The Efficiency of Chlorella vulgaris in Heavy Metal Removal: A Comparative Study of Mono- and Multi-Component Metal Systems
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microalga Cultivation
2.2. Single- and Multi-Metal Treatment of C. vulgaris
2.3. Estimation of Culture Biomass
2.4. Pigment Content Estimation
2.5. Fourier Transform Infrared Spectroscopy
2.6. Atomic Absorption Spectroscopy (AAS)
2.7. Application of the Langmuir Isotherm Model to Mono- and Multi-MT Cultures
3. Results and Discussion
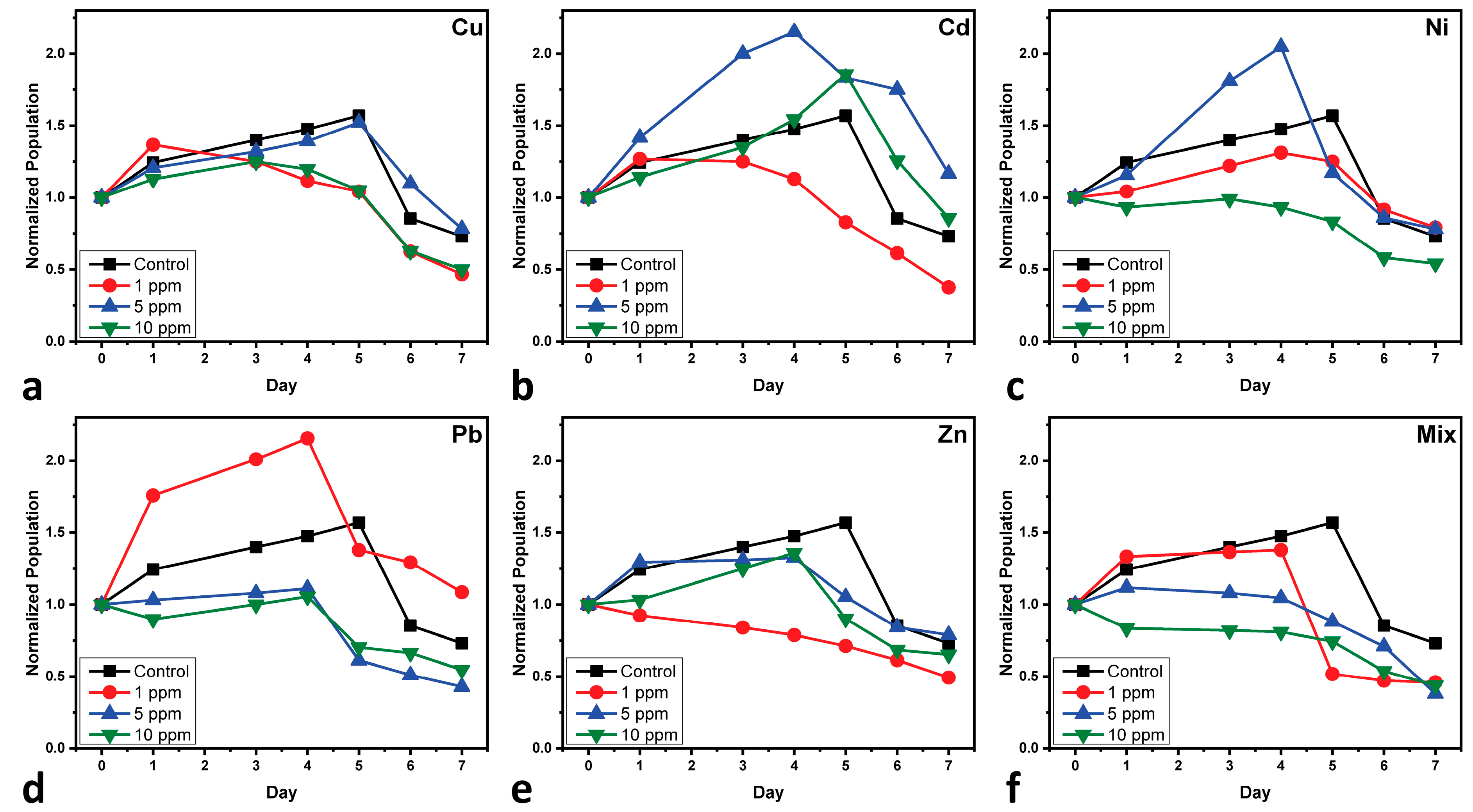
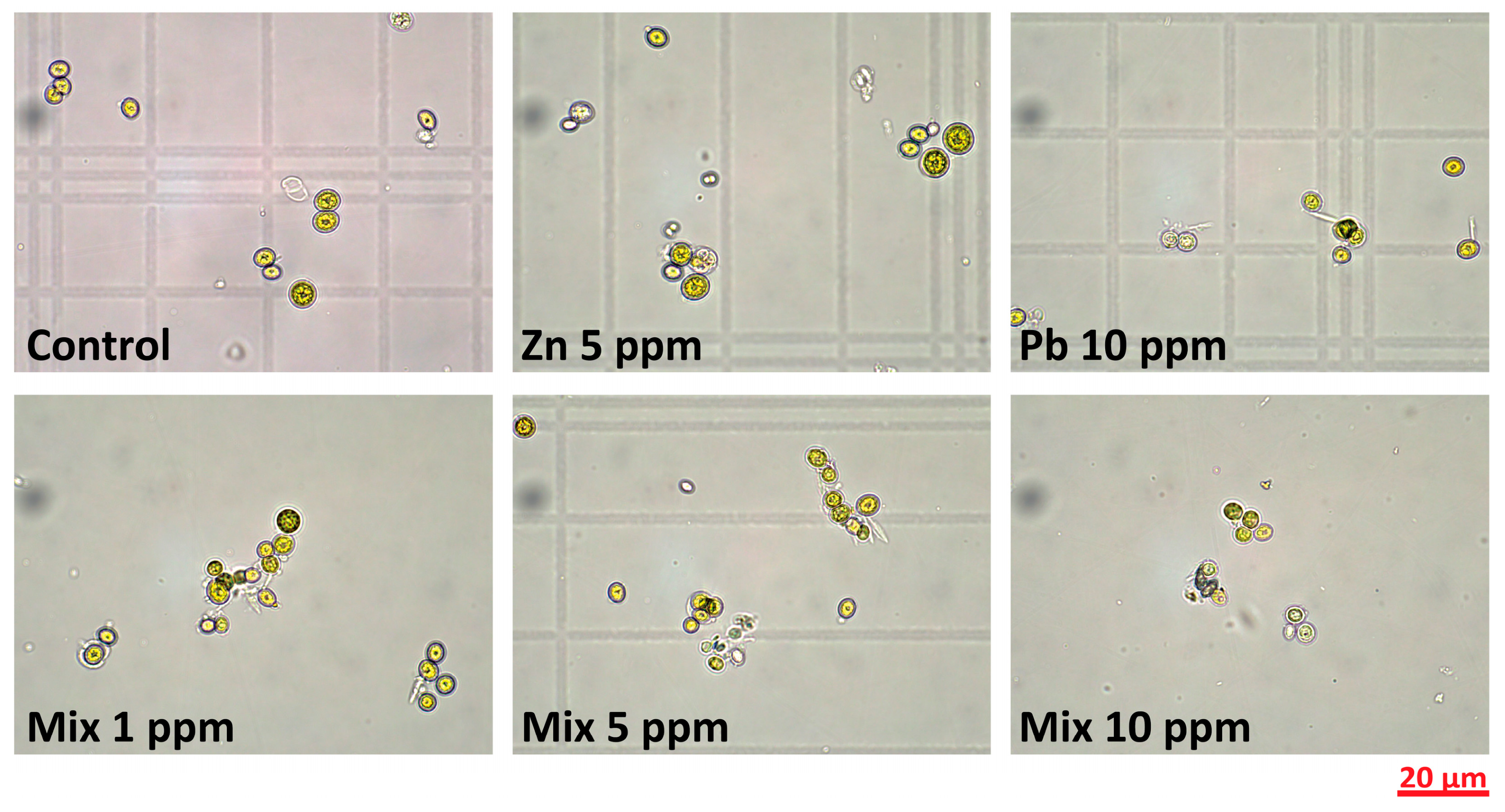
3.1. Cultures’ Monitoring During Cultivation
3.2. Pigments
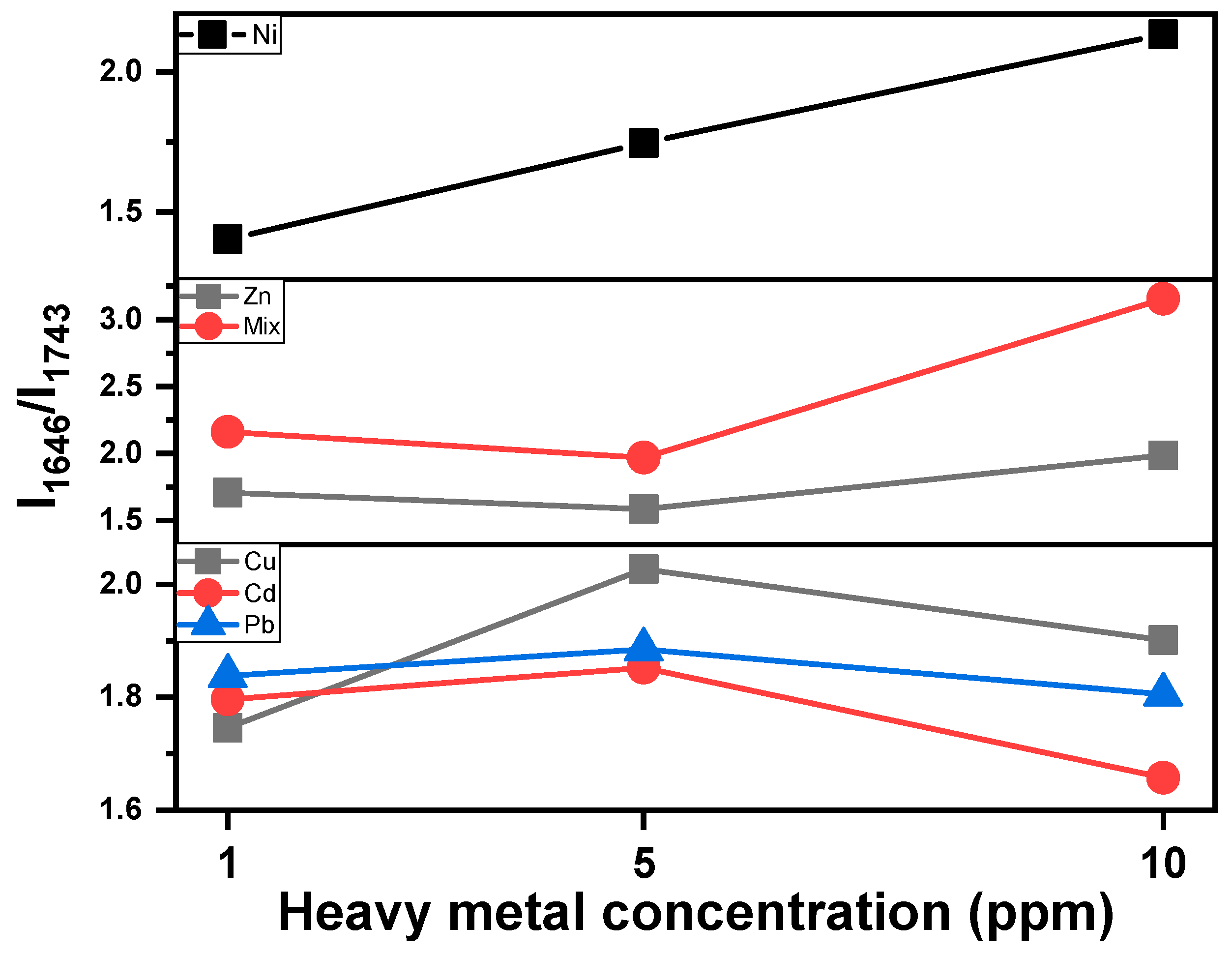
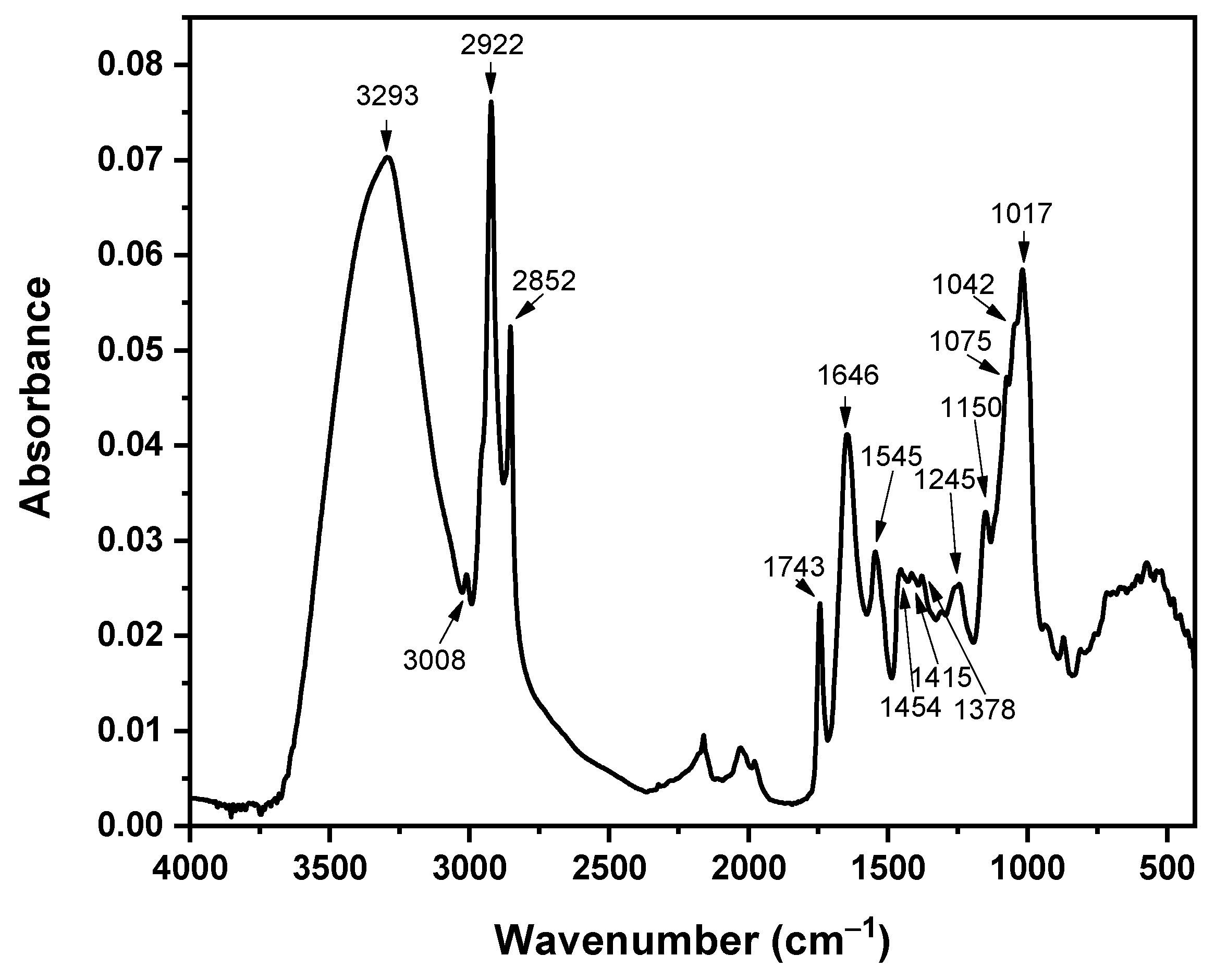
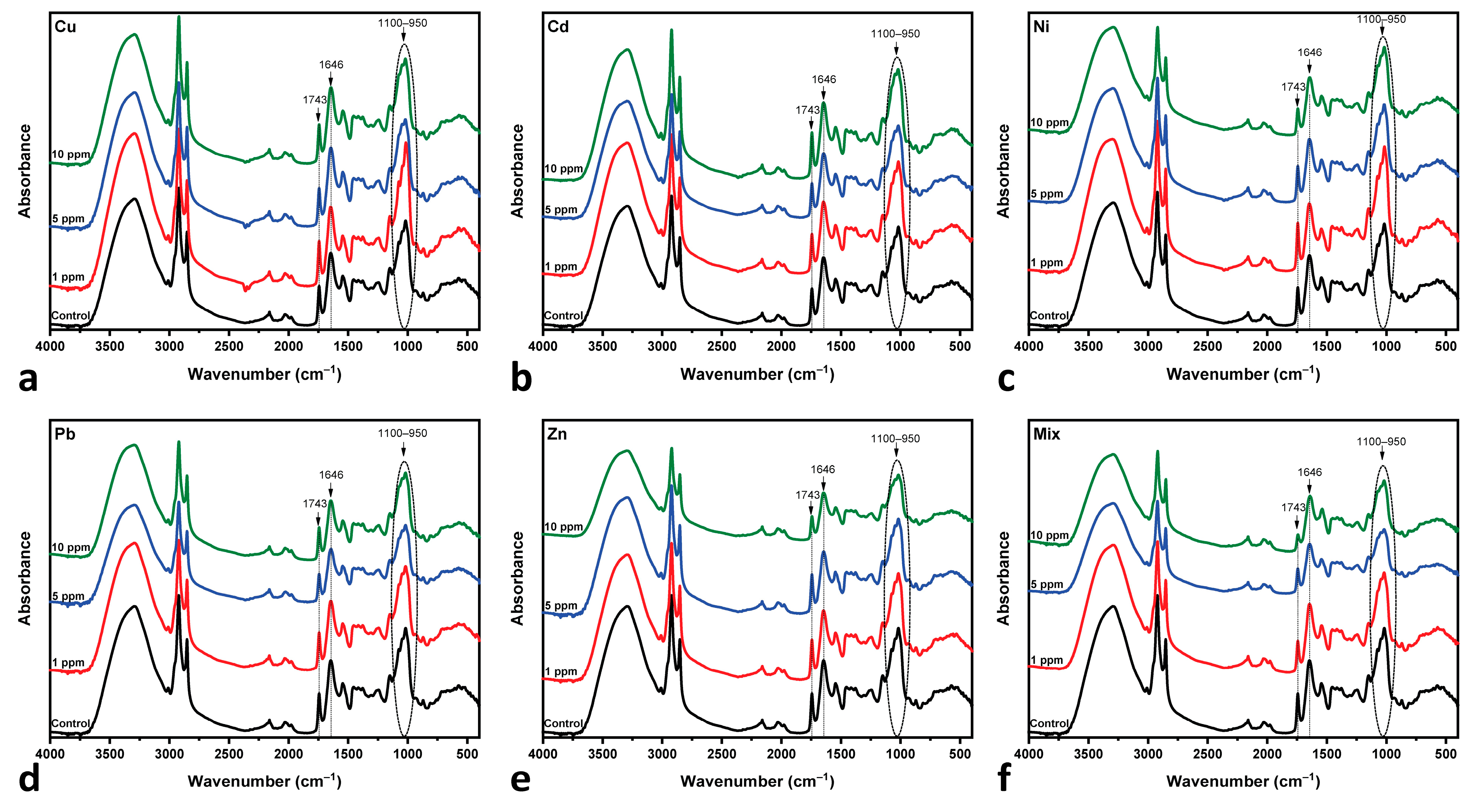
3.3. Fourier Transform Infrared Analysis
| Wavenumber (cm−1) | Attribution | References |
|---|---|---|
