.K.; Habashy, M.M.; Abdeldayem, O.M.; Mostafa, S.R.; El-Sheltawy, S.T.; Abadir, M.F.; Mostafa, M.K.; Rene, E.R.; Elnashaie, S.S.E.H. A bi-functional alginate-based composite for catalyzing one-pot methyl esters synthesis from waste cooking oil having high acidity. Fuel 2021, 306, 121637. [Google Scholar] [CrossRef]
- Encinar, J.M.; González, J.F.; Martínez, G.; Nogales-Delgado, S. Use of NaNO3/SiAl as Heterogeneous Catalyst for Fatty Acid Methyl Ester Production from Rapeseed Oil. Catalysts 2021, 11, 1405. [Google Scholar] [CrossRef]
- Naeem, A.; Wali Khan, I.; Farooq, M.; Mahmood, T.; Ud Din, I.; Ali Ghazi, Z.; Saeed, T. Kinetic and optimization study of sustainable biodiesel production from waste cooking oil using novel heterogeneous solid base catalyst. Bioresour. Technol. 2021, 328, 124831. [Google Scholar] [CrossRef] [PubMed]
- Jamil, U.; Husain Khoja, A.; Liaquat, R.; Raza Naqvi, S.; Nor Nadyaini Wan Omar, W.; Aishah Saidina Amin, N. Copper and calcium-based metal organic framework (MOF) catalyst for biodiesel production from waste cooking oil: A process optimization study. Energy Convers. Manag. 2020, 215, 112934. [Google Scholar] [CrossRef]
- Tamjidi, S.; Kamyab Moghadas, B.; Esmaeili, H. Ultrasound-assisted biodiesel generation from waste edible oil using CoFe2O4@GO as a superior and reclaimable nanocatalyst: Optimization of two-step transesterification by RSM. Fuel 2022, 327, 125170. [Google Scholar] [CrossRef]
- Baskar, G.; Aberna Ebenezer Selvakumari, I.; Aiswarya, R. Biodiesel production from castor oil using heterogeneous Ni doped ZnO nanocatalyst. Bioresour. Technol. 2018, 250, 793–798. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Wu, H.B.; Xie, Y.; Lou, X.W. Mixed Transition-Metal Oxides: Design, Synthesis, and Energy-Related Applications. Angew. Chem. Int. Ed. 2014, 53, 1488–1504. [Google Scholar] [CrossRef] [PubMed]
- Dantas, J.; Leal, E.; Cornejo, D.R.; Kiminami, R.H.G.A.; Costa, A.C.F.M. Biodiesel production evaluating the use and reuse of magnetic nanocatalysts Ni0.5Zn0.5Fe2O4 synthesized in pilot-scale. Arab J. Chem. 2020, 13, 3026–3042. [Google Scholar] [CrossRef]
- Raj, J.V.A.; Bharathiraja, B.; Vijayakumar, B.; Arokiyaraj, S.; Iyyappan, J.; Kumar, R.P. Biodiesel production from microalgae Nannochloropsis oculata using heterogeneous Poly Ethylene Glycol (PEG) encapsulated ZnOMn2+ nanocatalyst. Bioresour. Technol. 2019, 282, 348–352. [Google Scholar] [CrossRef]
- Singh, D.; Ganesh, A.; Mahajani, S. Heterogeneous catalysis for biodiesel synthesis and valorization of glycerol. Clean. Techn. Environ. Policy 2015, 17, 1103–1110. [Google Scholar] [CrossRef]
- Kalavathy, G.; Baskar, G. Synergism of clay with zinc oxide as nanocatalyst for production of biodiesel from marine Ulva lactuca. Bioresour. Technol. 2019, 281, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, S.; Yang, R.; Yan, Y. Biodiesel production from vegetable oil using heterogenous acid and alkali catalyst. Fuel 2010, 89, 2939–2944. [Google Scholar] [CrossRef]
- Vujicic, D.j.; Comic, D.; Zarubica, A.; Micic, R.; Boskovic, G. Kinetics of biodiesel synthesis from sunflower oil over CaO heterogeneous catalyst. Fuel 2010, 89, 2054–2061. [Google Scholar] [CrossRef]