| 3293 | Hydroxyl and amino groups -OH, -NH, and -OH from carboxyl groups of lipid origin | [25] |
| 3008 | C-H stretching | [34] |
| 2922 and 2852 | C-H asymmetric and symmetric stretching | [25,34] |
| 1743 | C=O of esters | [25,83,86,87] |
| 1646 | C=O amide I | [25,88] |
| 1545 | C-N, N-H amide II | [25,88] |
| 1454 | CH3 asymmetric stretching or CH2 scissoring | [87,88] |
| 1378 | OH symmetric stretch of mono-, di-, and tri-glycerides, CH2 and CH3 proteins, the COO− of carboxylates, and the N(CH3)3 of lipids | [83,86,87,88] |
| 1245 | C-O stretch of COOH, PO, or OCO | [26,89] |
| 1150 | C-O-C of polysaccharides | |
| 1075 | C-O stretching and C-O-C, P=O, nucleic acid, or other phosphates and stretching of phosphodiesters | [83,86,87,88] |
| 1042 | C-O-C ether of polysaccharides | [25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83] |
| 1017 | C-H | [25] |
- Cu-, Cd- and Pb-MT cultures: The amide I/lipid ratio increases at 5 ppm, followed by a slight decrease at the 10 ppm concentration. This behavior is indicative of lipid degradation or protein loss under metal-induced stress. Pb appears to have moderate changes in comparison to Cu and Cd.
- Zn-MT and Mix (multi-MT) cultures: The ratio decreases slightly up to the 5 ppm concentration. This is followed by an increase up to 10 ppm, while Mix increases sharper than that of the Zn-MT culture. Both cases present similar spectral behavior, as shown in Figure 6. The overall increase in the amide I/lipid ratio suggests a cumulative effect of both protein and lipid changes.
- Ni-MT culture: in this case, the steady upward trend of the amide I/lipid ratio over the metal concentration suggests that structural changes increase with the concentration of metals.
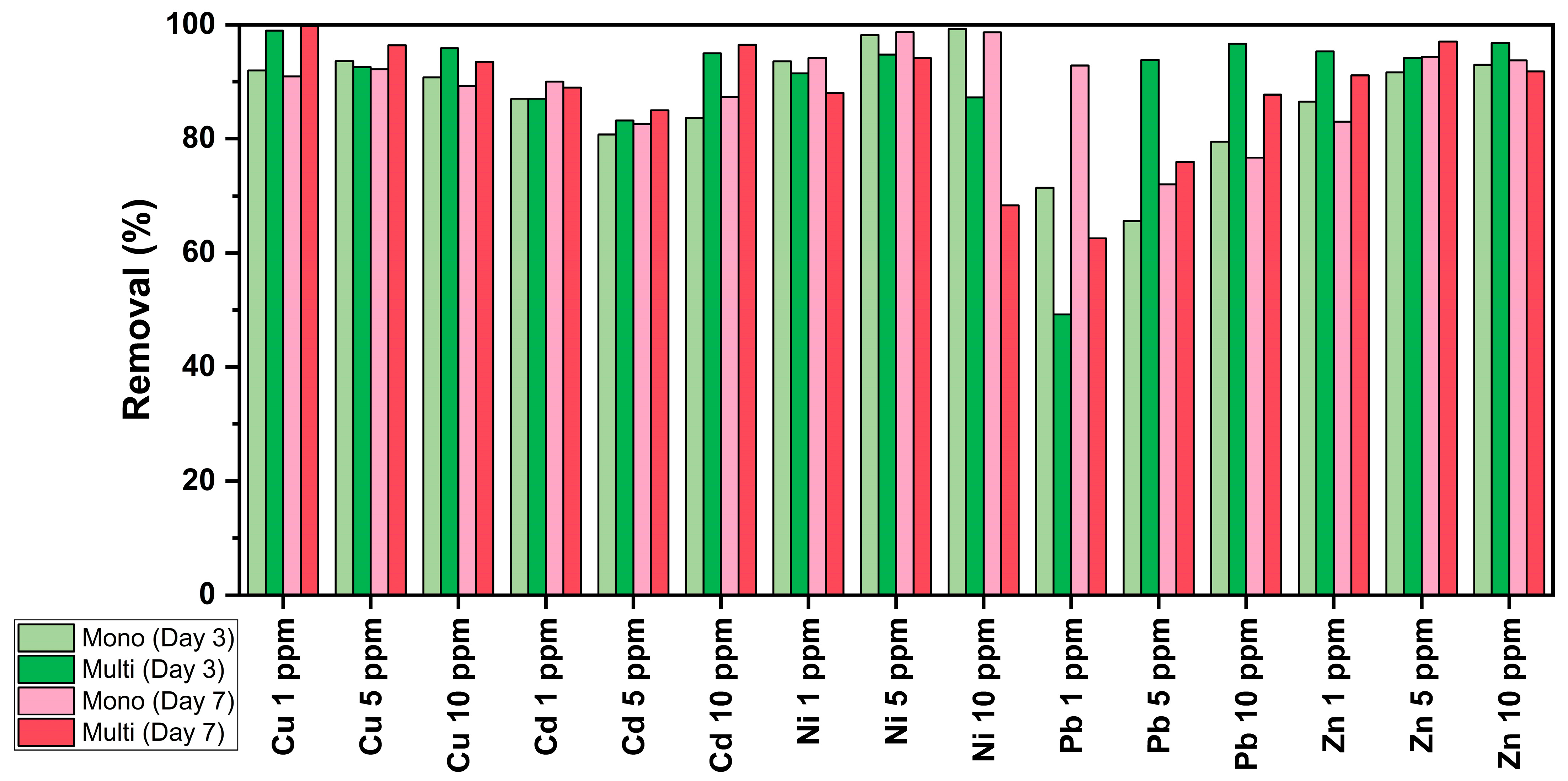
3.4. Removal Efficiency of C. vulgaris
3.5. Biosorption Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AAS | Atomic absorption spectroscopy |
| ATR | Attenuated total reflectance |
| BBM | Bold’s Basal Medium |
| CAT | Catalase |
| C. vulgaris | Chlorella vulgaris |
| DNA | Deoxyribonucleic acid |
| FTIR | Fourier transform infrared |
| MT | Metal treated |
| ROS | Reactive oxygen species |
| SL | Stock Solution(s) |
| SOD | Superoxide dismutase |
References
- Kalita, N.; Baruah, P.P. Cyanobacteria as a Potent Platform for Heavy Metals Biosorption: Uptake, Responses and Removal Mechanisms. J. Hazard. Mater. Adv. 2023, 11, 100349. [Google Scholar] [CrossRef]
- Suresh Kumar, K.; Dahms, H.U.; Won, E.J.; Lee, J.S.; Shin, K.H. Microalgae—A Promising Tool for Heavy Metal Remediation. Ecotoxicol. Environ. Saf. 2015, 113, 329–352. [Google Scholar] [CrossRef] [PubMed]
- Saavedra, R.; Muñoz, R.; Taboada, M.E.; Vega, M.; Bolado, S. Comparative Uptake Study of Arsenic, Boron, Copper, Manganese and Zinc from Water by Different Green Microalgae. Bioresour. Technol. 2018, 263, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Arunakumara, K.K.I.U.; Zhang, X. Heavy Metal Bioaccumulation and Toxicity with Special Reference to Microalgae. J. Ocean Univ. China 2007, 7, 60–64. [Google Scholar] [CrossRef]
- Alloway, B.J. (Ed.) Heavy Metals in Soils. Trace Metals and Metalloids in Soils and Their Bioavailability, 3rd ed.; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012; Volume 22, ISBN 9780128243152. [Google Scholar]
- Velarde, L.; Nabavi, M.S.; Escalera, E.; Antti, M.L.; Akhtar, F. Adsorption of Heavy Metals on Natural Zeolites: A Review. Chemosphere 2023, 328, 138508. [Google Scholar] [CrossRef]
- Abadin, H.; Ashizawa, A.; Stevens, Y.-W.; Llados, F.; Diamond, G.; Sage, G.; Citra, M.; Quinones, A.; Bosch, S.J.; Swarts, S.G. Toxicological Profile for Lead; Agency for Toxic Substances and Disease Registry (US): Atlanta, GA, USA, 2020; pp. 1–583. [Google Scholar]
- Dettori, M.; Arghittu, A.; Deiana, G.; Castiglia, P.; Azara, A. The Revised European Directive 2020/2184 on the Quality of Water Intended for Human Consumption. A Step Forward in Risk Assessment, Consumer Safety and Informative Communication. Environ. Res. 2022, 209, 112773. [Google Scholar] [CrossRef]
- Xu, K.; Du, M.; Yao, R.; Luo, J.; Chen, Z.; Li, C.; Lei, A.; Wang, J. Microalgae-Mediated Heavy Metal Removal in Wastewater Treatment: Mechanisms, Influencing Factors, and Novel Techniques. Algal Res. 2024, 82, 103645. [Google Scholar] [CrossRef]
- European Parliament and Council. European Pollutant Release and Transfer Register. Off. J. Eur. Union 2006, L33, 1–17. [Google Scholar]
- Li, H.; Watson, J.; Zhang, Y.; Lu, H.; Liu, Z. Environment-Enhancing Process for Algal Wastewater Treatment, Heavy Metal Control and Hydrothermal Biofuel Production: A Critical Review. Bioresour. Technol. 2020, 298, 122421. [Google Scholar] [CrossRef]
- Arora, R. Adsorption of Heavy Metals—A Review. Mater. Today Proc. 2019, 18, 4745–4750. [Google Scholar] [CrossRef]
- Anastopoulos, I.; Kyzas, G.Z. Progress in Batch Biosorption of Heavy Metals onto Algae. J. Mol. Liq. 2015, 209, 77–86. [Google Scholar] [CrossRef]
- Rodrigues, M.S.; Ferreira, L.S.; de Carvalho, J.C.M.; Lodi, A.; Finocchio, E.; Converti, A. Metal Biosorption onto Dry Biomass of Arthrospira (Spirulina) platensis and Chlorella Vulgaris: Multi-Metal Systems. J. Hazard. Mater. 2012, 217–218, 246–255. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, V.; Govindaradjane, S.; Rajamohan, N.; Rajasimman, M. Biosorption Potential of Brown Algae, Sargassum polycystum, for the Removal of Toxic Metals, Cadmium and Zinc. Environ. Sci. Pollut. Res. 2022, 29, 41909–41922. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Li, W.; Jin, M.; Zhang, L.; Qin, L.; Geng, W. Responses and Tolerance Mechanisms of Microalgae to Heavy Metal Stress: A Review. Mar. Environ. Res. 2023, 183, 105805. [Google Scholar] [CrossRef]
- Mehta, S.K.; Gaur, J.P. Use of Algae for Removing Heavy Metal Ions from Wastewater: Progress and Prospects. Crit. Rev. Biotechnol. 2005, 25, 113–152. [Google Scholar] [CrossRef]
- Rangabhashiyam, S.; Balasubramanian, P. Characteristics, Performances, Equilibrium and Kinetic Modeling Aspects of Heavy Metal Removal Using Algae. Bioresour. Technol. Rep. 2019, 5, 261–279. [Google Scholar] [CrossRef]
- Khan, Z.; Anees, A.; Khan, I.; Shahwar, D. Advancements in Microalgal Bioremediation of Heavy Metal-Contaminated Water. In Bio-Organic Amendments for Heavy Metal Remediation: Water, Soil and Plant Approaches and Technologies; Elsevier: Amsterdam, The Netherlands, 2024; pp. 33–55. ISBN 9780443216107. [Google Scholar]
- El-Sheekh, M.M.; El-Kassas, H.Y.; Ali, S.S. Microalgae-Based Bioremediation of Refractory Pollutants: An Approach towards Environmental Sustainability. Microb. Cell Factories 2025, 24, 19. [Google Scholar] [CrossRef]
- Edris, G.; Alhamed, Y.; Alzahrani, A. Biosorption of Cadmium and Lead from Aqueous Solutions by Chlorella vulgaris Biomass: Equilibrium and Kinetic Study. Arab. J. Sci. Eng. 2014, 39, 87–93. [Google Scholar] [CrossRef]