- Choudhury, H.A.; Chakma, S.; Moholkar, V.S. Mechanistic insight into sonochemical biodiesel synthesis using heterogeneous base catalyst. Ultrason. Sonochem. 2014, 21, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Caballero, J.M.; Santamaría-González, J.; Mérida-Robles, J.; Moreno-Tost, R.; Jiménez-López, A.; Maireles-Torres, P. Calcium zincate as precursor of active catalysts for biodiesel production under mild conditions. Appl. Catal. B Environ. 2009, 91, 339–346. [Google Scholar] [CrossRef]
- Ho, W.W.S.; Ng, H.K.; Gan, S. Development and characterisation of novel heterogeneous palm oil mill boiler ash-based catalysts for biodiesel production. Bioresour. Technol. 2012, 125, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Sharma, R. Real-time monitoring of physicochemical parameters in water using big data and smart IoT sensors. Environ. Dev. Sustain. 2022, 26, 22013–22060. [Google Scholar] [CrossRef]
- Uprety, B.K.; Chaiwong, W.; Ewelike, C.; Rakshit, S.K. Biodiesel production using heterogeneous catalysts including wood ash and the importance of enhancing byproduct glycerol purity. Energy Convers. Manag. 2016, 115, 191–199. [Google Scholar] [CrossRef]
- Degirmenbasi, N.; Coskun, S.; Boz, N.; Kalyon, D.M. Biodiesel synthesis from canola oil via heterogeneous catalysis using functionalized CaO nanoparticles. Fuel 2015, 153, 620–627. [Google Scholar] [CrossRef]
- Torkzaban, S.; Feyzi, M.; Norouzi, L. A novel robust CaO/ZnFe2O4 hollow magnetic microspheres heterogenous catalyst for synthesis biodiesel from waste frying sunflower oil. Renew. Energy 2022, 200, 996–1007. [Google Scholar] [CrossRef]
- Witoon, T.; Bumrungsalee, S.; Vathavanichkul, P.; Palitsakun, S.; Saisriyoot, M.; Faungnawakij, K. Biodiesel production from transesterification of palm oil with methanol over CaO supported on bimodal meso-macroporous silica catalyst. Bioresour. Technol. 2014, 156, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Anastopoulos, G.; Dodos, G.S.; Kalligeros, S.; Zannikos, F. CaO loaded with Sr(NO3)2 as a heterogeneous catalyst for biodiesel production from cottonseed oil and waste frying oil. Biomass Conv. Bioref. 2013, 3, 169–177. [Google Scholar] [CrossRef]
- Lei, X.; Lu, W.; Peng, Q.; Li, H.; Chen, T.; Xu, S.; Zhang, F. Activated MgAl-layered double hydroxide as solid base catalysts for the conversion of fatty acid methyl esters to monoethanolamides. Appl. Catal. A Gen. 2011, 399, 87–92. [Google Scholar] [CrossRef]
- Lima, J.R.D.O.; Ghani, Y.A.; Da Silva, R.B.; Batista, F.M.C.; Bini, R.A.; Varanda, L.C.; De Oliveira, J.E. Strontium zirconate heterogeneous catalyst for biodiesel production: Synthesis, characterization and catalytic activity evaluation. Appl. Catal. A Gen. 2012, 445–446, 76–82. [Google Scholar] [CrossRef]