- Rachlin, J.W.; Grosso, A. The Effects of PH on the Growth of Chlorella vulgaris and Its Interactions with Cadmium Toxicity. Arch. Environ. Contam. Toxicol. 1991, 20, 505–508. [Google Scholar] [CrossRef]
- Jones, L.A.; Ogden, K.L.; Jia, F. Comparative Study of Biosorption of Copper(II) by Lipid Extracted and Non-Extracted Chlorella sorokiniana. CLEAN Soil Air Water 2015, 43, 73–78. [Google Scholar] [CrossRef]
- Zeng, Y.; Chen, X.; Zhu, J.; Long, D.; Jian, Y.; Tan, Q.; Wang, H. Effects of Cu (II) on the Growth of Chlorella vulgaris and Its Removal Efficiency of Pollutants in Synthetic Piggery Digestate. Toxics 2024, 12, 56. [Google Scholar] [CrossRef]
- Soto-Ramírez, R.; Lobos, M.G.; Córdova, O.; Poirrier, P.; Chamy, R. Effect of Growth Conditions on Cell Wall Composition and Cadmium Adsorption in Chlorella vulgaris: A New Approach to Biosorption Research. J. Hazard. Mater. 2021, 411, 125059. [Google Scholar] [CrossRef]
- Ferreira, L.S.; Rodrigues, M.S.; de Carvalho, J.C.M.; Lodi, A.; Finocchio, E.; Perego, P.; Converti, A. Adsorption of Ni2+, Zn2+ and Pb2+ onto Dry Biomass of Arthrospira (Spirulina) platensis and Chlorella vulgaris. I. Single Metal Systems. Chem. Eng. J. 2011, 173, 326–333. [Google Scholar] [CrossRef]
- Manzoor, F.; Karbassi, A.; Golzary, A. Removal of Heavy Metal Contaminants from Wastewater by Using Chlorella vulgaris Beijerinck: A Review. Curr. Environ. Manag. 2019, 6, 174–187. [Google Scholar] [CrossRef]
- El-Sayed, A.E.K.B.; Reda, M.M.; Almutairi, A.W.; Mavromatis, C. Biomass Production and Biochemical Composition of Chlorella vulgaris Grown in Net-House Photobioreactor (NHPBR) Using Sugarcane Press Mud Waste. J. Taibah Univ. Sci. 2023, 17, 2194843. [Google Scholar] [CrossRef]
- Spain, O.; Funk, C. Detailed Characterization of the Cell Wall Structure and Composition of Nordic Green Microalgae. J. Agric. Food Chem. 2022, 70, 9711–9721. [Google Scholar] [CrossRef]
- Spolaore, P.; Joannis-Cassan, C.; Duran, E.; Isambert, A. Commercial Applications of Microalgae. J. Biosci. Bioeng. 2006, 101, 87–96. [Google Scholar] [CrossRef]
- Lam, M.K.; Lee, K.T. Potential of Using Organic Fertilizer to Cultivate Chlorella vulgaris for Biodiesel Production. Appl. Energy 2012, 94, 303–308. [Google Scholar] [CrossRef]
- Ali, S.S.; Hassan, L.H.S. Microalgae-Mediated Bioremediation: Current Trends and Opportunities-a Review. Arch. Microbiol. 2024, 206, 343. [Google Scholar] [CrossRef]
- Chia, M.A.; Lombardi, A.T.; da Melão, M.G.G. Growth and Biochemical Composition of Chlorella vulgaris in Different Growth Media. An. Acad. Bras. Cienc. 2013, 85, 1427–1438. [Google Scholar] [CrossRef]
- Yang, J.S.; Cao, J.; Xing, G.L.; Yuan, H.L. Lipid Production Combined with Biosorption and Bioaccumulation of Cadmium, Copper, Manganese and Zinc by Oleaginous Microalgae Chlorella minutissima UTEX2341. Bioresour. Technol. 2015, 175, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Qiu, H.; Chang, Z.; Jiang, Z.; Yin, W. The Effect of Cadmium on the Growth and Antioxidant Response for Freshwater Algae Chlorella vulgaris. SpringerPlus 2016, 5, 1290. [Google Scholar] [CrossRef] [PubMed]
- Qasem, N.A.A.; Mohammed, R.H.; Lawal, D.U. Removal of Heavy Metal Ions from Wastewater: A Comprehensive and Critical Review. npj Clean Water 2021, 4, 1–15. [Google Scholar] [CrossRef]
- Expósito, N.; Carafa, R.; Kumar, V.; Sierra, J.; Schuhmacher, M.; Papiol, G.G. Performance of Chlorella vulgaris Exposed to Heavy Metal Mixtures: Linking Measured Endpoints and Mechanisms. Int. J. Environ. Res. Public Health 2021, 18, 1037. [Google Scholar] [CrossRef]
- Liu, Z.W.; Zhang, L.S.; Feng, X.Y.; He, Z.X.; Sun, Y.; Tao, X.Y.; Yin, Q.; Yang, L.M.; Zhou, R.J.; He, X.Q.; et al. Near-Infrared Spectroscopy Bioprobe Estimation of Metabolites’ Responses to Pb2+ in Cladophora rupestris. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2024, 306, 123544. [Google Scholar] [CrossRef]
- Rinaldi, K.L.; Senhorinho, G.N.A.; Laamanen, C.A.; Scott, J.A. A Review of Extremophilic Microalgae: Impacts of Experimental Cultivation Conditions for the Production of Antimicrobials. Algal Res. 2024, 78, 103427. [Google Scholar] [CrossRef]
- Bajguz, A. Suppression of Chlorella vulgaris Growth by Cadmium, Lead, and Copper Stress and Its Restoration by Endogenous Brassinolide. Arch. Environ. Contam. Toxicol. 2011, 60, 406–416. [Google Scholar] [CrossRef]
- Hasanuzzaman, M. Approaches to the Remediation of Inorganic Pollutants; Springer: Hyderabad, India, 2021; ISBN 9789811562211. [Google Scholar]
- Tripathi, S.; Poluri, K.M. Heavy Metal Detoxification Mechanisms by Microalgae: Insights from Transcriptomics Analysis. Environ. Pollut. 2021, 285, 117443. [Google Scholar] [CrossRef]
- Liu, S.; Jiang, M.; Wu, J.; Li, X.; Zhu, J. Mechanism of Biological Transport and Transformation of Copper, Cadmium, and Zinc in Water by Chlorella. Water 2024, 16, 1906. [Google Scholar] [CrossRef]
- Santos, F.M.; Mazur, L.P.; Mayer, D.A.; Vilar, V.J.P.; Pires, J.C.M. Inhibition Effect of Zinc, Cadmium, and Nickel Ions in Microalgal Growth and Nutrient Uptake from Water: An Experimental Approach. Chem. Eng. J. 2019, 366, 358–367. [Google Scholar] [CrossRef]
- Geng, W.; Xiao, X.; Zhang, L.; Ni, W.; Li, N.; Li, Y. Response and Tolerance Ability of Chlorella vulgaris to Cadmium Pollution Stress. Environ. Technol. 2022, 43, 4391–4401. [Google Scholar] [CrossRef] [PubMed]
- Lalhmunsiama; Gupta, P.L.; Jung, H.; Tiwari, D.; Kong, S.H.; Lee, S.M. Insight into the Mechanism of Cd(II) and Pb(II) Removal by Sustainable Magnetic Biosorbent Precursor to Chlorella vulgaris. J. Taiwan Inst. Chem. Eng. 2017, 71, 206–213. [Google Scholar] [CrossRef]
- Kusuma, H.S.; Illiyanasafa, N.; Jaya, D.E.C.; Darmokoesoemo, H.; Putra, N.R. Utilization of the Microalga Chlorella vulgaris for Mercury Bioremediation from Wastewater and Biomass Production. Sustain. Chem. Pharm. 2024, 37, 101346. [Google Scholar] [CrossRef]
- Dmytryk, A.; Saeid, A.; Chojnacka, K. Biosorption of Microelements by Spirulina: Towards Technology of Mineral Feed Supplements. Sci. World J. 2014, 2014, 356328. [Google Scholar] [CrossRef]
- Zinicovscaia, I.; Safonov, A.; Tregubova, V.; Ilin, V.; Cepoi, L.; Chiriac, T.; Rudi, L.; Frontasyeva, M.V. Uptake of Metals from Single and Multi-Component Systems by Spirulina platensis Biomass. Ecol. Chem. Eng. S 2016, 23, 401–412. [Google Scholar] [CrossRef]
- Malletzidou, L.; Kyratzopoulou, E.; Kyzaki, N.; Nerantzis, E.; Kazakis, N.A. Towards the Sustainable Removal of Heavy Metals from Wastewater Using Arthrospira platensis: A Laboratory-Scale Approach in the Context of a Green Circular Economy. Appl. Sci. 2025, 15, 791. [Google Scholar] [CrossRef]
- Birungi, Z.S.; Chirwa, E.M.N.; Botai, O.J. Competitive Adsorption in a Ternary System of Toxic Metals and Rare Earth Elements Using Desmodesmus multivariabilis: Empirical and Kinetic Modelling. J. Appl. Phycol. 2017, 29, 2899–2910. [Google Scholar] [CrossRef]
- Kazakis, N.A. Green Approaches to Heavy Metal Removal from Wastewater: Microalgae Solutions in a Circular Economy Framework. Soc. Impacts 2025, 5, 100103. [Google Scholar] [CrossRef]
- Bischoff, H.W. Phycological Studies IV. Some Soil Algae from Enchanted Rock and Related Algal Species; University of Texas Publication: Austin, TX, USA, 1963; No. 6318; pp. 1–95. [Google Scholar]
- BBM Medium. CCCryo Culture Collection of Cryophilic Algae, 06/2020. 2020. Available online: http://cccryo.fraunhofer.de/sources/files/medien/BBM.pdf (accessed on 28 January 2024).
- Challagulla, V.; Walsh, K.B.; Subedi, P. Biomass and Total Lipid Content Assessment of Microalgal Cultures Using Near and Short Wave Infrared Spectroscopy. Bioenergy Res. 2014, 7, 306–318. [Google Scholar] [CrossRef]
- Gille, A.; Trautmann, A.; Posten, C.; Briviba, K. Bioaccessibility of Carotenoids from Chlorella vulgaris and Chlamydomonas reinhardtii. Int. J. Food Sci. Nutr. 2016, 67, 507–513. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and Carotenoids: Pigments of Photosynthetic Biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar] [CrossRef]
- Parsons, T.R.; Strickland, J.D. Discussion of Spectrophotometric Determination of Marine-Plant Pigments, with Revised Equations for Ascertaining Chlorophylls and Carotenoids. J. Mar. Res. 1963, 21, 155–163. [Google Scholar] [CrossRef]
- Plöhn, M.; Escudero-Oñate, C.; Funk, C. Biosorption of Cd(II) by Nordic Microalgae: Tolerance, Kinetics and Equilibrium Studies. Algal Res. 2021, 59, 102471. [Google Scholar] [CrossRef]
- Rajfur, M.; Kłos, A. Sorption of Heavy Metals in the Biomass of Alga Palmaria palmata. Water Sci. Technol. 2013, 68, 1543–1549. [Google Scholar] [CrossRef]
- Omolabake Abiodun, O.-A.; Oluwaseun, O.; Kayode Oladayo, O.; Abayomi, O.; Arubi George, A.; Opatola, E.; Friday Orah, R.; Jeffery Isukuru, E.; Chiamaka Ede, I.; Temitayo Oluwayomi, O.; et al. Remediation of Heavy Metals Using Biomass-Based Adsorbents: Adsorption Kinetics and Isotherm Models. Clean Technol. 2023, 5, 934–960. [Google Scholar] [CrossRef]
- Abdulaziz, M.; Musayev, S. Multicomponent Biosorption of Heavy Metals from Aqueous Solutions: A Review. Pol. J. Environ. Stud. 2017, 26, 1433–1441. [Google Scholar] [CrossRef]
- Cheng, J.; Yin, W.; Chang, Z.; Lundholm, N.; Jiang, Z. Biosorption Capacity and Kinetics of Cadmium(II) on Live and Dead Chlorella vulgaris. J. Appl. Phycol. 2017, 29, 211–221. [Google Scholar] [CrossRef]
- Langmuir, I. The Adsorption of Gases on Plane Surfaces of Glass, Mica and Platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Rangsayatorn, N.; Upatham, E.S.; Kruatrachue, M.; Pokethitiyook, P.; Lanza, G.R. Phytoremediation Potential of Spirulina (Arthrospira) platensis: Biosorption and Toxicity Studies of Cadmium. Environ. Pollut. 2002, 119, 45–53. [Google Scholar] [CrossRef]
- Jiang, L.; Zhou, W.; Liu, D.; Liu, T.; Wang, Z. Biosorption Isotherm Study of Cd2 +, Pb2 + and Zn2 + Biosorption onto Marine Bacterium Pseudoalteromonas sp. SCSE709-6 in Multiple Systems. J. Mol. Liq. 2017, 247, 230–237. [Google Scholar] [CrossRef]