- Rashtizadeh, E.; Farzaneh, F.; Talebpour, Z. Synthesis and characterization of Sr3Al2O6 nanocomposite as catalyst for biodiesel production. Bioresour. Technol. 2014, 154, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-L.; Huang, C.-C.; Tran, D.-T.; Chang, J.-S. Biodiesel synthesis via heterogeneous catalysis using modified strontium oxides as the catalysts. Bioresour. Technol. 2012, 113, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Kumar, A.; Sharma, Y.C. Biodiesel synthesis from microalgae (Anabaena PCC 7120) by using barium titanium oxide (Ba2TiO4) solid base catalyst. Bioresour. Technol. 2019, 287, 121357. [Google Scholar] [CrossRef] [PubMed]
- Betiku, E.; Akintunde, A.M.; Ojumu, T.V. Banana peels as a biobase catalyst for fatty acid methyl esters production using Napoleon’s plume (Bauhinia monandra) seed oil: A process parameters optimization study. Energy 2016, 103, 797–806. [Google Scholar] [CrossRef]
- Shahraki, H.; Entezari, M.H.; Goharshadi, E.K. Sono-synthesis of biodiesel from soybean oil by KF/γ-Al2O3 as a nano-solid-base catalyst. Ultrason. Sonochem. 2015, 23, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Boz, N.; Degirmenbasi, N.; Kalyon, D.M. Conversion of biomass to fuel: Transesterification of vegetable oil to biodiesel using KF loaded nano-γ-Al2O3 as catalyst. Appl. Catal. B Environ. 2009, 89, 590–596. [Google Scholar] [CrossRef]
- Vyas, A.P.; Subrahmanyam, N.; Patel, P.A. Production of biodiesel through transesterification of Jatropha oil using KNO3/Al2O3 solid catalyst. Fuel 2009, 88, 625–628. [Google Scholar] [CrossRef]
- Liang, X.; Gao, S.; Wu, H.; Yang, J. Highly efficient procedure for the synthesis of biodiesel from soybean oil. Fuel Process. Technol. 2009, 90, 701–704. [Google Scholar] [CrossRef]
- Pukale, D.D.; Maddikeri, G.L.; Gogate, P.R.; Pandit, A.B.; Pratap, A.P. Ultrasound assisted transesterification of waste cooking oil using heterogeneous solid catalyst. Ultrason. Sonochem. 2015, 22, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Al-Zaini, E.O.; Olsen, J.; Nguyen, T.H.; Adesina, A. Transesterification of Waste Cooking Oil in Presence of Crushed Seashell as a Support for Solid Heterogeneous Catalyst. SAE Int. J. Fuels Lubr. 2011, 4, 139–157. [Google Scholar] [CrossRef]
- Zaki, A.H.; Naeim, A.A.; EL-Dek, S.I. Sodium titanate nanotubes for efficient transesterification of oils into biodiesel. Environ. Sci. Pollut. Res. 2019, 26, 36388–36400. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.W.; Naeem, A.; Farooq, M.; Din, I.U.; Ghazi, Z.A.; Saeed, T. Reusable Na-SiO2@CeO2 catalyst for efficient biodiesel production from non-edible wild olive oil as a new and potential feedstock. Energy Convers. Manag. 2021, 231, 113854. [Google Scholar] [CrossRef]
- Umdu, E.S.; Tuncer, M.; Seker, E. Transesterification of Nannochloropsis oculata microalga’s lipid to biodiesel on Al2O3 supported CaO and MgO catalysts. Bioresour. Technol. 2009, 100, 2828–2831. [Google Scholar] [CrossRef] [PubMed]
- Mello, V.M.; Pousa, G.P.A.G.; Pereira, M.S.C.; Dias, I.M.; Suarez, P.A.Z. Metal oxides as heterogeneous catalysts for esterification of fatty acids obtained from soybean oil. Fuel Process. Technol. 2011, 92, 53–57. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, J.; Choi, S.; Zhu, R.; Tang, S.; Bond, J.Q.; Tavlarides, L.L. Heterogeneous catalytic esterification of oleic acid under subsupercritical methanol over γ-Al2O3. Fuel 2020, 268, 117359. [Google Scholar] [CrossRef]
- Benjapornkulaphong, S.; Ngamcharussrivichai, C.; Bunyakiat, K. Al2O3-supported alkali and alkali earth metal oxides for transesterification of palm kernel oil and coconut oil. Chem. Eng. J. 2009, 145, 468–474. [Google Scholar] [CrossRef]
- Gurunathan, B.; Ravi, A. Biodiesel production from waste cooking oil using copper doped zinc oxide nanocomposite as heterogeneous catalyst. Bioresour. Technol. 2015, 188, 124–127. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wei, F.; Zhang, Y.; Wei, F.; Ma, P.; Zheng, W.; Zhao, Y.; Chen, H. Biodiesel Production by Catalytic Esterification of Oleic Acid over Copper (II)–Alginate Complexes. J. Oleo. Sci. 2017, 66, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Jamil, F.; Al-Muhtaseb, A.; Myint, M.T.Z.; Al-Hinai, M.; Al-Haj, L.; Baawain, M.; Al-Abri, M.; Kumar, G.; Atabani, A.E. Biodiesel production by valorizing waste Phoenix dactylifera L. Kernel oil in the presence of synthesized heterogeneous metallic oxide catalyst (Mn@MgO-ZrO2). Energy Convers. Manag. 2018, 155, 128–137. [Google Scholar] [CrossRef]
- Gombotz, K.; Parette, R.; Austic, G.; Kannan, D.; Matson, J.V. MnO and TiO solid catalysts with low-grade feedstocks for biodiesel production. Fuel 2012, 92, 9–15. [Google Scholar] [CrossRef]
- Jaiswal, S.; Sharma, Y.C. Ni modified distillation waste derived heterogeneous catalyst utilized for the production of glycerol carbonate from a biodiesel by-product glycerol: Optimization and green metric studies. Waste Manag. 2023, 156, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Fereidooni, L.; Pirkarami, A.; Ghasemi, E.; Kasaeian, A. Using ZnAl–LDH@SiO2 as a catalyst for the electrocatalytic conversion of waste frying oil into biodiesel. Energy Convers. Manag. 2023, 296, 117646. [Google Scholar] [CrossRef]
- Marso, T.M.M.; Kalpage, C.S.; Udugala-Ganehenege, M.Y. ZnO/CuO composite catalyst to pre-esterify waste coconut oil for producing biodiesel in high yield. Reac. Kinet. Mech. Cat. 2021, 132, 935–966. [Google Scholar] [CrossRef]
- Corro, G.; Pal, U.; Tellez, N. Biodiesel production from Jatropha curcas crude oil using ZnO/SiO2 photocatalyst for free fatty acids esterification. Appl. Catal. B Environ. 2013, 129, 39–47. [Google Scholar] [CrossRef]
- Loy, A.C.M.; Quitain, A.T.; Lam, M.K.; Yusup, S.; Sasaki, M.; Kida, T. Development of high microwave-absorptive bifunctional graphene oxide-based catalyst for biodiesel production. Energy Convers. Manag. 2019, 180, 1013–1025. [Google Scholar] [CrossRef]
- Mukhtar, A.; Saqib, S.; Lin, H.; Hassan Shah, M.U.; Ullah, S.; Younas, M.; Rezakazemi, M.; Ibrahim, M.; Mahmood, A.; Asif, S.; et al. Current status and challenges in the heterogeneous catalysis for biodiesel production. Renew. Sust. Energy Rev. 2022, 157, 112012. [Google Scholar] [CrossRef]
- Feyzi, M.; Hosseini, N.; Yaghobi, N.; Ezzati, R. Preparation, characterization, kinetic and thermodynamic studies of MgO-La2O3 nanocatalysts for biodiesel production from sunflower oil. Chem. Phys. Lett. 2017, 677, 19–29. [Google Scholar] [CrossRef]