- Zhou, G.J.; Peng, F.Q.; Zhang, L.J.; Ying, G.G. Biosorption of Zinc and Copper from Aqueous Solutions by Two Freshwater Green Microalgae Chlorella pyrenoidosa and Scenedesmus obliquus. Environ. Sci. Pollut. Res. 2012, 19, 2918–2929. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.K.; Rastogi, A. Biosorption of Lead(II) from Aqueous Solutions by Non-Living Algal Biomass Oedogonium sp. and Nostoc sp.-A Comparative Study. Colloids Surf. B Biointerfaces 2008, 64, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Nowicka, B. Heavy Metal–Induced Stress in Eukaryotic Algae—Mechanisms of Heavy Metal Toxicity and Tolerance with Particular Emphasis on Oxidative Stress in Exposed Cells and the Role of Antioxidant Response. Environ. Sci. Pollut. Res. 2022, 29, 16860–16911. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, E.J.; Baldwin, L.A. Hormesis: U-Shaped Dose Responses and Their Centrality in Toxicology. Trends Pharmacol. Sci. 2001, 22, 285–291. [Google Scholar] [CrossRef]
- Iqhrammullah, M.; Saudah, S.; Monalisa, M.; Fahrurrozi, F.; Akbar, S.A.; Lubis, S.S. Network Meta-Analysis of Cadmium Toxicity against Chlorella vulgaris and the Role of Growth Stimulants and Macronutrients. Glob. J. Environ. Sci. Manag. 2024, 10, 1561–1572. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Baldwin, L.A. Applications of Hormesis in Toxicology, Risk Assessment and Chemotherapeutics. Trends Pharmacol. Sci. 2002, 23, 331–337. [Google Scholar] [CrossRef]
- Nagajyoti, P.C.; Lee, K.D.; Sreekanth, T.V.M. Heavy Metals, Occurrence and Toxicity for Plants: A Review. Environ. Chem. Lett. 2010, 8, 199–216. [Google Scholar] [CrossRef]
- Gikas, P. Single and Combined Effects of Nickel (Ni(II)) and Cobalt (Co(II)) Ions on Activated Sludge and on Other Aerobic Microorganisms: A Review. J. Hazard. Mater. 2008, 159, 187–203. [Google Scholar] [CrossRef]
- Monteiro, C.M.; Castro, P.M.L.; Malcata, F.X. Metal Uptake by Microalgae: Underlying Mechanisms and Practical Applications. Biotechnol. Prog. 2012, 28, 299–311. [Google Scholar] [CrossRef]
- Zeraatkar, A.K.; Ahmadzadeh, H.; Talebi, A.F.; Moheimani, N.R.; McHenry, M.P. Potential Use of Algae for Heavy Metal Bioremediation, a Critical Review. J. Environ. Manag. 2016, 181, 817–831. [Google Scholar] [CrossRef]
- Kondzior, P.; Butarewicz, A. Effect of Heavy Metals (Cu and Zn) on the Content of Photosynthetic Pigments in the Cells of Algae Chlorella vulgaris. J. Ecol. Eng. 2018, 19, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Rocha, G.S.; Parrish, C.C.; Espíndola, E.L.G. Effects of Copper on Photosynthetic and Physiological Parameters of a Freshwater Microalga (Chlorophyceae). Algal Res. 2021, 54, 102223. [Google Scholar] [CrossRef]
- Sytar, O.; Kumar, A.; Latowski, D.; Kuczynska, P.; Strzałka, K.; Prasad, M.N.V. Heavy Metal-Induced Oxidative Damage, Defense Reactions, and Detoxification Mechanisms in Plants. Acta Physiol. Plant. 2013, 35, 985–999. [Google Scholar] [CrossRef]
- Wang, M.; Zou, J.; Duan, X.; Jiang, W.; Liu, D. Cadmium Accumulation and Its Effects on Metal Uptake in Maize (Zea Mays L.). Bioresour. Technol. 2007, 98, 82–88. [Google Scholar] [CrossRef]
- Ekmekçi, Y.; Tanyolaç, D.; Ayhan, B. Effects of Cadmium on Antioxidant Enzyme and Photosynthetic Activities in Leaves of Two Maize Cultivars. J. Plant Physiol. 2008, 165, 600–611. [Google Scholar] [CrossRef]
- Qian, H.; Li, J.; Sun, L.; Chen, W.; Sheng, G.D.; Liu, W.; Fu, Z. Combined Effect of Copper and Cadmium on Chlorella vulgaris Growth and Photosynthesis-Related Gene Transcription. Aquat. Toxicol. 2009, 94, 56–61. [Google Scholar] [CrossRef]
- Duygu, D.; Udoh, A.; Ozer, T.; Akbulut, A.; Erkaya, I.; Yildiz, K.; Guler, D. Fourier Transform Infrared (FTIR) Spectroscopy for Identification of Chlorella vulgaris Beijerinck 1890 and Scenedesmus obliquus (Turpin) Kützing 1833. Afr. J. Biotechnol. 2012, 11, 3817–3824. [Google Scholar] [CrossRef]
- Sukhikh, S.; Prosekov, A.; Ivanova, S.; Maslennikov, P.; Andreeva, A.; Budenkova, E.; Kashirskikh, E.; Tcibulnikova, A.; Zemliakova, E.; Samusev, I.; et al. Identification of Metabolites with Antibacterial Activities by Analyzing the FTIR Spectra of Microalgae. Life 2022, 12, 1395. [Google Scholar] [CrossRef]
- Dao, L.; Beardall, J.; Heraud, P. Characterisation of Pb-Induced Changes and Prediction of Pb Exposure in Microalgae Using Infrared Spectroscopy. Aquat. Toxicol. 2017, 188, 33–42. [Google Scholar] [CrossRef]
- El-Naggar, N.E.A.; Hussein, M.H.; Shaaban-Dessuuki, S.A.; Dalal, S.R. Production, Extraction and Characterization of Chlorella vulgaris Soluble Polysaccharides and Their Applications in AgNPs Biosynthesis and Biostimulation of Plant Growth. Sci. Rep. 2020, 10, 3011. [Google Scholar] [CrossRef]
- Baldev, E.; MubarakAli, D.; Sivasubramanian, V.; Pugazhendhi, A.; Thajuddin, N. Unveiling the Induced Lipid Production in Chlorella vulgaris under Pulsed Magnetic Field Treatment. Chemosphere 2021, 279, 130673. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Saxena, A.; Song, B.; Ward, B.B.; Beveridge, T.J.; Myneni, S.C.B. Elucidation of Functional Groups on Gram-Positive and Gram-Negative Bacterial Surfaces Using Infrared Spectroscopy. Langmuir 2004, 20, 11433–11442. [Google Scholar] [CrossRef] [PubMed]