- Mawlid, O.A.; Abdelhady, H.H.; El-Deab, M.S. Boosted biodiesel production from waste cooking oil using novel SrO/MgFe2O4 magnetic nanocatalyst at low temperature: Optimization process. Energy Convers. Manag. 2022, 273, 116435. [Google Scholar] [CrossRef]
- Qu, T.; Niu, S.; Zhang, X.; Han, K.; Lu, C. Preparation of calcium modified Zn-Ce/Al2O3 heterogeneous catalyst for biodiesel production through transesterification of palm oil with methanol optimized by response surface methodology. Fuel 2021, 284, 118986. [Google Scholar] [CrossRef]
- Abedin, A.; Kanitkar, S.; Kumar, N.; Wang, Z.; Ding, K.; Hutchings, G.; Spivey, J. Probing the Surface Acidity of Supported Aluminum Bromide Catalysts. Catalysts 2020, 10, 869. [Google Scholar] [CrossRef]
- Patiño, Y.; Faba, L.; Peláez, R.; Cueto, J.; Marín, P.; Díaz, E.; Ordóñez, S. The Role of Ion Exchange Resins for Solving Biorefinery Catalytic Processes Challenges. Catalysts 2023, 13, 999. [Google Scholar] [CrossRef]
- Alsultan, A.G.; Asikin-Mijan, N.; Ibrahim, Z.; Yunus, R.; Razali, S.Z.; Mansir, N.; Islam, A.; Seenivasagam, S.; Taufiq-Yap, Y.H. A Short Review on Catalyst, Feedstock, Modernised Process, Current State and Challenges on Biodiesel Production. Catalysts 2021, 11, 1261. [Google Scholar] [CrossRef]
- Chen, S.; Hu, Y.H. Chemical recycling of plastic wastes with alkaline earth metal oxides: A review. Sci. Total. Environ. 2023, 905, 167251. [Google Scholar] [CrossRef] [PubMed]
- Faruque, M.O.; Razzak, S.A.; Hossain, M.M. Application of Heterogeneous Catalysts for Biodiesel Production from Microalgal Oil—A Review. Catalysts 2020, 10, 1025. [Google Scholar] [CrossRef]
- Wachs, I.E. Recent conceptual advances in the catalysis science of mixed metal oxide catalytic materials. Catal. Today 2005, 100, 79–94. [Google Scholar] [CrossRef]
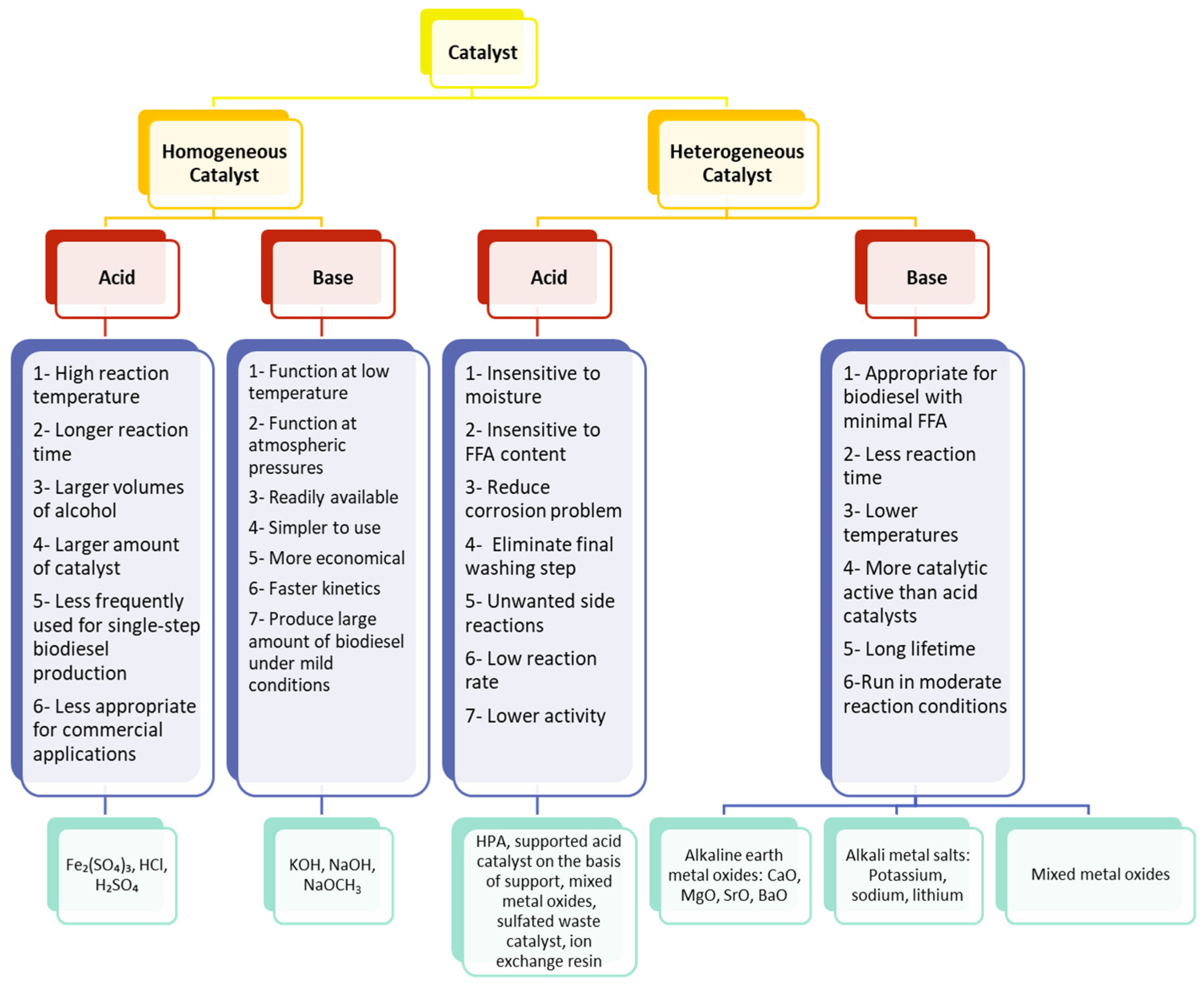
| Advantages | Disadvantages | Reference |
|---|---|---|
| HPA catalysts | ||
| Chemical stability Economically and environmentally attractive Convenient synthesis Easily separated, thus improving reusability HPAs on acceptable support increase the surface area and thermal stability of the catalyst Supported HPAs increase active sites’ accessibility and limit the formation of bulky residues High mechanical and thermal stabilities, easy handling, nontoxicity, high reactivity and recyclability Strong acidity, high redox properties, good thermal stability, easy separation, good reusability, less side products, less waste generation, non-toxicity, and easy handling Limited surface area | The requirement of using more demanding reaction conditions Leaching of active catalyst from the supporting material Deactivation of the catalytic sites The recyclability of HPA can be challenging Leaching of the catalyst, leading to a decrease in the activity Acidification procedure coupled with ether extraction could lower the yield of some HPAs Substituted HPA catalysts can have low target product yield and low selectivity due to the resistance of mass transfer Some HPAs require a high dosage and lower activity | [24,25,26,27,34,37] |
| Supported acid catalyst on the basis of support | ||
| Minimal waste Ease of product separation The occurrence of transesterification and esterification processes simultaneously Insensitive to water and FFA content in the feedstock | Lower catalytic activity Catalyst neutralization required Additional step required for the removal of the catalyst | [8,248] |
| Ion exchange resin | ||
| Possibility of continuous or repeated use without renewal Easy removal through decantation or otherwise | Lack of stability Low exchange capacity Reduced acid resistance Low thermal stability | [249] |
| Sulphated waste catalysts | ||
| High stability High acidity Good catalytic efficiency Excellent activity Recycled several times | High amounts of inputs and high energy demand High catalyst production cost and difficulty industrializing | [51,250] |
| Alkali metal salt catalysts | ||
| Less corrosive High reaction rate Less expensive Simpler to separate than water during the transesterification procedure Good morphology Easy separation Easy recovery Environmental acceptance | Leaching of the catalyst High loss of active catalyst Lower activity Lack of stability | [19,129,187] |
| Alkaline earth metal oxide catalysts | ||
| These catalysts are readily available, affordable, non-corrosive, and recyclable Some of these catalysts have higher basic strength and therefore higher catalytic activity Highly stable Easily separated | FFA and water adsorption may poison basic sites, deactivating the catalyst Low surface of some catalysts Soap formation | [128,251,252] |
| Mixed metal oxide catalysts | ||
| Highly active Easy separation Effective catalysis of different reactions: acid–base catalysis, oxidation reactions, photocatalysis, or biomass conversion Many potential applications Low cost Highly stable Environmental benignity | Mixed metal oxides are much more complex than metal-based catalysts High mass and heat transfer resistance and small contact areas Insufficient utilization, quick deactivation, difficult regeneration | [3,65,201,253] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yaghi, M.; Chidiac, S.; Awad, S.; El Rayess, Y.; Zgheib, N. An Overview of Biodiesel Production via Heterogeneous Catalysts: Synthesis, Current Advances, and Challenges. Clean Technol. 2025, 7, 62. https://doi.org/10.3390/cleantechnol7030062
Yaghi M, Chidiac S, Awad S, El Rayess Y, Zgheib N. An Overview of Biodiesel Production via Heterogeneous Catalysts: Synthesis, Current Advances, and Challenges. Clean Technologies. 2025; 7(3):62. https://doi.org/10.3390/cleantechnol7030062
Chicago/Turabian StyleYaghi, Maya, Sandra Chidiac, Sary Awad, Youssef El Rayess, and Nancy Zgheib. 2025. "An Overview of Biodiesel Production via Heterogeneous Catalysts: Synthesis, Current Advances, and Challenges" Clean Technologies 7, no. 3: 62. https://doi.org/10.3390/cleantechnol7030062
APA StyleYaghi, M., Chidiac, S., Awad, S., El Rayess, Y., & Zgheib, N. (2025). An Overview of Biodiesel Production via Heterogeneous Catalysts: Synthesis, Current Advances, and Challenges. Clean Technologies, 7(3), 62. https://doi.org/10.3390/cleantechnol7030062