- Yalçın, S.; Sezer, S.; Apak, R. Characterization and Lead(II), Cadmium(II), Nickel(II) Biosorption of Dried Marine Brown Macro Algae Cystoseira barbata. Environ. Sci. Pollut. Res. 2012, 19, 3118–3125. [Google Scholar] [CrossRef] [PubMed]
- Salama, E.S.; Roh, H.S.; Dev, S.; Khan, M.A.; Abou-Shanab, R.A.I.; Chang, S.W.; Jeon, B.H. Algae as a Green Technology for Heavy Metals Removal from Various Wastewater. World J. Microbiol. Biotechnol. 2019, 35, 75. [Google Scholar] [CrossRef]
- Fitri, W.E.; Putra, A.; Febria, F.A. Removal of Heavy Metals Using Chlorella vulgaris: A Review. J. Katalisator 2024, 9, 148–162. [Google Scholar] [CrossRef]
- Mitrogiannis, D.; Markou, G.; Çelekli, A.; Bozkurt, H. Biosorption of Methylene Blue onto Arthrospira platensis Biomass: Kinetic, Equilibrium and Thermodynamic Studies. J. Environ. Chem. Eng. 2015, 3, 670–680. [Google Scholar] [CrossRef]
- Hussain, M.K.; Khatoon, S.; Nizami, G.; Fatma, U.K.; Ali, M.; Singh, B.; Quraishi, A.; Assiri, M.A.; Ahamad, S.; Saquib, M. Unleashing the Power of Bio-Adsorbents: Efficient Heavy Metal Removal for Sustainable Water Purification. J. Water Process Eng. 2024, 64, 105705. [Google Scholar] [CrossRef]
- Kiran Marella, T.; Saxena, A.; Tiwari, A. Diatom Mediated Heavy Metal Remediation: A Review. Bioresour. Technol. 2020, 305, 123068. [Google Scholar] [CrossRef]
- Safi, C.; Zebib, B.; Merah, O.; Pontalier, P.Y.; Vaca-Garcia, C. Morphology, Composition, Production, Processing and Applications of Chlorella vulgaris: A Review. Renew. Sustain. Energy Rev. 2014, 35, 265–278. [Google Scholar] [CrossRef]
- Soeprobowati, T.R. Phycoremediation of Pb+2, Cd+2, Cu+2, and Cr+3 by Spirulina platensis (Gomont) Geitler. Am. J. Biosci. 2014, 2, 165–170. [Google Scholar] [CrossRef]
- Goswami, R.K.; Agrawal, K.; Shah, M.P.; Verma, P. Bioremediation of Heavy Metals from Wastewater: A Current Perspective on Microalgae-based Future. Lett. Appl. Microbiol. 2022, 75, 701–717. [Google Scholar] [CrossRef]
- Aung, W.L.; Hlaing, N.; Aye, K.N. Biosorption of Lead (Pb2+) by Using Chlorella vulgaris. Int. J. Chem. Environ. Biol. Sci. 2013, 1, 2320–4087. [Google Scholar]

| Stock Solutions (SLs) | Volume (mL) | Component | Concentration in SL |
|---|---|---|---|
| 1 | 10 | NaNO3 | 2.50 g·100 mL−1 |
| 2 | 10 | MgSO4·7H2O | 0.75 g·100 mL−1 |
| 3 | 10 | NaCl | 0.25 g·100 mL−1 |
| 4 | 10 | K2HPO4 | 0.75 g·100 mL−1 |
| 5 | 10 | KH2PO4 | 1.75 g·100 mL−1 |
| 6 | 10 | CaCl2·2H2O | 0.25 g·100 mL−1 |
| 7 | 1 | ZnSO4·7H2O | 8.82 g·L−1 |
| MnCl2·4H2O | 1.44 g·L−1 | ||
| MoO3 | 0.71 g·L−1 | ||
| CuSO4·5H2O | 1.57 g·L−1 | ||
| Co(NO3)2·6H2O | 0.49 g·L−1 | ||
| 8 | 1 | H3BO3 | 1.14 g·100 mL−1 |
| 9 | 1 | Na2EDTA·2H2O | 5.00 g·100 mL−1 |
| KOH | 3.10 g·100 mL−1 | ||
| 10 | 1 | FeSO4·7H2O | 4.98 g·L−1 |
| H2SO4 (conc.) | 1 ml |
| Metal | qm (×10−8 mg/cell) | KL (L/mg) | R2 |
|---|---|---|---|
| Cu | 3.84 ± 0.59 | 0.35 ± 0.05 | 0.995 |
| Cd | 1.55 ± 0.11 | 0.72 ± 0.06 | 0.987 |
| Ni | (6.50 ± 0.83) × 10−2 | 11.05 ± 0.18 | 0.488 |
| Pb | 7.93 ± 1.21 | 0.04 ± 0.01 | 0.996 |
| Zn | 1.16 ± 0.21 | 0.77 ± 0.17 | 0.999 |
| Metal | qe,i (×10−10 mg/cell) | ||
|---|---|---|---|
| 1 ppm Multi-MT Culture | 5 ppm Multi-MT Culture | 10 ppm Multi-MT Culture | |
| Cu | 0.64 ± 0.06 | 10.3 ± 1.80 | 3.48 ± 0.92 |
| Cd | 6.90 ± 0.44 | 19.3 ± 1.30 | 3.51 ± 1.06 |
| Ni | 2.92 ± 0.39 | 3.86 ± 0.35 | 5.78 ± 0.69 |
| Pb | 7.67 ± 0.63 | 2.54 ± 0.29 | 0.67 ± 0.08 |
| Zn | 1.97 ± 0.22 | 5.32 ± 1.09 | 1.80 ± 0.48 |
| Concentration (ppm) | Mono-MT Cultures | |
|---|---|---|
| Day 3 | Day 7 | |
| 1 | Ni > Cu > Zn = Cd > Pb | Ni > Pb > Cu > Cd > Zn |
| 5 | Ni > Cu > Zn > Cd > Pb | Ni > Zn > Cu > Cd > Pb |
| 10 | Ni > Zn > Cu > Cd > Pb | Ni > Zn > Cu > Cd > Pb |
| Multi-MT Cultures | ||
| Day 3 | Day 7 | |
| 1 | Cu > Zn > Ni > Cd > Pb | Cu > Zn > Cd > Ni > Pb |
| 5 | Zn = Ni > Pb > Cu > Cd | Zn > Cu > Ni > Cd > Pb |
| 10 | Zn = Pb > Cu > Cd > Ni | Cd > Cu > Zn > Pb > Ni |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kyratzopoulou, E.; Kyzaki, N.; Malletzidou, L.; Nerantzis, E.; Kazakis, N.A. The Efficiency of Chlorella vulgaris in Heavy Metal Removal: A Comparative Study of Mono- and Multi-Component Metal Systems. Clean Technol. 2025, 7, 35. https://doi.org/10.3390/cleantechnol7020035
Kyratzopoulou E, Kyzaki N, Malletzidou L, Nerantzis E, Kazakis NA. The Efficiency of Chlorella vulgaris in Heavy Metal Removal: A Comparative Study of Mono- and Multi-Component Metal Systems. Clean Technologies. 2025; 7(2):35. https://doi.org/10.3390/cleantechnol7020035
Chicago/Turabian StyleKyratzopoulou, Eleni, Nikoletta Kyzaki, Lamprini Malletzidou, Evangelos Nerantzis, and Nikolaos A. Kazakis. 2025. "The Efficiency of Chlorella vulgaris in Heavy Metal Removal: A Comparative Study of Mono- and Multi-Component Metal Systems" Clean Technologies 7, no. 2: 35. https://doi.org/10.3390/cleantechnol7020035
APA StyleKyratzopoulou, E., Kyzaki, N., Malletzidou, L., Nerantzis, E., & Kazakis, N. A. (2025). The Efficiency of Chlorella vulgaris in Heavy Metal Removal: A Comparative Study of Mono- and Multi-Component Metal Systems. Clean Technologies, 7(2), 35. https://doi.org/10.3390/cleantechnol7020035







